The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry
Abstract
1. Introduction
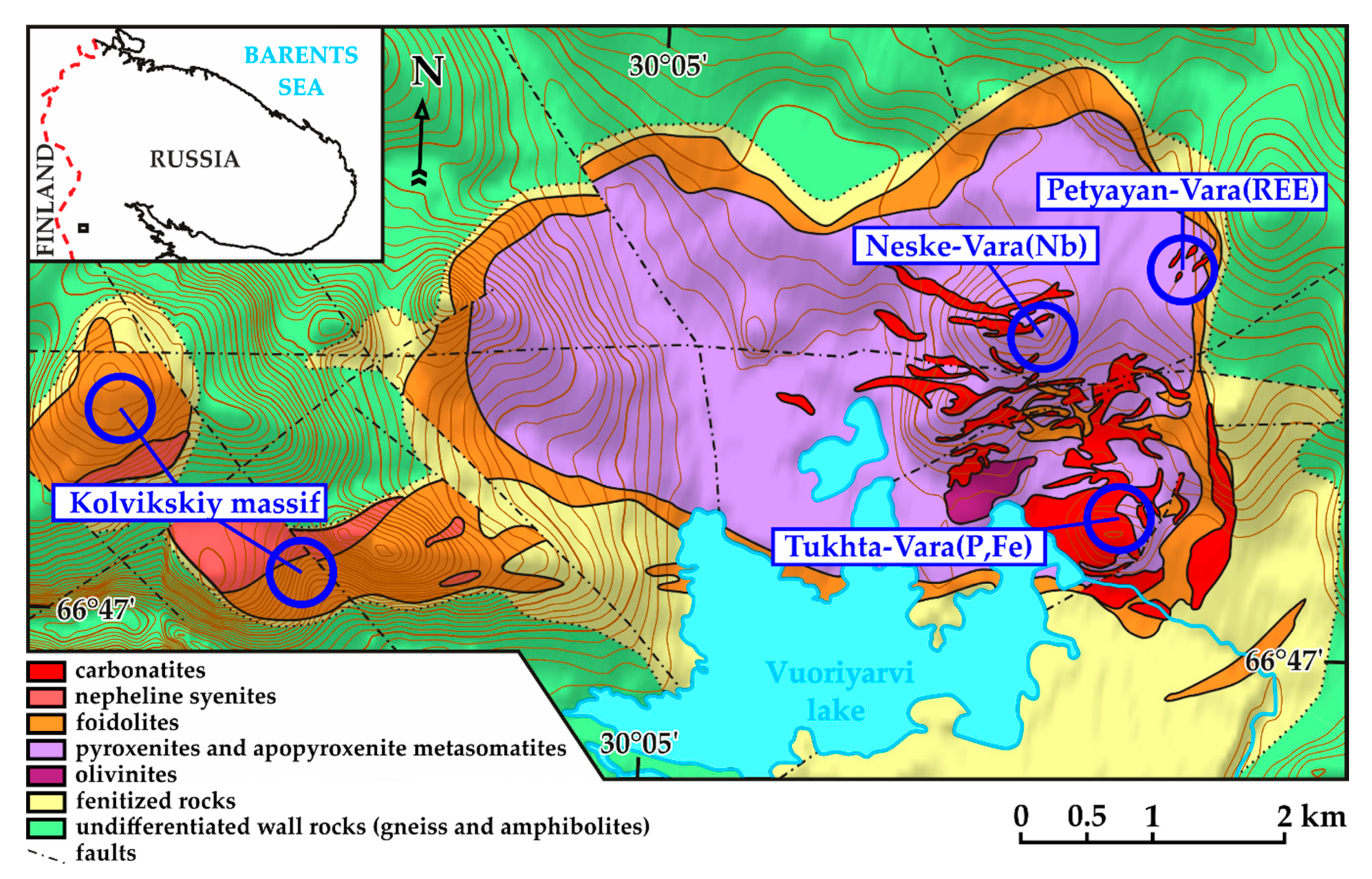
2. Geological Setting
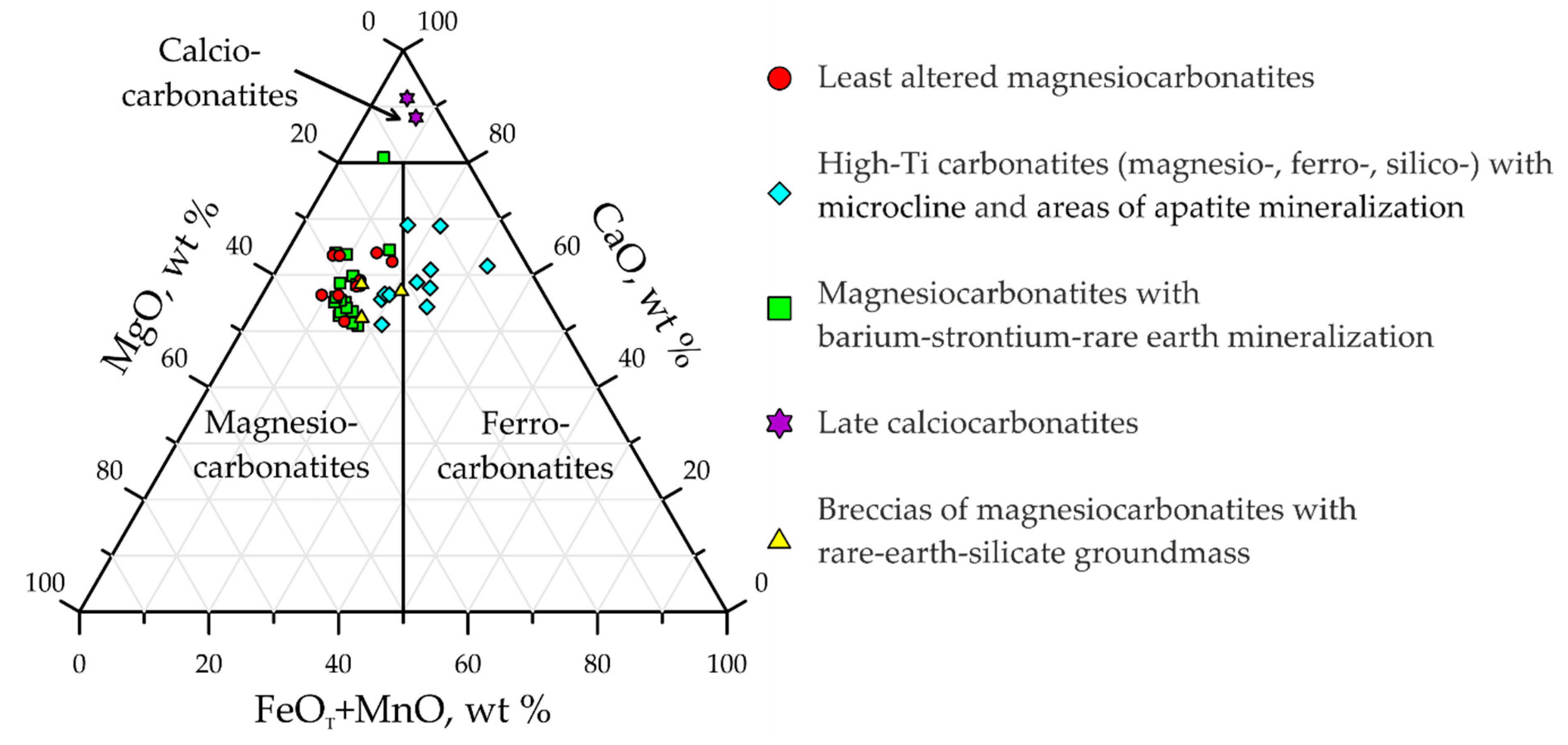
- Least altered magnesiocarbonatites;
- High-Ti magnesiocarbonatites and silicocarbonatites with microcline and areas of apatite mineralization;
- Magnesiocarbonatites with barium–strontium–rare earth mineralization;
- Late calciocarbonatites;
- Breccias of magnesiocarbonatites with rare-earth-silicate groundmass.
3. Sampling Procedure and Analytical Methods
3.1. Petrographic Studies
3.2. Scanning Electron Microscope (SEM)
3.3. Whole Rock Analysis
3.3.1. Major Elements
3.3.2. Trace Elements
4. Results
4.1. Carbonatite Mineralogy and Mineral Chemistry
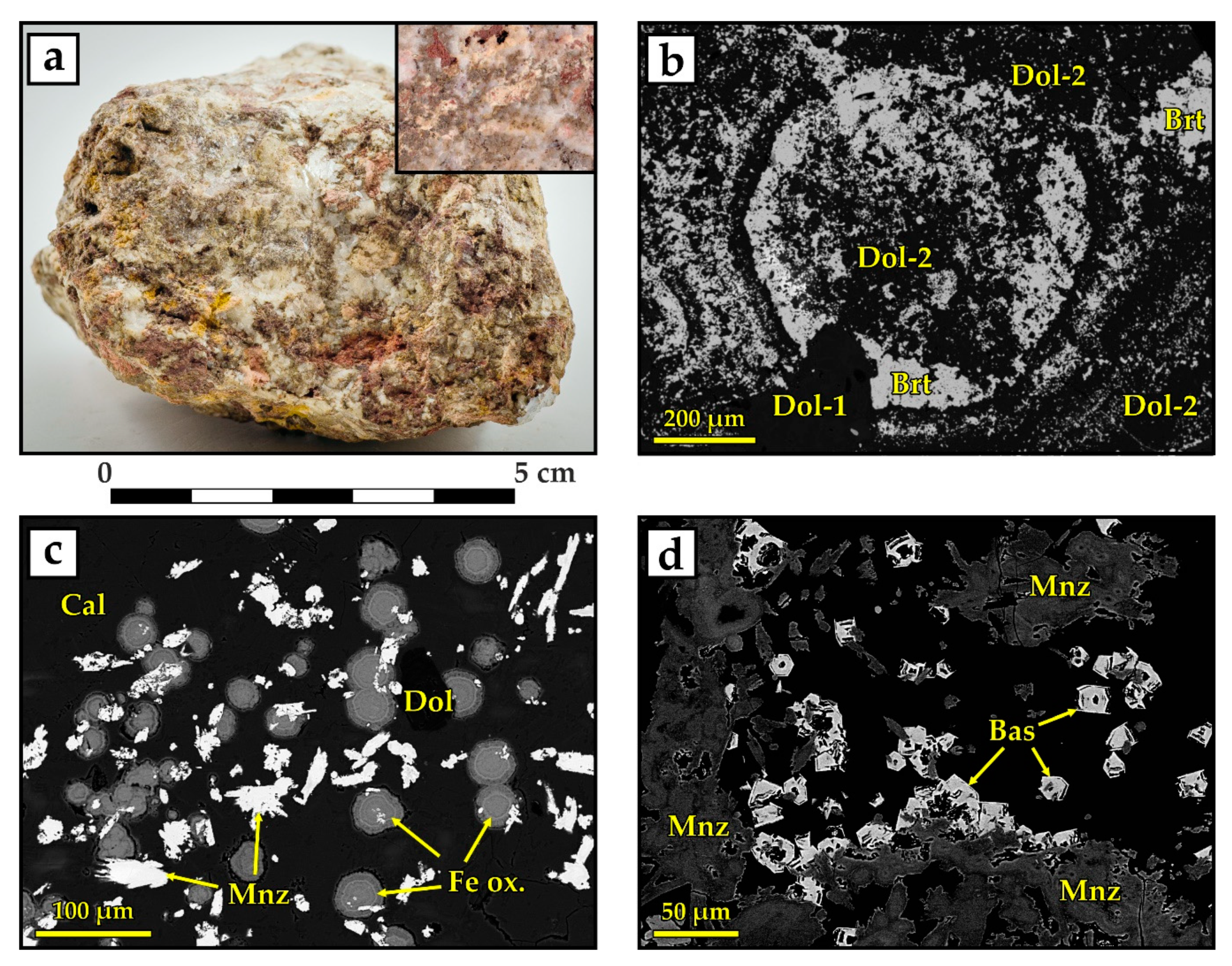
4.1.1. Least Altered Magnesiocarbonatites
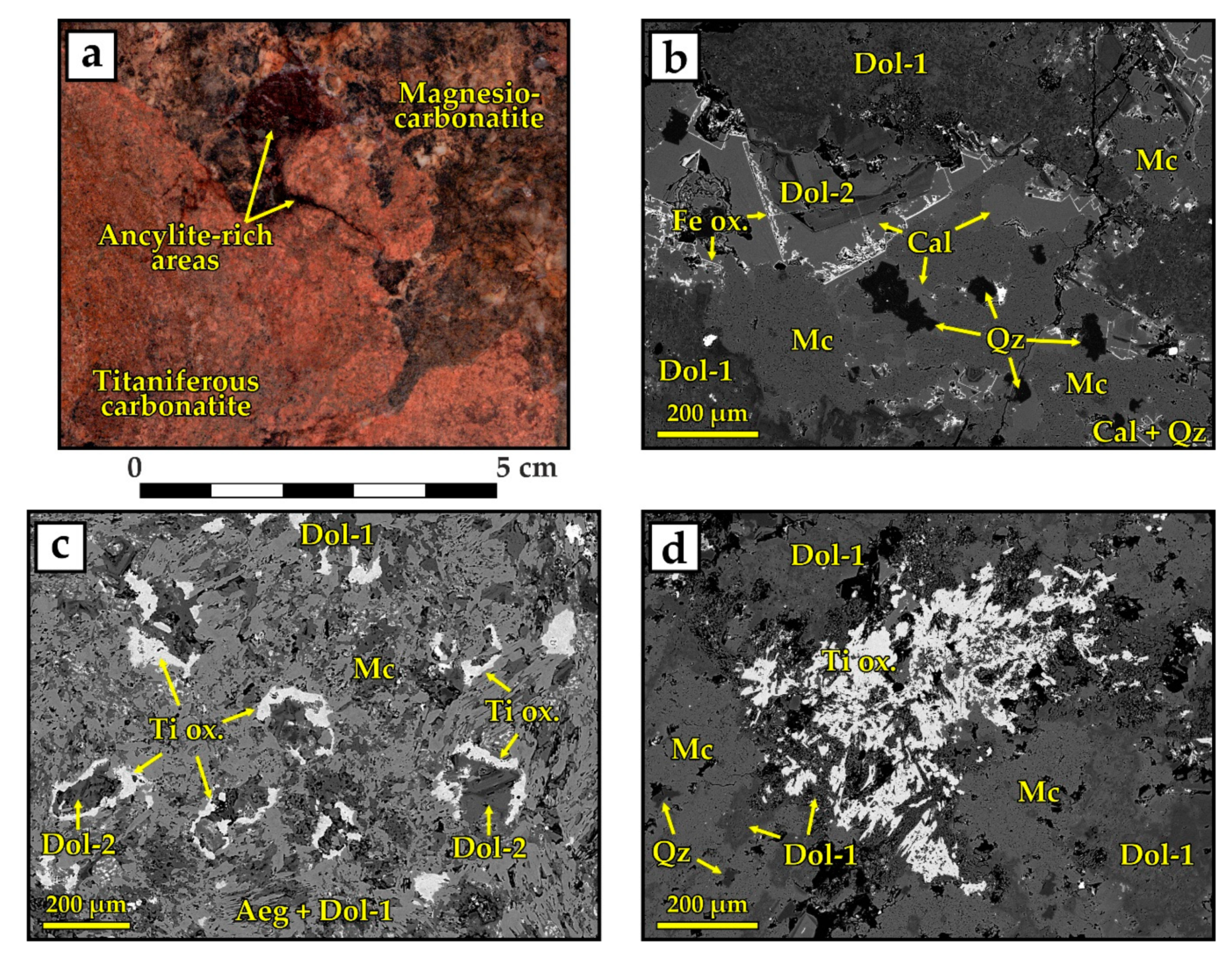
4.1.2. High-Ti Carbonatites with Areas of Apatite Mineralization
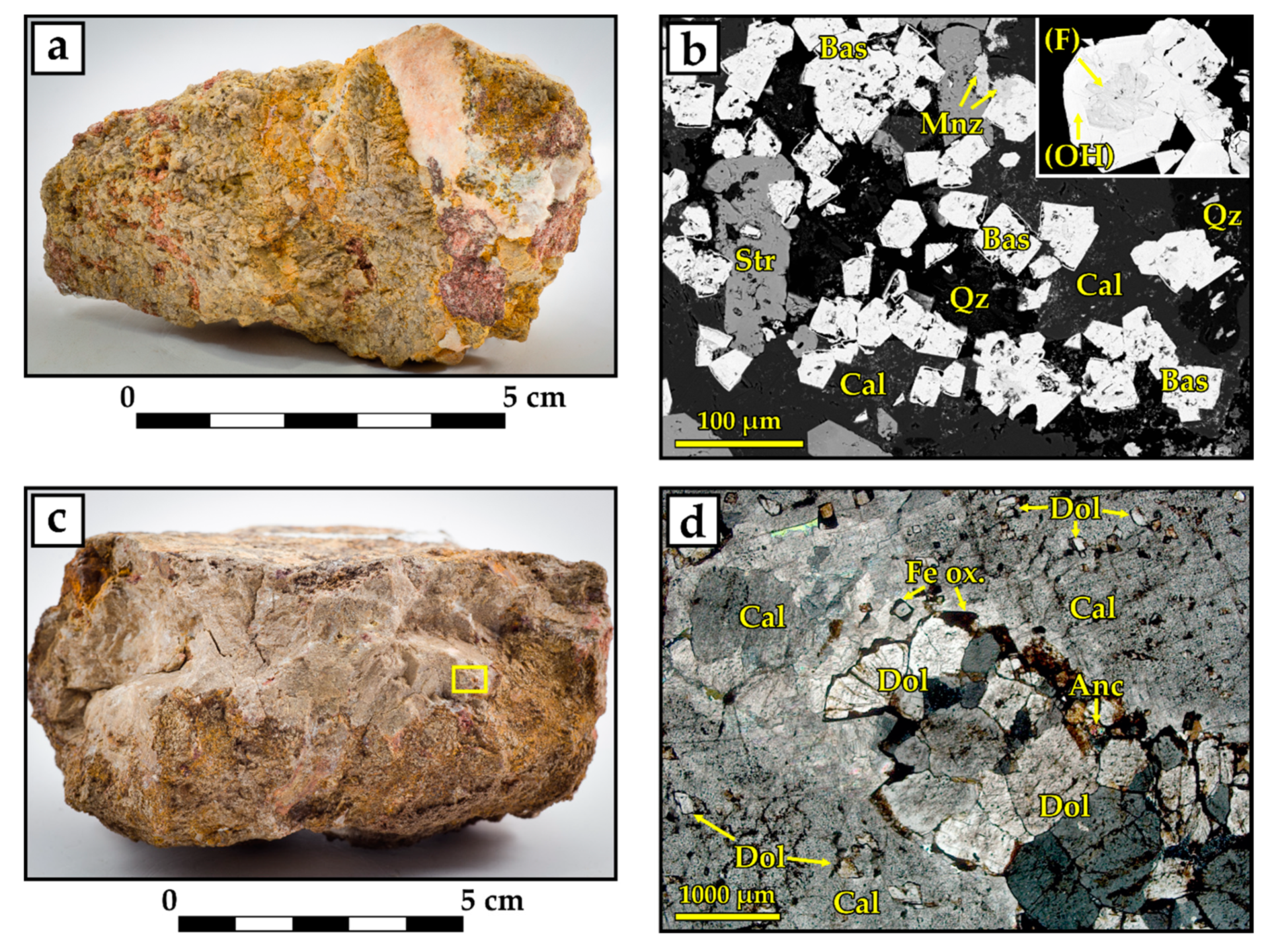
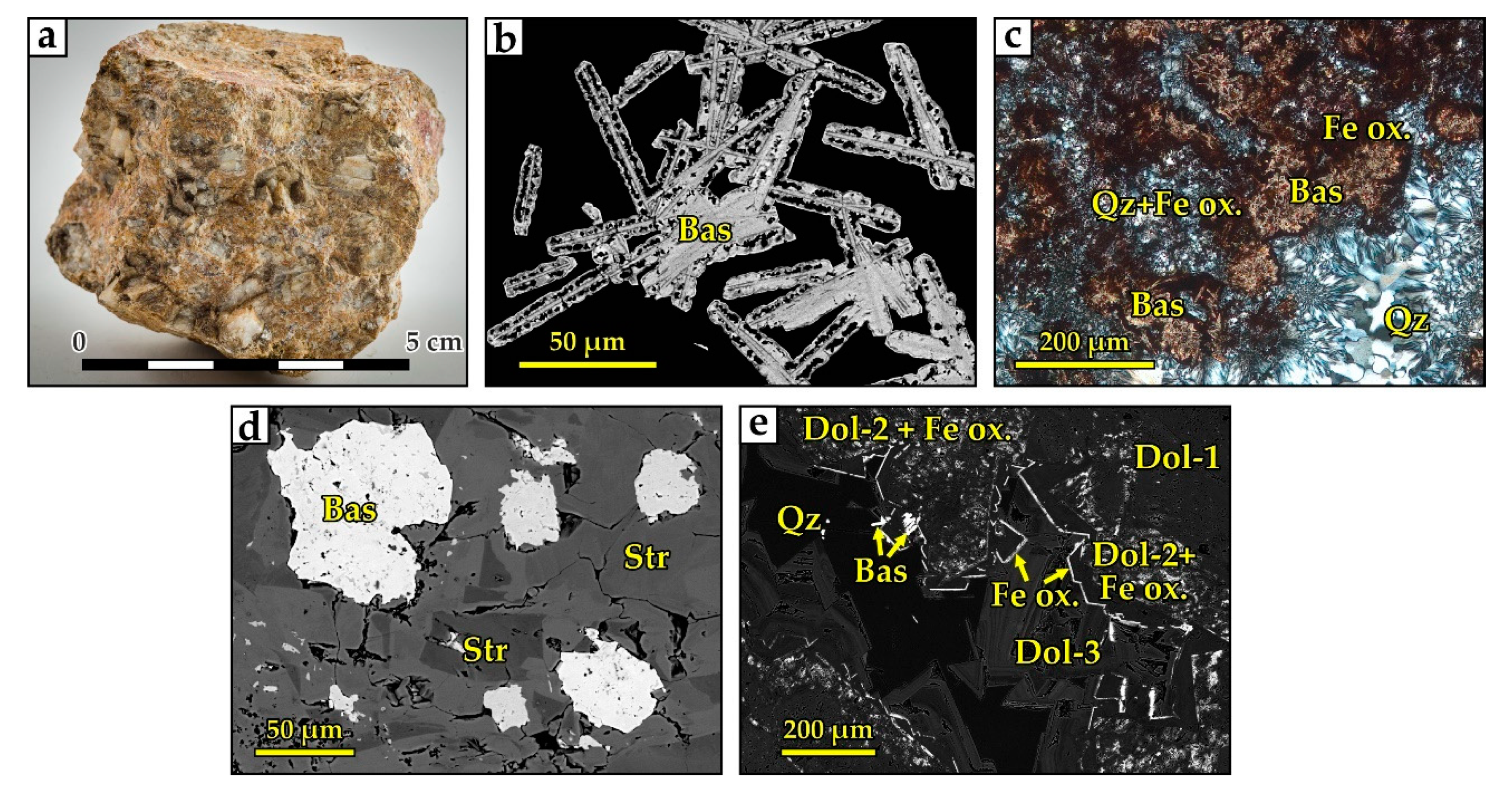
4.1.3. Barium–Strontium–Rare Earth Carbonatites
4.1.4. Late Calciocarbonatites
4.1.5. Magnesiocarbonatite Breccias with REE and Silicate Mineral Matrix
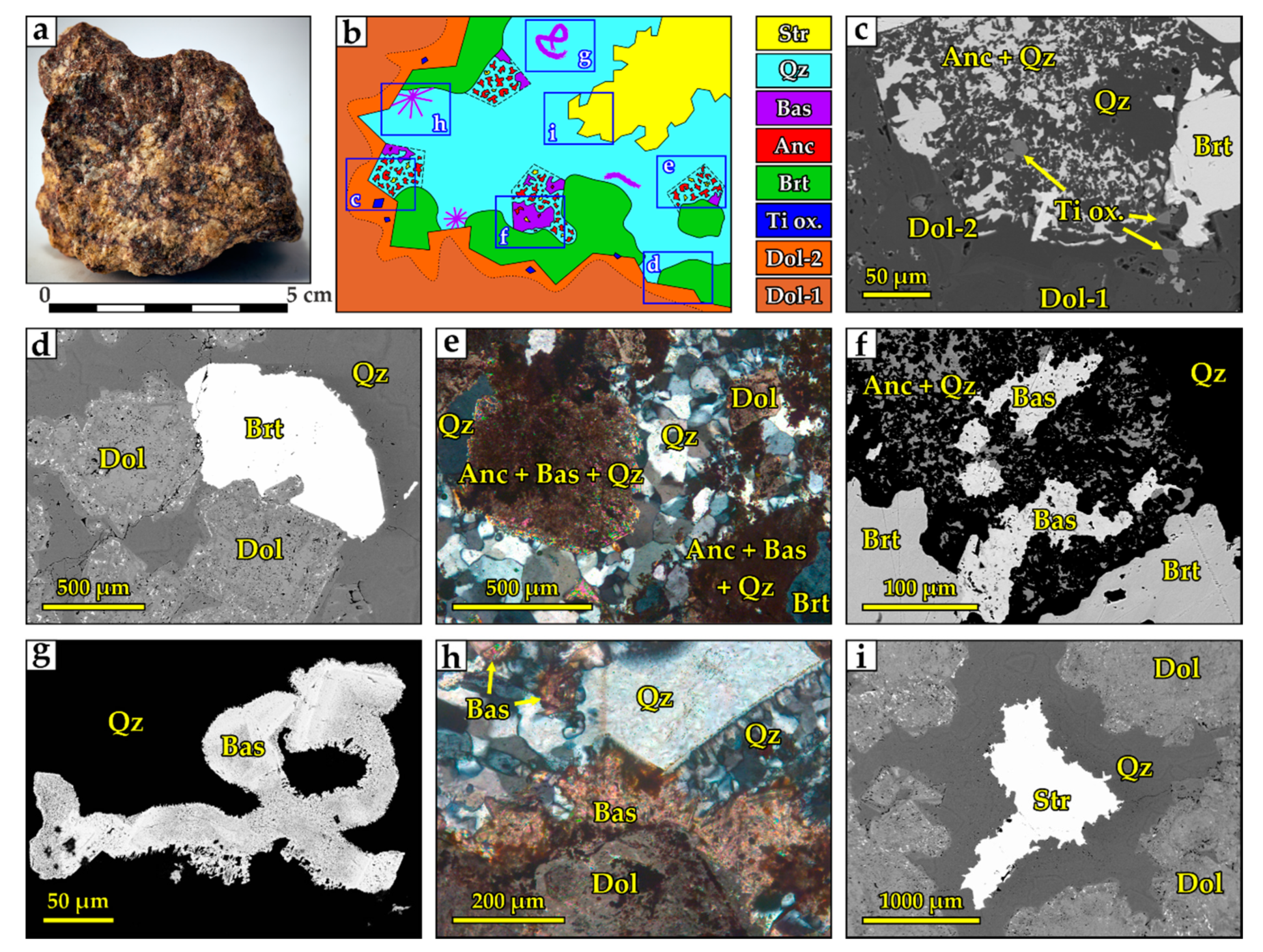
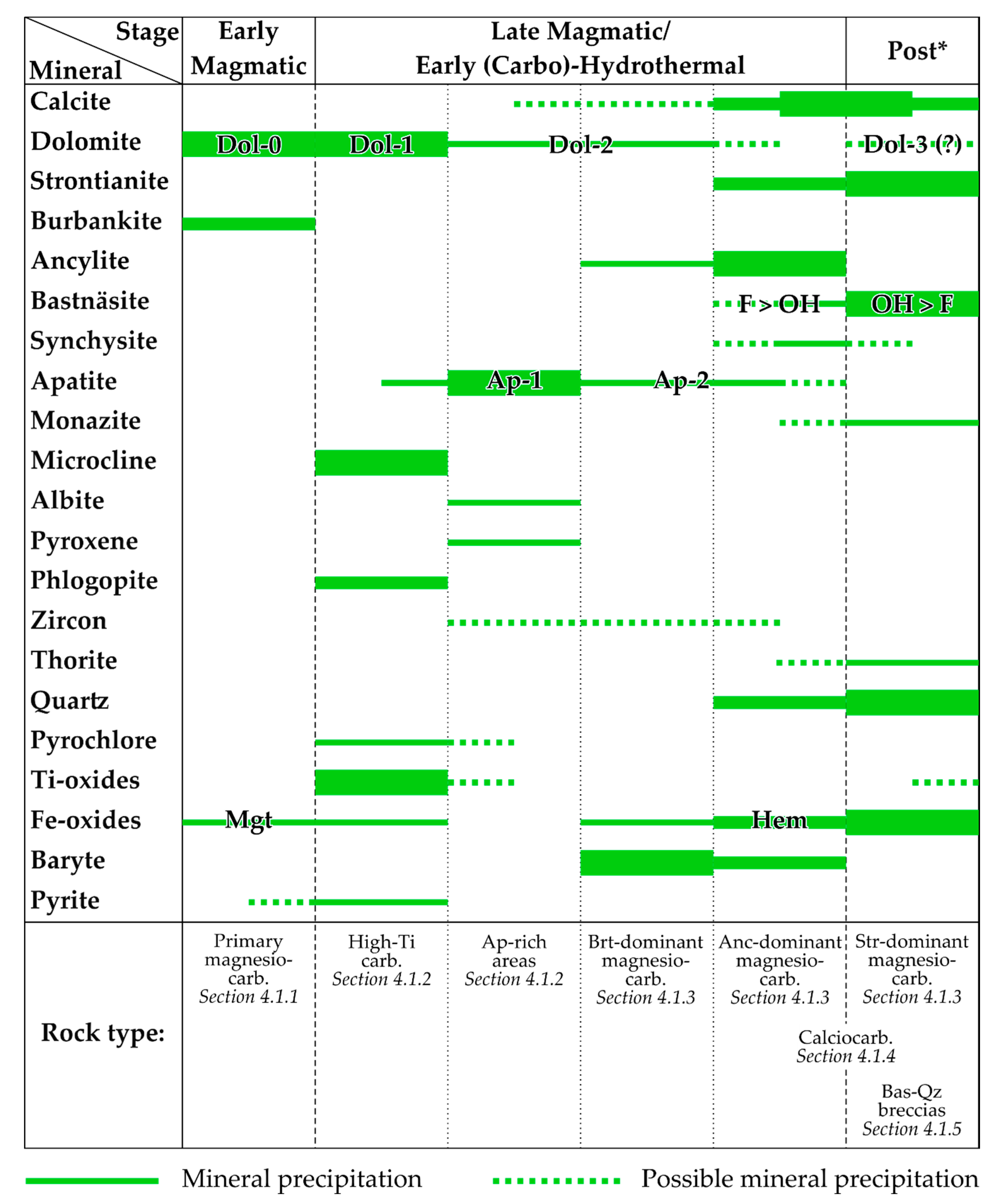
4.1.6. “Hybrid Rocks” and the Mineral Paragenesis
- Sharp-angled fragments (30–40% of the rock volume) composed of spongy dolomite Dol-0 and Dol-1 with zoned Dol-2 rims. Dol-2 includes idiomorphic crystals of brookite (Figure 15c). These are characteristic of high-Ti carbonatite;
- In the space between the dolomite fragments separated by brecciation, minerals were deposited in several stages. The earliest mineral is baryte, representing the assemblage of baryte-dominant carbonatites. Baryte is not as idiomorphic as dolomite (Figure 15c) but is more euhedral than all other minerals;
- After baryte and dolomite, euhedral crystals of irregular polygonal and rhombic shapes were crystallized (Figure 15e). This morphology is characteristic of ancylite from the studied carbonatites. Ancylite crystals correspond to the formation stages of ancylite-dominant carbonatites. A detailed study of these crystals showed that they represent pseudomorphs consisting of anhedral bastnäsite fragments inside a matrix of intergrown ancylite and quartz (Figure 15f). Inclusions of ancylite and strontianite also occur in bastnäsite;
- On the walls of the cavities that remained after the quartz deposition, quartz crystals with well-crystallized tips were formed (Figure 15i). In the final stage, all remaining empty space was filled with strontianite assemblage.
4.2. Geochemistry
5. Discussion
5.1. The Protolith of Carbonatites of the Petyayan-Vara Field
5.2. Formation of HFSE-Rich (High-Ti) Carbonatites
5.3. Connection between the Local HREE Enrichment and Apatitization
5.4. The Main Stage of REE Mineralization (the Formation of Ancylite Ores)
- Since the formation of ancylite is controlled by aREE3+, aCa2+, aSr2+, aBa2+, aNa+, aF− [65], aCO32− [68,142], and a(OH)− [78] (where “a” is activity), the most important factor for its deposition is fluid chemistry. Ancylite crystallization is possible at relatively low values of aREE3+/(aCa2+ + aSr2+ + aBa2+ + aNa+) (otherwise, carbocernaite is formed [65]), at low values of aF− (otherwise, cordylite is formed [65]), and at high values of aCO32− and a(OH)− (otherwise, synchysite and/or bastnäsite is formed [68,78,142]).
- The absence of supergene processes, which often destabilize ancylite. For example, in the Bear Lodge alkaline complex, a clear vertical zonation was discovered [68,71]. Ancylite-strontianite–baryte mineralization occurs in deeper parts of the section and is replaced by REE fluorocarbonates in the shallower oxide zone (100 m from the surface). Carbonatite samples of the Petyayan-Vara field were collected from the surface, where most of their mineral assemblages are similar to those found only in the deeper parts of the Bear Lodge alkaline complex. The weak influence of supergene alteration on the rocks of the Vuoriyarvi massif is explained by specific aspects of the recent geological history of the Kola region. The Svecofennian shield has undergone several periods of glaciation. Any weathering crusts that may have formed before the latest glaciations were eroded by glacial abrasion. Subsequently, the exposed rocks were protected from aggressive chemical weathering by the subarctic climate. This facilitated the preservation of ancylite ores in the near-surface parts of the Vuoriyarvi massif.
5.5. Late-Stage Processes
6. Conclusions and Further Perspectives
- The primary rocks of the Petyayan-Vara field were magmatic magnesiocarbonatites, consisting of dolomite with burbankite, minor magnetite, and possibly sulfides. These rocks were the protolith of all other carbonatites;
- Mineralogical and geochemical features, including high REE contents that are characteristic of the rocks of the Petyayan-Vara field, are the result of several metasomatic events;
- The introduction of a carbonatitic fluid (or fluid-saturated melt) rich in K, Al, Si, Fe, and Ti has resulted in the formation of high-Ti carbonatites containing Nb-rich Ti-oxides. Local apatitization is superimposed on high-Ti carbonatites and the surrounding rocks;
- The most pervasive alteration of magnesiocarbonatites was caused by a magmatic S (+Cl?)-rich fluid with high concentrations of Ba, Sr, and REE. At this stage, baryte-strontianite-ancylite ores with high REE contents (up to 11 wt. % total REE) were formed;
- Initially, Ba–Sr–REE–S fluid had a high capacity to transport REE, and, consequently, no REE minerals were deposited. Cooling caused the crystallization of a large amount of baryte, which captured the bulk of the S from the fluid. As a result, the capacity of the latter to transport REE decreased, triggering ancylite precipitation in the waning stage of (carbo)- hydrothermal activity. This mechanism explains the formation of the baryte mineral assemblage instead of the ancylite assemblage;
- Interaction with the Ba–Sr–REE–S fluid caused replacement of early-formed apatite with a secondary HREE-rich generation of apatite;
- Late-stage processes yielded breccias of magnesiocarbonatites with a quartz–bastnäsite matrix, minerals of the strontianite assemblage, monazite, Fe hydroxides, quartz, as well as late dolomite and calcite generations.
- The universality of the proposed HREE-enrichment mechanisms for late carbonatites;
- Ancylite stability field limits;
- The mechanism(s) of joint fluid migration of REE and Ba.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Wall, F. Rare earth elements. In Critical Metals Handbook; John Wiley & Sons: Oxford, UK, 2013; pp. 312–339. ISBN 9781118755341. [Google Scholar]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks: A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Le Maitre, R.W., Ed.; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521619486. [Google Scholar]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Amores-Casals, S.; Gonçalves, A.O.; Melgarejo, J.-C.; Martí Molist, J. Nb and REE Distribution in the Monte Verde Carbonatite–Alkaline–Agpaitic Complex (Angola). Minerals 2019, 10, 5. [Google Scholar] [CrossRef]
- Casals, A.-C.; Melgarejo, J.-C.; Bambi, A.; Gonçalves, A.O.; Morais, E.A.; Manuel, J.; Neto, A.B.; Costanzo, A.; Molist, J.M.; Amores-Casals, S.; et al. Lamprophyre-Carbonatite Magma Mingling and Subsolidus Processes as Key Controls on Critical Element Concentration in Carbonatites—The Bonga Complex (Angola). Minerals 2019, 9, 601. [Google Scholar] [CrossRef]
- Downes, P.J.; Demény, A.; Czuppon, G.; Jaques, A.L.; Verrall, M.; Sweetapple, M.; Adams, D.; McNaughton, N.J.; Gwalani, L.G.; Griffin, B.J. Stable H–C–O isotope and trace element geochemistry of the Cummins Range Carbonatite Complex, Kimberley region, Western Australia: Implications for hydrothermal REE mineralization, carbonatite evolution and mantle source regions. Min. Depos. 2014, 49, 905–932. [Google Scholar] [CrossRef]
- Anenburg, M.; Burnham, A.D.; Mavrogenes, J.A. REE Redistribution Textures in Altered Fluorapatite: Symplectites, Veins, and Phosphate-Silicate-Carbonate Assemblages from the Nolans Bore P-REE-Th Deposit, Northern Territory, Australia. Can. Miner. 2018, 56, 331–354. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A. Carbonatitic versus hydrothermal origin for fluorapatite REE-Th deposits: Experimental study of REE transport and crustal “antiskarn” metasomatism. Am. J. Sci. 2018, 318, 335–366. [Google Scholar] [CrossRef]
- Guarino, V.; Wu, F.-Y.; Melluso, L.; de Barros Gomes, C.; Tassinari, C.C.G.; Ruberti, E.; Brilli, M. U–Pb ages, geochemistry, C–O–Nd–Sr–Hf isotopes and petrogenesis of the Catalão II carbonatitic complex (Alto Paranaíba Igneous Province, Brazil): Implications for regional-scale heterogeneities in the Brazilian carbonatite associations. Int. J. Earth Sci. 2017, 106, 1963–1989. [Google Scholar] [CrossRef]
- Giovannini, A.L.; Bastos Neto, A.C.; Porto, C.G.; Pereira, V.P.; Takehara, L.; Barbanson, L.; Bastos, P.H.S. Mineralogy and geochemistry of laterites from the Morro dos Seis Lagos Nb (Ti, REE) deposit (Amazonas, Brazil). Ore Geol. Rev. 2017, 88, 461–480. [Google Scholar] [CrossRef]
- Dalsin, M.L.; Groat, L.A.; Creighton, S.; Evans, R.J. The mineralogy and geochemistry of the Wicheeda Carbonatite Complex, British Columbia, Canada. Ore Geol. Rev. 2015, 64, 523–542. [Google Scholar] [CrossRef]
- Nadeau, O.; Cayer, A.; Pelletier, M.; Stevenson, R.; Jébrak, M. The Paleoproterozoic Montviel carbonatite-hosted REE–Nb deposit, Abitibi, Canada: Geology, mineralogy, geochemistry and genesis. Ore Geol. Rev. 2015, 67, 314–335. [Google Scholar] [CrossRef]
- Trofanenko, J.; Williams-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The Nature and Origin of the REE Mineralization in the Wicheeda Carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Smith, D.L. Geology and mineralogy of the Ashram Zone carbonatite, Eldor Complex, Québec. Ore Geol. Rev. 2017, 86, 784–806. [Google Scholar] [CrossRef]
- Néron, A.; Bédard, L.; Gaboury, D. The Saint-Honoré Carbonatite REE Zone, Québec, Canada: Combined Magmatic and Hydrothermal Processes. Minerals 2018, 8, 397. [Google Scholar] [CrossRef]
- Pandur, K.; Ansdell, K.M.; Kontak, D.J. Graphic-textured inclusions in apatite: Evidence for pegmatitic growth in a REE-enriched carbonatitic system. Geology 2015, 43, 547–550. [Google Scholar] [CrossRef]
- Smith, M.P.; Campbell, L.S.; Kynicky, J. A review of the genesis of the world class Bayan Obo Fe–REE–Nb deposits, Inner Mongolia, China: Multistage processes and outstanding questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Hou, Z.; Cooke, D.R.; Danyushevsky, L.; Dominy, S.C.; Yin, S. A model for carbonatite hosted REE mineralisation—The Mianning-Dechang REE belt, Western Sichuan Province, China. Ore Geol. Rev. 2015, 70, 595–612. [Google Scholar] [CrossRef]
- Shu, X.; Liu, Y. Fluid inclusion constraints on the hydrothermal evolution of the Dalucao Carbonatite-related REE deposit, Sichuan Province, China. Ore Geol. Rev. 2019, 107, 41–57. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y. Mechanisms of element precipitation in carbonatite-related rare-earth element deposits: Evidence from fluid inclusions in the Maoniuping deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2019, 107, 218–238. [Google Scholar] [CrossRef]
- Bai, T.; Chen, W.; Jiang, S.-Y. Evolution of the carbonatite Mo-HREE deposits in the Lesser Qinling Orogen: Insights from in situ geochemical investigation of calcite and sulfate. Ore Geol. Rev. 2019, 113, 103069. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Pan, J.; Dai, T.; Bayless, R.C. Mineralogical and geochemical characteristics of the Miaoya REE prospect, Qinling orogenic Belt, China: Insights from Sr-Nd-C-O isotopes and LA-ICP-MS mineral chemistry. Ore Geol. Rev. 2019, 110, 102932. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Y. REE Enrichment during Magmatic–Hydrothermal Processes in Carbonatite-Related REE Deposits: A Case Study of the Weishan REE Deposit, China. Minerals 2019, 10, 25. [Google Scholar] [CrossRef]
- Su, J.-H.; Zhao, X.-F.; Li, X.-C.; Hu, W.; Chen, M.; Xiong, Y.-L. Geological and geochemical characteristics of the Miaoya syenite-carbonatite complex, Central China: Implications for the origin of REE-Nb-enriched carbonatite. Ore Geol. Rev. 2019, 113, 103101. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Zhang, H.; Zhang, X.; Zhang, D.; Xi, Z.; Wang, Z. Geochronology and mineralogy of the Weishan carbonatite in Shandong province, eastern China. Geosci. Front. 2019, 10, 769–785. [Google Scholar] [CrossRef]
- Song, W.; Xu, C.; Smith, M.P.; Chakhmouradian, A.R.; Brenna, M.; Kynický, J.; Chen, W.; Yang, Y.; Deng, M.; Tang, H. Genesis of the world’s largest rare earth element deposit, Bayan Obo, China: Protracted mineralization evolution over ~1 b.y. Geology 2018, 46. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Jiang, S.-Y.; Ying, Y.-C.; Liu, Y.-S. Radiogenic Pb reservoir contributes to the rare earth element (REE) enrichment in South Qinling carbonatites. Chem. Geol. 2018, 494, 80–95. [Google Scholar] [CrossRef]
- Song, W.; Xu, C.; Smith, M.P.; Kynicky, J.; Huang, K.; Wei, C.; Zhou, L.; Shu, Q. Origin of unusual HREE-Mo-rich carbonatites in the Qinling orogen, China. Sci. Rep. 2016, 6, 37377. [Google Scholar] [CrossRef]
- Fan, H.-R.; Yang, K.-F.; Hu, F.-F.; Liu, S.; Wang, K.-Y. The giant Bayan Obo REE–Nb–Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Feng, M.; Xu, C.; Kynicky, J.; Zeng, L.; Song, W. Rare earth element enrichment in Palaeoproterozoic Fengzhen carbonatite from the North China block. Int. Geol. Rev. 2016, 58, 1940–1950. [Google Scholar] [CrossRef]
- Deng, M.; Xu, C.; Song, W.; Tang, H.; Liu, Y.; Zhang, Q.; Zhou, Y.; Feng, M.; Wei, C. REE mineralization in the Bayan Obo deposit, China: Evidence from mineral paragenesis. Ore Geol. Rev. 2017, 91, 100–109. [Google Scholar] [CrossRef]
- Yang, X.; Lai, X.; Pirajno, F.; Liu, Y.; Mingxing, L.; Sun, W. Genesis of the Bayan Obo Fe-REE-Nb formation in Inner Mongolia, North China Craton: A perspective review. Precambrian Res. 2017, 288, 39–71. [Google Scholar] [CrossRef]
- Smith, M.; Kynicky, J.; Xu, C.; Song, W.; Spratt, J.; Jeffries, T.; Brtnicky, M.; Kopriva, A.; Cangelosi, D. The origin of secondary heavy rare earth element enrichment in carbonatites: Constraints from the evolution of the Huanglongpu district, China. Lithos 2018, 308–309, 65–82. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Aibai, A.; Kong, W.; Holtz, F. The role of magmatic and post-magmatic hydrothermal processes on rare-earth element mineralization: A study of the Bachu carbonatites from the Tarim Large Igneous Province, NW China. Lithos 2018, 314–315, 71–87. [Google Scholar] [CrossRef]
- Liu, Y.; Chakhmouradian, A.R.; Hou, Z.; Song, W.; Kynický, J. Development of REE mineralization in the giant Maoniuping deposit (Sichuan, China): Insights from mineralogy, fluid inclusions, and trace-element geochemistry. Min. Depos. 2018. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y. Occurrence and geochemistry of bastnäsite in carbonatite-related REE deposits, Mianning–Dechang REE belt, Sichuan Province, SW China. Ore Geol. Rev. 2019, 107, 266–282. [Google Scholar] [CrossRef]
- Al-Ani, T.; Molnár, F.; Lintinen, P.; Leinonen, S.; Al-Ani, T.; Molnár, F.; Lintinen, P.; Leinonen, S. Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland. Minerals 2018, 8, 356. [Google Scholar] [CrossRef]
- Bhushan, S.K. Geology of the Kamthai Rare Earth Deposit. J. Geol. Soc. India 2015, 85, 537–546. [Google Scholar] [CrossRef]
- Krishnamurthy, P. Carbonatites of India. J. Geol. Soc. India 2019, 94, 117–138. [Google Scholar] [CrossRef]
- Nagabhushanam, B.; Durai Raju, S.; Mundra, K.L.; Rai, S.D.; Purohit, R.K.; Verma, M.B.; Nanda, L.K. LREE–Nb Mineralization in the South Western Part of Ambadongar Carbonatite Complex, Chhota Udepur District, Gujarat, India. Curr. Sci. 2018, 114, 1608. [Google Scholar] [CrossRef]
- Viladkar, S.G. Ferrocarbonatites in the Amba Dongar Diatreme, Gujarat, India. J. Geol. Soc. India 2018, 92, 141–144. [Google Scholar] [CrossRef]
- Stoppa, F.; Pirajno, F.; Schiazza, M.; Vladykin, N.V. State of the art: Italian carbonatites and their potential for critical-metal deposits. Gondwana Res. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Stoppa, F.; Schiazza, M.; Rosatelli, G.; Castorina, F.; Sharygin, V.V.; Ambrosio, F.A.; Vicentini, N. Italian carbonatite system: From mantle to ore-deposit. Ore Geol. Rev. 2019, 114. [Google Scholar] [CrossRef]
- Duraiswami, R.; Shaikh, T. Fluid-rock interaction in the Kangankunde Carbonatite Complex, Malawi: SEM based evidence for late stage pervasive hydrothermal mineralisation. Open Geosci. 2014, 6. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Heaton, T.; Wall, F.; Gunn, G. Tracing the fluid source of heavy REE mineralisation in carbonatites using a novel method of oxygen-isotope analysis in apatite: The example of Songwe Hill, Malawi. Chem. Geol. 2016, 440, 275–287. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Brady, A.E.; Horstwood, M.S.A.; Woolley, A.R.; Mtegha, J.; Wall, F.; Dawes, W.; Gunn, G. Geology, geochemistry and geochronology of the Songwe Hill carbonatite, Malawi. J. Afr. Earth Sci. 2017, 134, 10–23. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Brady, A.E.; Wall, F.; Gunn, G.; Dawes, W. REE minerals at the Songwe Hill carbonatite, Malawi: HREE-enrichment in late-stage apatite. Ore Geol. Rev. 2017, 81, 23–41. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Wall, F.; Spiro, B.; Ullmann, C.V. Deducing the source and composition of rare earth mineralising fluids in carbonatites: Insights from isotopic (C, O, 87Sr/86Sr) data from Kangankunde, Malawi. Contrib. Mineral. Petrol. 2017, 172, 96. [Google Scholar] [CrossRef]
- Dowman, E.; Wall, F.; Treloar, P.J.J.; Rankin, A.H.H. Rare-earth mobility as a result of multiple phases of fluid activity in fenite around the Chilwa Island Carbonatite, Malawi. Mineral. Mag. 2017, 81, 1367–1395. [Google Scholar] [CrossRef]
- Chikanda, F.; Otake, T.; Ohtomo, Y.; Ito, A.; Yokoyama, T.D.; Sato, T. Magmatic-Hydrothermal Processes Associated with Rare Earth Element Enrichment in the Kangankunde Carbonatite Complex, Malawi. Minerals 2019, 9, 442. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Song, W.; Chakhmouradian, A.R.; Xu, C.; Kopriva, A.; Galiova, M.V.; Brtnicky, M. The role of carbonate-fluoride melt immiscibility in shallow REE deposit evolution. Geosci. Front. 2018. [Google Scholar] [CrossRef]
- Feng, M.; Song, W.; Kynicky, J.; Smith, M.; Cox, C.; Kotlanova, M.; Brtnicky, M.; Fu, W.; Wei, C. Primary rare earth element enrichment in carbonatites: Evidence from melt inclusions in Ulgii Khiid carbonatite, Mongolia. Ore Geol. Rev. 2019, 1675, 103294. [Google Scholar] [CrossRef]
- Bodeving, S.; Williams-Jones, A.E.; Swinden, S. Carbonate–silicate melt immiscibility, REE mineralising fluids, and the evolution of the Lofdal Intrusive Suite, Namibia. Lithos 2017, 268–271, 383–398. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Smith, M.; Andrade, M.B.; Ray, S.; Banks, D.A.; Loye, E.; Atencio, D.; Pickles, J.R.; Wall, F. Sulfur-bearing monazite-(Ce) from the Eureka carbonatite, Namibia: Oxidation state, substitution mechanism, and formation conditions. Mineral. Mag. 2019, 1–44. [Google Scholar] [CrossRef]
- Cangelosi, D.; Broom-Fendley, S.; Banks, D.; Morgan, D.; Yardley, B. Light rare earth element redistribution during hydrothermal alteration at the Okorusu carbonatite complex, Namibia. Mineral. Mag. 2019, 1–16. [Google Scholar] [CrossRef]
- Cooper, A.F.; Collins, A.K.; Palin, J.M.; Spratt, J. Mineralogical evolution and REE mobility during crystallisation of ancylite-bearing ferrocarbonatite, Haast River, New Zealand. Lithos 2015, 216–217, 324–337. [Google Scholar] [CrossRef]
- Marien, C.; Dijkstra, A.H.; Wilkins, C. The hydrothermal alteration of carbonatite in the Fen Complex, Norway: Mineralogy, geochemistry, and implications for rare-earth element resource formation. Mineral. Mag. 2018, 82, S115–S131. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Terry Williams, C.; Jeffries, T.E.; Strekopytov, S.; Moutte, J.; Ivashchenkova, O.V.; Spratt, J.; Petrov, S.V.; Wall, F.; Seltmann, R.; et al. ‘Rare earth elements in phoscorites and carbonatites of the Devonian Kola Alkaline Province, Russia: Examples from Kovdor, Khibina, Vuoriyarvi and Turiy Mys complexes’. Ore Geol. Rev. 2015, 64, 477–498. [Google Scholar] [CrossRef]
- Lazareva, E.V.; Zhmodik, S.M.; Dobretsov, N.L.; Tolstov, A.V.; Shcherbov, B.L.; Karmanov, N.S.; Gerasimov, E.Y.; Bryanskaya, A.V. Main minerals of abnormally high-grade ores of the Tomtor deposit (Arctic Siberia). Russ. Geol. Geophys. 2015, 56, 844–873. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Veksler, I.V.; Izbrodin, I.A.; Ripp, G.S.; Khromova, E.A.; Posokhov, V.F.; Travin, A.V.; Vladykin, N.V. Stable isotope composition of minerals in the Belaya Zima plutonic complex, Russia: Implications for the sources of the parental magma and metasomatizing fluids. J. Asian Earth Sci. 2016, 116, 81–96. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Borisenko, A.S.; Borovikov, A.A.; Pavlova, G.G. Origin of REE-rich ferrocarbonatites in southern Siberia (Russia): Implications based on melt and fluid inclusions. Mineral. Petrol. 2016, 110, 845–859. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Chebotarev, D.A.; Sharygin, V.V.; Prokopyev, I.R.; Nikolenko, A.M. Petrology of alkaline silicate rocks and carbonatites of the Chuktukon massif, Chadobets upland, Russia: Sources, evolution and relation to the Triassic Siberian LIP. Lithos 2019, 332–333, 245–260. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Redina, A.A.; Obukhov, A.V. Magnetite-apatite-dolomitic rocks of Ust-Chulman (Aldan shield, Russia): Seligdar-type carbonatites? Mineral. Petrol. 2018, 112, 257–266. [Google Scholar] [CrossRef]
- Giebel, R.J.; Gauert, C.D.K.; Marks, M.A.W.; Costin, G.; Markl, G. Multi-stage formation of REE minerals in the Palabora Carbonatite Complex, South Africa. Am. Mineral. 2017, 102, 1218–1233. [Google Scholar] [CrossRef]
- Milani, L.; Bolhar, R.; Frei, D.; Harlov, D.E.; Samuel, V.O. Light rare earth element systematics as a tool for investigating the petrogenesis of phoscorite-carbonatite associations, as exemplified by the Phalaborwa Complex, South Africa. Miner. Depos. 2017, 52, 1105–1125. [Google Scholar] [CrossRef]
- Witt, W.K.; Hammond, D.P.; Hughes, M. Geology of the Ngualla carbonatite complex, Tanzania, and origin of the Weathered Bastnaesite Zone REE ore. Ore Geol. Rev. 2019, 105, 28–54. [Google Scholar] [CrossRef]
- Moore, M.; Chakhmouradian, A.R.; Mariano, A.N.; Sidhu, R. Evolution of rare-earth mineralization in the Bear Lodge carbonatite, Wyoming: Mineralogical and isotopic evidence. Ore Geol. Rev. 2015, 64, 499–521. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Neill, O.K. Mineral chemistry and petrogenesis of a HFSE(+HREE) occurrence, peripheral to carbonatites of the Bear Lodge alkaline complex, Wyoming. Am. Mineral. 2016, 101, 1604–1623. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Cooper, M.A.; Reguir, E.P.; Moore, M.A. Carbocernaite from Bear Lodge, Wyoming: Crystal chemistry, paragenesis, and rare-earth fractionation on a microscale. Am. Mineral. 2017, 102, 1340–1352. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Donovan, J.J. REE fractionation, mineral speciation, and supergene enrichment of the Bear Lodge carbonatites, Wyoming, USA. Ore Geol. Rev. 2017, 89, 780–807. [Google Scholar] [CrossRef]
- Andersen, A.K.; Larson, P.B.; Cosca, M.A. C–O stable isotope geochemistry and 40Ar/39Ar geochronology of the Bear Lodge carbonatite stockwork, Wyoming, USA. Lithos 2019, 324–325, 640–660. [Google Scholar] [CrossRef]
- Nguyen Thi, T.; Wada, H.; Ishikawa, T.; Shimano, T. Geochemistry and petrogenesis of carbonatites from South Nam Xe, Lai Chau area, northwest Vietnam. Mineral. Petrol. 2014, 108, 371–390. [Google Scholar] [CrossRef]
- Harmer, R.E.; Nex, P.A.M. Rare Earth Deposits of Africa. Episodes 2016, 39, 381. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S. Carbonatites: Related ore deposits, resources, footprint, and exploration methods. Appl. Earth Sci. Trans. Inst. Min. Metall. 2018, 127, 123–152. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Wall, F.; Bas, M.J. Le REE–Sr–Ba minerals from the Khibina carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Ripp, G.S.; Moore, K.R. Genesis of the Khaluta alkaline-basic Ba-Sr carbonatite complex (West Transbaikala, Russia). Mineral. Petrol. 2010, 98, 245–268. [Google Scholar] [CrossRef]
- Ngwenya, B.T. Hydrothermal rare earth mineralisation in carbonatites of the Tundulu complex, Malawi: Processes at the fluid/rock interface. Geochim. Cosmochim. Acta 1994, 58, 2061–2072. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Mineral. 2016, 101, 596–611. [Google Scholar] [CrossRef]
- Nadeau, O.; Stevenson, R.; Jébrak, M. Evolution of Montviel alkaline-carbonatite complex by coupled fractional crystallization, fluid mixing and metasomatism-part I: Petrography and geochemistry of metasomatic aegirine-augite and biotite: Implications for REE-Nb mineralization. Ore Geol. Rev. 2016, 72, 1143–1162. [Google Scholar] [CrossRef]
- Afanasyev, B.V. Mineral Resources of the Alkaline–Ultramafic Massifs of the Kola Peninsula; Roza Vetrov: St. Petersburg, Russia, 2011. (In Russian) [Google Scholar]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kressall, R.D.; Crozier, J.; Pisiak, L.K.; Sidhu, R.; Yang, P. Carbonatite-hosted niobium deposit at Aley, northern British Columbia (Canada): Mineralogy, geochemistry and petrogenesis. Ore Geol. Rev. 2015, 64, 642–666. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average chemical compositions, and element distribution. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Kravchenko-Berezhnoy, R.A.; Medvedeva, E.M.; Pakhomovsky, Y.A.; Polezhaeva, L.I.; Rezhenova, S.A. Combined usage of microprobe MS-46 and computer ‘Nairi-2’. In Instrumental Methods of Mineral Studies and Usage of Electronic Computing Devices; Kravchenko-Berezhnoy, R.A., Ed.; Kola Branch USSR Academy of Sciences: Apatity, Russia, 1976; pp. 46–69. (In Russian) [Google Scholar]
- Andersen, T. Secondary processes in carbonatites: Petrology of “rødberg” (hematite-calcite-dolomite carbonatite) in the Fen central complex, Telemark (South Norway). Lithos 1984, 17, 227–245. [Google Scholar] [CrossRef]
- Atencio, D.; Gieré, R.; Andrade, M.B.; Christy, A.G.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–678. [Google Scholar] [CrossRef]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V. Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia). Geosciences 2018, 8, 281. [Google Scholar] [CrossRef]
- Flohr, M.J.K. Titanium, vanadium, and niobium mineralization and alkali metasomatism from the Magnet Cove Complex, Arkansas. Econ. Geol. 1994, 89, 105–130. [Google Scholar] [CrossRef]
- Werner, M.; Cook, N.J. Nb-rich brookite from Gross Brukkaros, Namibia: Substitution mechanisms and Fe2+/Fe3+ ratios. Mineral. Mag. 2001, 65, 437–440. [Google Scholar] [CrossRef]
- Verwoerd, W.J.; Viljoen, E.A.; Chevallier, L. Rare metal mineralization at the Salpeterkop carbonatite complex, Western Cape Province, South Africa. J. Afr. Earth Sci. 1995, 21, 171–186. [Google Scholar] [CrossRef]
- Bulakh, A.G.; Nesterov, A.R.; Zaitsev, A.N.; Pilipiuk, A.N.; Wall, F.; Kirillov, A.S. Sulfur-containing monazite-(Ce) from late-stage mineral assemblages at the Kandaguba and Vuoriyarvi carbonatite complexes, Kola peninsula, Russia. Neues Jahrb. Fur Mineral. Mon. 2000, 5, 217–233. [Google Scholar]
- Torró, L.; Villanova, C.; Castillo, M.; Campeny, M.; Gonçalves, A.O.; Melgarejo, J.C. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Mineral. Mag. 2012, 76, 393–409. [Google Scholar] [CrossRef]
- Savelyeva, V.B.; Bazarova, E.P.; Sharygin, V.V.; Karmanov, N.S.; Kanakin, S.V. Altered rocks of the Onguren carbonatite complex in the Western Tansbaikal Region: Geochemistry and composition of accessory minerals. Geol. Ore Depos. 2017, 59, 315–340. [Google Scholar] [CrossRef]
- Wall, F.; Zaitsev, A.; Jones, A.P.; Mariano, A.N. Rare-earth rich carbonatites: A review and latest results. J. Czech Geol. Soc. 1997, 42, 49. [Google Scholar]
- Silva, E.N.; Ayala, A.P.; Guedes, I.; Paschoal, C.W.A.; Moreira, R.L.; Loong, C.-K.; Boatner, L.A. Vibrational spectra of monazite-type rare-earth orthophosphates. Opt. Mater. 2006, 29, 224–230. [Google Scholar] [CrossRef]
- Clavier, N.; Mesbah, A.; Szenknect, S.; Dacheux, N. Monazite, rhabdophane, xenotime & churchite: Vibrational spectroscopy of gadolinium phosphate polymorphs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 85–94. [Google Scholar] [CrossRef]
- Heuser, J.; Bukaemskiy, A.A.; Neumeier, S.; Neumann, A.; Bosbach, D. Raman and infrared spectroscopy of monazite-type ceramics used for nuclear waste conditioning. Prog. Nucl. Energy 2014, 72, 149–155. [Google Scholar] [CrossRef]
- Assaaoudi, H.; Ennaciri, A.; Rulmont, A. Vibrational spectra of hydrated rare earth orthophosphates. Vib. Spectrosc. 2001, 25, 81–90. [Google Scholar] [CrossRef]
- Valenzano, L.; Noël, Y.; Orlando, R.; Zicovich-Wilson, C.M.; Ferrero, M.; Dovesi, R. Ab initio vibrational spectra and dielectric properties of carbonates: Magnesite, calcite and dolomite. Theor. Chem. Acc. 2007, 117, 991–1000. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Yang, H.; Dembowski, R.F.; Conrad, P.G.; Downs, R.T. Crystal structure and Raman spectrum of hydroxyl-bastnasite-(Ce), CeCO3(OH). Am. Mineral. 2008, 93, 698–701. [Google Scholar] [CrossRef]
- Frost, R.L.; Dickfos, M.J. Raman spectroscopy of halogen-containing carbonates. J. Raman Spectrosc. 2007, 38, 1516–1522. [Google Scholar] [CrossRef]
- Kirillov, A.S. Hydroxyl bastnäsite, a new variety of basnäsite. Dokl. Akad. Nauk SssrEarth Sci. Sect. 1964, 159, 93–95. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; DE GRUYTER: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Ruberti, E.; Enrich, G.E.R.; Gomes, C.B.; Comin-Chiaramonti, P. Hydrothermal REE fluorocarbonate mineralization at Barra do Itapirapuã, a multiple stockwork carbonatite, southern Brazil. Can. Mineral. 2008, 46, 901–914. [Google Scholar] [CrossRef]
- Andrade, F.R.D.; Möller, P.; Lüders, V.; Dulski, P.; Gilg, H.A. Hydrothermal rare earth elements mineralization in the Barra do Itapirapuã carbonatite, southern Brazil: Behavior of selected trace elements and stable isotopes (C,O). Chem. Geol. 1999, 155, 91–113. [Google Scholar] [CrossRef]
- Sitnikova, M.A.; Zaitsev, A.N.; Wall, F.; Chakhmouradian, A.R.; Subbotin, V.V. Evolution of chemical composition of rock-forming carbonates in Sallanlatvi carbonatites, Kola Peninsula, Russia. J. Afr. Earth Sci. 2001, 32, A34. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Castor, S.B. The Mountain Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Couëslan, C.; Yang, P. Calcite and dolomite in intrusive carbonatites. II. Trace-element variations. Mineral. Petrol. 2016, 110, 361–377. [Google Scholar] [CrossRef]
- Jöreskog, K.G.; Klovan, J.E.; Reyment, R.A. Geological Factor Analysis; Elsevier Scientific Pub. Co: Amsterdam, The Netherlands, 1976; ISBN 0-444-41367-7. [Google Scholar]
- Le Maitre, R.W. Numerical Petrology: Ststistical Interpretation of Geochemical Data; Elsevier Scientific Pub. Co: Amsterdam, The Netherlands, 1982; ISBN 9780444420985. [Google Scholar]
- Fomina, E.; Kozlov, E.; Ivashevskaja, S. Study of diffraction data sets using factor analysis: A new technique for comparing mineralogical and geochemical data and rapid diagnostics of the mineral composition of large collections of rock samples. Powder Diffr. 2019, 34, 1–12. [Google Scholar] [CrossRef]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate Melts and Carbonatites. Rev. Mineral. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Zaitsev, A.N. Calcite and dolomite in intrusive carbonatites. I. Textural variations. Mineral. Petrol. 2016, 110, 333–360. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Demény, A.; Sindern, S.; Wall, F. Burbankite group minerals and their alteration in rare earth carbonatites—source of elements and fluids (evidence from C–O and Sr–Nd isotopic data). Lithos 2002, 62, 15–33. [Google Scholar] [CrossRef]
- Guzmics, T.; Mitchell, R.H.; Szabó, C.; Berkesi, M.; Milke, R.; Ratter, K. Liquid immiscibility between silicate, carbonate and sulfide melts in melt inclusions hosted in co-precipitated minerals from Kerimasi volcano (Tanzania): Evolution of carbonated nephelinitic magma. Contrib. Mineral. Petrol. 2012, 164, 101–122. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Schmidt, M.W.; Mattsson, H.B.; Guenther, D. Element Partitioning between Immiscible Carbonatite and Silicate Melts for Dry and H2O-bearing Systems at 1–3 GPa. J. Petrol. 2013, 54, 2301–2338. [Google Scholar] [CrossRef]
- Jones, J.H.; Walker, D.; Pickett, D.A.; Murrell, M.T.; Beattie, P. Experimental investigations of the partitioning of Nb, Mo, Ba, Ce, Pb, Ra, Th, Pa, and U between immiscible carbonate and silicate liquids. Geochim. Cosmochim. Acta 1995, 59, 1307–1320. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Dulski, P.; Kamenetsky, V.S.; Danyushevsky, L.V.; Jeffries, T.; Dingwell, D.B. Partitioning of elements between silicate melt and immiscible fluoride, chloride, carbonate, phosphate and sulfate melts, with implications to the origin of natrocarbonatite. Geochim. Cosmochim. Acta 2012, 79, 20–40. [Google Scholar] [CrossRef]
- Veksler, I.V.; Petibon, C.; Jenner, G.A.; Dorfman, A.M.; Dingwell, D.B. Trace Element Partitioning in Immiscible Silicate-Carbonate Liquid Systems: An Initial Experimental Study Using a Centrifuge Autoclave. J. Petrol. 1998, 39, 2095–2104. [Google Scholar] [CrossRef]
- Barker, D.S. Calculated silica activities in carbonatite liquids. Contrib. Mineral. Petrol. 2001, 141, 704–709. [Google Scholar] [CrossRef]
- Giebel, R.J.; Parsapoor, A.; Walter, B.F.; Braunger, S.; Marks, M.A.W.; Wenzel, T.; Markl, G. Evidence for Magma–Wall Rock Interaction in Carbonatites from the Kaiserstuhl Volcanic Complex (Southwest Germany). J. Petrol. 2019, 60, 1163–1194. [Google Scholar] [CrossRef]
- Ray, J.S. Radiogenic Isotopic Ratio Variations in Carbonatites and Associated Alkaline Silicate Rocks: Role of Crustal Assimilation. J. Petrol. 2009, 50, 1955–1971. [Google Scholar] [CrossRef]
- Kapustin, Y.L. Mineralogy of Carbonatites; Amerind Publishing Company Pvt. Ltd.: New Dehli, India, 1980. [Google Scholar]
- Logvinova, A.M.; Wirth, R.; Tomilenko, A.A.; Afanas’ev, V.P.; Sobolev, N.V. The phase composition of crystal-fluid nanoinclusions in alluvial diamonds in the northeastern Siberian Platform. Russ. Geol. Geophys. 2011, 52, 1286–1297. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Origin of K-rich diamond-forming immiscible melts and CO2 fluid via partial melting of carbonated pelites at a depth of 180–200 km. Gondwana Res. 2019, 75. [Google Scholar] [CrossRef]
- Wall, F.; Niku-Paavola, V.N.; Storey, C.; Muller, A.; Jeffries, T. Xenotime-(Y) from carbonatite dykes at Lofdal, Namibia: Unusually low LREE:HREE ratio in carbonatite, and the first dating of xenotime overgrowths on zircon. Can. Mineral. 2008, 46, 861–877. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Zaitsev, A.N.; Couëslan, C.; Xu, C.; Kynický, J.; Mumin, A.H.; Yang, P. Apatite in carbonatitic rocks: Compositional variation, zoning, element partitioning and petrogenetic significance. Lithos 2017, 274–275, 188–213. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Nikolenko, A.M.; Redina, A.A.; Doroshkevich, A.G.; Prokopyev, I.R.; Ragozin, A.L.; Vladykin, N.V. The origin of magnetite-apatite rocks of Mushgai-Khudag Complex, South Mongolia: Mineral chemistry and studies of melt and fluid inclusions. Lithos 2018, 320–321, 567–582. [Google Scholar] [CrossRef]
- Louvel, M.; Bordage, A.; Testemale, D.; Zhou, L.; Mavrogenes, J. Hydrothermal controls on the genesis of REE deposits: Insights from an in situ XAS study of Yb solubility and speciation in high temperature fluids (T < 400 °C). Chem. Geol. 2015, 417, 228–237. [Google Scholar] [CrossRef]
- Shivaramaiah, R.; Anderko, A.; Riman, R.E.; Navrotsky, A. Thermodynamics of bastnaesite: A major rare earth ore mineral. Am. Mineral. 2016, 101, 1129–1134. [Google Scholar] [CrossRef]
- Hsu, L.C. Synthesis and stability of bastnaesites in a part of the system (Ce,La)–F–H–C–O. Mineral. Petrol. 1992, 47, 87–101. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. The thermodynamic properties of bastnäsite-(Ce) and parisite-(Ce). Chem. Geol. 2015, 392, 87–101. [Google Scholar] [CrossRef]
- Kutty, T.R.N.; Tareen, J.A.K.; Mohammed, I. Correlation between the stability of carbonates in ternary Ln2O3-H2O-CO2 hydrothermal systems and lanthanide systematics. J. Less Common Met. 1985, 105, 197–209. [Google Scholar] [CrossRef]
- Tucker, R.D.; Belkin, H.E.; Schulz, K.J.; Peters, S.G.; Horton, F.; Buttleman, K.; Scott, E.R. A major light rare-earth element (LREE) resource in the Khanneshin carbonatite complex, southern Afghanistan. Econ. Geol. 2012, 107, 197–208. [Google Scholar] [CrossRef]
- Samson, I.M.; Wood, S.A.; Finucane, K. Fluid Inclusion Characteristics and Genesis of the Fluorite-Parisite Mineralization in the Snowbird Deposit, Montana. Econ. Geol. 2004, 99, 1727–1744. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- Borisenko, A.S.; Borovikov, A.A.; Vasyukova, E.A.; Pavlova, G.G.; Ragozin, A.L.; Prokop’ev, I.R.; Vladykin, N.V. Oxidized magmatogene fluids: Metal-bearing capacity and role in ore formation. Russ. Geol. Geophys. 2011, 52, 144–164. [Google Scholar] [CrossRef]
- Blount, C.W. Barite solubilities and thermodynamic quantities up to 300 °C and 1400 bars. Am. Mineral. 1977, 62, 942–957. [Google Scholar]
- Benaouda, R.; Devey, C.W.; Badra, L.; Ennaciri, A. Light rare-earth element mineralization in hydrothermal veins related to the Jbel Boho alkaline igneous complex, AntiAtlas/Morocco: The role of fluid-carbonate interactions in the deposition of synchysite-(Ce). J. Geochem. Explor. 2017, 177, 28–44. [Google Scholar] [CrossRef]
- Manfredi, T.R.; Bastos Neto, A.C.; Pereira, V.P.; Barbanson, L.; Schuck, C. The parisite-(Ce) mineralization associated with the Fazenda Varela carbonatite (Correia Pinto, SC). Pesqui. Em Geociências 2013, 40, 295–307. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; Bolonin, A.V.; Sugorakova, A.M.; Popov, V.A.; Lykhin, D.A. Carbonatites of Central Tuva: Geological Structure and Mineral and Chemical Composition. Geol. Ore Depos. 2005, 47, 326–345. [Google Scholar]
- Nisbet, H.; Migdisov, A.; Xu, H.; Guo, X.; van Hinsberg, V.; Williams-Jones, A.E.; Boukhalfa, H.; Roback, R. An experimental study of the solubility and speciation of thorium in chloride-bearing aqueous solutions at temperatures up to 250 °C. Geochim. Cosmochim. Acta 2018, 239, 363–373. [Google Scholar] [CrossRef]
- Al Ani, T.; Sarapää, O. Geochemistry and mineral phases of REE in Jammi carbonatite veins and fenites, southern end of the Sokli complex, NE Finland. Geochem. Explor. Environ. Anal. 2013, 13, 217–224. [Google Scholar] [CrossRef]
- Lottermoser, B.G.G. Rare-earth element mineralisation within the Mt. Weld carbonatite laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- Andersen, T. A model for the evolution of hematite carbonatite, based on whole-rock major and trace element data from the Fen complex, southeast Norway. Appl. Geochem. 1987, 2, 163–180. [Google Scholar] [CrossRef]









| Mineral, Mineral Formula | Abbr. | (1) * | (2) | (3) | (4) | (5) |
|---|---|---|---|---|---|---|
| Calcite, CaCO3 | Cal | + | ++ | +++ | +++ | + |
| Dolomite, CaMg(CO3)2 | Dol | +++ | +++ | +++ | + | +++ |
| Ankerite, Fe2+Mg(CO3)2 | Ank | + | ||||
| Kutnohorite, Mn2+Mg(CO3)2 | Kut | + | ||||
| Norsethite, BaMg(CO3)2 | Nor | + | ||||
| Strontianite, Sr(CO3) | Str | + | +++ | + | ||
| Ancylite-(Ce), Sr(Ce,La)(CO3)2(OH)∙H2O | Anc | + | + | +++ | ||
| Burbankite **, (Na,Ca)3(Sr,Ba,Ce)3(CO3)5 | Brb | (++) | ||||
| Carbocernaite, (Sr,Ce,La)(Ca,Na)(CO3)2 | Cbc | + | ||||
| Bastnäsite-(Ce), (Ce,La)CO3F | Bas | + | + | + | ||
| Hydroxylbastnäsite-(Ce), (Ce,La)CO3(OH) | Bas | + | + | ++ | ||
| Synchysite-(Ce), CaCe(CO3)2F | Syn | + | ||||
| Baryte, BaSO4 | Brt | ++ | + | +++ | + | |
| Fluorapatite, Ca5(PO4)3F | Ap | + | ++ | |||
| Monazite, CePO4 | Mnz | + | + | + | ||
| Microcline, KAlSi3O8 | Mc | +++ | ||||
| Phlogopite, KMg3(AlSi3O10)(F,OH)2 | Phl | + | ++ | |||
| Aegirine, NaFe3+Si2O6 | Aeg | ++ | ||||
| Albite, NaAlSi3O8 | Ab | + | ||||
| Zircon, ZrSiO4 | Zrn | + | ||||
| Thorite, ThSiO4 | Thr | + | ||||
| Quartz, SiO2 | Qz | ++ | ++ | +++ | ||
| Fluorcalciopyrochlore, (Ca, Na)Nb2O6F | Pcl | + | + | |||
| Hydroxycalciopyrochlore, (Ca,□)2Nb2(O,OH)6(OH) | Pcl | + | + | |||
| Kenoplumbopyrochlore, (Pb,□)2Nb2O6(□,OH) | Pcl | + | + | |||
| Anatase, brookite, rutile ***, TiO2 | Ti ox. | + | ++ | + | ||
| Hollandite, Ba(Mn4+6Mn3+2)O16 | Hol | + | + | |||
| Fe oxides/hydroxides | Fe ox. | + | ++ | ++ | + | ++ |
| Pyrite ****, FeS2 | Py | + |
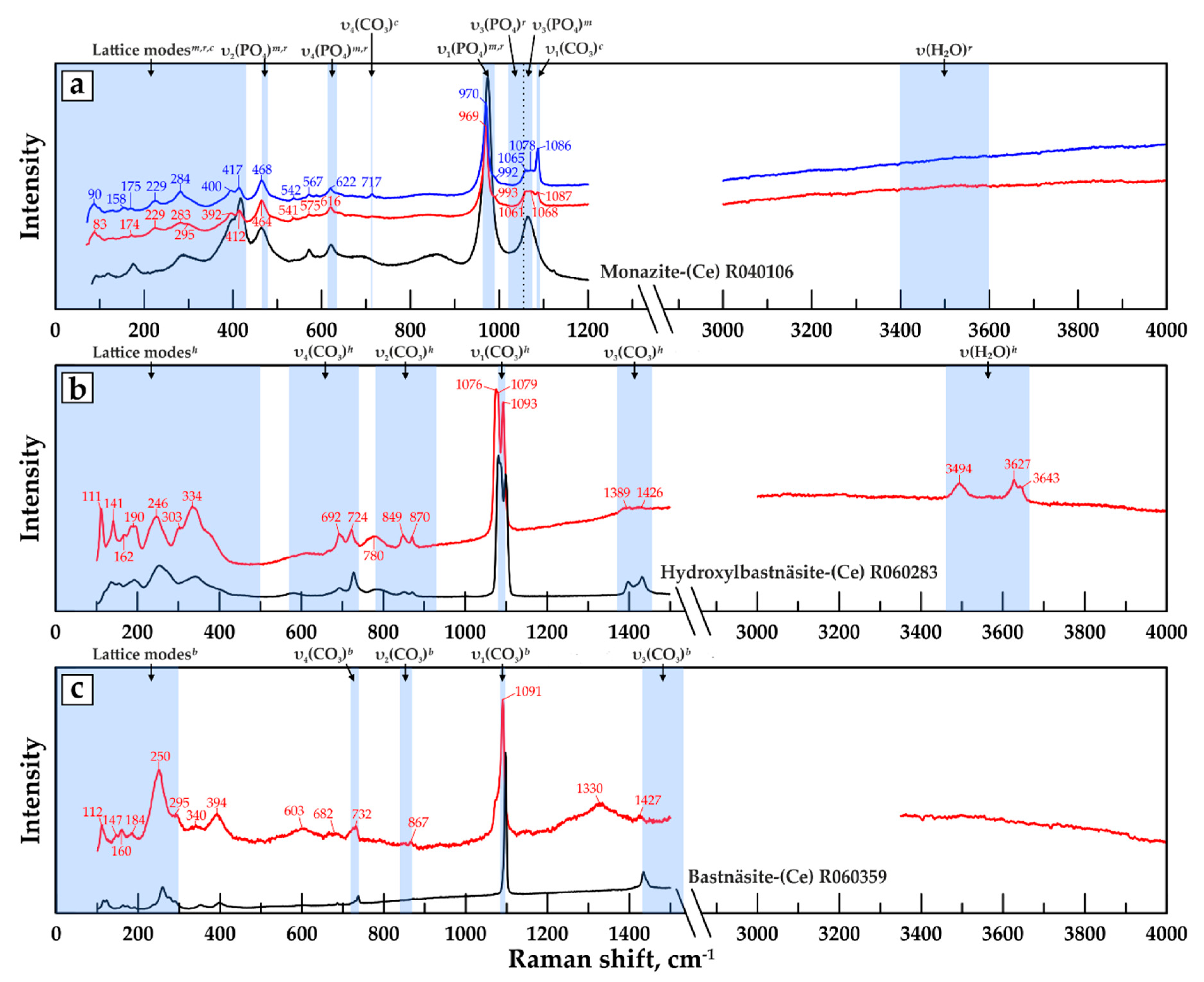
| Band Assignment | Monazite | Rhabdo-Phane | Calcite | Bastnäsite | Hydroxyl-Bastnäsite |
|---|---|---|---|---|---|
| Lattice modes | up to 430 | up to 300 | up to 320 | up to 300 | up to 500 |
| υ2 (PO4) out-of-plane bending | 465–478 | 468–474 | - | - | - |
| υ4 (PO4) in-plane bending | 618–634 | 613–622 | - | - | - |
| υ4 (CO3) in-plane bending | - | - | 711–716 | 720–740 | 570–740 |
| υ2 (CO3) out-of-plane bending | - | - | - | 840–870 | 780–930 |
| υ1 (PO4) symmetric stretching | 965–990 | 963–985 | - | - | - |
| υ3 (PO4) asymmetric stretching | 1054–1075 | 1020–1056 | - | - | - |
| υ1 (CO3) symmetric stretching * | - | - | 1084–1092 | 1085–1098 | 1080–1098 |
| υ3 (CO3) asymmetric stretching | - | - | 1432–1437 | 1432–1538 | ~1400 |
| υ (H2O) stretching | - | ~3500 | - | - | ~3500 |
| References: | [98,99,100] | [99,101] | [102,103] | [104,105] | [104,105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V.; Bocharov, V.; Chernyavsky, A.; Huber, M. The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry. Minerals 2020, 10, 73. https://doi.org/10.3390/min10010073
Kozlov E, Fomina E, Sidorov M, Shilovskikh V, Bocharov V, Chernyavsky A, Huber M. The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry. Minerals. 2020; 10(1):73. https://doi.org/10.3390/min10010073
Chicago/Turabian StyleKozlov, Evgeniy, Ekaterina Fomina, Mikhail Sidorov, Vladimir Shilovskikh, Vladimir Bocharov, Alexey Chernyavsky, and Miłosz Huber. 2020. "The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry" Minerals 10, no. 1: 73. https://doi.org/10.3390/min10010073
APA StyleKozlov, E., Fomina, E., Sidorov, M., Shilovskikh, V., Bocharov, V., Chernyavsky, A., & Huber, M. (2020). The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry. Minerals, 10(1), 73. https://doi.org/10.3390/min10010073







