Chiral Buckybowl Molecules
Abstract
:1. Introduction
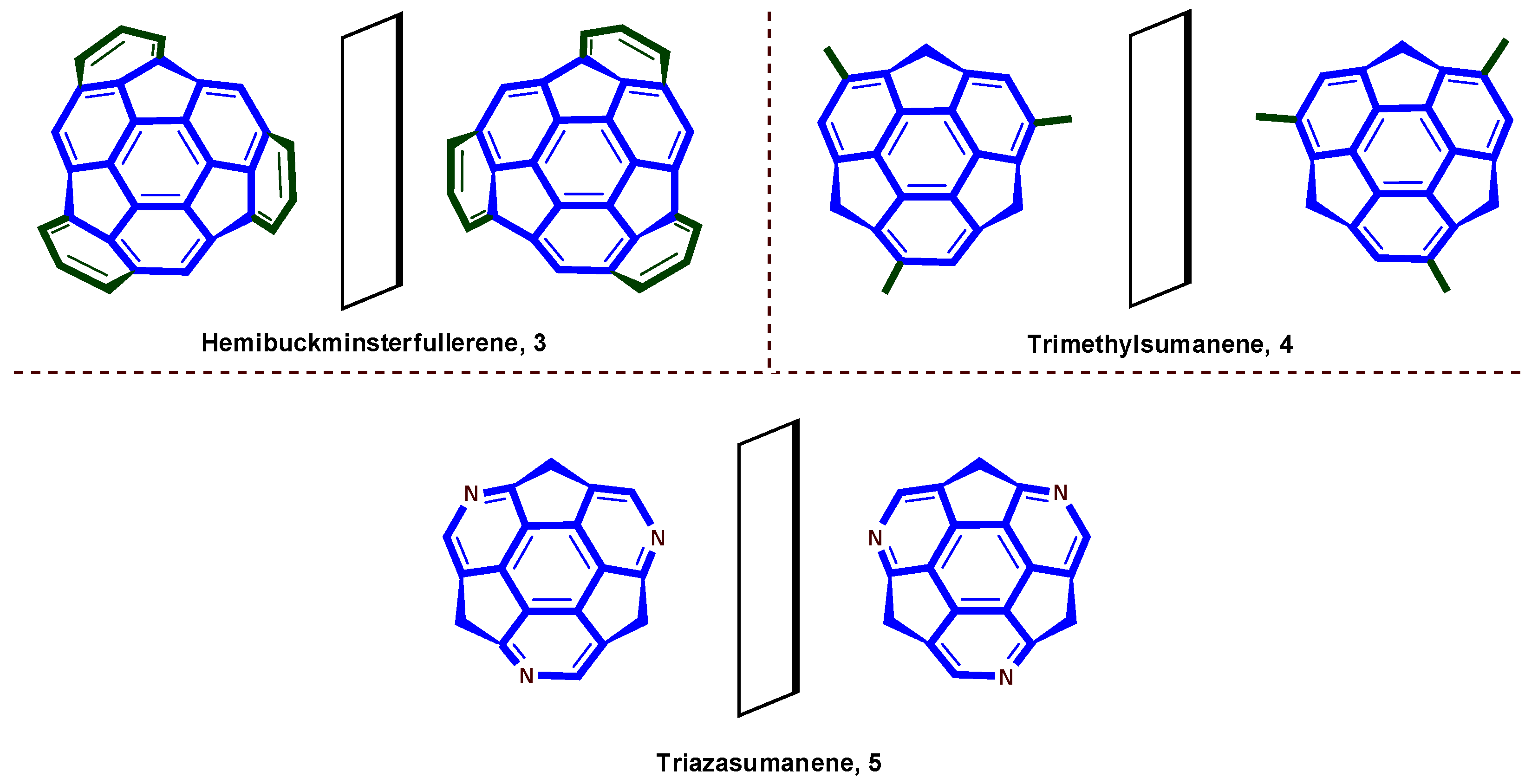
2. Classification of Bowl Chirality
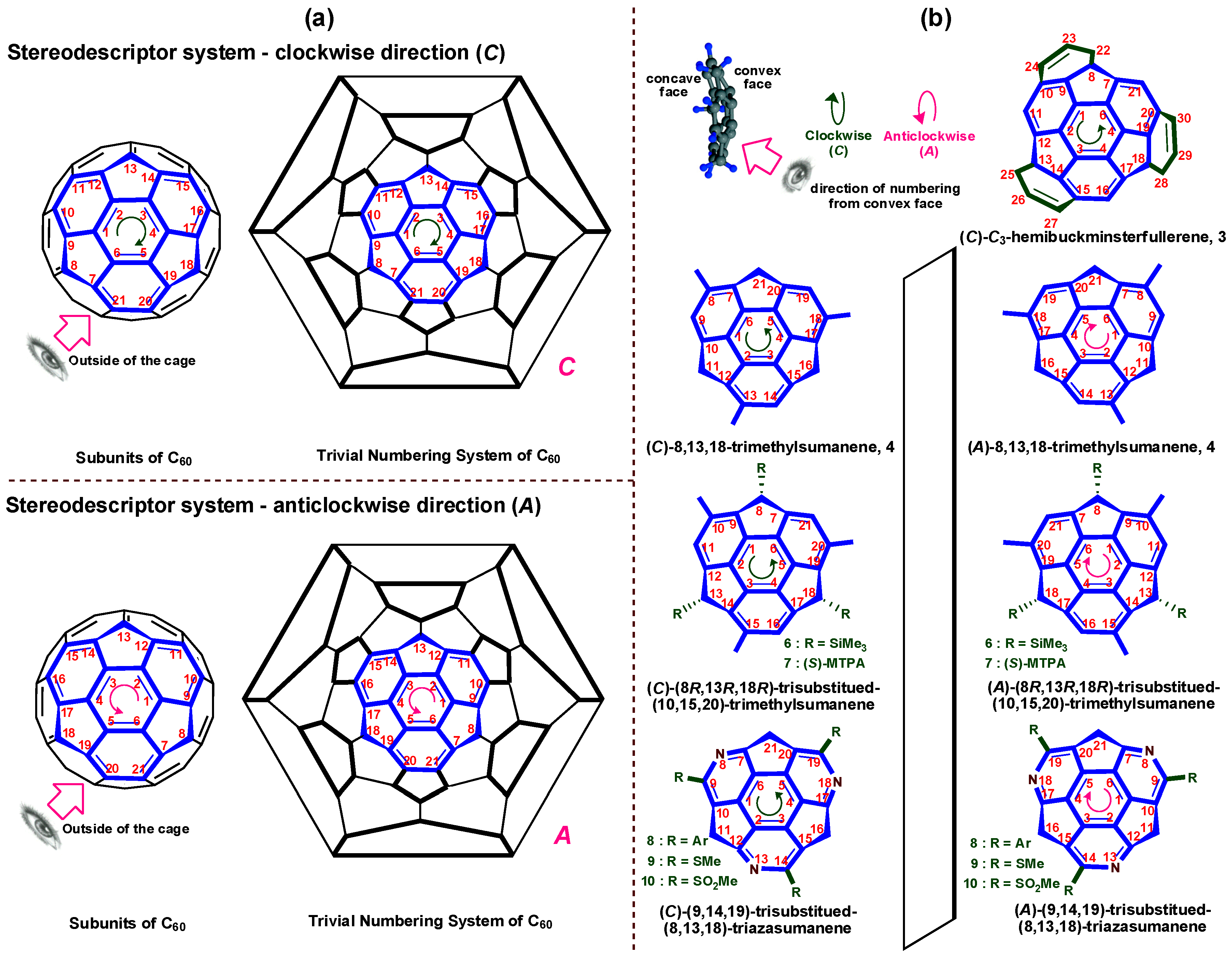
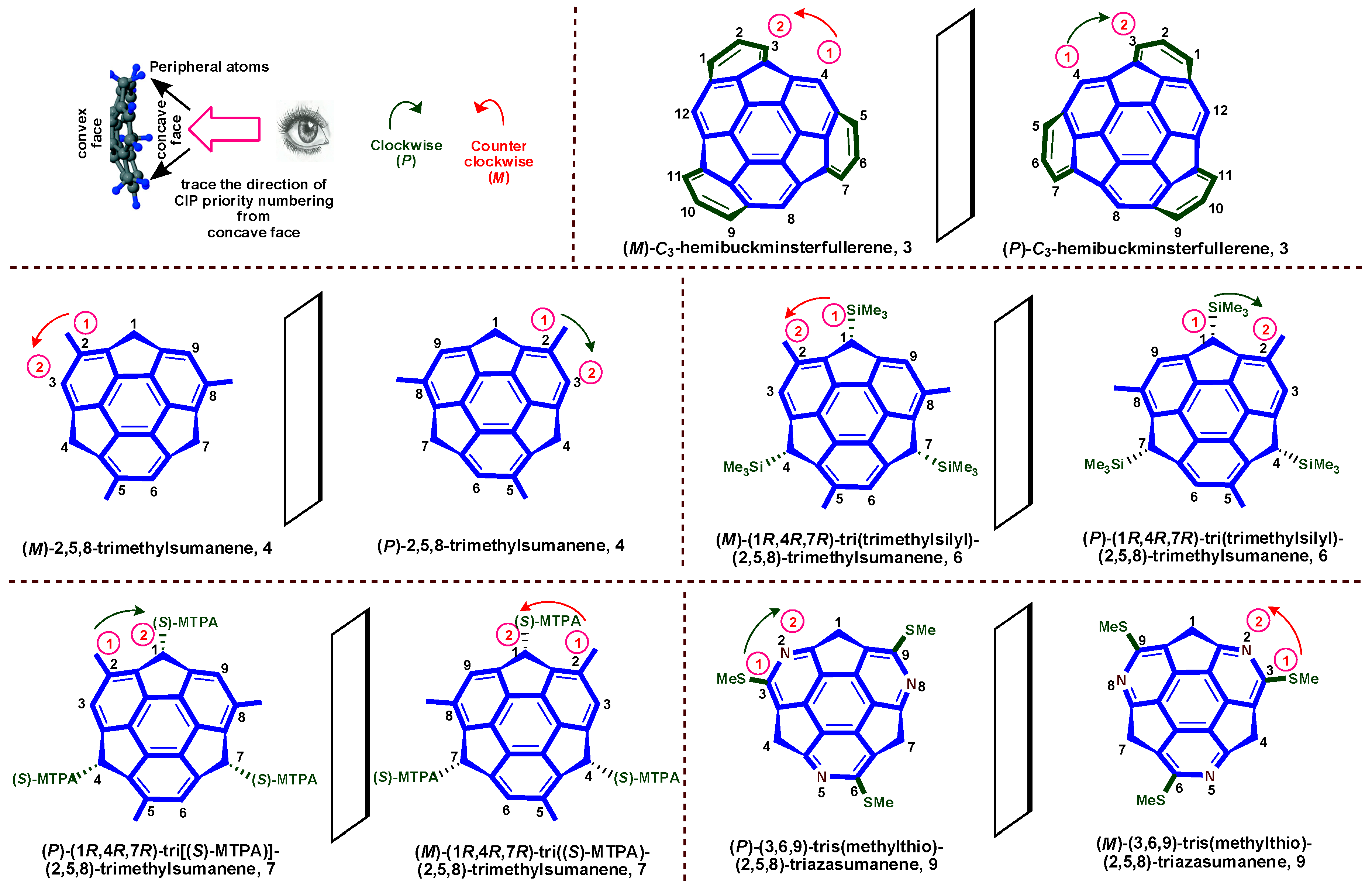
3. Stereodescriptor System of Buckybowls
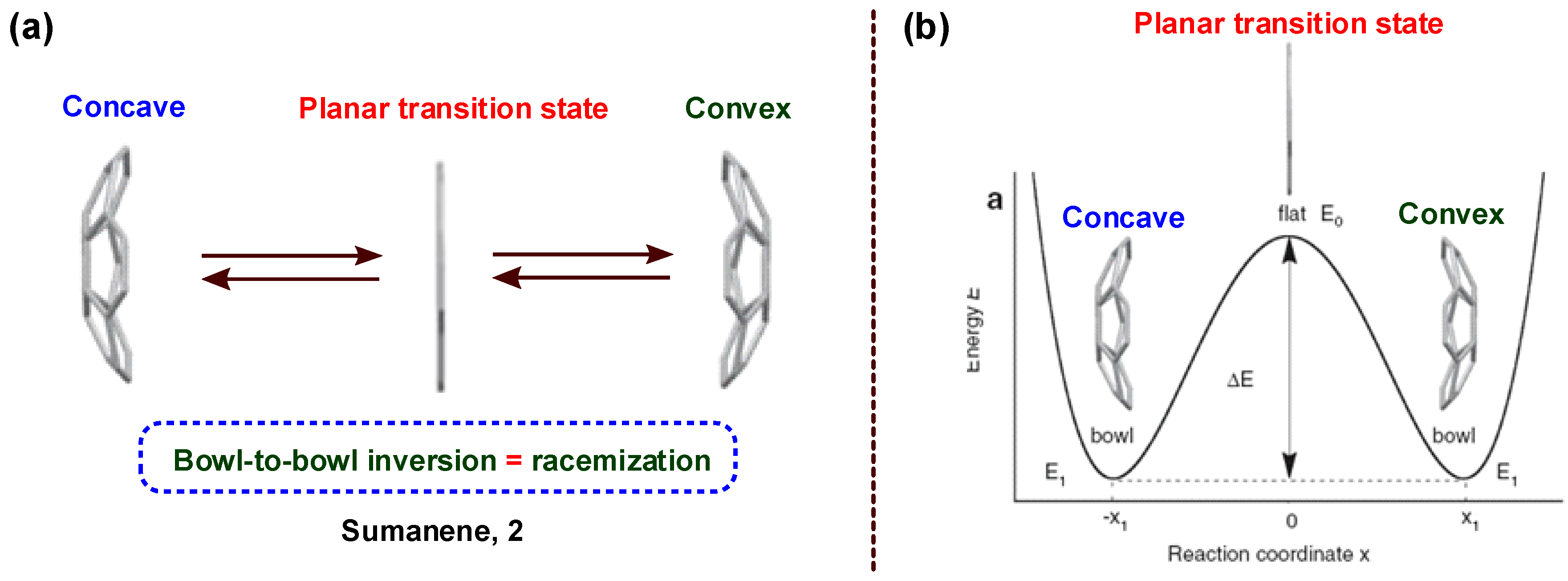
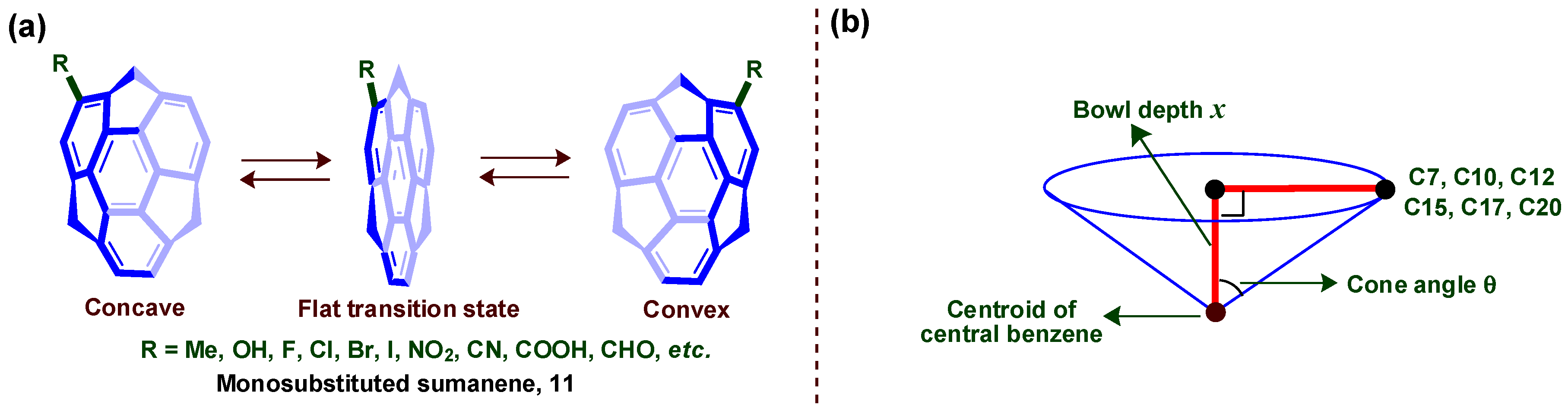
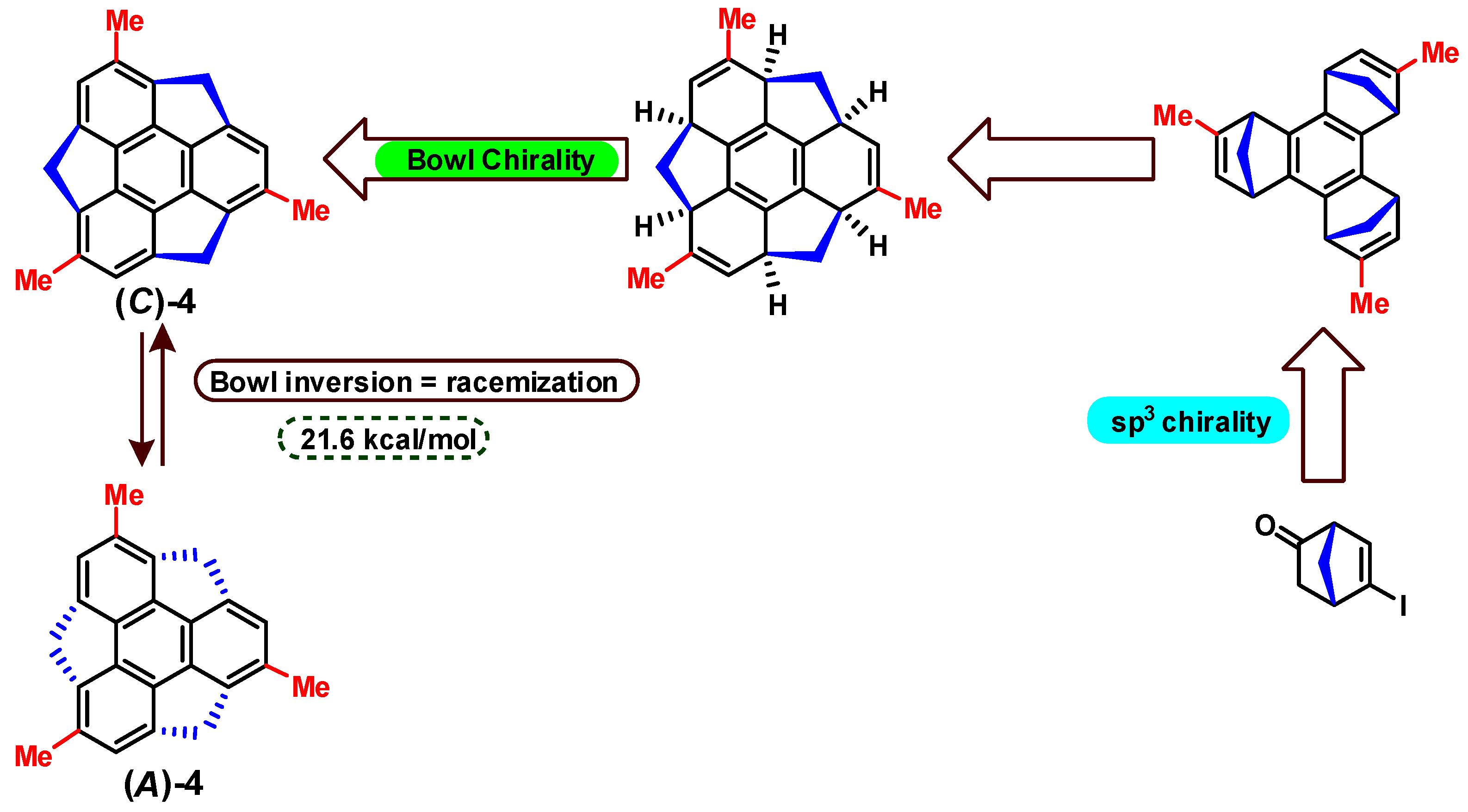
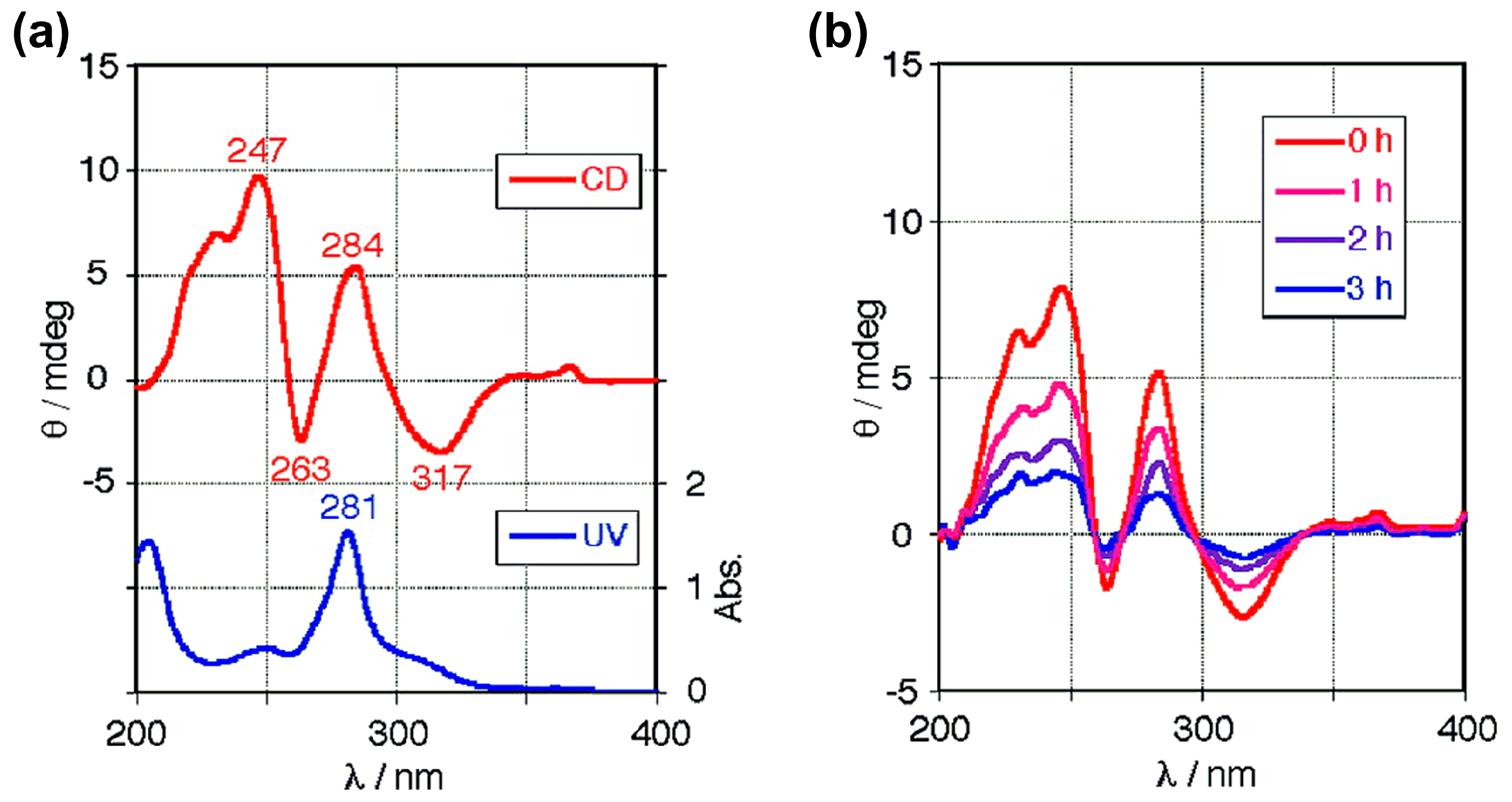
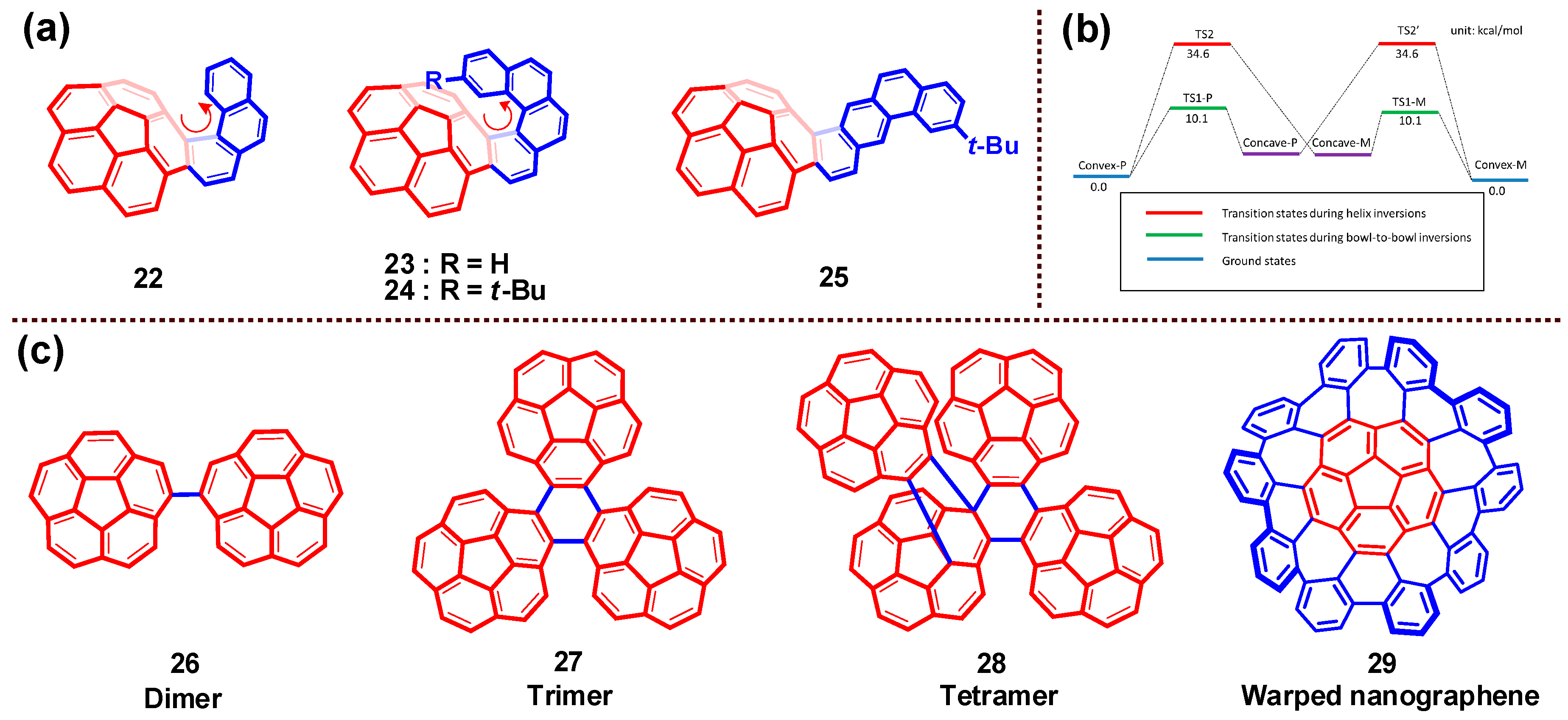
4. Bowl Inversion/Racemization of Chiral Buckybowls
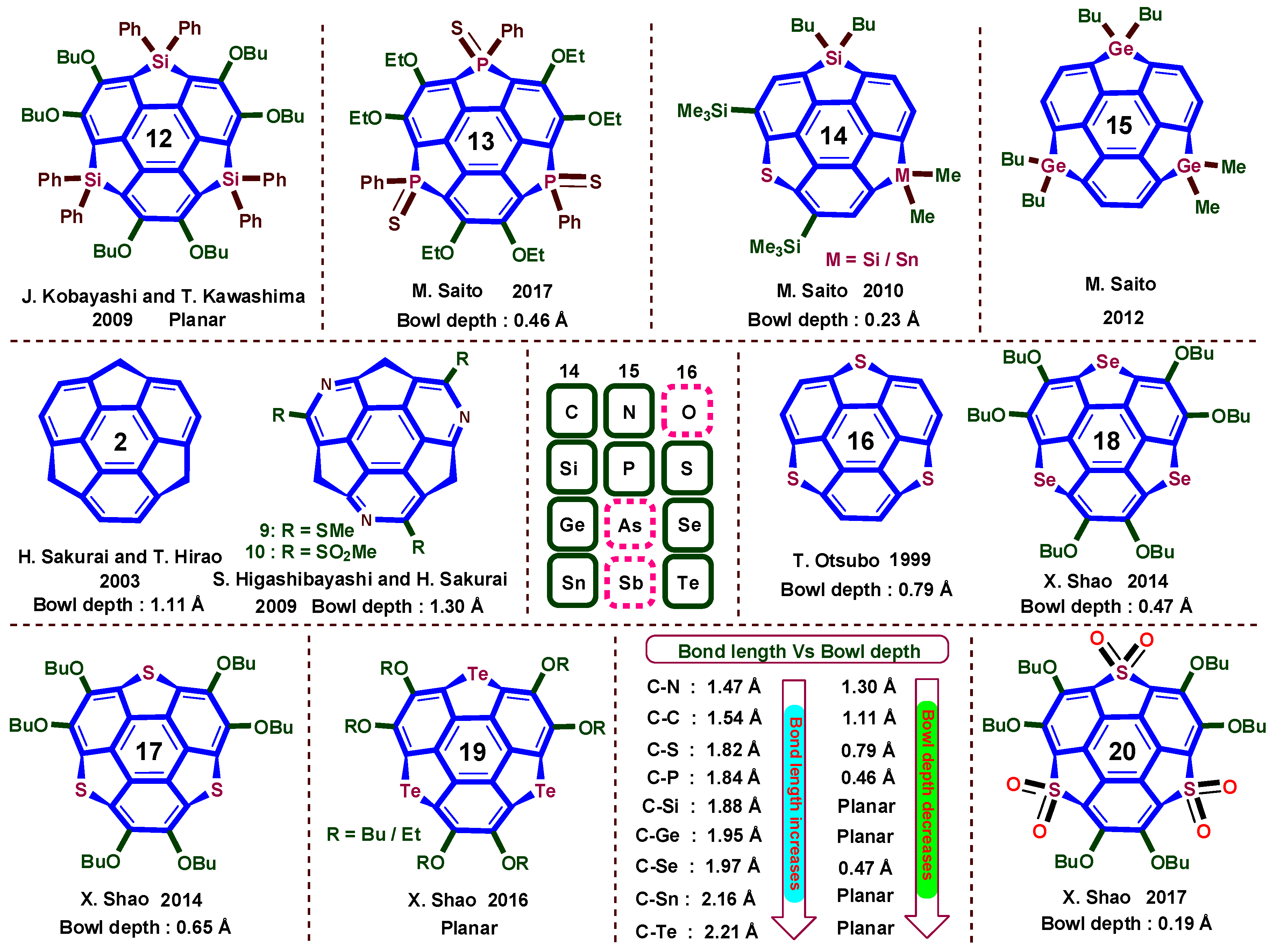
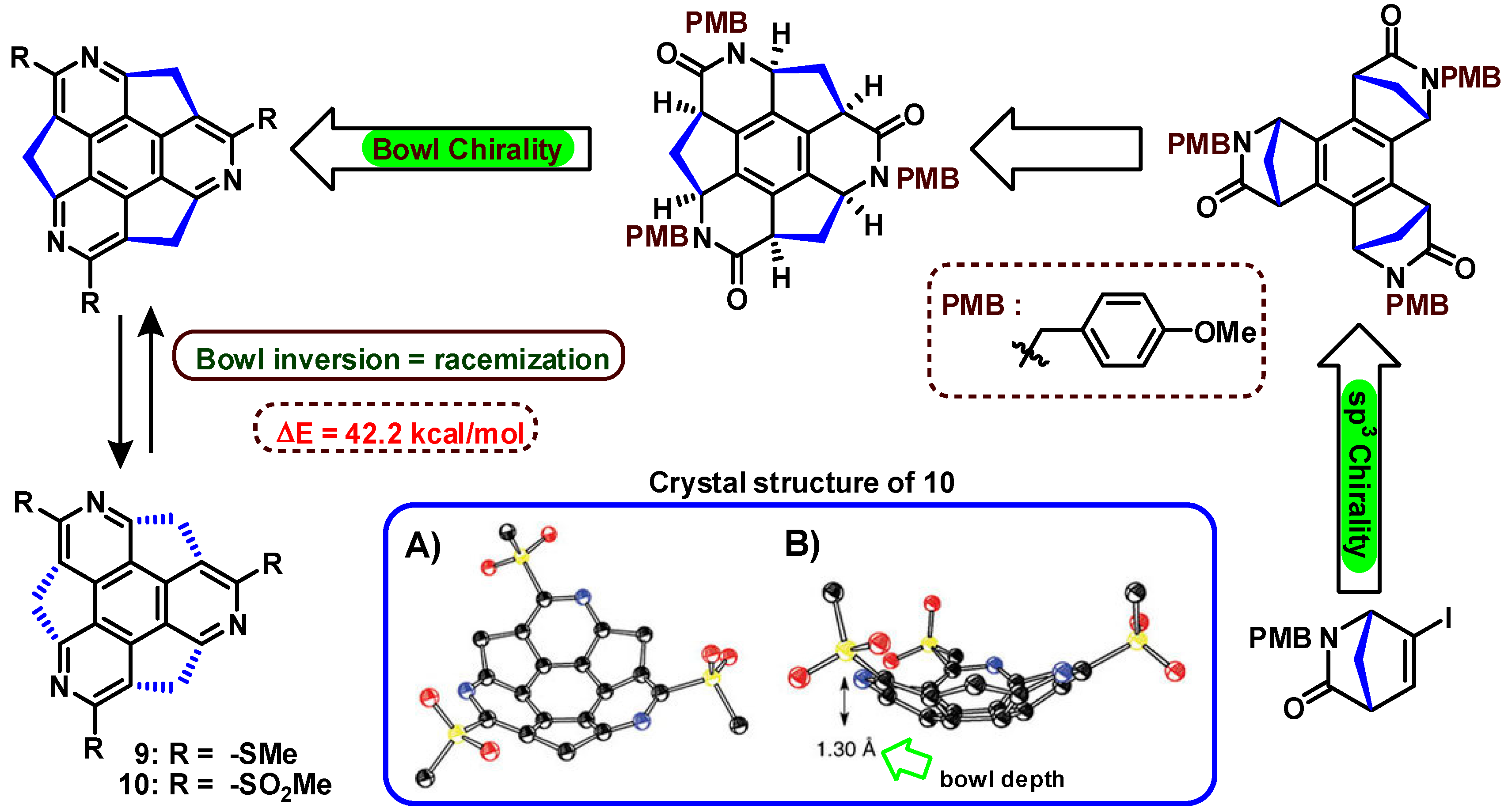
5. Heterobuckybowls
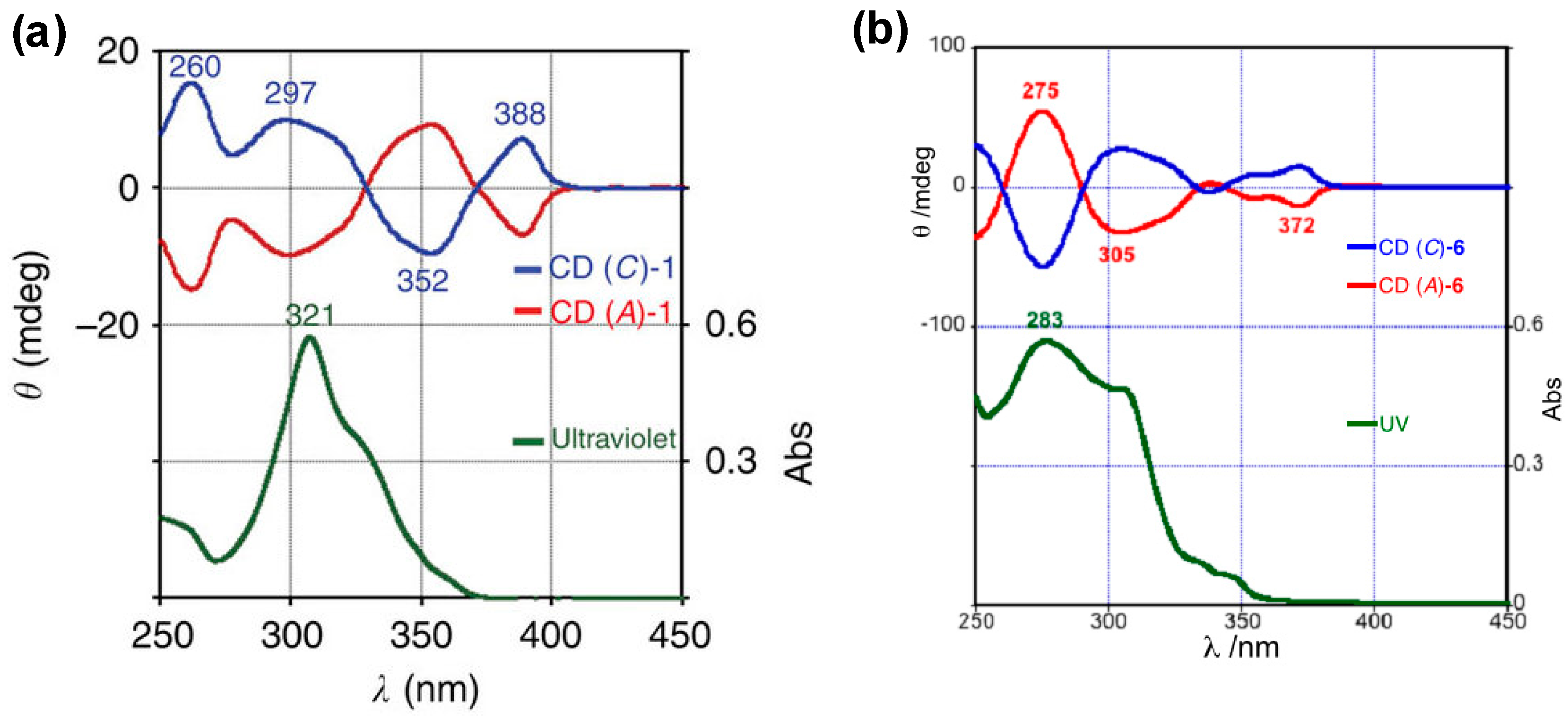
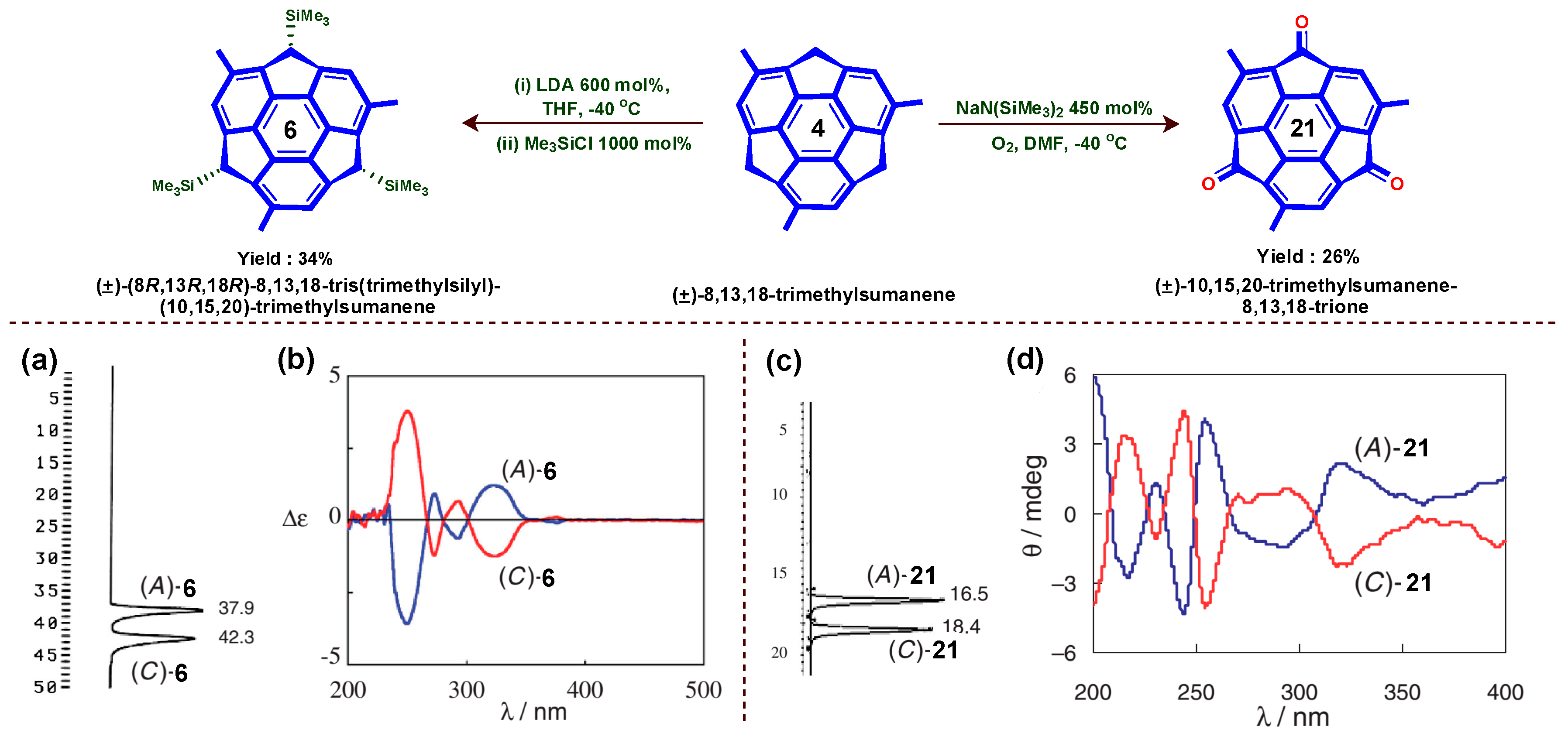
6. Asymmetric Synthesis of Buckybowl and Azabuckybowl
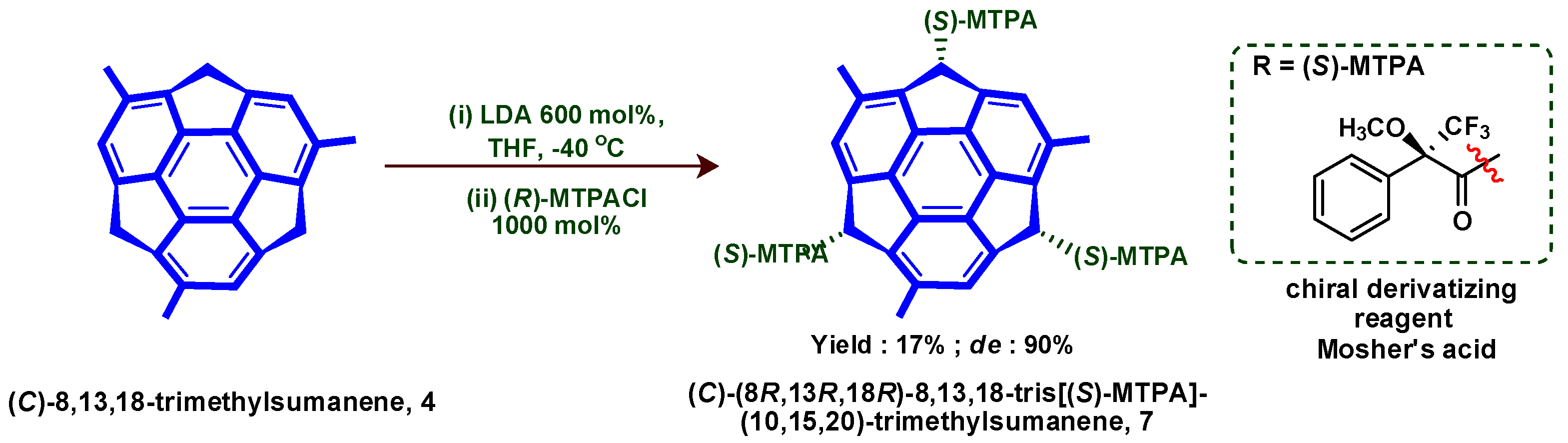
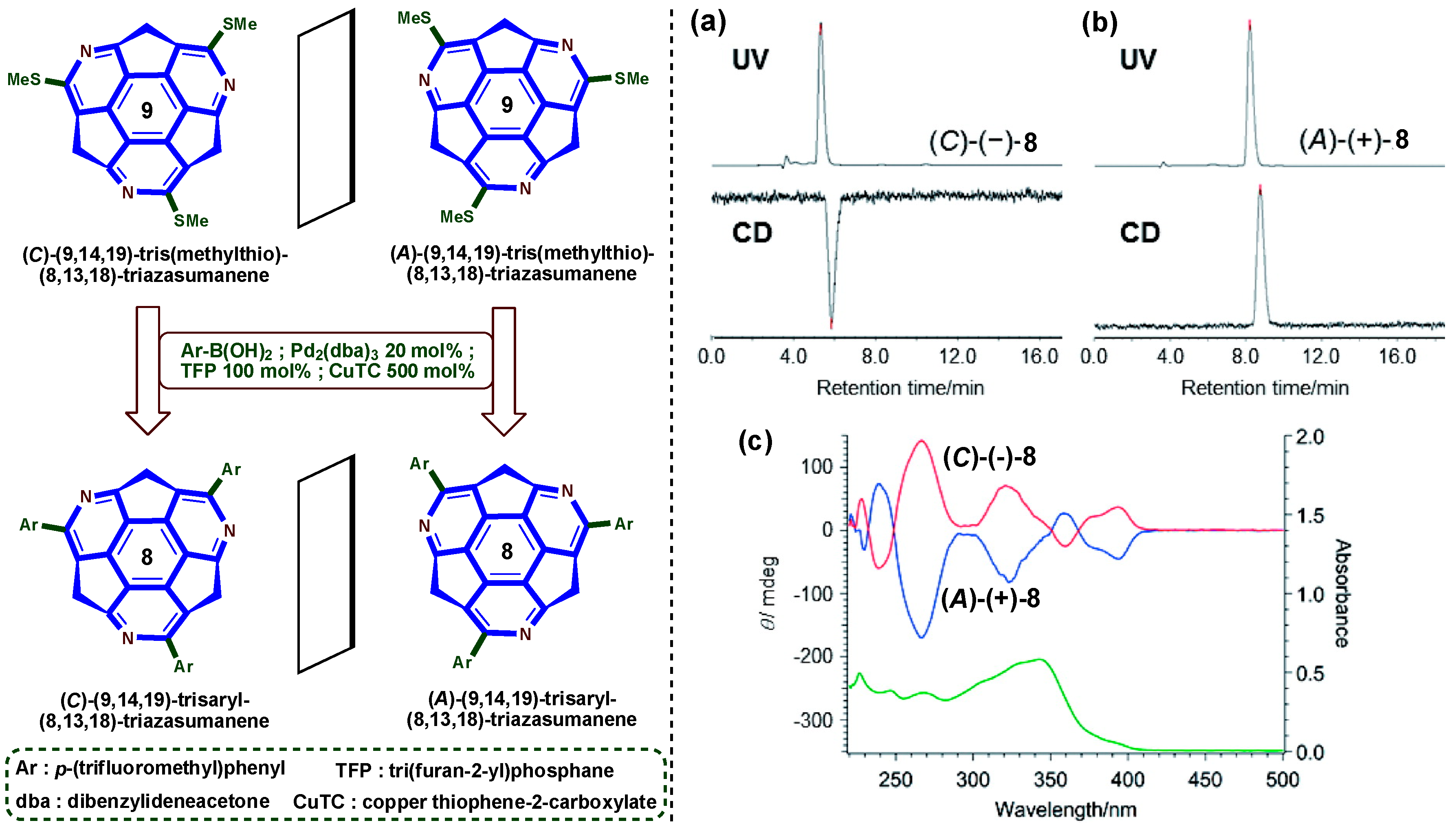
7. Chiral Resolution of Buckybowls
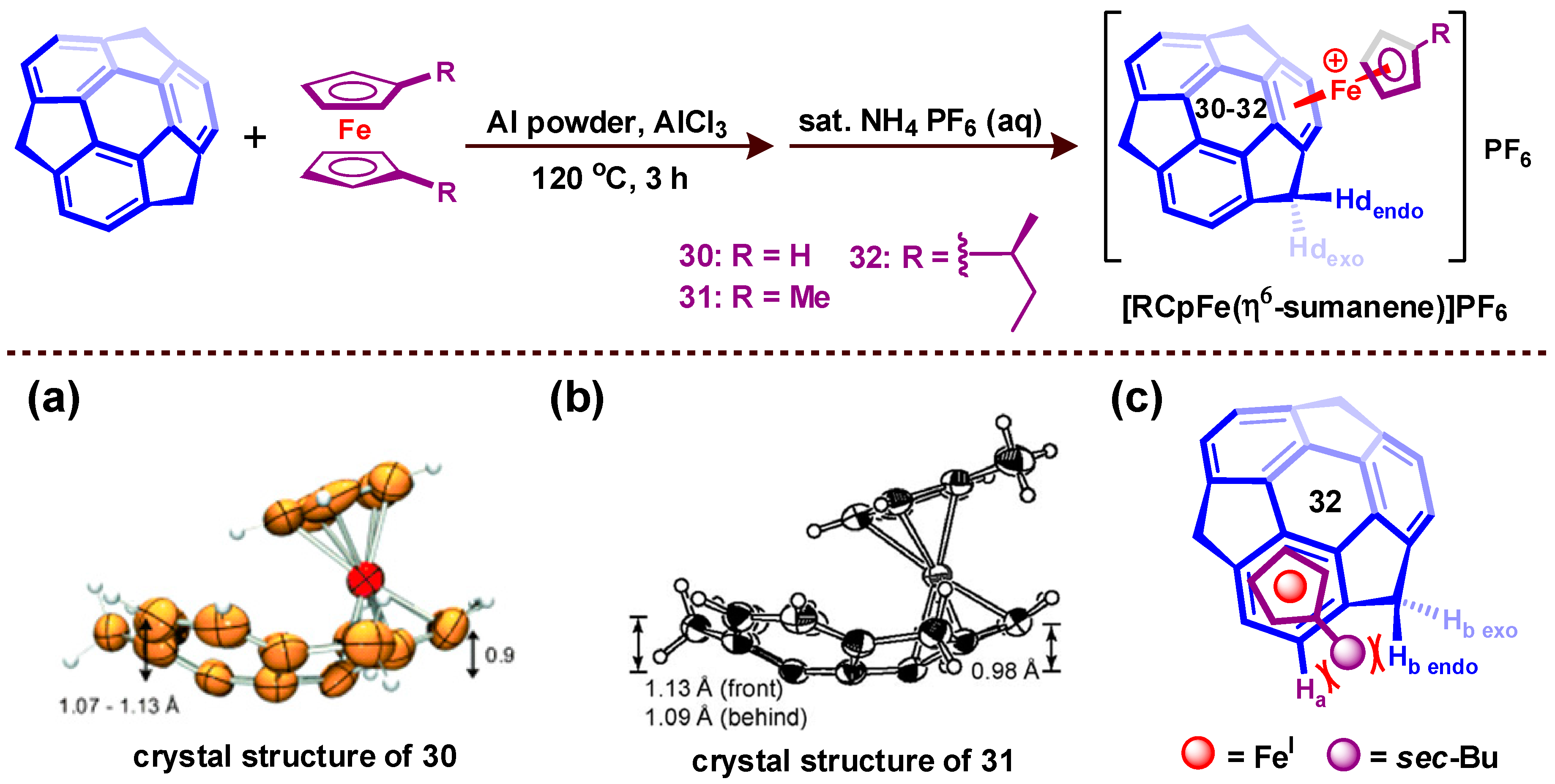
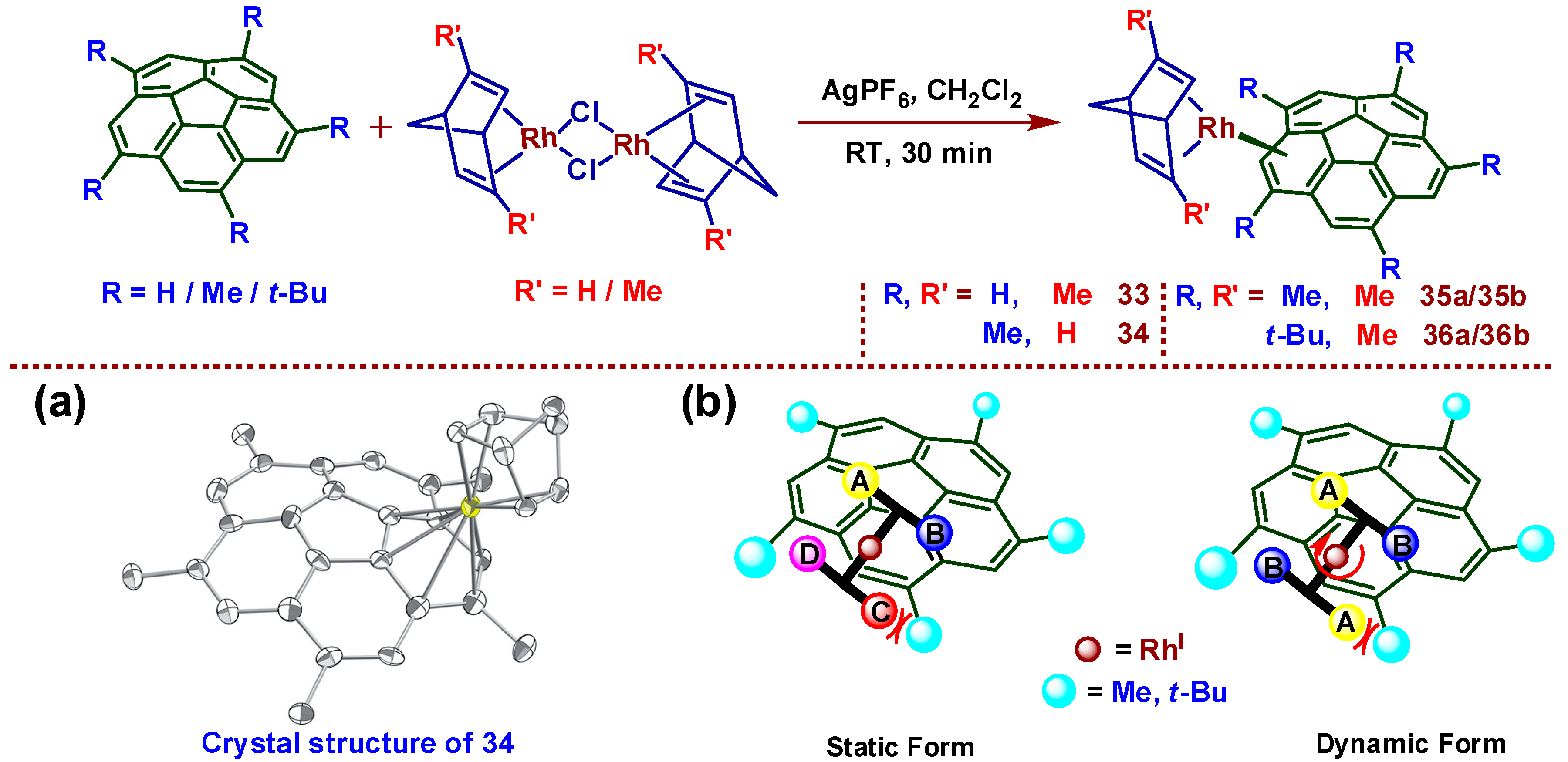
8. Chiral Metal Complexes of Buckybowls
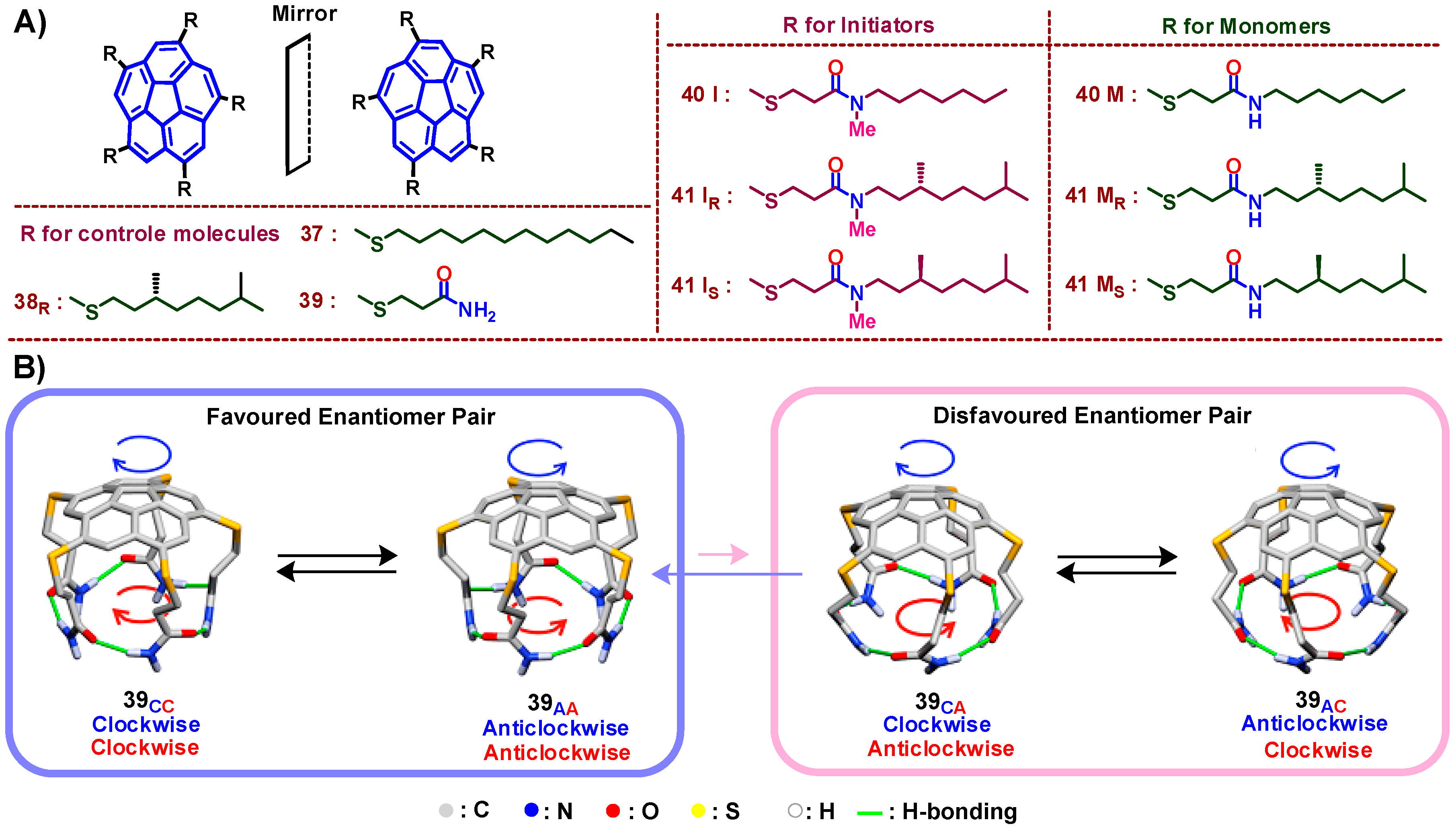
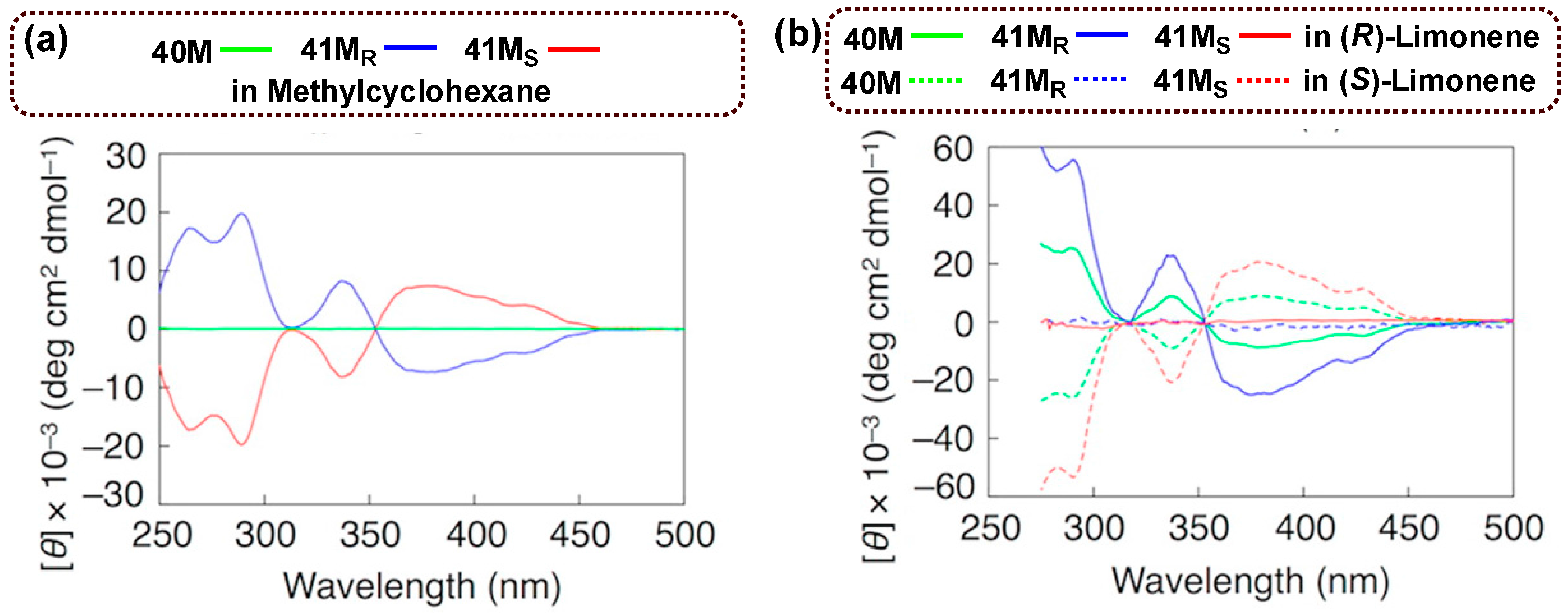
9. Chiral Assembly of Buckybowls
10. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, K.; Lei, T.; Wang, X.-Y.; Wang, J.-Y.; Pei, J. A bowl-shaped molecule for organic field-effect transistors: Crystal engineering and charge transport switching by oxygen doping. Chem. Sci. 2014, 5, 1041–1045. [Google Scholar] [CrossRef]
- Xiao, W.; Passerone, D.; Ruffieux, P.; Aït-Mansour, K.; Gröning, O.; Tosatti, E.; Siegel, J.S.; Fasel, R. C60/corannulene on Cu(110): A surface-supported bistable buckybowl-buckyball host-guest system. J. Am. Chem. Soc. 2008, 130, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, D.; Tashiro, K.; Araoka, F.; Takezoe, H.; Kim, J.; Kato, K.; Takata, M.; Aida, T. Liquid crystalline corannulene responsive to electric field. J. Am. Chem. Soc. 2009, 131, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Lovas, F.J.; McMahon, R.J.; Grabow, J.-U.; Schnell, M.; Mack, J.; Scott, L.T.; Kuczkowski, R.L. Interstellar chemistry: A strategy for detecting polycyclic aromatic hydrocarbons in space. J. Am. Chem. Soc. 2005, 127, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, L.; Wang, X.; Cao, H.; Liu, M. Supramolecular chirality in self-assembled soft materials: Regulation of chiral nanostructures and chiral functions. Adv. Mater. 2014, 26, 6959–6964. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, F.; Inagaki, M. Buckybowls: Corannulene and its derivatives. Small 2016, 12, 3206–3223. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Hirao, T. Chemistry of sumanene. Chem. Rec. 2015, 15, 310–321. [Google Scholar] [CrossRef] [PubMed]
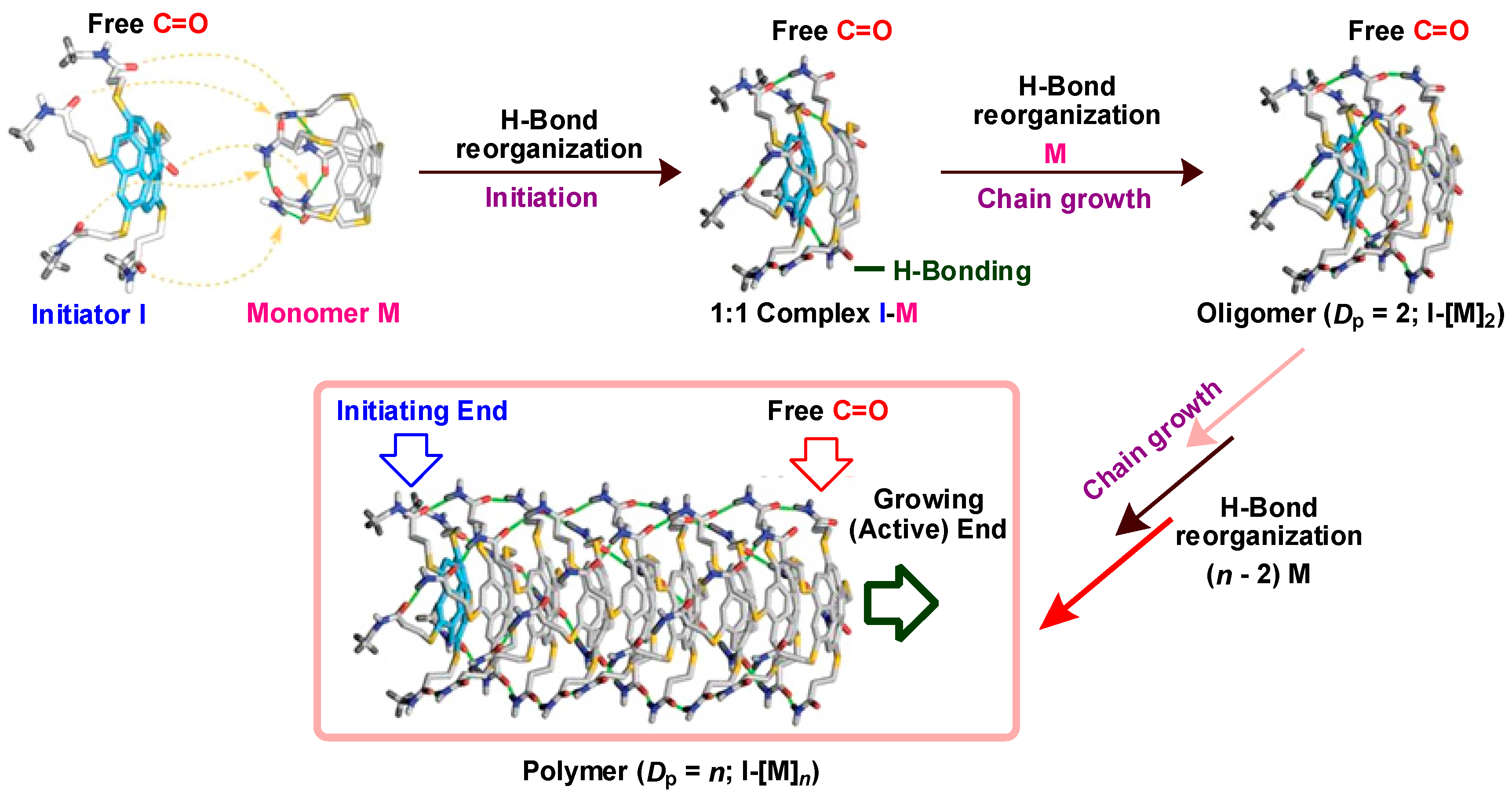
- Kang, J.; Miyajima, D.; Mori, T.; Inoue, Y.; Itoh, Y.; Aida, T. A rational strategy for the realization of chain-growth supramolecular polymerization. Science 2015, 347, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Higashibayashi, S.; Sakurai, H. Synthesis of sumanene and related buckybowls. Chem. Lett. 2011, 40, 122–128. [Google Scholar] [CrossRef]
- Scott, L.T.; Jackson, E.A.; Zhang, Q.; Steinberg, B.D.; Bancu, M.; Li, B. A short, rigid, structurally pure carbon nanotube by stepwise chemical synthesis. J. Am. Chem. Soc. 2012, 134, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Higashibayashi, S.; Karanjit, S.; Sakurai, H. Enantioselective synthesis of a chiral nitrogen-doped buckybowl. Nat. Commun. 2012, 3, 891. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.T.; Hashemi, M.M.; Bratcher, M.S. Corannulene bowl-to-bowl inversion is rapid at room temperature. J. Am. Chem. Soc. 1992, 114, 1920–1921. [Google Scholar] [CrossRef]
- Seiders, T.J.; Baldridge, K.K.; Grube, G.H.; Siegel, J.S. Structure/energy correlation of bowl depth and inversion barrier in corannulene derivatives: Combined experimental and quantum mechanical analysis. J. Am. Chem. Soc. 2001, 123, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Sakane, H.; Muneishi, T.; Hirao, T. Bowl-to-bowl inversion of sumanene derivatives. Chem. Commun. 2008, 765–767. [Google Scholar] [CrossRef]
- Hayama, T.; Baldridge, K.K.; Wu, Y.-T.; Linden, A.; Siegel, J.S. Steric isotope effects gauged by the bowl-inversion barrier in selectively deuterated pentaarylcorannulenes. J. Am. Chem. Soc. 2008, 130, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Higashibayashi, S.; Sakurai, H. Asymmetric synthesis of a chiral buckybowl, trimethylsumanene. J. Am. Chem. Soc. 2008, 130, 8592–8593. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, R.; Higashibayashi, S.; Ishikawa, T.; Toyota, S.; Sakurai, H. Optical resolution of chiral buckybowls by chiral HPLC. Chem. Lett. 2010, 39, 646–647. [Google Scholar] [CrossRef]
- Higashibayashi, S.; Tsuruoka, R.; Soujanya, Y.; Purushotham, U.; Sastry, G.N.; Seki, S.; Ishikawa, T.; Toyota, S.; Sakurai, H. Trimethylsumanene: Enantioselective synthesis, substituent effect on bowl structure, inversion energy, and electron conductivity. Bull. Chem. Soc. Jpn. 2012, 85, 450–467. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Karanjit, S.; Higashibayashi, S.; Sakurai, H. Correlation between bowl-inversion energy and bowl depth in substituted sumanenes. Pure Appl. Chem. 2014, 86, 747–753. [Google Scholar] [CrossRef]
- Kang, J.; Miyajima, D.; Itoh, Y.; Mori, T.; Tanaka, H.; Yamauchi, M.; Inoue, Y.; Harada, S.; Aida, T. C5-symmetric chiral corannulenes: Desymmetrization of bowl inversion equilibrium via “intramolecular” hydrogen-bonding network. J. Am. Chem. Soc. 2014, 136, 10640–10644. [Google Scholar] [CrossRef] [PubMed]
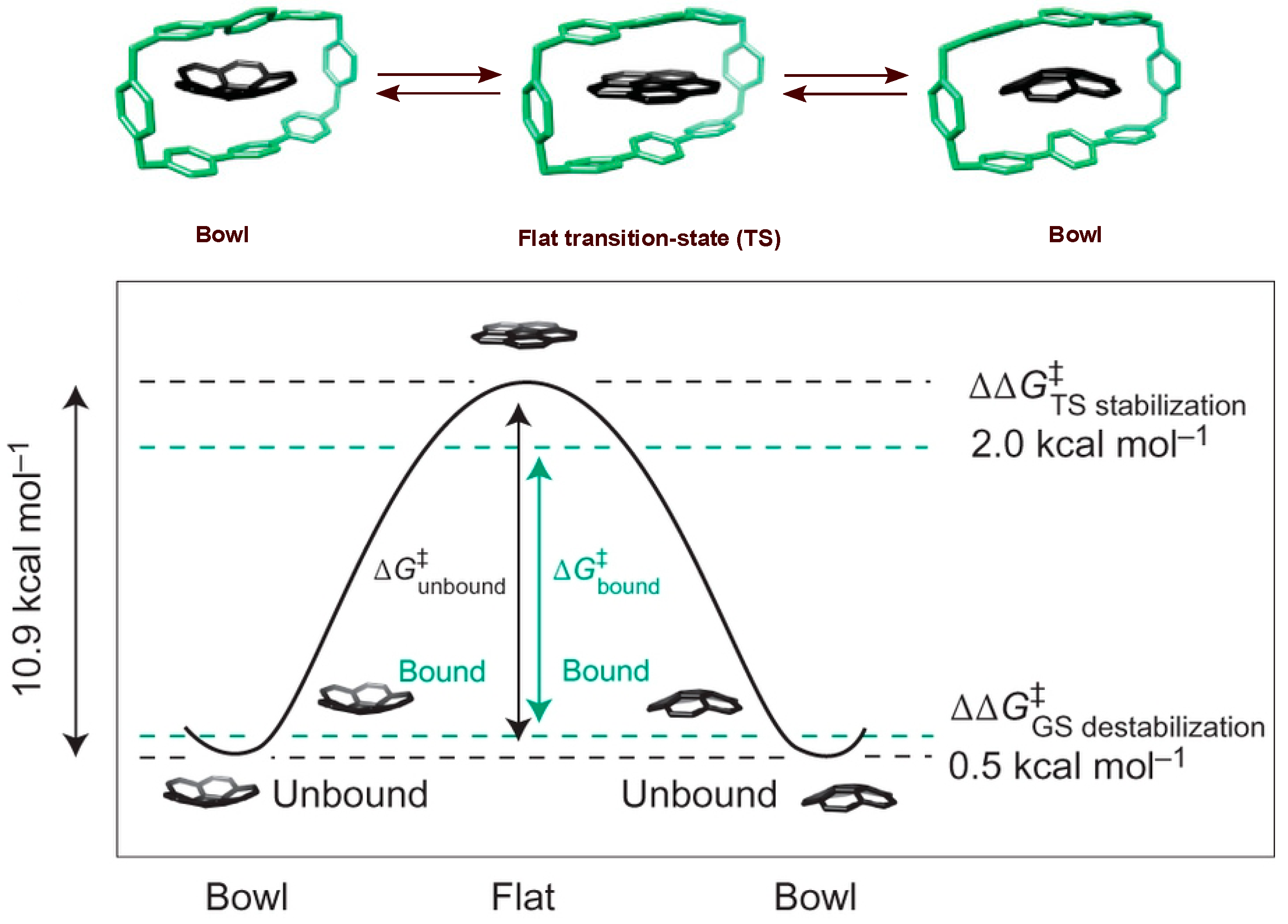
- Juríček, M.; Strutt, N.L.; Barnes, J.C.; Butterfield, A.M.; Dale, E.J.; Baldridge, K.K.; Stoddart, J.F.; Siegel, J.S. Induced-fit catalysis of corannulene bowl-to-bowl inversion. Nat. Chem. 2014, 6, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Seiders, T.J.; Baldridge, K.K.; O’Connor, J.M.; Siegel, J.S. Hexahapto metal coordination to curved polyaromatic hydrocarbon surfaces: The first transition metal corannulene complex. J. Am. Chem. Soc. 1997, 119, 4781–4782. [Google Scholar] [CrossRef]
- Sakurai, H.; Daiko, T.; Sakane, H.; Amaya, T.; Hirao, T. Structural elucidation of sumanene and generation of its benzylic anions. J. Am. Chem. Soc. 2005, 127, 11580–11581. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.B.; Morita, Y.; Kojima, T.; Kawano, M.; Higashibayashi, S.; Sakurai, H. Eclipsed columnar packing in crystal structure of sumanenetrione. Chem. Lett. 2014, 43, 1294–1296. [Google Scholar] [CrossRef]
- Sygula, A.; Yanney, M.; Henry, W.P.; Fronczek, F.R.; Zabula, A.V.; Petrukhina, M.A. Inclusion complexes and solvates of buckycatcher, a versatile molecular host with two corannulene pincers. Cryst. Growth Des. 2014, 14, 2633–2639. [Google Scholar] [CrossRef]
- Yamada, M.; Ohkubo, K.; Shionoya, M.; Fukuzumi, S. Photoinduced electron transfer in a charge-transfer complex formed between corannulene and li+@C60 by concave–convex π–π interactions. J. Am. Chem. Soc. 2014, 136, 13240–13248. [Google Scholar] [CrossRef] [PubMed]
- Merz, L.; Parschau, M.; Zoppi, L.; Baldridge, K.K.; Siegel, J.S.; Ernst, K.-H. Reversible phase transitions in a buckybowl monolayer. Angew. Chem. Int. Ed. 2009, 48, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Bauert, T.; Merz, L.; Bandera, D.; Parschau, M.; Siegel, J.S.; Ernst, K.-H. Building 2d crystals from 5-fold-symmetric molecules. J. Am. Chem. Soc. 2009, 131, 3460–3461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Xia, D.; Li, B.-W.; Zhang, Q.-Y.; Sakurai, T.; Tan, Y.-Z.; Seki, S.; Xie, S.-Y.; Zheng, L.-S. Functional sulfur-doped buckybowls and their concave–convex supramolecular assembly with fullerenes. Angew. Chem. Int. Ed. 2016, 55, 13047–13051. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, P.A.; Alvarez, C.M.; Ellern, A.; Angelici, R.J.; Sygula, A.; Sygula, R.; Rabideau, P.W. Synthesis and structure of a dimetallated buckybowl: Coordination of one {cp*ru}+ unit to each side of corannulene. Angew. Chem. Int. Ed. 2004, 43, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Petrukhina, M.A.; Andreini, K.W.; Peng, L.; Scott, L.T. Hemibuckminsterfullerene C30H12: X-ray crystal structures of the parent hydrocarbon and of the two-dimensional organometallic network {[Rh2(O2CCF3)4]3(C30H12)}. Angew. Chem. Int. Ed. 2004, 43, 5477–5481. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Sakane, H.; Hirao, T. A concave-bound cpfe complex of sumanene as a metal in a π-bowl. Angew. Chem. Int. Ed. 2007, 46, 8376–8379. [Google Scholar] [CrossRef] [PubMed]
- Sakane, H.; Amaya, T.; Moriuchi, T.; Hirao, T. A chiral concave-bound cyclopentadienyl iron complex of sumanene. Angew. Chem. Int. Ed. 2009, 48, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Wang, W.-Z.; Sakane, H.; Moriuchi, T.; Hirao, T. A dynamically inverting π-bowl complex. Angew. Chem. Int. Ed. 2010, 49, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Bandera, D.; Baldridge, K.K.; Linden, A.; Dorta, R.; Siegel, J.S. Stereoselective coordination of C5-symmetric corannulene derivatives with an enantiomerically pure [rhi(nbd*)] metal complex. Angew. Chem. Int. Ed. 2011, 50, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Seki, S.; Moriuchi, T.; Nakamoto, K.; Nakata, T.; Sakane, H.; Saeki, A.; Tagawa, S.; Hirao, T. Anisotropic electron transport properties in sumanene crystal. J. Am. Chem. Soc. 2009, 131, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.M.; Seki, S.; Topolinski, B.; Ohkubo, K.; Fukuzumi, S.; Sakurai, H.; Lentz, D. Electronic properties of trifluoromethylated corannulenes. Angew. Chem. Int. Ed. 2012, 51, 11385–11388. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Siegel, J.S. Aromatic molecular-bowl hydrocarbons: Synthetic derivatives, their structures, and physical properties. Chem. Rev. 2006, 106, 4843–4867. [Google Scholar] [CrossRef] [PubMed]
- Tsefrikas, V.M.; Scott, L.T. Geodesic polyarenes by flash vacuum pyrolysis. Chem. Rev. 2006, 106, 4868–4884. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Hirao, T. A molecular bowl sumanene. Chem. Commun. 2011, 47, 10524–10535. [Google Scholar] [CrossRef] [PubMed]
- Sygula, A. Chemistry on a half-shell: Synthesis and derivatization of buckybowls. Eur. J. Org. Chem. 2011, 2011, 1611–1625. [Google Scholar] [CrossRef]
- Filatov, A.S.; Petrukhina, M.A. Probing the binding sites and coordination limits of buckybowls in a solvent-free environment: Experimental and theoretical assessment. Coord. Chem. Rev. 2010, 254, 2234–2246. [Google Scholar] [CrossRef]
- Zoppi, L.; Martin-Samos, L.; Baldridge, K.K. Structure–property relationships of curved aromatic materials from first principles. Acc. Chem. Res. 2014, 47, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valencia, J.R.; Dienel, T.; Groning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.; Ruffieux, P.; Fasel, R. Controlled synthesis of single-chirality carbon nanotubes. Nature 2014, 512, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Kudo, S.; Kawakami, J.; Sakai, A.; Ikeda, H.; Nakamura, H.; Ohta, K.; Ito, S. Preparation of a cyclic polyphenylene array for a chiral-type carbon nanotube segment. Bull. Chem. Soc. Jpn. 2016, 89, 1260–1275. [Google Scholar] [CrossRef]
- Liu, B.; Wu, F.; Gui, H.; Zheng, M.; Zhou, C. Chirality-controlled synthesis and applications of single-wall carbon nanotubes. ACS Nano 2017, 11, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.R.; Salerno, F.; Fuchter, M.J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 2017, 1, 0045. [Google Scholar] [CrossRef]
- Yang, C.; Inoue, Y. Supramolecular photochirogenesis. Chem. Soc. Rev. 2014, 43, 4123–4143. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ke, C.; Liang, W.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Dual supramolecular photochirogenesis: Ultimate stereocontrol of photocyclodimerization by a chiral scaffold and confining host. J. Am. Chem. Soc. 2011, 133, 13786–13789. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, W.; Chruma, J.J.; Zhao, J.; Yang, C. Enhanced triplet-triplet energy transfer and upconversion fluorescence through host-guest complexation. J. Am. Chem. Soc. 2016, 138, 15405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhao, W.-Y.; Zhou, D.-Y.; Huang, Y.; Tao, Y.; Wu, W.-H.; Yang, C. Recent advance of photochromic diarylethenes-containing supramolecular systems. Chin. Chem. Lett. 2015, 26, 817–824. [Google Scholar] [CrossRef]
- Yang, C. Recent progress in supramolecular chiral photochemistry. Chin. Chem. Lett. 2013, 24, 437–441. [Google Scholar] [CrossRef]
- Thilgen, C.; Diederich, F. Structural aspects of fullerene chemistrya journey through fullerene chirality. Chem. Rev. 2006, 106, 5049–5135. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
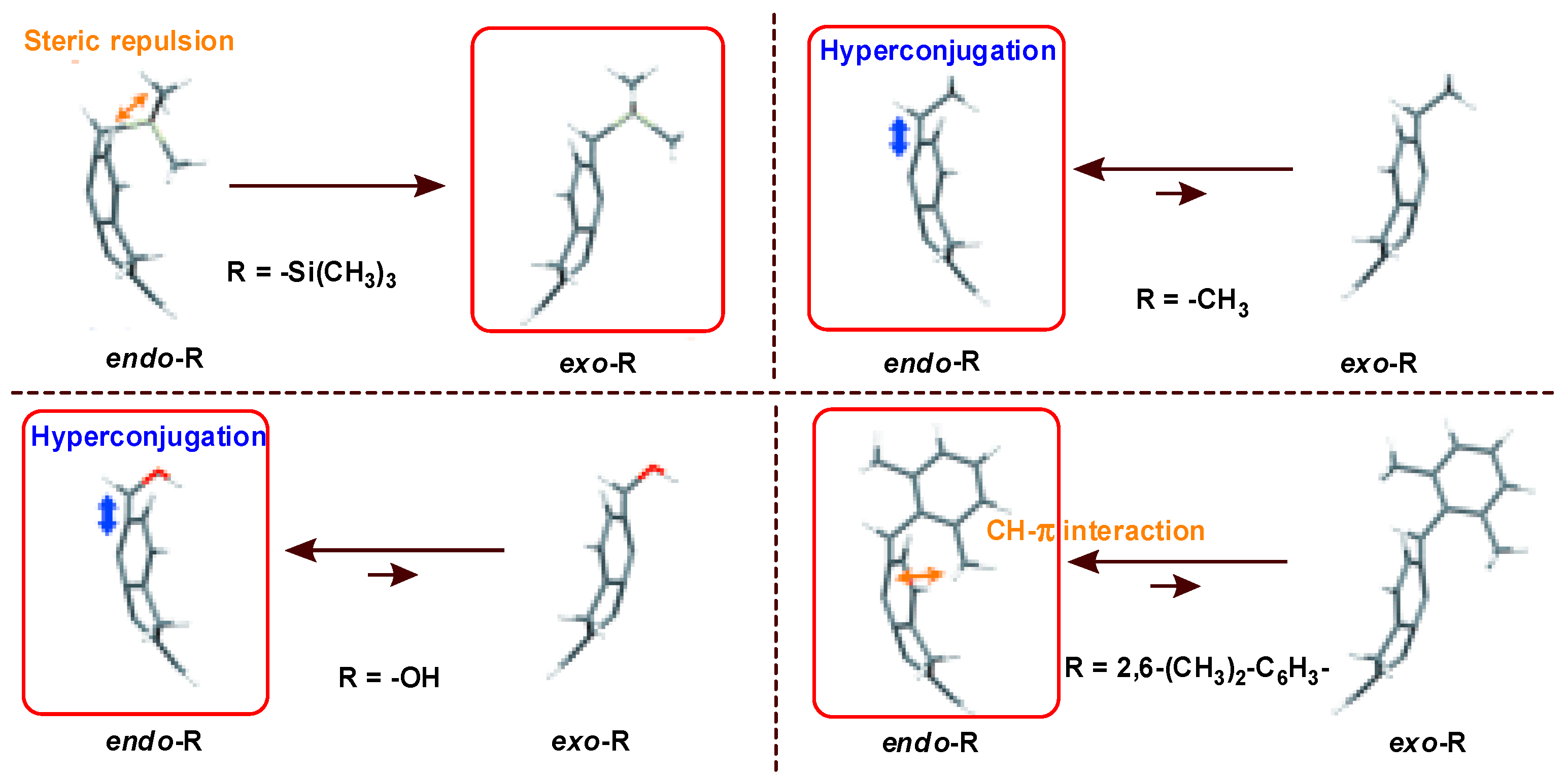
- Higashibayashi, S.; Onogi, S.; Srivastava, H.K.; Sastry, G.N.; Wu, Y.-T.; Sakurai, H. Stereoelectronic effect of curved aromatic structures: Favoring the unexpected endo conformation of benzylic-substituted sumanene. Angew. Chem. Int. Ed. 2013, 52, 7314–7316. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Filatov, A.S.; Jackson, E.A.; Rabinovitz, M.; Petrukhina, M.A.; Scott, L.T.; Shenhar, R. Bicorannulenyl: Stereochemistry of a c40h18 biaryl composed of two chiral bowls. J. Org. Chem. 2008, 73, 6073–6078. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Takimiya, K.; Otsubo, T.; Aso, Y. Triphenyleno[1,12-bcd:4,5-b[prime or minute]c[prime or minute]d[prime or minute]:8,9-b[double prime]c[double prime]d[double prime]]trithiophene: The first bowl-shaped heteroaromatic. Chem. Commun. 1999, 1859–1860. [Google Scholar] [CrossRef]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Development of a sila-friedel-crafts reaction and its application to the synthesis of dibenzosilole derivatives. J. Am. Chem. Soc. 2009, 131, 14192–14193. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Application of the sila-friedel-crafts reaction to the synthesis of [small pi]-extended silole derivatives and their properties. Dalton Trans. 2010, 39, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tanikawa, T.; Tajima, T.; Guo, J.D.; Nagase, S. Synthesis and structures of heterasumanenes having different heteroatom functionalities. Tetrahedron Lett. 2010, 51, 672–675. [Google Scholar] [CrossRef]
- Tanikawa, T.; Saito, M.; Guo, J.D.; Nagase, S. Synthesis, structures and optical properties of trisilasumanene and its related compounds. Org. Biomol. Chem. 2011, 9, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Saito, M.; Guo, J.D.; Nagase, S.; Minoura, M. Synthesis, structures, and optical properties of heterasumanenes containing group 14 elements and their related compounds. Eur. J. Org. Chem. 2012, 2012, 7135–7142. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Shao, J.; Wang, B.; Zhang, S.; Shao, Y.; Jin, X.; Yao, X.; Fang, R.; Shao, X. Non-pyrolytic, large-scale synthesis of trichalcogenasumanene: A two-step approach. Angew. Chem. Int. Ed. 2014, 53, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Hou, X.; Sun, Y.; Shao, X. Tritellurasumanene: Ultrasound assisted one-pot synthesis and extended valence adducts with bromine. Chem. Commun. 2016, 52, 14486–14489. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhu, Y.; Qin, Y.; Chen, L.; Li, X.; Zhang, H.-L.; Xu, W.; Zhu, D.; Shao, X. Tris(s,s-dioxide)-trithiasumanene: Strong fluorescence and cocrystal with 1,2,6,7,10,11-hexabutoxytriphenylene. Chem. Commun. 2017, 53, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Suda, Y.; Kobayashi, J.; Kawashima, T.; Tada, T.; Fujii, S.; Kiguchi, M.; Saito, M. Triphosphasumanene trisulfide: High out-of-plane anisotropy and janus-type π-surfaces. J. Am. Chem. Soc. 2017, 139, 5787–5792. [Google Scholar] [CrossRef] [PubMed]
- Barth, W.E.; Lawton, R.G. Dibenzo[ghi,mno]fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. [Google Scholar] [CrossRef]
- Scott, L.T.; Hashemi, M.M.; Meyer, D.T.; Warren, H.B. Corannulene. A convenient new synthesis. J. Am. Chem. Soc. 1991, 113, 7082–7084. [Google Scholar] [CrossRef]
- Borchardt, A.; Fuchicello, A.; Kilway, K.V.; Baldridge, K.K.; Siegel, J.S. Synthesis and dynamics of the corannulene nucleus. J. Am. Chem. Soc. 1992, 114, 1921–1923. [Google Scholar] [CrossRef]
- Abdourazak, A.H.; Marcinow, Z.; Sygula, A.; Sygula, R.; Rabideau, P.W. Buckybowls 2. Toward the total synthesis of buckminsterfullerene (C60): Benz[5,6]-as-indaceno[3,2,1,8,7-mnopqr]indeno[4,3,2,1-cdef]chrysene. J. Am. Chem. Soc. 1995, 117, 6410–6411. [Google Scholar] [CrossRef]
- Mehta, G.; Shahk, S.R.; Ravikumarc, K. Towards the design of tricyclopenta [def, jkl, pqr] triphenylene (‘sumanene’): A ‘bowl-shaped’ hydrocarbon featuring a structural motif present in C60(buckminsterfullerene). J. Chem. Soc. Chem. Commun. 1993, 1006–1008. [Google Scholar] [CrossRef]
- Seiders, T.J.; Baldridge, K.K.; Siegel, J.S. Synthesis and characterization of the first corannulene cyclophane. J. Am. Chem. Soc. 1996, 118, 2754–2755. [Google Scholar] [CrossRef]
- Sakurai, H.; Daiko, T.; Hirao, T. A synthesis of sumanene, a fullerene fragment. Science 2003, 301, 1878. [Google Scholar] [CrossRef] [PubMed]
- Higashibayashi, S.; Sakurai, H. Synthesis of an enantiopure syn-benzocyclotrimer through regio-selective cyclotrimerization of a halonorbornene derivative under palladium nanocluster conditions. Chem. Lett. 2007, 36, 18–19. [Google Scholar] [CrossRef]
- Reza, A.F.G.M.; Higashibayashi, S.; Sakurai, H. Preparation of C3-symmetric homochiral syn-trisnorbornabenzenes through regioselective cyclotrimerization of enantiopure iodonorbornenes. Chem. Asian J. 2009, 4, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Priyakumar, U.D.; Sastry, G.N. Heterobuckybowls: A theoretical study on the structure, bowl-to-bowl inversion barrier, bond length alternation, structure-inversion barrier relationship, stability, and synthetic feasibility. J. Org. Chem. 2001, 66, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.; Bratcher, M.S.; Erickson, M.S.; Zimmermann, G.; Scott, L.T. Novel syntheses of three C30H12 bowl-shaped polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 1997, 36, 406–408. [Google Scholar] [CrossRef]
- Amaya, T.; Nakata, T.; Hirao, T. Synthesis of highly strained π-bowls from sumanene. J. Am. Chem. Soc. 2009, 131, 10810–10811. [Google Scholar] [CrossRef] [PubMed]
- Tsefrikas, V.M.; Arns, S.; Merner, P.M.; Warford, C.C.; Merner, B.L.; Scott, L.T.; Bodwell, G.J. Benzo[a]acecorannulene: Surprising formation of a new bowl-shaped aromatic hydrocarbon from an attempted synthesis of 1,2-diazadibenzo[d,m]corannulene. Org. Lett. 2006, 8, 5195–5198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Hayama, T.; Baldridge, K.K.; Linden, A.; Siegel, J.S. Synthesis of fluoranthenes and indenocorannulenes: Elucidation of chiral stereoisomers on the basis of static molecular bowls. J. Am. Chem. Soc. 2006, 128, 6870–6884. [Google Scholar] [CrossRef] [PubMed]
- Rickhaus, M.; Mayor, M.; Juricek, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Branda, N.; Lehn, J.-M.; Decian, A.; Fischer, J. Supramolecular chirality: Chiral hydrogen-bonded supermolecules from achiral molecular components. Helv. Chim. Acta 1998, 81, 1–13. [Google Scholar] [CrossRef]
- Dalla Cort, A.; Mandolini, L.; Pasquini, C.; Schiaffino, L. “Inherent chirality” and curvature. New J. Chem. 2004, 28, 1198–1199. [Google Scholar] [CrossRef]
- Szumna, A. Inherently chiral concave molecules-from synthesis to applications. Chem. Soc. Rev. 2010, 39, 4274–4285. [Google Scholar] [CrossRef] [PubMed]
- Supramolecular chirality. In Topics in Current Chemistry; Crego-Calama, M.; Reinhoudt, D.N. (Eds.) Springer: Berlin/Heidelberg, Germany, 2006; Volume 265, p. 312. [Google Scholar]
- Yao, J.; Wu, W.; Liang, W.; Feng, Y.; Zhou, D.; Chruma, J.J.; Fukuhara, G.; Mori, T.; Inoue, Y.; Yang, C. Temperature-driven planar chirality switching of a pillar[5]arene-based molecular universal joint. Angew. Chem. Int. Ed. 2017, 56, 6869–6873. [Google Scholar] [CrossRef] [PubMed]
- Kaewmati, P.; Tan, Q.; Higashibayashi, S.; Yakiyama, Y.; Sakurai, H. Synthesis of triaryltriazasumanenes. Chem. Lett. 2017, 46, 146–148. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, L.; Liang, W.; Gui, J.; Xu, D.; Wu, W.; Nakai, Y.; Nishijima, M.; Fukuhara, G.; Mori, T.; et al. Inherently chiral azonia[6]helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in water. J. Org. Chem. 2016, 81, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, Q.; Liang, W.; Yu, X.; Zhou, D.; Wu, W.; Chruma, J.J.; Yang, C. Enantiodifferentiation in the photoisomerization of (z,z)-1,3-cyclooctadiene in the cavity of γ-cyclodextrin–curcubit[6]uril-wheeled [4]rotaxanes with an encapsulated photosensitizer. Org. Lett. 2017, 19, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.-C.; Yan, Z.-Q.; Peng, Y.; Yi, J.-G.; Zhou, D.-Y.; Su, D.; Zhong, Z.-H.; Gao, G.-W.; Wu, W.-H.; Yang, C. Enhanced head-to-head photodimers in the photocyclodimerization of anthracenecarboxylic acid with a cationic pillar [6] arene. Chin. Chem. Lett. 2016, 27, 1017–1021. [Google Scholar] [CrossRef]
- Yao, J.; Yan, Z.; Ji, J.; Wu, W.; Yang, C.; Nishijima, M.; Fukuhara, G.; Mori, T.; Inoue, Y. Ammonia-driven chirality inversion and enhancement in enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate mediated by diguanidino-γ-cyclodextrin. J. Am. Chem. Soc. 2014, 136, 6916–6919. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, C.; Nishijima, M.; Fukuhara, G.; Mori, T.; Mele, A.; Castiglione, F.; Caldera, F.; Trotta, F.; Inoue, Y. Cyclodextrin nanosponge-sensitized enantiodifferentiating photoisomerization of cyclooctene and 1,3-cyclooctadiene. Beilstein J. Org. Chem. 2012, 8, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, C.; Ke, C.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrins. Chem. Commun. 2011, 47, 6849–6851. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Preda, D.V.; Segawa, Y.; Itami, K.; Scott, L.T. Corannulene–helicene hybrids: Chiral π-systems comprising both bowl and helical motifs. Org. Lett. 2016, 18, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Yanney, M.; Fronczek, F.R.; Sygula, A. Corannulene subunit acts as a diene in a cycloaddition reaction: Synthesis of C80H32 corannulyne tetramer. Org. Lett. 2012, 14, 4942–4945. [Google Scholar] [CrossRef] [PubMed]
- Yanney, M.; Fronczek, F.R.; Henry, W.P.; Beard, D.J.; Sygula, A. Cyclotrimerization of corannulyne: Steric hindrance tunes the inversion barriers of corannulene bowls. Eur. J. Org. Chem. 2011, 2011, 6636–6639. [Google Scholar] [CrossRef]
- Kawasumi, K.; Zhang, Q.; Segawa, Y.; Scott, L.T.; Itami, K. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects. Nat. Chem. 2013, 5, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Mori, K.; Wu, H.-L.; Ishida, S.; Nakamura, J.-I.; Murata, K.; Hirao, T. Synthesis and characterization of [small pi]-extended bowl-shaped [small pi]-conjugated molecules. Chem. Commun. 2007, 1902–1904. [Google Scholar] [CrossRef]
- Chen, R.; Lu, R.-Q.; Shi, P.-C.; Cao, X.-Y. Corannulene derivatives for organic electronics: From molecular engineering to applications. Chin. Chem. Lett. 2016, 27, 1175–1183. [Google Scholar] [CrossRef]
- Kobayashi, S.-i.; Mori, S.; Iida, S.; Ando, H.; Takenobu, T.; Taguchi, Y.; Fujiwara, A.; Taninaka, A.; Shinohara, H.; Iwasa, Y. Conductivity and field effect transistor of la2@C80 metallofullerene. J. Am. Chem. Soc. 2003, 125, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanazawa, Y.; Okumura, M.; Taninaka, A.; Yokawa, T.; Shinohara, H. Lanthanoid endohedral metallofullerenols for MRI contrast agents. J. Am. Chem. Soc. 2003, 125, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Petrukhina, M.A.; Scott, L.T. Coordination chemistry of buckybowls: From corannulene to a hemifullerene. Dalton Trans. 2005, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, P.A.; Alvarez, C.M.; Ellern, A.; Angelici, R.J.; Sygula, A.; Sygula, R.; Rabideau, P.W. Flattening of a curved-surface buckybowl (corannulene) by η6 coordination to {cp*ru}+. Organometallics 2005, 24, 4543–4552. [Google Scholar] [CrossRef]
- Zhu, B.; Ellern, A.; Sygula, A.; Sygula, R.; Angelici, R.J. η6-Coordination of the curved carbon surface of corannulene (C20H10) to (η6-arene)M2+ (M = Ru, Os). Organometallics 2007, 26, 1721–1728. [Google Scholar] [CrossRef]
- Zabula, A.V.; Spisak, S.N.; Filatov, A.S.; Rogachev, A.Y.; Clerac, R.; Petrukhina, M.A. Supramolecular trap for a transient corannulene trianion. Chem. Sci. 2016, 7, 1954–1961. [Google Scholar] [CrossRef]
- Kameno, Y.; Ikeda, A.; Nakao, Y.; Sato, H.; Sakaki, S. Theoretical study of M(PH3)2 complexes of C60, corannulene (C20H10), and sumanene (C21H12) (M = Pd or Pt). Unexpectedly large binding energy of M(PH3)2(C60). J. Phys. Chem. A 2005, 109, 8055–8063. [Google Scholar] [CrossRef] [PubMed]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Vander Griend, D.A.; Pasini, D. A chiroptical probe for sensing metal ions in water. Eur. J. Org. Chem. 2013, 2013, 6078–6083. [Google Scholar] [CrossRef]
- Caricato, M.; Coluccini, C.; Dondi, D.; Vander Griend, D.A.; Pasini, D. Nesting complexation of C60 with large, rigid d2 symmetrical macrocycles. Org. Biomol. Chem. 2010, 8, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Sharma, A.K.; Coluccini, C.; Pasini, D. Nanostructuring with chirality: Binaphthyl-based synthons for the production of functional oriented nanomaterials. Nanoscale 2014, 6, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Pacini, A.; Pasini, D. Chiral nanotubes. Nanomaterials 2017, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Bancu, M.; Rai, A.K.; Cheng, P.; Gilardi, R.D.; Scott, L.T. Corannulene polysulfides: Molecular bowls with multiple arms and flaps. Synlett 2004, 2004, 173–176. [Google Scholar]
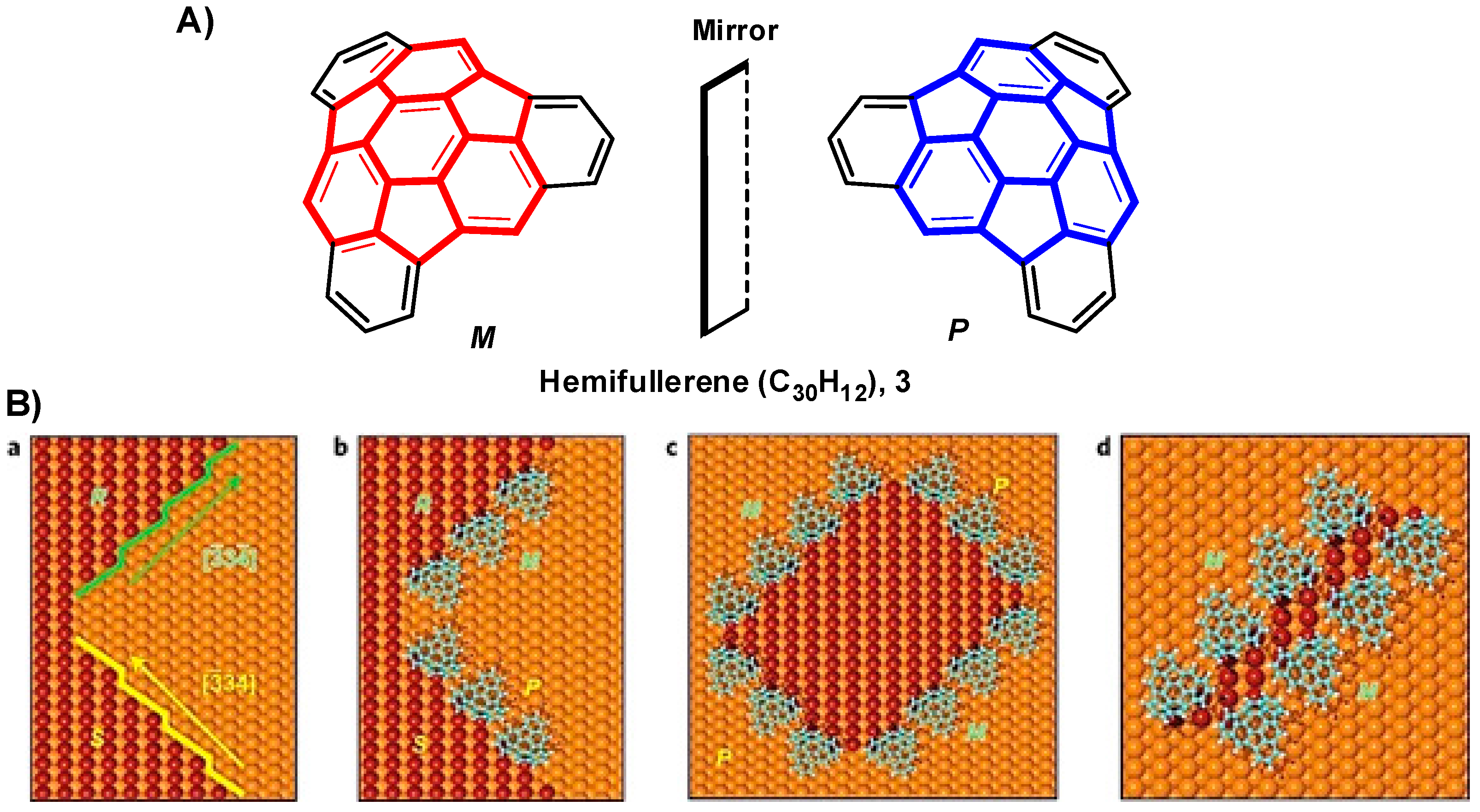
- Xiao, W.; Ernst, K.-H.; Palotas, K.; Zhang, Y.; Bruyer, E.; Peng, L.; Greber, T.; Hofer, W.A.; Scott, L.T.; Fasel, R. Microscopic origin of chiral shape induction in achiral crystals. Nat. Chem. 2016, 8, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Schunack, M.; Lægsgaard, E.; Stensgaard, I.; Johannsen, I.; Besenbacher, F. A chiral metal surface. Angew. Chem. Int. Ed. 2001, 40, 2623–2626. [Google Scholar] [CrossRef]
- Karageorgaki, C.; Ernst, K.-H. A metal surface with chiral memory. Chem. Commun. 2014, 50, 1814–1816. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanagaraj, K.; Lin, K.; Wu, W.; Gao, G.; Zhong, Z.; Su, D.; Yang, C. Chiral Buckybowl Molecules. Symmetry 2017, 9, 174. https://doi.org/10.3390/sym9090174
Kanagaraj K, Lin K, Wu W, Gao G, Zhong Z, Su D, Yang C. Chiral Buckybowl Molecules. Symmetry. 2017; 9(9):174. https://doi.org/10.3390/sym9090174
Chicago/Turabian StyleKanagaraj, Kuppusamy, Kangjie Lin, Wanhua Wu, Guowei Gao, Zhihui Zhong, Dan Su, and Cheng Yang. 2017. "Chiral Buckybowl Molecules" Symmetry 9, no. 9: 174. https://doi.org/10.3390/sym9090174
APA StyleKanagaraj, K., Lin, K., Wu, W., Gao, G., Zhong, Z., Su, D., & Yang, C. (2017). Chiral Buckybowl Molecules. Symmetry, 9(9), 174. https://doi.org/10.3390/sym9090174






