Abstract
In this study, the effects of four fine minerals, which were fine diaspore (FDIA), kaolinite, illite, and pyrophyllite (D50 is about 4.55 μm, D80 is about 10.78 μm), on the pulp rheology of the diaspore and pyrite mixed ores (D50 is about 120.53 μm, D80 is about 187.36 μm) and the recovery of pyrite were investigated through flotation tests, pulp rheology measurements, and sedimentation tests. It was found that fine minerals could change the pulp rheology and affect the pyrite recovery. The apparent viscosity of the mixed ores slurry increased with the addition of FDIA, kaolinite, and illite and the pyrite recovery decreased in varying degrees. When the addition was 15 wt.%, the recovery of pyrite decreased from 92.3% to 60.8%, 81.4%, and 84.7%, respectively. The addition of pyrophyllite had a significant deteriorating effect on flotation. When the addition of pyrophyllite was 5 wt.%, the pyrite recovery was reduced to 49.2%, and when the addition was further increased to 15 wt.%, the pyrite recovery reduced to 28.5%. However, the effect of pyrophyllite addition on the pulp rheology of the mixed ore was not remarkable. Pyrophyllite affected pyrite recovery not only by affecting the rheological behavior of the pulp, but also because pyrophyllite was adsorbed on the surface of pyrite and diaspore, producing hetero-aggregation, which made it difficult for the pyrite particles to collide with the bubbles effectively. This was the main reason for the reduction of pyrite recovery. Generally, the order in which the reduction of pyrite recovery was affected by the additions of fine minerals was pyrophyllite > FDIA > kaolinite > illite.
1. Introduction
In China, about 11% of high-sulfur bauxite resources are diaspore-type bauxite. With the decrease of high-quality bauxite resources, high-sulfur bauxite has gradually become the main raw material for alumina production by Bayer process in China [1]. Too much sulfur impurities in bauxite will have a strongly negative influence on the Bayer process. Thus, high-sulfur bauxite cannot be used directly in the Bayer process to produce alumina. At present, there are two main methods of bauxite desulfurization: one is desulfurization in the Bayer process [2,3], which is called wet desulfurization, and the other is pre-desulfurization of high-sulfur bauxite [4]. For high-sulfur bauxite, flotation is the most economical and efficient desulfurization method at present [5].
In the mineral processing industry, the existence of clay minerals will not only make it difficult to transport materials, but will also affect the pulp rheology and flotation [6,7]. Clay mineral is a natural mineral. Due to its small hardness, fine clay mineral is produced during grinding [8] and two different crystallographic surfaces are exposed, i.e., the flat basal surface and the edge surface. These two surfaces are charged and fine clay minerals will cover the surface of floating minerals by electrostatic attraction [9], thus, reducing the hydrophobicity of the floating mineral surface. Furthermore, fine clay minerals can enter the concentrate through entrainment and reduce the product quality [10]. In the flotation process of clay-rich minerals, not just the reagent system and flotation process affect the flotation performance [11]. In recent years, many studies have showed that the existence of clay minerals has a certain impact on pulp rheology and flotation effect [12]. Because of the anisotropic structure and charge properties of clay minerals, they can form three kinds of combination modes: side-to-side, side-to-face, and face-to-face [13]. Side-to-side and side-to-face structures cause a large number of three-dimensional high-capacity “house of cards” structures, which lead to increased pulp viscosity [14]. However, the face-to-face structure produces thicker and larger flakes, which reduce the pulp viscosity contrarily [15]. Generally, higher pulp viscosity will lead to lower recovery of floating minerals. In the flotation cell, the high viscosity of pulp will cause the increase of turbulence damping, the poor dispersion of bubbles, the decrease of fluidity of mineral particles and bubbles, and the decrease of collision efficiency of mineral particles and bubbles [16,17,18].
Not only does the increase in slurry viscosity reduce the collision efficiency of mineral particles and bubbles, but it is also another negative factor that cannot be avoided during flotation that will also reduce the collision efficiency. The surface of fine clay minerals is generally negatively charged and will cause hetero-aggregation with floating minerals [19,20]. This factor will cause fine-grained gangue minerals to adsorb on the surface of the floating minerals and enter the concentrate and will also prevent the floating minerals from contacting the air bubbles, thereby reducing the flotation rate and recovery [21]. Researchers believe that the electrostatic attraction between mineral particles is due to the opposite charge on their surfaces [22], such as the electrostatic attraction of negatively-charged iron hydroxide and galena [23]. Positively-charged serpentine adsorbed on the surface of pentlandite [24]. Many researchers believe that minerals with the same symbolic charge will not have electrostatic attraction, which makes the flotation process quite complicated [25]. For example, the dissolution of minerals and the addition of agents cause flotation chemical reactions. This process may alter the original charge properties of the mineral surface. Feng Bo et al. [26] studied the hetero-aggregation of chlorite and pyrite and found that, when pH > 4.5, both the surface of chlorite and pyrite were negatively charged, but the hetero-aggregation still occurred. The reason for this phenomenon is because that in the presence of oxygen, when oxygen is available, the surface of pyrite is oxidized and iron is dissolved from the surface of pyrite and enters into the solution in the form of iron trivalent, forming the hydroxyl iron and iron hydroxide. The hydroxyl iron and iron hydroxide are adsorbed or precipitated on the surface of the mineral, making the surface of pyrite and chlorite electrochemically opposite, resulting in hetero-aggregation condensation.
Kaolinite, illite, and pyrophyllite are common clay minerals in bauxite. They have different structures and have distinct effects on the pulp rheology. The structures of clay minerals are usually divided into two types: one is composed of alumina octahedron and silica tetrahedron connected by common oxygen atom (i.e., T–O structure), and the other is two tetrahedral silicon oxygen structures enclose an aluminum oxygen octahedron (i.e., T–O–T structure). The T–O and T–O–T structures are shown in Figure 1. Kaolinite is a typical, non-swelling clay mineral with a 1:1 alumina to silica layered structure [27] and it has low chemical reactivity. In the flotation process, due to the kaolinite structure and surface electrical properties, it may interact with many components and form aggregates or network structures with water, flotation reagents, and other minerals in pulp, so as to change the pulp rheology [28,29]. It has been reported that the charge properties of the flat basal surface and the edge surface of kaolinite are determined by pH [27]. Illite is a non-swelling clay mineral with a 2:1 alumina to silica layered structure. Christian Blachier [30] used the concept of effective volume fraction to analyze the rheological properties of illite, kaolinite, and montmorillonite, and connected the morphological characteristics of suspended solids with the hydrodynamic behavior. It was found that different types of clay minerals can affect the pulp rheology of calcareous sand. Like illite, pyrophyllite is a 2:1 non-swelling clay mineral with T–O–T structure. However, pyrophyllite has good natural hydrophobicity and is difficult to separate from pyrite [31,32]. At present, the influence of pyrophyllite on pulp rheology is rarely reported, which is also one significance of this paper.
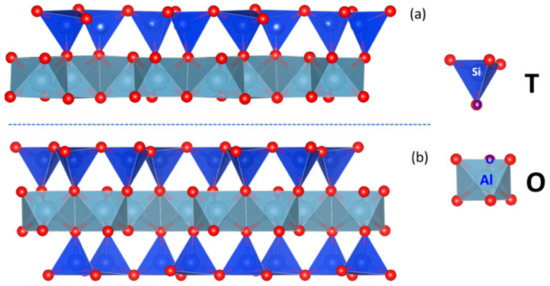
Figure 1.
Schematic diagram of clay type (a) 1:1 and (b) 2:1; O and T represent octahedral and tetrahedral layers, respectively.
In this study, three common clay minerals (kaolinite, illite, and pyrophyllite) with high content in bauxite and FDIA were selected to study the influence of fine minerals on pulp rheology and flotation. These four minerals can be collectively referred to as fine minerals. In order to eliminate the influence of dissociation degree on flotation, a series of artificial mixed ores of diaspore, pyrite, and fine minerals were used to measure the pulp rheology and to test flotation. Pulp rheology measurement, sedimentation tests, and flotation tests were used to study the effects of fine minerals on the pulp rheology and flotation of diaspore-pyrite mixture pulp in order to provide theoretical guidance for the flotation desulfurization of bauxite with different contents and kinds of fine minerals.
2. Materials and Methods
2.1. Mineral Preparation and Reagents
Diaspore was mined in Zunyi, Guizhou Province; pyrite was mined in Maanshan, Anhui Province; kaolinite was purchased from Up star chemical Co., Ltd.; illite was mined in Ningde, Fujian Province; and pyrophyllite was mined in Qingtian, Zhejiang Province. All minerals were crushed to about 2 mm after manual selection. The mineral compositions of minerals were analyzed by X-ray diffraction (XRD) (X’Pert PRO, PANalytical Company, Almelo, The Netherlands), where the scanning step was 0.013°, the scanning time per step was 50 s, and the scanning type was continuous. The results are shown in Figure 2. Quantitative XRD analysis showed that the diaspore sample contained 94.8% diaspore, 3.2% anatase, 1.7% goethite, and 0.4% annite. The pyrite sample contained 98.3% pyrite, 1.2% hematite, and 0.5% iron sulfide. The kaolinite sample contained 98.2% kaolinite and 1.8% dickite. The pyrophyllite sample contained 94.5% pyrophyllite and 5.5% cristobalite. The illite sample contained 60.8% illite and 39.2% quartz. Illite is closely associated with quartz and there is no relatively mature purification method at present. The presence of quartz does not significantly change the clay properties of illite, so illite with a purity of 60.8% could be used as a pure mineral in this experiment. No chemical reagent was added during the selection of all pure minerals.
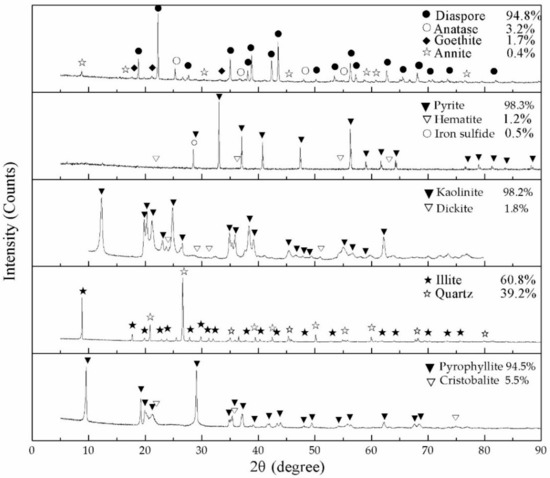
Figure 2.
XRD patterns of diaspore, pyrite, kaolinite, illite, and pyrophyllite.
Through grinding the six minerals, the particle size of diaspore and pyrite were similar and the particle sizes of FDIA, kaolinite, illite, and pyrophyllite were similar. Dry screening of diaspore and pyrite with 400-mesh sieve was conducted and the sample on the sieve (+38 μm) was taken for particle size distribution measurement. The particle size distribution of the six kinds of minerals was measured by laser particle size analyzer (Beckman Coulter, Inc, Miami, FL, USA). The six minerals were mixed individually with water to form very thin suspensions, which were then dispersed by ultrasound for 30 s and poured into the diluter of the laser particle size analyzer for particle size distribution measurement. The particle size distributions of four fine minerals, diaspore, and pyrite are shown in Figure 3. The particle size distribution of the fine minerals was similar (D50 was about 4.55 μm, D80 was about 10.78 μm) and the particle size distribution of diaspore and pyrite were similar (D50 was about 120.53 μm, D80 was about 187.36 μm).
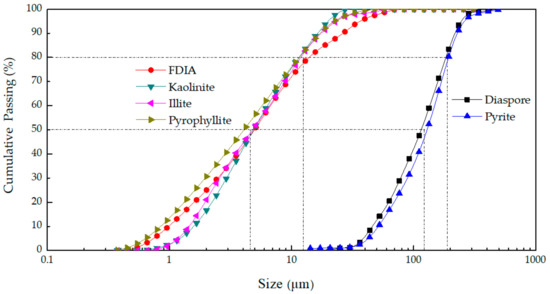
Figure 3.
Particle size distribution of the four fine minerals, diaspore, and pyrite.
In this study, sodium butyl xanthate (Coal Preparation Plant, Guizhou, China) was used as the collector, terpineol (Coal Preparation Plant, Guizhou, China) was used as the frother, sodium carbonate (Yongda Chemical Reagent Co., Ltd., Tianjin, China) was used as the pH regulator, copper sulfate (Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China) was used as the activator, and soluble starch (Jinshan Chemical Reagent Co., Ltd., Chengdu, China) was used as the inhibitor. Terpineol is a chemically-pure reagent, the other reagents were analytically pure, and the experimental water was deionized water.
2.2. Flotation Tests
In order to study the effects of the different kinds and contents of fine-grained minerals on the flotation of diaspore and pyrite mixture, a series of artificially mixed ores were prepared and the ratios are shown in Table 1. The weight of each artificial mixed ore was 20 g, the fixed proportion of pyrite was 10 wt.%, and the proportions of fine minerals were 5, 10, and 15 wt.% in the three trials, respectively. With the increase in the proportion of fine minerals, the proportion of diaspore decreased to 85, 80, and 75 wt.%, respectively, ensuring that the sum of the mass of fine minerals, diaspore, and pyrite in each artificial mixed ore was 100 wt.%.

Table 1.
Composition of artificially mixed ore.
The 20 g of artificially mixed ore was added into 100 mL flotation cell (XFG‖ 100 mL), then added to 80 mL of deionized water, and then fully mixed. According to our previous exploratory experiment, the suitable reagent system was determined as follows: pulp pH was 8.5, inhibitor dosage was 150 g/t, activator dosage was 100 g/t, collector dosage was 300 g/t, frother dosage was 150 g/t, flotation pulp mixing speed was 1920 rpm, air rate was 0.1L/min, and its type was air. Flotation was carried our according to the above flotation parameters.
2.3. Rheology Measurements
An ARES-G2 rheometer (TA Instruments Co., Ltd., New Castle, DE, USA) was used for rheological measurements. All rheology measurements were performed at a room temperature of 20 °C. The rheometer computer program can calculate the shear stress and viscosity directly. A geometric model with stationary inner cylinder and an external rotating outer cylinder was adopted. The gap between the inner cylinder (diameter = 32 mm) and the outer cylinder (diameter = 34 mm) was 1 mm, which is very small compared to the cup (<3%). Therefore, the error caused by non-uniform shearing in this small gap can be ignored. In this study, the rheological models were the Newtonian fluid model and Bingham fluid model.
According to the parameters during flotation, the slurry was prepared again and, then, the 20 mL syringe without attached needles was inserted into the middle of the slurry to extract 20 mL for rheological measurements. Rheograms were generated for the shearing rate ranging between 4 and 300 s−1 for 100 s to obtain complete rheological curves. The rheological property of pulp is generally characterized by the change of shear stress with shear rate.
2.4. Sedimentation Tests
Addition of fine minerals affected the dispersion of ore particles in the slurry. Therefore, the turbidity of the slurry was used to characterize the dispersion. The higher the turbidity, the better the slurry dispersion will be. The sedimentation test is different from the flotation test. The sedimentation tests were performed in a 100 mL sedimentation graduated cylinder. The mass concentration of the four fine minerals was 0.5 g/L and the mass concentration of diaspore and pyrite was 10 g/L [26]. The mixing function of the flotation machine was used to mix the slurry, which contained fine mineral and diaspore or pyrite, and, then, the slurry was transferred quickly to the sedimentation graduated cylinder and precipitated for 5 min. Then, the 20 mL suspension at the top of the settling liquid was extracted and its turbidity was measured in the turbidimeter (HACH 2100Q, Loveland, CO, USA; turbidimetric unit is NTU and the turbidimetric of 1mg/L SiO2 suspension is 1 NTU). In each test, the amount of fine minerals used was 0.05 g and the amount of diaspore or pyrite was 1 g. The turbidity of each test was measured 3 times and then averaged.
2.5. Optical Microscope Observations
Mixing the fine minerals with pyrite or diaspore was conducted according to the sedimentation test conditions. In the stirring state, a small amount of mineral slurry was absorbed by the rubber head dropper and dropped on the glass slide. The mineral dispersion state was observed under the microscope and the photos were obtained by the camera connected to the optical microscope.
3. Results and Discussion
3.1. Effect of Fine Minerals on Pulp Rheology
The effects of FDIA, kaolinite, illite, and pyrophyllite on the pulp rheology were studied, as shown in Figure 4, Figure 5, Figure 6 and Figure 7. When the pulp concentration was about 20 wt.%, the presence or absence of fine minerals will not change the Newtonian properties of the pulp. The relationship between shear stress and shear rate is a function of one variable that passes through the original point. With the increase in shear rate, the shear stress of different fine minerals is different. In pace with the increase of FDIA or kaolinite, the shear stress increased rapidly, but the pulp was Newtonian fluid as usual. However, with the increase of illite and pyrophyllite, the shear stress slightly increased at the same shear rate and the pulp also behaved as a Newtonian fluid. Munro et al. [33] studied the rheological properties of coal-oil slurry and thought that the coal-oil slurry with concentrations lower than 30 wt.% was a Newtonian fluid, while the coal-oil slurry with concentrations higher than 30 wt.% was a Bingham fluid. Zhang et al. [6] found that the relatively low concentration of Q38 (kaolinite with poor crystallinity) and relatively high concentration of snobrite would not change the Newtonian properties of the pulp when they studied the rheological properties of clay minerals to copper and gold minerals. However, the relatively high concentration of Q38 and relatively low concentration of bentonite would change the pulp into a non-Newtonian fluid with yield stress. But, it should be noted that the pulp concentration was about 30 wt.%. In this study, pulp concentration was about 20 wt.%, so it is reasonable to believe Newtonian properties of pulp should exist.
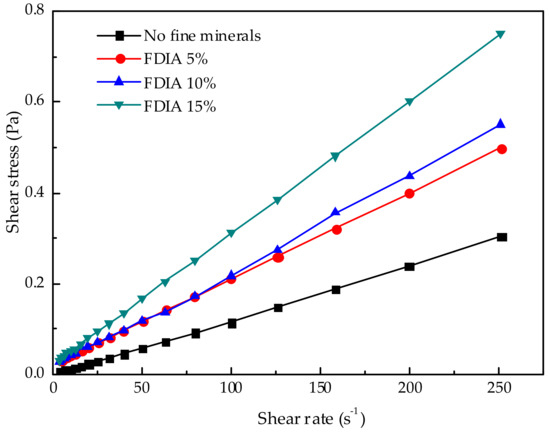
Figure 4.
Effect of different FDIA additions on pulp rheology.
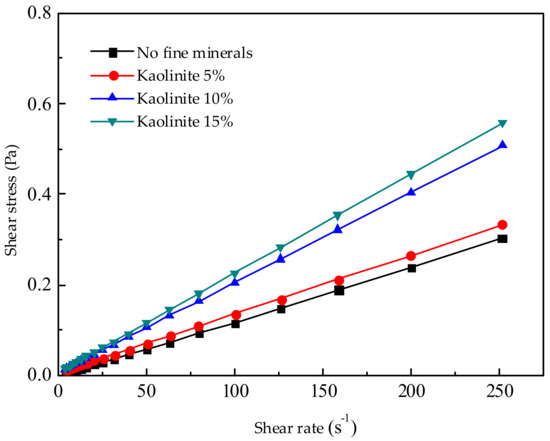
Figure 5.
Effect of different kaolinite additions on pulp rheology.
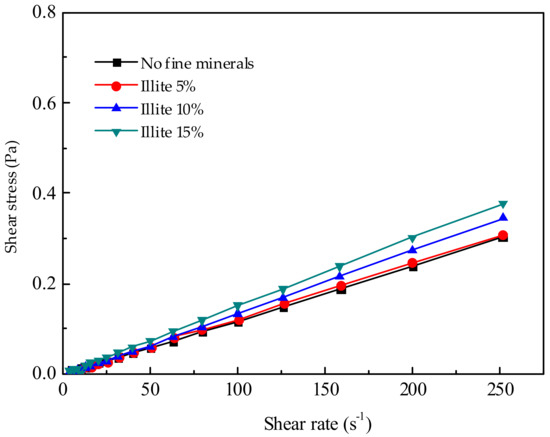
Figure 6.
Effect of different illite additions on pulp rheology.
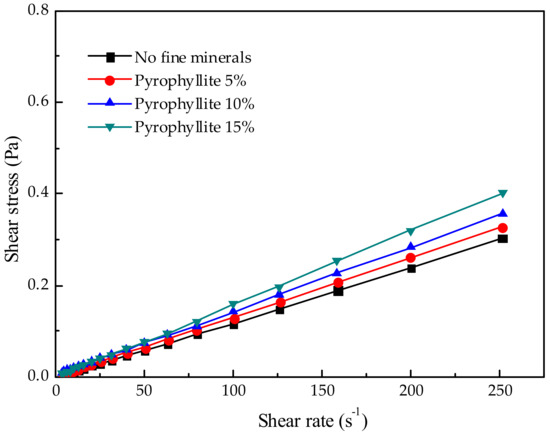
Figure 7.
Effect of different pyrophyllite additions on pulp rheology.
In the presence of different fine minerals, the change in the rheological property of pulp can also be explained by the aggregation form of mineral particles. When the pH is 8.5, the surface and edge of both diaspore and clay mineral particles have negative points [34] and the electrostatic repulsion between particles is large, thus there is no or little aggregation in the pulp, so the pulp has a Newtonian property. If the pulp at a low concentration (about 20 wt.%) does not reach the critical point of aggregation, fine minerals will not produce aggregates and will not change the original Newtonian properties of pulp.
In order to further study the effect of fine minerals on the rheological properties of pulp, the apparent viscosity with a shear rate of 100 s−1 was analyzed. The apparent viscosity at 100 s−1 was chosen because this may be the average shear rate in the flotation cell [35]. Figure 8 shows the effects of the content of fine minerals in the shear rate of 100 s−1 on the apparent viscosity. With the increase in the content of fine minerals, all associated apparent viscosity increased, but the increase in the apparent viscosity was quite different between minerals. The apparent viscosity of illite and pyrophyllite did not change much with the increase in the content, while the increase in the content of FDIA and kaolinite had a greater impact on the apparent viscosity. The apparent viscosity of the pulp increased rapidly with the increase in the addition of FDIA and kaolinite. When the addition amount was 15 wt.%, the apparent viscosity of FDIA pulp was about twice that of the illite or pyrophyllite pulp and the apparent viscosity of kaolinite pulp was about 1.5 times that of the illite or pyrophyllite pulp.
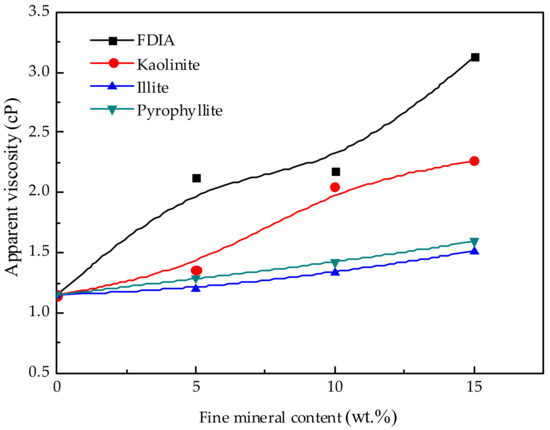
Figure 8.
Effect of fine mineral addition on apparent viscosity at shear rate of 100 s−1.
3.2. Effect of Fine Minerals on Diaspore-Pyrite Mixture Flotation
The flotation of artificially mixed ores with different contents of fine mineral was carried out and the rheological parameters and the recovery of pyrite were analyzed together. Figure 9 shows the effect of different fine minerals on the recovery of pyrite. Without the addition of fine minerals, the recovery of pyrite was 92.3%. When fine minerals were added, the recovery of pyrite changed greatly. When the addition of FDIA was 5 wt.%, the recovery of pyrite decreased to 65.7%. When the addition of FDIA increased to 15 wt.%, the recovery of pyrite decreased to 60.8%. Figure 8 shows that the apparent viscosity of the pulp increased with the increase in the amount of FDIA added. The effect of kaolinite addition on the recovery of pyrite was also remarkable. After adding 5 wt.% kaolinite, the recovery of pyrite decreased to 74.7%. With the increase of kaolinite addition to 10 wt.% and 15 wt.%, the recovery of pyrite was 77.5% and 81.4%, respectively, which shows that kaolinite has a significant impact on the pulp rheology and the recovery of pyrite. Adding 5–15 wt.% illite slightly increased the viscosity of pulp. Compared with the addition of other fine minerals, adding illite had little effect on the recovery of pyrite; the recovery was only reduced from 92.3% to 90.5%, 86.4%, and 84.7%. Nevertheless, the addition of these three fine minerals showed a trend. With the addition of fine minerals, the apparent viscosity of the pulp increased and the recovery of pyrite decreased. The recovery rate of pyrite decreased with the increase in the apparent viscosity of pulp. There was a good correlation between the change in pyrite recovery and the change in pulp viscosity.
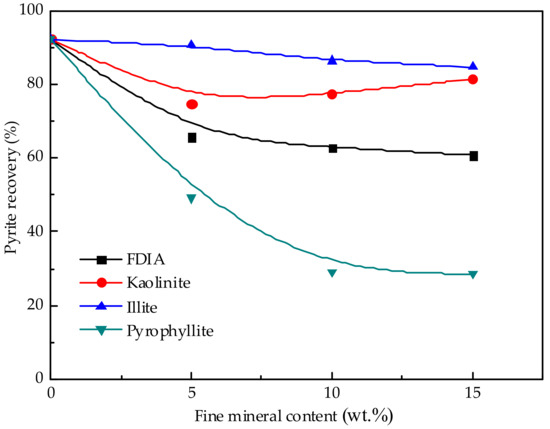
Figure 9.
Effect of different fine mineral additions on pyrite recovery.
Nevertheless, when pyrophyllite was added into the pulp, the apparent viscosity of the pulp did not change much, which was similar to the effect of illite addition on the apparent viscosity of the pulp. The addition of these two fine minerals did not cause a significant increase in the apparent viscosity of the pulp. Yet, the effect of pyrophyllite on the recovery of pyrite was noticeable. When pyrophyllite was added at 5 wt.%, the recovery of pyrite reduced to 49.2%. When pyrophyllite was added at 10 wt.% and 15 wt.%, the recovery of pyrite reduced to 29.1% and 28.5%, respectively. This shows that the addition of pyrophyllite reduces the recovery of pyrite, not only because pyrophyllite changes the recovery of pyrite by changing the pulp rheology, but also for other reasons.
3.3. Rheological Properties of the Six Minerals Added into Water
In order to better research the rheological property of pulp, six kinds of minerals were added into water separately to prepare pulp with a concentration of 20 wt.% for rheological measurements. The results are shown in Figure 10. At 20 wt.% diaspore or pyrite, pulps were Newtonian fluids. Different from other minerals, with the increase in shear rate, the shear stress decreased and the values were almost the same. Similarly, illite pulp with 20 wt.% concentration was a Newtonian fluid. Yet, the shear stress was slightly higher than that of the diaspore pulp or pyrite pulp. The shear stress of the 20 wt.% FDIA slurry is the largest with the increase of shear rate, which also shows that the former addition of FDIA has the greatest impact on the pulp rheology of artificially mixed ore, but the difference is that FDIA slurry was not a Newtonian fluid but a Bingham fluid with a certain yield stress. This condition may have resulted from that the pulp containing only one kind of FDIA mineral, but the former artificially mixed ore contained diaspore, pyrite, and FDIA, and diaspore and pyrite account for the majority of the addition [6]. When there were large number of fine minerals, the viscosity of the pulp increased and the pulp behaved as a Bingham fluid with yield stress. The 20 wt.% kaolinite slurry was also a Bingham fluid for similar reasons as FDIA. However, 20 wt.% kaolinite pulp showed less shear stress than the 20 wt.% diaspore pulp. In the previous study, the increase in shear stress caused by adding kaolinite was smaller than that caused by adding FDIA.
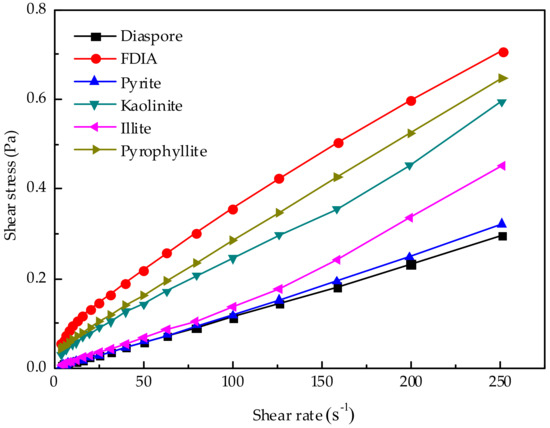
Figure 10.
Rheological properties of pulp when six minerals were added to water alone.
It is remarkable that the pyrophyllite pulp with a concentration of 20 wt.% showed the highest shear stress. In this moment, the pyrophyllite pulp is a Bingham fluid. However, in the previous study on the pulp rheology of the artificially mixed ore, the addition of pyrophyllite did not cause an increase in the shear stress or apparent viscosity. Compared with the addition of other fine minerals, the effect of pyrophyllite on the pulp rheology was smaller, but the addition of pyrophyllite led to the lowest recovery of pyrite. Therefore, it can be concluded that the reduction in pyrite recovery by adding pyrophyllite is not only due to the influence of the rheological properties of pulp, but also due to other more important factors, such as complex particle interactions in the pulp. Adding pyrophyllite to change the rheological property of pulp is not the main factor to reduce the pyrite recovery.
3.4. Hetero-Aggregation of Fine Minerals with Pyrite or Diaspore
The correlation analysis between rheological behavior and pyrite recovery found that the 20 wt.% of pyrophyllite pulp showed the highest shear stress. However, in the previous study of rheological behavior of artificially mixed ore, the addition of pyrophyllite did not cause an increase in shear stress and apparent viscosity of artificially mixed ore pulp, which may be caused by other more important factors. Therefore, the effect of fine minerals on the properties of artificially mixed ore pulp was further studied by sedimentation tests.
Turbidity can characterize the dispersion behavior of particles in the pulp. The larger the turbidity, the easier it is for the particles to disperse in the pulp and form a stable suspension. The smaller the turbidity, then the aggregation has occurred between the mineral particles. In this experiment, the initial concentration of diaspore and pyrite was 10 g/L and the diameters of diaspore and pyrite were large, so they were able to easily settle in the pulp. Their turbidity is 50.4 NTU and 69.7 NTU, respectively. The initial concentration of the fine minerals was 0.05 g/L, but the turbidity was relatively large, with an average value around 600 NTU. The sum of the turbidity of diaspore or pyrite and the turbidity of fine minerals was the theoretical turbidity. The change in pulp turbidity reflects the aggregation phenomenon between fine minerals and diaspore or pyrite. A schematic diagram of the mineral particle aggregation phenomenon is shown in Figure 11.
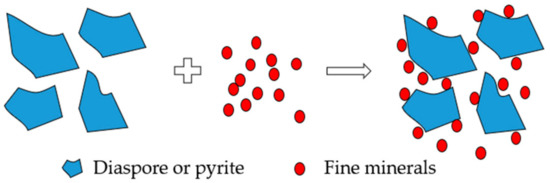
Figure 11.
Schematic diagram of the mineral particle aggregation phenomenon.
Figure 12 shows the turbidity of the fine minerals with diaspore or pyrite. The study found that the actual turbidity of FDIA mixed with pyrite or diaspore was slightly less than the theoretical turbidity, indicating that FDIA and diaspore have slight homo-aggregation. FDIA and pyrite have slight hetero-aggregation. Like FDIA, illite produced slight hetero-aggregation with pyrite or diaspore and the actual turbidity was not much different from the theoretical turbidity. This shows that these two fine minerals indeed affect the flotation effect by affecting the pulp rheology. On the contrary, when kaolinite was mixed with diaspore or pyrite, the actual turbidity was slightly higher than the theoretical turbidity, indicating that there was no hetero-aggregation. The actual turbidity increase may have resulted from the presence of fine kaolinite in the pulp, which is beneficial for the dispersion of diaspore or pyrite. Generally, the better the particles are dispersed in the pulp, the more helpful they are for flotation [36]. However, in the flotation test, with the addition of kaolinite, the recovery of pyrite was reduced. The addition of kaolinite has an impact on the pulp rheology, which in turn affects the recovery of pyrite, and it is also possible that the fine kaolinite adsorbed a large amount of flotation agents, which resulted in a decrease in the amounts of agents adsorbed on the surface of pyrite. Furthermore, the recovery of pyrite was reduced. The specific reason for this needs further study.
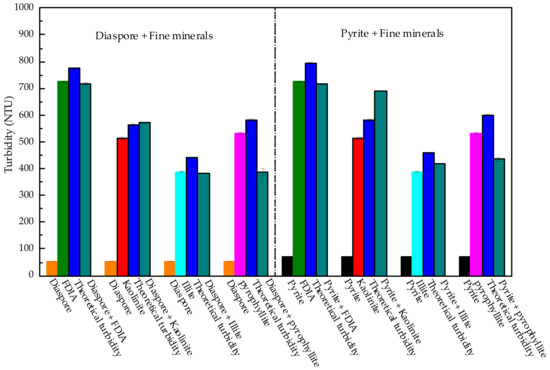
Figure 12.
Turbidity of mixture of fine minerals and diaspore or pyrite.
The sedimentation test of pyrophyllite with diaspore or pyrite well-explained why pyrophyllite did not increase the viscosity of the artificially mixed ore pulp when added to the artificially mixed ore. The turbidity of the pyrophyllite and diaspore mixture decreased from 582 NTU (theoretical turbidity) to 386 NTU (actual turbidity). The turbidity of pyrophyllite and pyrite mixture decreased from 601 NTU (theoretical turbidity) to 435 NTU (actual turbidity). Pure pyrophyllite pulp has a very high viscosity, but pyrophyllite and diaspore or pyrite produce distinct hetero-aggregation. It reduced the fine pyrophyllite content in the slurry. As to why the addition of pyrophyllite will sharply deteriorate pyrite recovery, it may be because pyrophyllite was adsorbed on the surface of pyrite, deteriorating the surface properties of pyrite and making it difficult for pyrite particles to collide with bubbles effectively. This is the main reason for the reduction of pyrite recovery.
In order to further confirm that the fine minerals had hetero-aggregation with diaspore or pyrite, the state of the slurry of the fine minerals with diaspore or pyrite was observed under a microscope. The results are shown in Figure 13. The aggregation of FDIA, kaolinite, and illite with diaspore or pyrite was not obvious, while pyrophyllite and pyrite or diaspore had significant hetero-aggregation, which is consistent with the results of the settlement test.
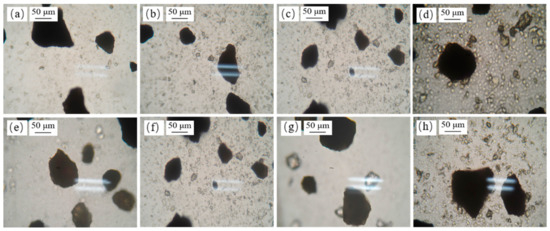
Figure 13.
Dispersion and aggregation of fine minerals with pyrite or diaspore. (a) FDIA + Diaspore; (b) Kaolinite + Diaspore; (c) Illite + Diaspore; (d) Pyrophyllite + Diaspore; (e) FDIA + Pyrite; (f) Kaolinite + Pyrite; (g) Illite + Pyrite; and (h) Pyrophyllite + Pyrite.
4. Conclusions
In the flotation process, pulp rheology is an important factor affecting the flotation effect. The type and content of fine minerals in pulp are the main factors affecting the rheological properties of pulp. The existence of fine minerals can change the rheological behavior of pulp and can cause agglomeration with pyrite. These two processes jointly affect the recovery of pyrite. Therefore, in the grinding operation before flotation, and on the premise of ensuring the dissociation of the minerals, over-grinding should be avoided as far as possible or the classified flotation process should be adopted to reduce the adverse effect of fine minerals on flotation. In this study, the main conclusions were as follows:
- (1)
- When the solid mass concentration of diaspore and pyrite mixture are about 20 wt.%, the pulp was a Newtonian fluid and the Newtonian property of the pulp will not be changed by adding fine minerals up to 15 wt.%;
- (2)
- The shear stress and apparent viscosity of artificially mixed ore pulp added with FDIA, kaolinite, or illite will increase with the increase in the amount of fine mineral added. With the increase in shear stress and apparent viscosity, the recovery of pyrite is reduced. The greater the amount of fine minerals added, the higher the shear stress and apparent viscosity of the slurry and the lower the recovery of pyrite. The order of influence of the three fine minerals on pulp rheology and pyrite recovery is: FDIA > kaolinite > illite. The change in the recovery of pyrite has a good correlation with the change in slurry viscosity;
- (3)
- The addition of pyrophyllite has little effect on the rheology of the mixed ore, but has a significant effect on the recovery of pyrite. With the increase in the amount of pyrophyllite, the recovery rate of pyrite is reduced significantly, indicating that pyrophyllite affects pyrite recovery, not just by changing the rheology of the slurry, but also by adsorption on the surface of pyrite and diaspore, making it difficult for the pyrite particles to collide with the bubbles effectively. This is the main reason for the reduction in pyrite recovery. Overall, the order of reduction in the pyrite recovery by the addition of fine minerals is pyrophyllite > FDIA > kaolinite > illite.
Author Contributions
C.W. and Q.Z. conceived and designed the experimental scheme; Q.Z. contributed experimental resources; C.W. performed all experimental work and wrote most of the manuscript; S.M. and S.Q. participated in data analysis and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key R&D Program of China (No. 2018YFC1903500).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Z.; Li, D.; Ma, W.; Yan, H.; Xie, K.; Zheng, L.; Li, P. Sulfur removal by adding aluminum in the bayer process of high-sulfur bauxite. Miner. Eng. 2018, 119, 76–81. [Google Scholar] [CrossRef]
- Liu, Z.W.; Li, W.X.; Ma, W.H.; Yin, Z.L.; Wu, G.B. Comparison of deep desulfurization methods in alumina production process. J. Cent. South Univ. 2015, 22, 3745–3750. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yin, J.G.; Chen, Y.L.; Xia, W.T.; Xiang, X.Y.; Yuan, X.L. Simultaneous removal of sulfur and iron by the seed precipitation of digestion solution for high-sulfur bauxite. Hydrometallurgy 2018, 181, 7–15. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Z.; Zhao, L.; Zhang, S.; Wang, D.; Wang, M. Competition of oxygen evolution and desulfurization for bauxite electrolysis. Ind. Eng. Chem. Res. 2017, 56, 6136–6144. [Google Scholar] [CrossRef]
- Chai, W.C.; Huang, Y.F.; Peng, W.J.; Han, G.H.; Cao, Y.J.; Liu, J.T. Enhanced separation of pyrite from high-sulfur bauxite using 2-mercaptobenzimidazole as chelate collector: Flotation optimization and interaction mechanisms. Miner. Eng. 2018, 129, 93–101. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zhang, G.F.; Wang, M.T.; Liu, D.Z. The Critical Role of Pulp Density on Flotation Separation of Nickel-Copper Sulfide from Fine Serpentine. Minerals 2018, 8, 317. [Google Scholar] [CrossRef]
- Tao, Q.; Su, L.; Frost, R.L.; Zhang, D.; Chen, M.; Shen, W.; He, H. Silylation of mechanically ground kaolinite. Clay Miner. 2014, 49, 559–568. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Vink, S. Diagnosis of the surface chemistry effects on fine coal flotation using saline water. Energy Fuels 2013, 27, 4869–4874. [Google Scholar] [CrossRef]
- Liu, D.; Peng, Y. Reducing the entrainment of clay minerals in flotation using tap and saline water. Powder Technol. 2014, 253, 216–222. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Chen, X.M.; Peng, Y.J. Managing clay minerals in froth flotation—A critical review. Miner. Process. Extr. Metall. Rev. 2018, 39, 289–307. [Google Scholar] [CrossRef]
- Mcfarlane, A.J.; Addai-Mensah, J.; Bremmell, K. Rheology of flocculated kaolinite dispersions. Korea-Aust. Rheol. J. 2005, 17, 181–190. [Google Scholar]
- Goh, R.; Leong, Y.K.; Lehane, B. Bentonite slurries—Zeta potential, yield stress, adsorbed additive and time-dependent behaviour. Rheol. Acta 2011, 50, 29–38. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Shabalala, N.Z.P.; Harris, M.; Filho, L.S.L.; Deglon, D.A. Effect of slurry rheology on gas dispersion in a pilot-scale mechanical flotation cell. Miner. Eng. 2011, 24, 1448–1453. [Google Scholar] [CrossRef]
- Becker, M.; Yorath, G.; Ndlovu, B.; Harris, M.; Deglon, D.; Franzidis, J.P. A rheological investigation of the behaviour of two Southern African platinum ores. Miner. Eng. 2013, 49, 92–97. [Google Scholar] [CrossRef]
- Meng, S.; Li, X.; Yan, X.; Wang, L.; Zhang, H.; Cao, Y. Turbulence models for single phase flow simulation of cyclonic flotation columns. Minerals 2019, 9, 464. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.; Lu, Y. The effect of lizardite surface characteristics on pyrite flotation. Appl. Surf. Sci. 2012, 259, 153–158. [Google Scholar] [CrossRef]
- Jiang, H.; Xiang, G.; Khoso, S.; Xie, J.; Huang, K.; Xu, L. Comparative studies of quaternary ammonium salts on the aggregation and dispersion behavior of kaolinite and quartz. Minerals 2019, 9, 473. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Feng, Q.; Long, T.; Ou, L.; Zhang, G. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferrous Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Forbes, E.; Davey, K.J.; Smith, L. Decoupling rehology and slime coatings effect on the natural flotability of chalcopyrite in a clay-rich flotation pulp. Miner. Eng. 2014, 56, 136–144. [Google Scholar] [CrossRef]
- Bandini, P.; Prestidge, C.A.; Ralston, J. Colloidal iron oxide slime coatings and galena particle flotation. Miner. Eng. 2001, 14, 487–497. [Google Scholar] [CrossRef]
- Edwards, C.R.; Kipkie, W.B.; Agar, G.E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, W.; Deng, R.; Xing, D.; Rao, F. Strengthening Sulfidation Flotation of Hemimorphite via Pretreatment with Pb2+. Minerals 2019, 9, 463. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.M.; Lu, Y. Mechanism of hetero-aggregation of chlorite and pyrite. J. Cent. South Univ. 2015, 46, 14–19. [Google Scholar]
- Schoonheydt, R.A.; Johnston, C.T. Chapter 3 Surface and Interface Chemistry of Clay Minerals. Dev. Clay Sci. 2006, 1, 87–113. [Google Scholar]
- Paineau, E.; Michot, L.J.; Bihannic, I.; Baravian, C. Aqueous suspensions of natural swelling clay minerals. 2. Rheological characterization. Langmuir 2011, 27, 7806–7819. [Google Scholar] [CrossRef]
- Lagaly, G. Principles of flow of kaolin and bentonite dispersions. Appl. Clay Sci. 1989, 4, 105–123. [Google Scholar] [CrossRef]
- Blachier, C.; Jacquet, A.; Mosquet, M.; Michot, L.; Baravian, C. Impact of clay mineral particle morphology on the rheological properties of dispersions: A combined X-ray scattering, transmission electronic microscopy and flow rheology study. Appl. Clay Sci. 2014, 87, 87–96. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Yan, W.; Gu, G.; Wang, C.; Wang, Z.; Xu, L.; Peng, T. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Miner. Eng. 2019, 132, 134–141. [Google Scholar] [CrossRef]
- Zhang, N.N.; Nguyen, A.V.; Zhou, C.C. A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv. Colloid Interface 2018, 254, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.M.; Lewellyn, M.M.; Crackel, P.R.; Bauer, L.G. A characterization of the rheological properties of Coal-Fuel oil slurries. AIChE J. 1979, 25, 355–358. [Google Scholar] [CrossRef]
- Wang, X.M.; Lu, Y. New progress in surface analysis technology and application in mineral engineering research-atomic force microscope. J. Guizhou Univ. 2018, 35, 1–12. [Google Scholar]
- Ralston, J.; Fornasiero, D.; Grano, S.; Duan, J.; Akroyd, T. Reducing uncertainty in mineral flotation—Flotation rate constant prediction for particles in an operating plant ore. Int. J. Miner. Process. 2007, 84, 89–98. [Google Scholar] [CrossRef]
- Chen, X.; Hadde, E.; Liu, S.; Peng, Y. The effect of amorphous silica on pulp rheology and copper flotation. Miner. Eng. 2017, 113, 41–46. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
