Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
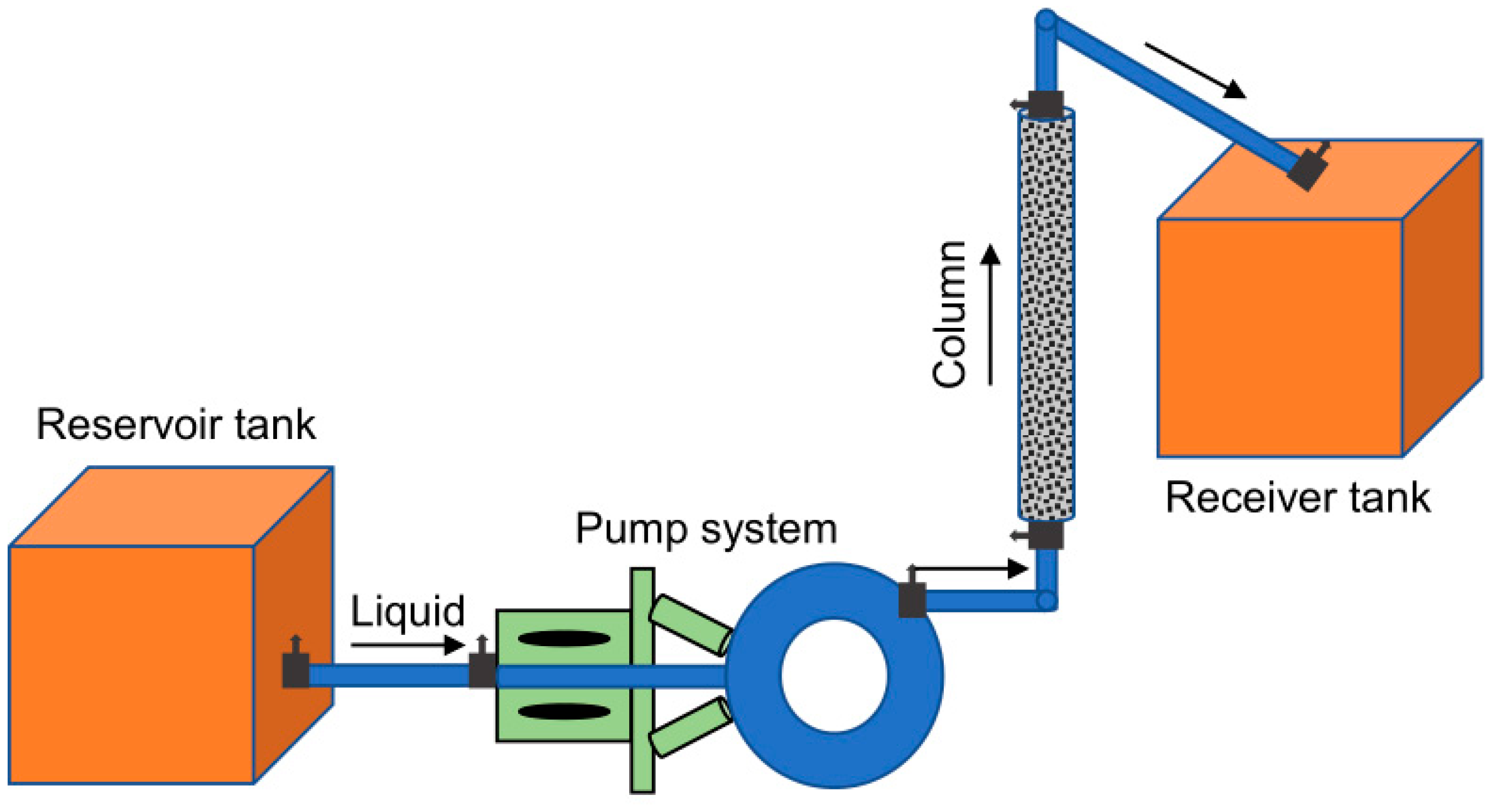
2.2. Column Experiments
2.3. Analysis and Characterization
3. Results and Discussion
3.1. Structure and Property of Modified Pyrite
3.2. Performance of MPy for Hg(II) Removal in the Column Experiment
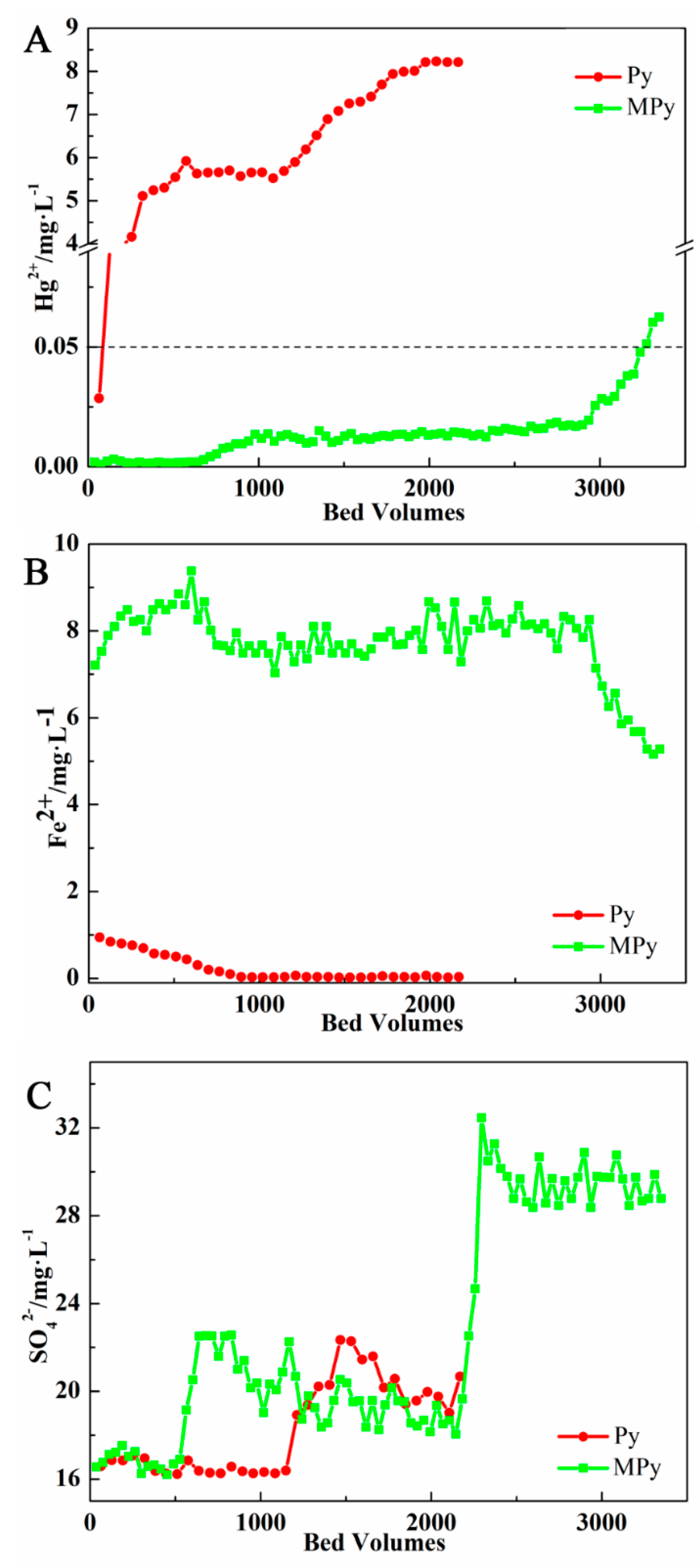
3.2.1. Effluent Analysis
3.2.2. Capacity Analysis
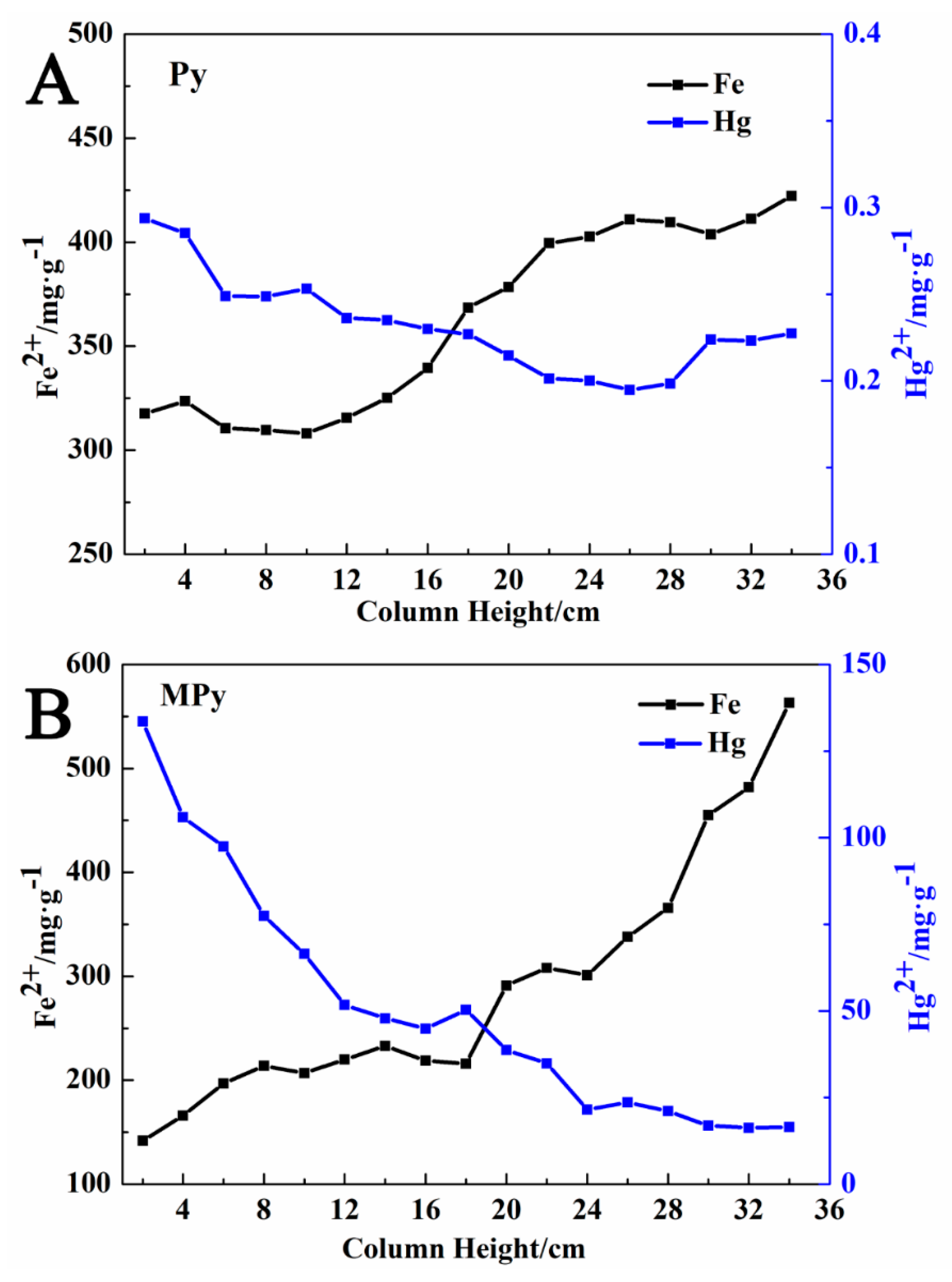
3.2.3. Variation of the Mercury and Iron Contents
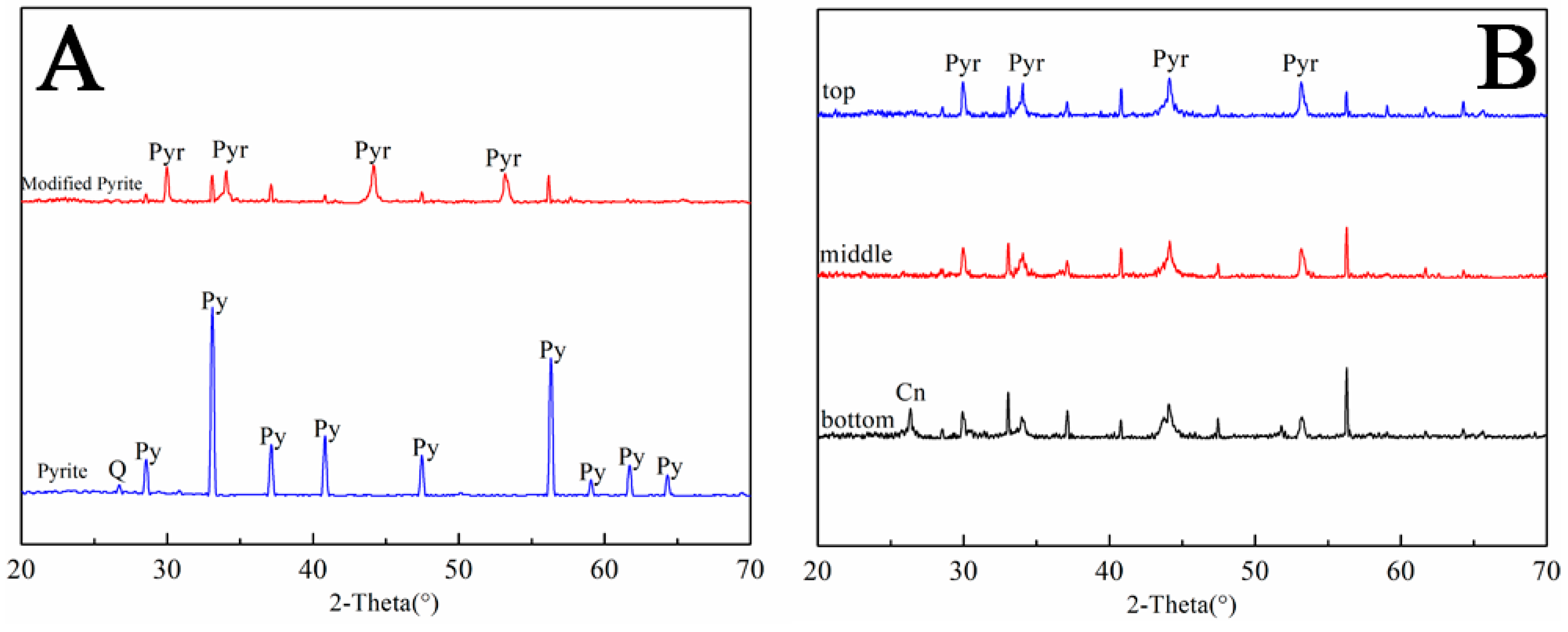
3.2.4. XRD Analysis
3.2.5. SEM and TEM Analysis
3.2.6. XPS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simões, M.R.; Fiorim, J.; de Batista, P.R.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. BioMed Res. Int. 2012, 2012, 949048. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am. J. Pathol. 2004, 165, 1385–1394. [Google Scholar] [CrossRef]
- Environmental Influences on the Immune System; Springer: Vienna, Austria, 2016.
- Rahaman, S.A.; Roy, B.; Mandal, S.; Bandyopadhyay, S. A Kamikaze Approach for Capturing Hg2+ Ions through the Formation of a One-Dimensional Metal–Organometallic Polymer. Inorg. Chem. 2016, 55, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Rissanen, T.H.; Voutilainen, S.; Tuomainen, T.-P. Mercury as a risk factor for cardiovascular diseases. J. Nutr. Biochem. 2007, 18, 75–85. [Google Scholar] [CrossRef]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef]
- Gundacker, C.; Gencik, M.; Hengstschläger, M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: Mercury and lead. Mutat. Res. 2010, 705, 130–140. [Google Scholar] [CrossRef]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110, 11–23. [Google Scholar] [CrossRef]
- Itri, F.M. Environmental Mercury Problem; CRC Press: Cleveland, OH, USA, 1972. [Google Scholar]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Gupta, S.; Kershaw, S.V.; Rogach, A.L. 25th anniversary article: Ion exchange in colloidal nanocrystals. Adv. Mater. 2013, 25, 6923–6944. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.; Jiang, X.; Chen, X. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 14. [Google Scholar]
- Dash, H.R.; Das, S. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrad. 2012, 75, 207–213. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sulochana, N. Mercury adsorption on a carbon sorbent derived from fruit shell of Terminalia catappa. J. Hazard. Mater. 2006, 133, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, M.; Asl, A.H.; Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, C.W.; Gullett, B.K. Importance of activated carbon’s oxygen surface functional groups on elemental mercury adsorption. Fuel 2003, 82, 451–457. [Google Scholar] [CrossRef]
- Mohan, D.; Gupta, V.K.; Srivastava, S.K.; Chander, S. Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids Surf. A 2001, 177, 169–181. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira ML, M.; Guibal, E.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Chandra, V.; Kim, K.S. Highly selective adsorption of Hg2+ by a polypyrrole–reduced graphene oxide composite. Chem. Commun. 2011, 47, 3942–3944. [Google Scholar] [CrossRef]
- Duan, Y.; Han, D.S.; Batchelor, B.; Abdel-Wahab, A. Synthesis, characterization, and application of pyrite for removal of mercury. Colloids Surf. A 2016, 490, 326–335. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Li, S.; Yan, L. Synthesis and adsorption/photocatalysis performance of pyrite FeS2. Appl. Surf. Sci. 2013, 268, 213–217. [Google Scholar] [CrossRef]
- Weisener, C.; Gerson, A. Cu (II) adsorption mechanism on pyrite: An XAFS and XPS study. Surf. Interface Anal. 2000, 30, 454–458. [Google Scholar] [CrossRef]
- Wang, X.; Forssberg, E.; Bolin, N.J. Adsorption of copper (II) by pyrite in acidic to neutral pH media. Scand. J. Metall. 1989, 18, 262–270. [Google Scholar]
- Erdem, M.; Ozverdi, A. Kinetics and thermodynamics of Cd (II) adsorption onto pyrite and synthetic iron sulphide. Sep. Purif. Technol. 2006, 51, 240–246. [Google Scholar] [CrossRef]
- Borah, D.; Senapati, K. Adsorption of Cd (II) from aqueous solution onto pyrite. Fuel 2006, 85, 1929–1934. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Li, P.; Liu, H.; Xie, J.; Xie, Q.; Zhan, X. Removal and recovery of Cu and Pb from single-metal and Cu–Pb–Cd–Zn multimetal solutions by modified pyrite: Fixed-bed columns. Ind. Eng. Chem. Res. 2014, 53, 18180–18188. [Google Scholar] [CrossRef]
- Toulmin, P., III.; Barton, P.B., Jr. A thermodynamic study of pyrite and pyrrhotite. Geochim. Cosmochim. Acta 1964, 28, 641–671. [Google Scholar] [CrossRef]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite reaction kinetics: Reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim. Cosmochim. Acta 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Lu, P.; Chen, T.; Liu, H.; Li, P.; Peng, S.; Yang, Y. Green Preparation of Nanoporous Pyrrhotite by Thermal Treatment of Pyrite as an Effective Hg (II) Adsorbent: Performance and Mechanism. Minerals 2019, 9, 74. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Liu, H.; Xu, B.; Xie, J. Kinetics and thermodynamics of Eu (III) and U (VI) adsorption onto palygorskite. J. Mol. Liq. 2016, 219, 272–278. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, J.Z.; Wang, J.; Xie, J.-J.; Zhu, C.-Z.; Zhan, X.-M. Phosphorus removal from aqueous solutions containing low concentration of phosphate using pyrite calcinate sorbent. Int. J. Environ. Sci. Technol. 2015, 12, 885–892. [Google Scholar] [CrossRef]
- Huang, S.; Ma, C.; Liao, Y.; Min, C.; Du, P.; Jiang, Y. Removal of Mercury(II) from Aqueous Solutions by Adsorption on Poly(1-amino-5-chloroanthraquinone) Nanofibrils: Equilibrium, Kinetics, and Mechanism Studies. J. Nanomater 2016, 2016, 7245829. [Google Scholar] [CrossRef]
- GB8978. Integrated Wastewater Discharge Standard; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 1996. Available online: http://kjs.mee.gov.cn/hjbhbz/bzwb/shjbh/swrwpfbz/199801/W020061027521858212955.pdf (accessed on 31 December 2019).
- Vieira, R.S.; Beppu, M.M. Dynamic and static adsorption and desorption of Hg (II) ions on chitosan membranes and spheres. Water Res. 2006, 40, 1726–1734. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Q.; Gong, F.; Sugita, S.; Shoya, M. Adsorption of lead and mercury by rice husk ash. J. Colloid Interface Sci. 2004, 278, 1–8. [Google Scholar] [CrossRef]
- Ni, J.A. Inorganic and Analytical Chemistry; Chemical Industry Press: Beijing, China, 1998. [Google Scholar]
- Mews, A.; Kadavanich, A.V.; Banin, U.; Alivisatos, A.P. Structural and spectroscopic investigations of CdS/HgS/CdS quantum-dot quantum wells. Phys. Rev. B 1996, 53, R13242. [Google Scholar] [CrossRef]
- Ehrhardt, J.J.; Behra, P.; Bonnissel-Gissinger, P.; Alnot, M. XPS study of the sorption of Hg(II) onto pyrite FeS2. Surf. Interface Anal. 2000, 30, 269–272. [Google Scholar] [CrossRef]
- Behra, P.; Bonnissel-Gissinger, P.; Alnot, M.; Revel, R.; Ehrhardt, J.J. XPS and XAS study of the sorption of Hg(II) onto pyrite. Langmuir 2001, 17, 3970–3979. [Google Scholar] [CrossRef]
- Mycroft, J.R.; Nesbitt, H.W.; Pratt, A.R. X-ray photoelectron and Auger electron spectroscopy of air-oxidized pyrrhotite: Distribution of oxidized species with depth. Geochim. Cosmochim. Acta 1995, 59, 721–733. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. X-ray photoelectron spectroscopy of oxidised pyrrhotite surfaces. Appl. Surf. Sci. 1985, 20, 472–480. [Google Scholar] [CrossRef]
- Pratt, A.R.; Muir, I.J.; Nesbitt, H.W. X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar] [CrossRef]

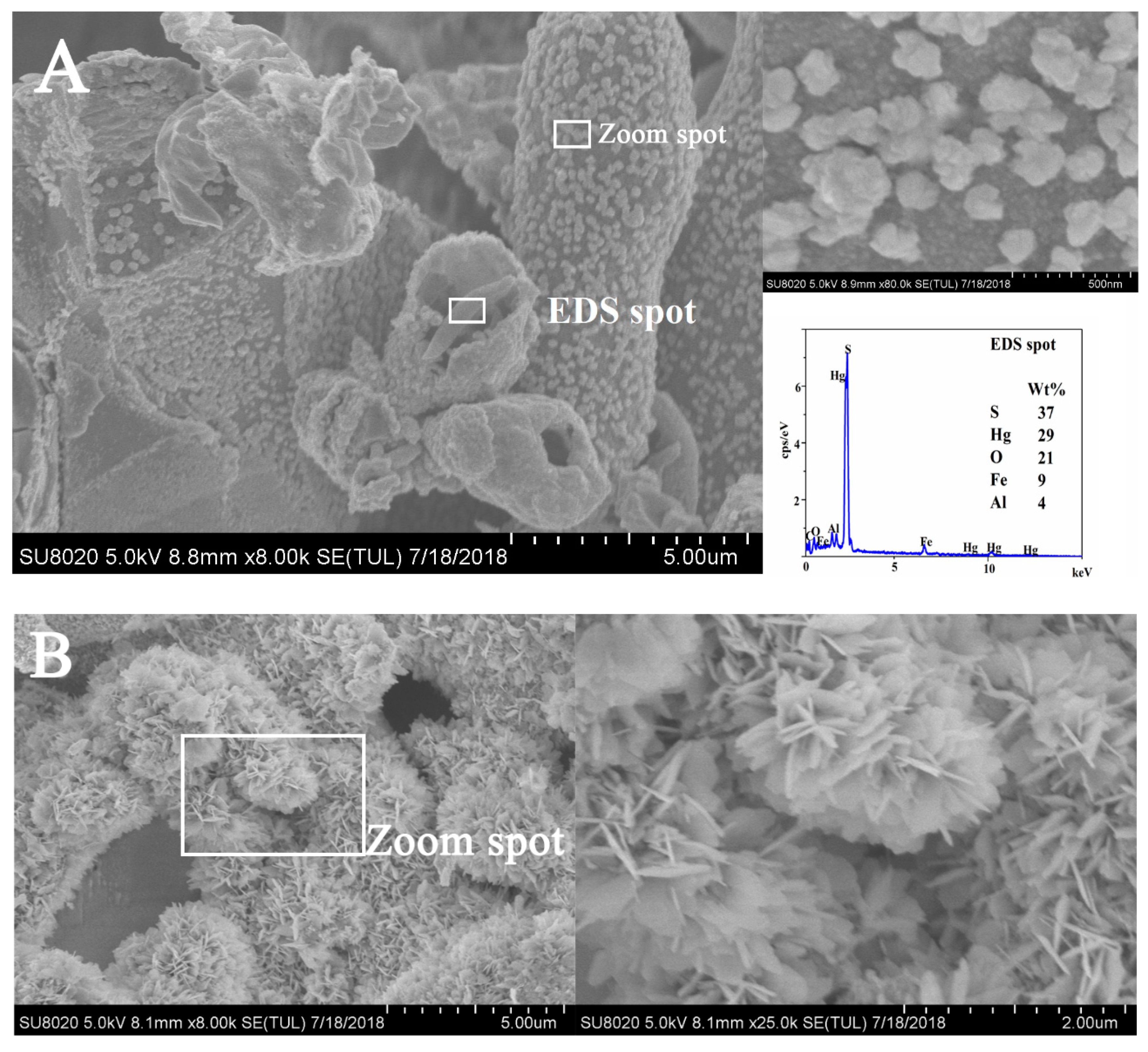


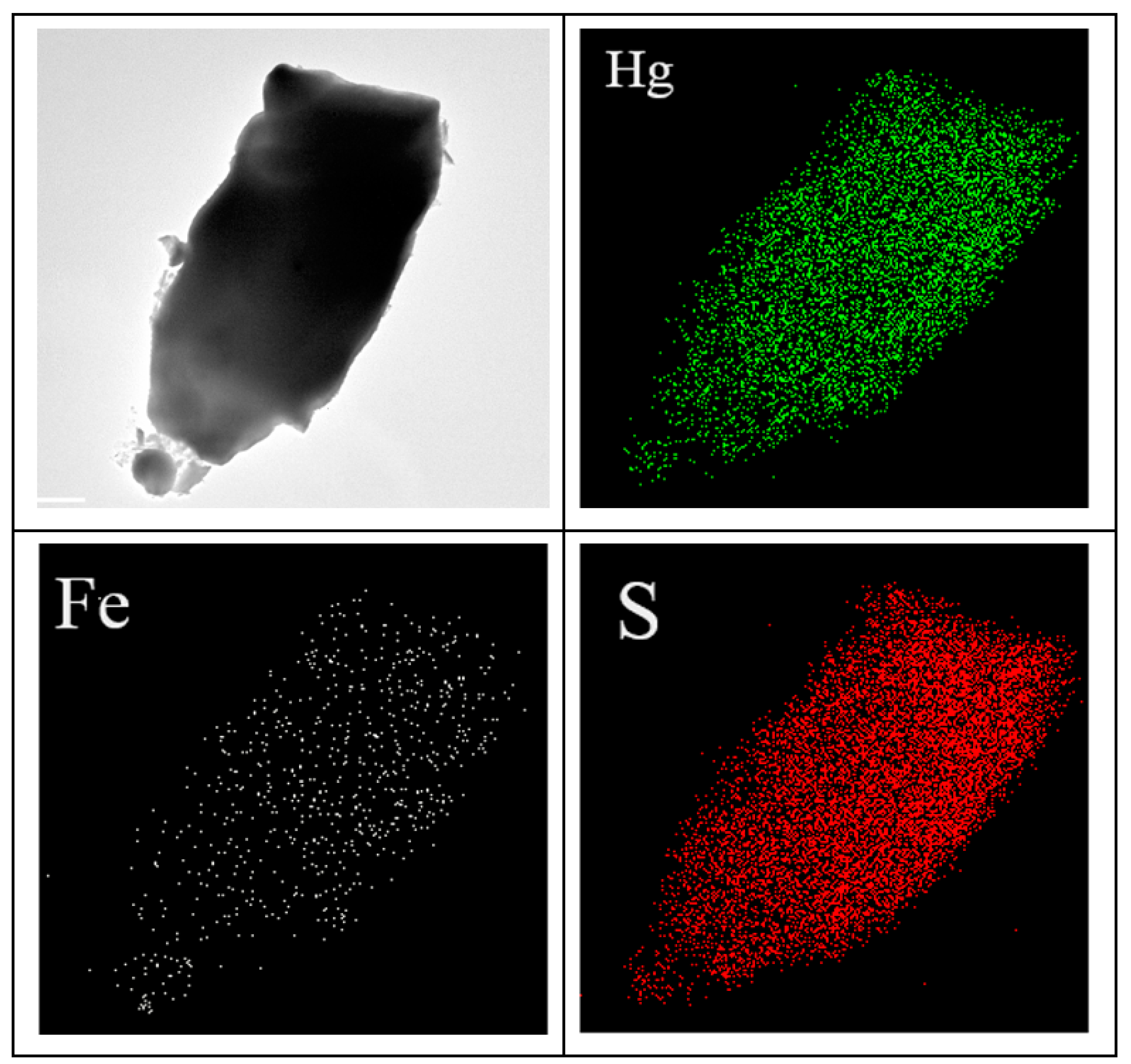
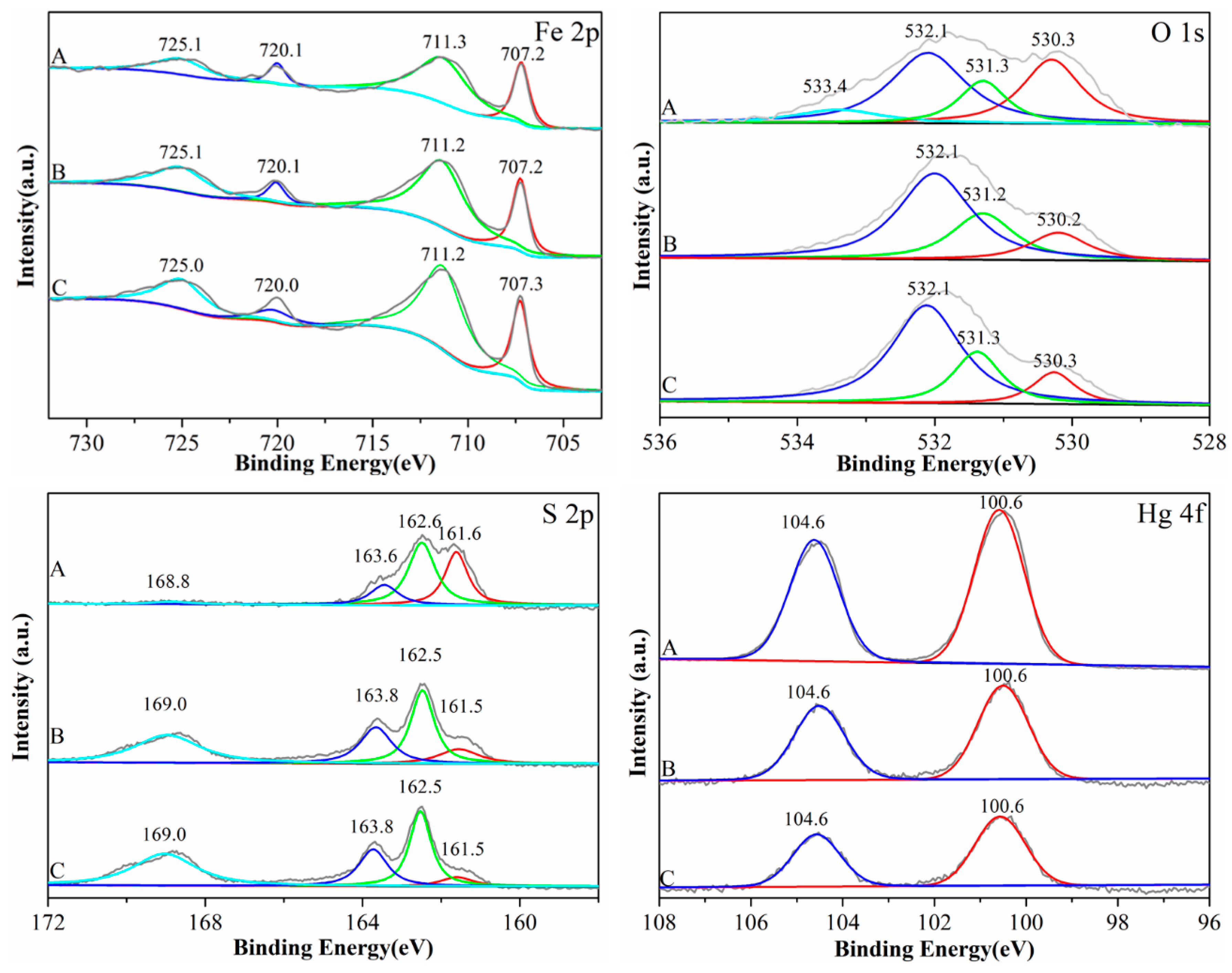
| Heavy Metal Pollutants | Optimum Calcination Temperature and Time | Experiment Form | Qmax(mg/g) | References |
|---|---|---|---|---|
| Cu(II) | 600 °C, 1 h | Column | 77.42 | [28] |
| Pb(II) | 600 °C, 1 h | Column | 73.68 | [28] |
| Cu(II)-Pb(II)-Cd(II)-Zn(II) multiple solution | 600 °C, 1 h | Column | 30.79 | [28] |
| Hg(II) | 600 °C, 0.5 h | Batch | 166.67 | [31] |
| Eu(III) | 650 °C, 1.5 h | Batch | 10.03 | [32] |
| Phosphorus | 600 °C, 1 h | Batch | 5.36 | [33] |
| Phosphorus | 600 °C, 1 h | Column | 0.17 | [33] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Peng, S.; Lu, P.; Chen, T.; Yang, Y. Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment. Minerals 2020, 10, 43. https://doi.org/10.3390/min10010043
Zhu Y, Peng S, Lu P, Chen T, Yang Y. Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment. Minerals. 2020; 10(1):43. https://doi.org/10.3390/min10010043
Chicago/Turabian StyleZhu, Yucheng, Shuchuan Peng, Ping Lu, Tianhu Chen, and Yan Yang. 2020. "Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment" Minerals 10, no. 1: 43. https://doi.org/10.3390/min10010043
APA StyleZhu, Y., Peng, S., Lu, P., Chen, T., & Yang, Y. (2020). Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment. Minerals, 10(1), 43. https://doi.org/10.3390/min10010043




