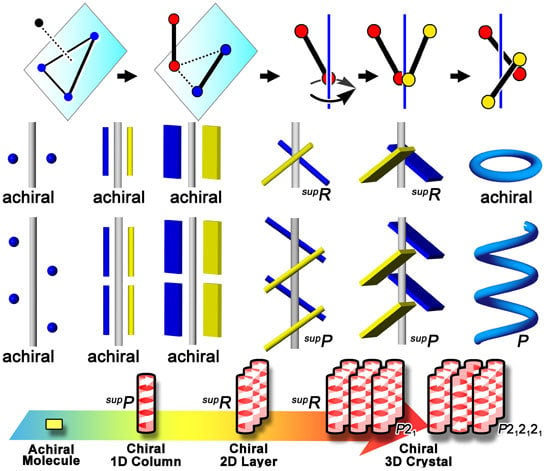
Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components
Abstract
:1. Introduction


2. Hitherto Overlooked Supramolecular Chirality in Organic Crystals
2.1. Supramolecular Chirality in Deformed Assemblies
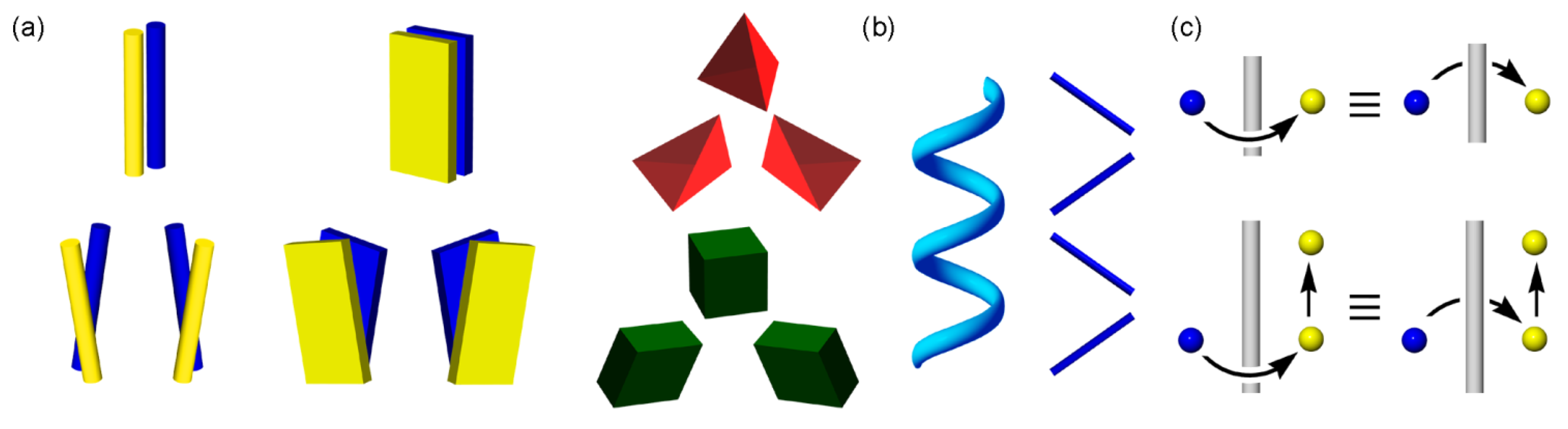
2.2. Conventional Idea for Chirality Generation in Major Crystals Involving 21 Helices
3. Chirality Generation from a Geometric Viewpoint
3.1. Four-point Assemblies and Their Handedness
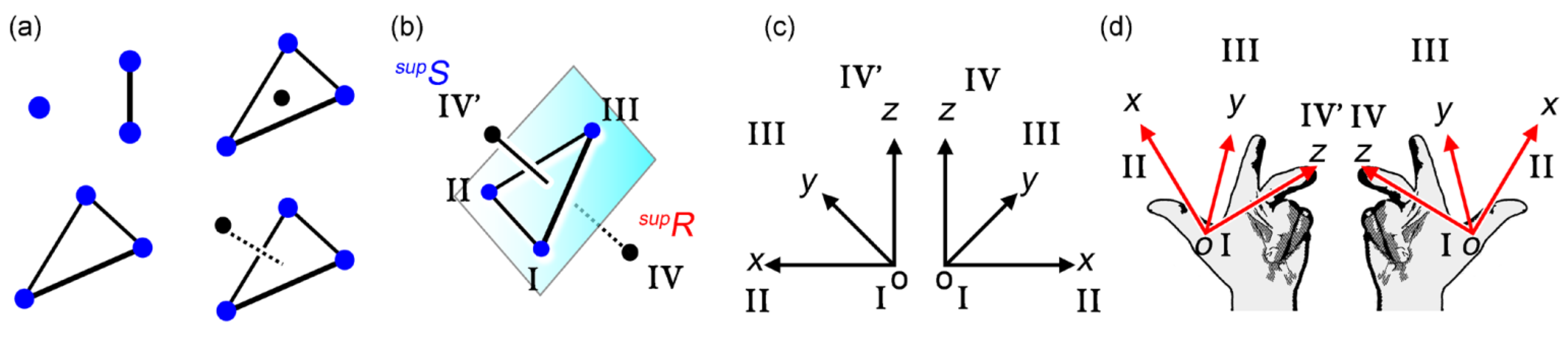
3.2. Multi-point Approximation and Edge-view Methods for Bars and Plates

3.3. Tilt-chirality around a Virtual Axis
4. Chirality Generation by Symmetry Operations
4.1. Rotation Operation
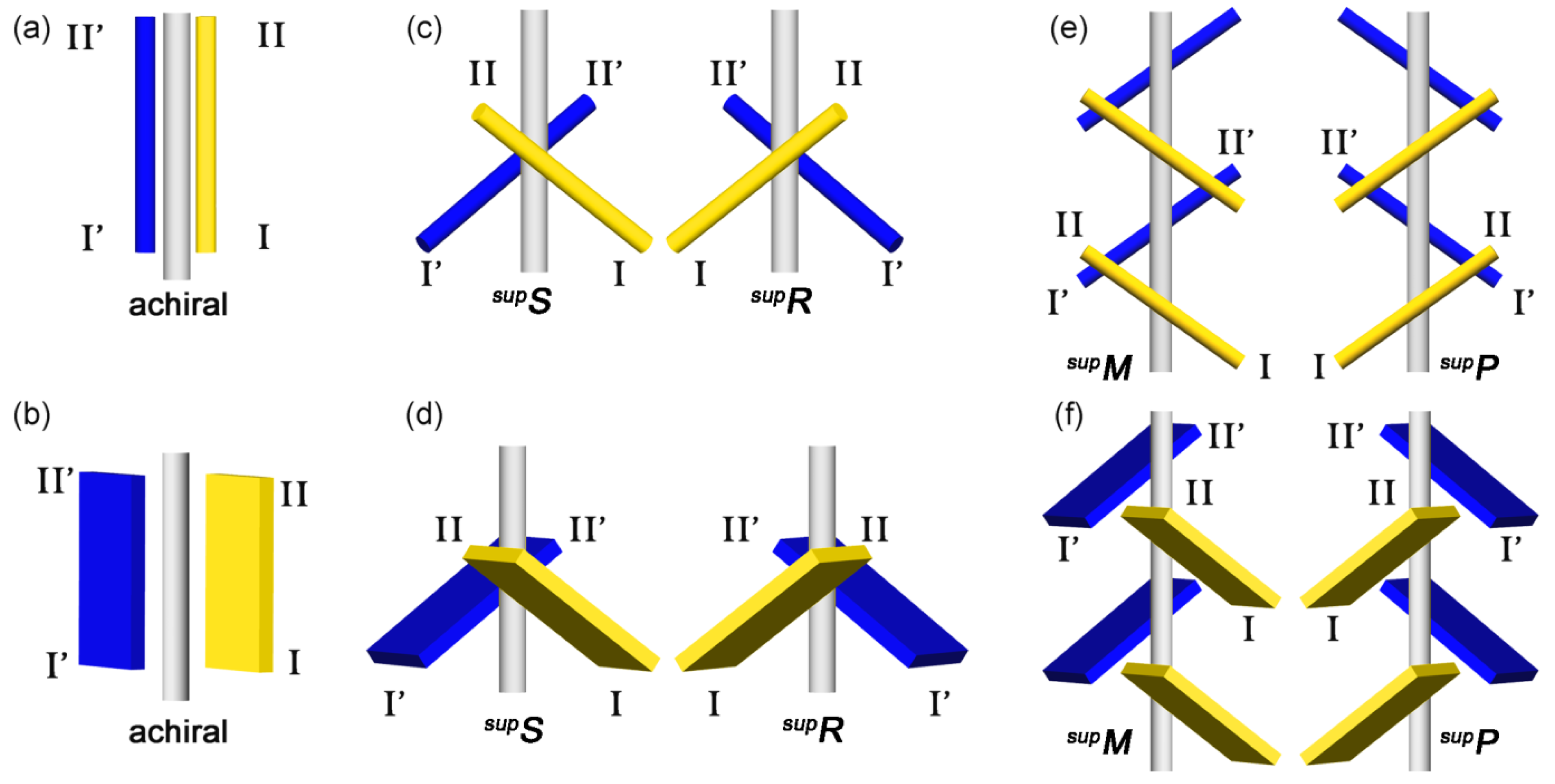
4.2. Helix (Screw) Operation
4.3. Translation Operation and Others
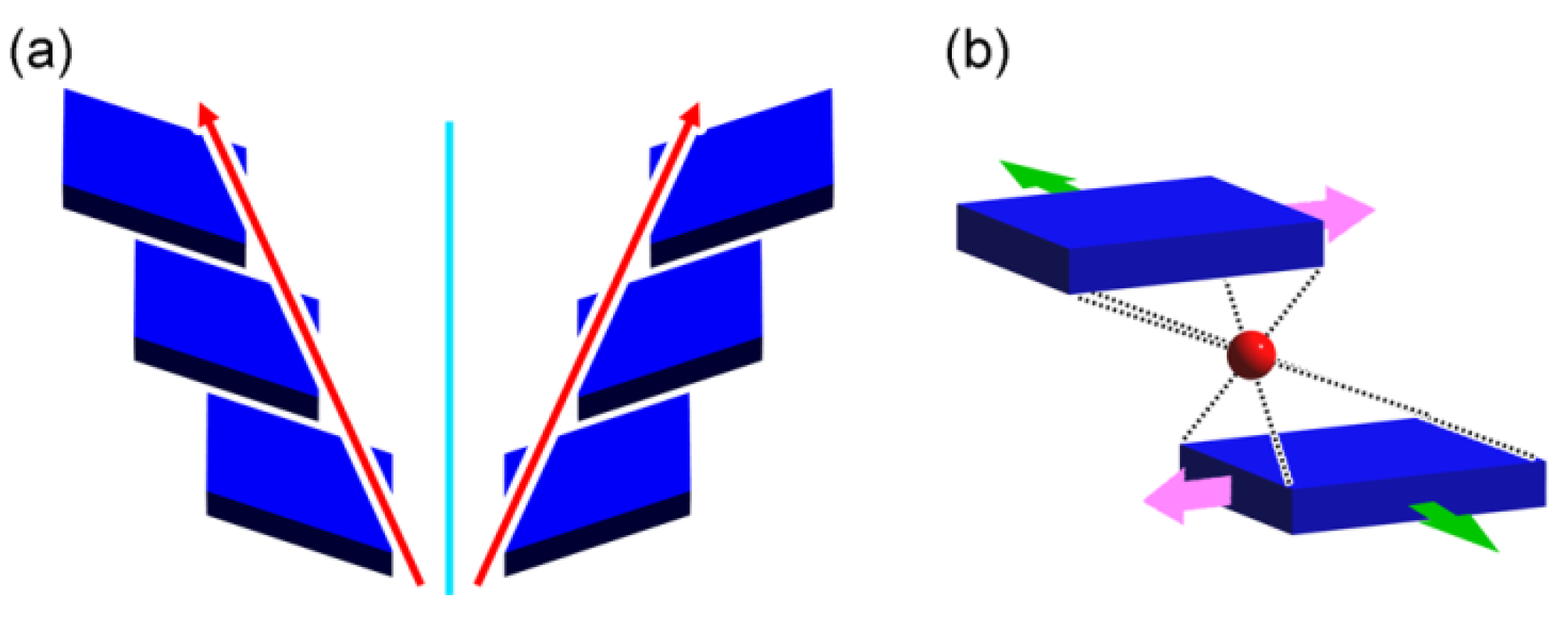
4.4. New Idea for Chirality Generation through Twofold Rotations
4.5. Supramolecular Chirality of 21 Helical Columns of Bars and Plates
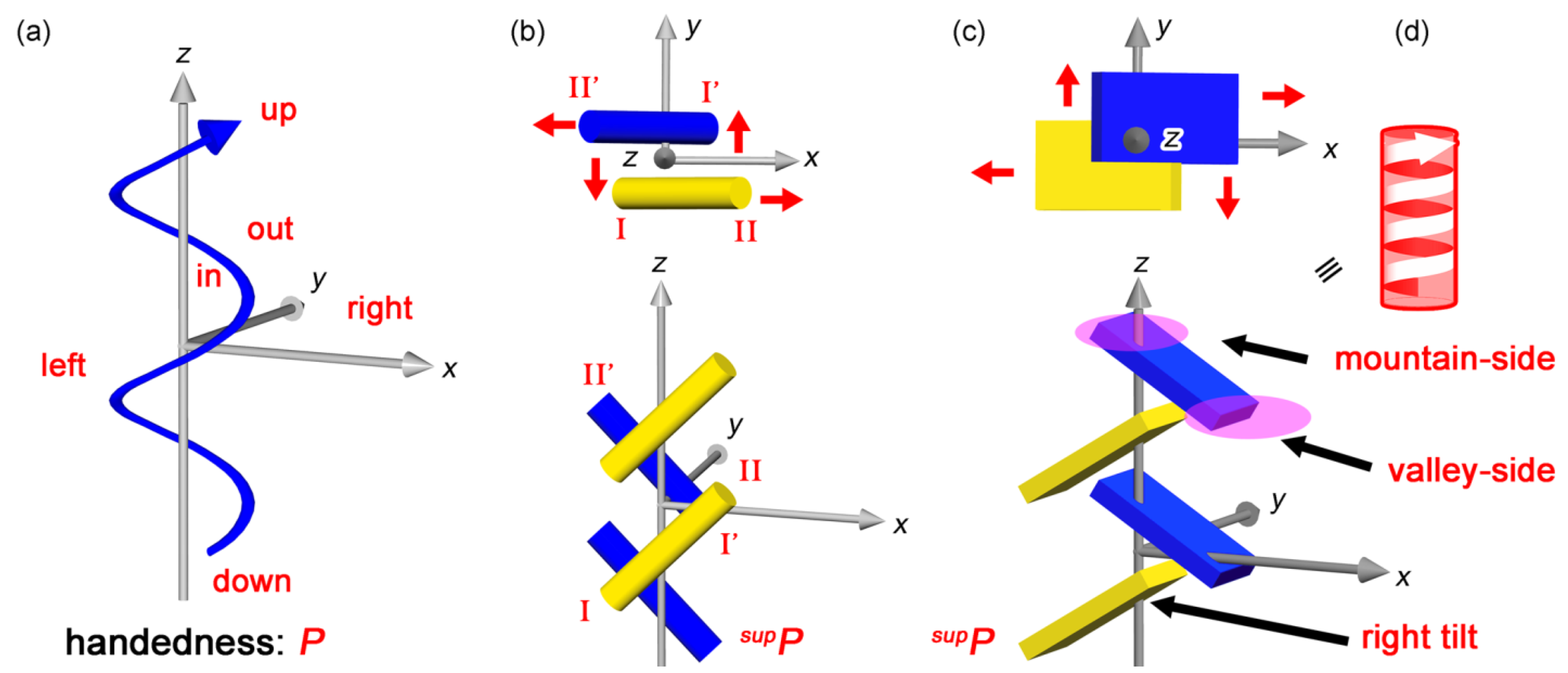
5. Chirality Generation by Bundling of 21 Helical Columns
5.1. Bundles of the Preferable 21 Columns
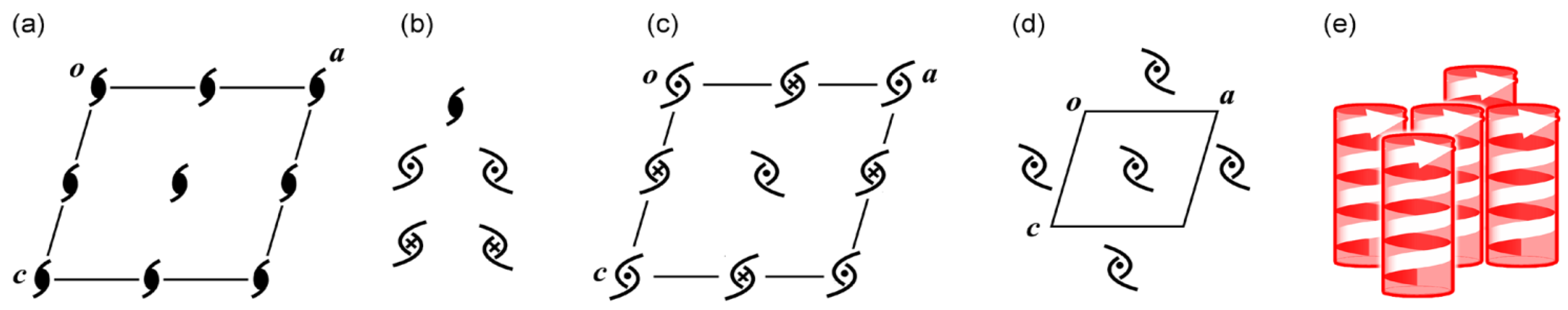
5.2. Chirality Generation in Bottom-up Construction toward Crystals from Achiral Molecules
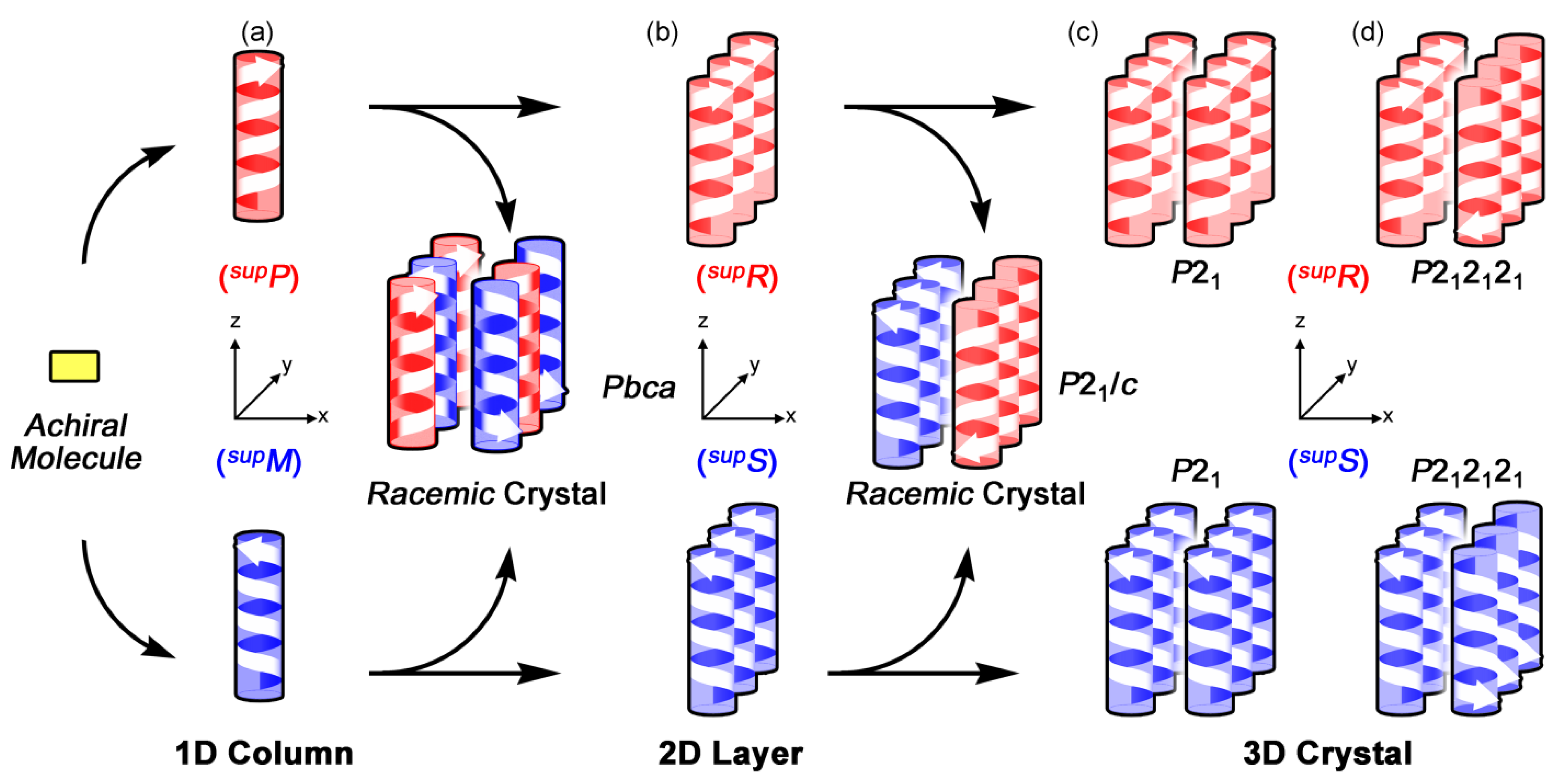
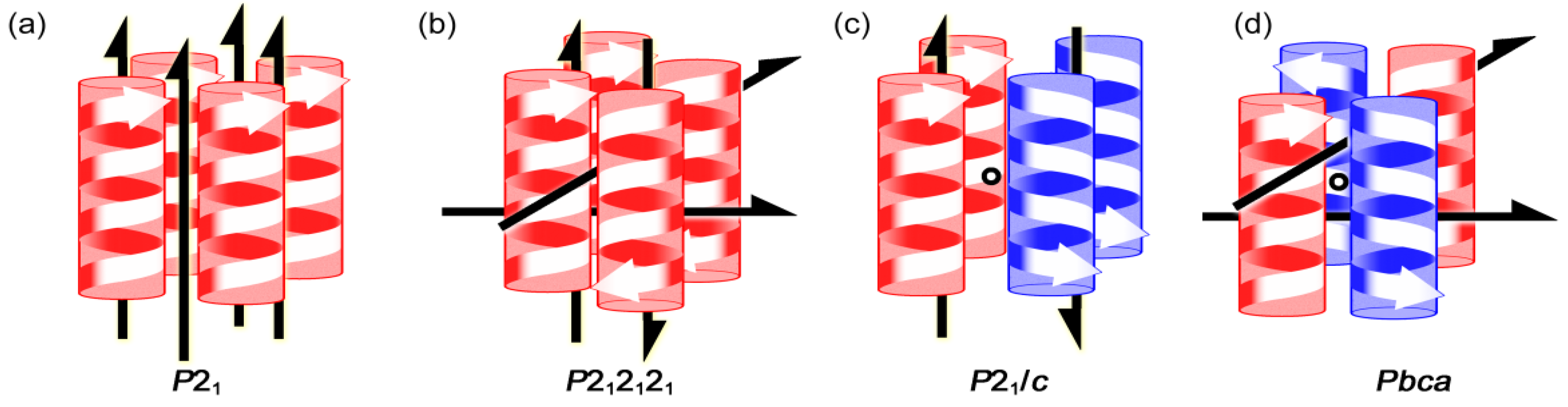
5.3. Chiral Space Groups
6. Linkage between Molecular and Supramolecular Chirality
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Miyata, M.; Shibakami, M.; Goonewardena, W.; Takemoto, K. Inclusion compounds of cholic acid with a variety of organic substances. Chem. Lett. 1987, 16, 605–608. [Google Scholar] [CrossRef]
- Miki, K.; Masui, A.; Kasai, N.; Miyata, M.; Shibakami, M.; Takemoto, K. New channel-type inclusion compound of steroidal bile acid. Structure of a 1:1 complex between cholic acid and acetophenone. J. Am. Chem. Soc. 1988, 110, 6594–6596. [Google Scholar] [CrossRef]
- Miyata, M.; Shibakami, M.; Chirachanchai, S.; Takemoto, K.; Kasai, N.; Miki, K. Guest-responsive structural changes in cholic acid intercalation crystals. Nature 1990, 343, 446–447. [Google Scholar] [CrossRef]
- Miyata, M.; Sada, K. Deoxycholic Acid and Related Hosts. In Solid-State Supramolecular Chemistry, Comprehensive Supramolecular Chemistry; MacNicol, D.D., Toda, F., Bishop, R., Eds.; Pergamon: Oxford, UK, 1996; Volume 6, pp. 147–176. [Google Scholar]
- Miyata, M.; Sada, K.; Yoswathananont, N. Deoxycholic, Cholic, and Apocholic Acids. In Encyclopedia of Supramolecular Chemistry; Atwood, J.L., Steed, J.W., Eds.; Marcel Dekker: New York, NY, USA, 2004; Volume 1, pp. 441–451. [Google Scholar]
- Miyata, M.; Yoswathananont, N.; Nakano, K.; Sada, K. Separation of Isomers and Enantiomers by Bile Acid Derivatives. In Separation and Reaction in Organic Supramolecular Chemistry, Perspectives in Supramolecuar Chemistry; Toda, F., Bishop, R., Eds.; John Wiley & Sons: England, UK, 2004; Volume 8, pp. 87–122. [Google Scholar]
- Miyata, M.; Tohnai, N.; Hisaki, I. Supramolecular chirality in crystalline assemblies of bile acids and their derivatives; Three-axial, tilt, helical, and bundle chirality. Molecules 2007, 12, 1973–2000. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Tohnai, N.; Hisaki, I. Crystalline host-guest assemblies of steroidal and related molecules: Diversity, hierarchy, and supramolecular chirality. Acc. Chem. Res. 2007, 40, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Aburaya, K.; Hisaki, I.; Tohnai, N.; Miyata, M. Flexible host frameworks with diverse cavities in inclusion crystals of bile acids and their derivatives. Chem. Rec. 2009, 9, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Sada, K.; Nakano, K.; Tohnai, N. Inclusion Compounds of Steroidal Molecules: Molecular Information and Their Expression through Molecular Assemblies. In Bottom-Up Nanofabrication; Nalwa, H.S., Ariga, K., Eds.; American Scientific Publisher: Valencia, CA, USA, 2009; Volume 3, pp. 147–169. [Google Scholar]
- Watabe, T.; Yoshikawa, D.; Hisaki, I.; Tohnai, N.; Miyata, M. Supramolecular chirality and isomerism in cinchonidine crystals: Hierarchical analysis on the basis of the asymmetric 21 helical columnar assembly. Chem. Lett. 2006, 35, 806–807. [Google Scholar] [CrossRef]
- Hisaki, I.; Watabe, T.; Kogami, Y.; Tohnai, N.; Miyata, M. 21 Helical assemblies of cinchona alkaloids in crystals: Definition of their handedness based on the molecular tilt. Chem. Lett. 2006, 35, 1274–1275. [Google Scholar] [CrossRef]
- Watabe, T.; Hisaki, I.; Tohnai, N.; Miyata, M. Four kinds of 21 helical assemblies with the molecular tilt as well as three-directional and facial chirality. Chem. Lett. 2007, 36, 234–235. [Google Scholar] [CrossRef]
- Watabe, T.; Kobayashi, K.; Hisaki, I.; Tohnai, N.; Miyata, M. Guest-induced supramolecular isomerism and chirality of brucine inclusion crystals with aliphatic alcohols: A hierarchical interpretation. Bull. Chem. Soc. Jap. 2007, 80, 464–475. [Google Scholar] [CrossRef]
- Hisaki, I.; Shizuki, N.; Aburaya, K.; Katsuta, M.; Tohnai, N.; Miyata, M. Structures of brucinium cholate: Bile acid and strychinine derivatives meet in the crystals. Cryst. Growth Des. 2009, 9, 1280–1283. [Google Scholar] [CrossRef]
- Hisaki, I.; Shizuki, N.; Sasaki, T.; Ito, Y.; Tohnai, N.; Miyata, M. Handedness determination of 21 helical motifs and hierarchical analysis of crystal structures based on the motifs: The case of cinchona alkaloid derivatives. Cryst. Growth Des. 2010, 10, 5262–5269. [Google Scholar] [CrossRef]
- Sasaki, T.; Shizuki, N.; Hiraishi, E.; Tohnai, N.; Miyata, M. Construction of multi-component supramolecular architectures of bile acids and cinchona alkaloids through helical-pitch-synchronized crystallization. Org. Biomol. Chem. 2012, 10, 5985–5992. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Hiraishi, E.; Sasaki, T.; Orita, H.; Tsuzuki, S.; Tohnai, N.; Miyata, M. Crystal structure of quinine: The effects of vinyl and methoxy groups on molecular assemblies of cinchona alkaloids cannot be ignored. Chem. Asian J. 2012, 7, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hisaki, I.; Tohnai, N.; Miyata, M. Supramolecular tilt-chirality derived from symmetric benzene molecules: Handedness of the 21 helical assembly. Chem. Asian J. 2007, 2, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Tohnai, N.; Miyata, M. Supramolecular tilt chirality in crystals of steroids and alkaloids. Chirality 2008, 20, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Sasaki, T.; Sakaguchi, K.; Liu, W.T.; Tohnai, N.; Miyata, M. Right- and left-handedness of 21 symmetrical herringbone assemblies of benzene. Chem. Commun. 2012, 48, 2219–2221. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Fisher, W.; Iler, U.M. International Tables for Crystallography, Vol. A, Space-Group Symmetry; Hahn, T., Ed.; Kluwer Academic Publishers: London, UK, 2002. [Google Scholar]
- Glusker, J.P.; Trueblood, K.N. Crystal Structure Analysis: A Primer, 3rd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Bennett, D.W. Understanding Single-Crystal X-ray Crystallography; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Hisaki, I.; Sasaki, T.; Tohnai, N.; Miyata, M. Supramolecular-tilt-chirality on twofold helical assemblies. Chem. Eur. J. 2012, 18, 10066–10073. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Sasaki, T.; Tohnai, N.; Miyata, M. Multipoint approximation method for handedness determination of two-fold helical assemblies and their bundles. J. Syn. Org. Chem. Jpn. 2012, 70, 908–917. [Google Scholar] [CrossRef]
- Miyata, M.; Hisaki, I. Twofold Helical Molecular Assemblies in Organic Crystals: Chirality Generation and Handedness Determination. In Advances in Organic Crystal Chemistry; Tamura, R., Miyata, M., Eds.; Springer Japan: Tokyo, Japan, 2015; Chapter 19; pp. 371–392. [Google Scholar]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π Interaction—Evidence, Nature, and Consequences; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Gilla, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Grell, J.; Bernstein, J.; Tinhofer, G. Graph-set analysis of hydrogen-bond patterns: Some mathematical concepts. Acta Crystallogr. B 1999, 55, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Desiraju, G.R. The Crystal as a Supramolecular Entity; John Wiley & Sons: Chichester, UK, 1995. [Google Scholar]
- Matsuura, T.; Koshima, H. Chiral crystallization of achiral organic compounds—Generation of chirality without chiral environment. Part 1. J. Syn. Org. Chem. Jpn. 1998, 29, 268–279. [Google Scholar]
- Matsuura, T.; Koshima, H. Chiral crystallization of achiral organic compounds—Generation of chirality without chiral environment. Part 2. J. Syn. Org. Chem. Jpn. 1998, 29, 466–477. [Google Scholar]
- Matsuura, T.; Koshima, H. Introduction to chiral crystallization of achiral organic compounds: Spontaneous generation of chirality. J. Photochem. Photobiol. C Photo. Rev. 2005, 6, 7–24. [Google Scholar] [CrossRef]
- Dryzun, C.; Avnir, D. On the abundance of chiral crystals. Chem. Commun. 2012, 48, 5874–5876. [Google Scholar] [CrossRef] [PubMed]
- Kitaigorodskii, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Cambridge Structural Database. Available online: http://www.ccdc.cam.ac.uk/products/csd/statistics/ (accessed on 16 February 2015).
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Krieger: Malabar, FL, USA, 1991. [Google Scholar]
- Cahn, R.S.; Ingold, C.K.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisaki, I.; Tsuzuki, S.; Tohnai, N.; Miyata, M. Halogen bond effect on bundling of hydrogen bonded 2-fold helical columns. CrystEngComm 2012, 14, 5749–5752. [Google Scholar] [CrossRef]
- Sasaki, T.; Ida, Y.; Tanaka, A.; Hisaki, I.; Tohnai, N.; Miyata, M. Chiral crystallization by non-parallel face contacts on the basis of three-axially asymmetric twofold helices. CrystEngComm 2013, 15, 8237–8240. [Google Scholar] [CrossRef]
- Yuge, T.; Tohnai, N.; Fukuda, T.; Hisaki, I.; Miyata, M. Topological study of pseudo-cubic hydrogen-bond networks in a binary system composed of primary ammonium carboxylates: An analog of an ice cube. Chem. Eur. J. 2007, 13, 4163–4168. [Google Scholar] [CrossRef] [PubMed]
- Yuge, T.; Sakai, T.; Kai, N.; Hisaki, I.; Miyata, M.; Tohnai, N. Topological classification and supramolecular chirality of 21-helical ladder-type hydrogen-bond networks composed of primary ammonium carboxylates: Bundle control in 21-helical assemblies. Chem. Eur. J. 2008, 14, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Inoue, K.; Hisaki, I.; Tohnai, N.; Miyata, M.; Matsumoto, A. supramolecular chirality in layered crystals of achiral ammonium salts and fatty acids: A hierarchical interpretation. Angew. Chem. Int. Ed. 2006, 45, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ida, Y.; Hisaki, I.; Yuge, T.; Uchida, Y.; Tohnai, N.; Miyata, M. Characterization of supramolecular hidden chirality of hydrogen-bonded networks by advanced graph set analysis. Chem. Eur. J. 2014, 20, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ida, Y.; Yuge, T.; Yamamoto, A.; Hisaki, I.; Tohnai, N.; Miyata, M. Chirality generation in supramolecular clusters: Analogues of octacoordinated polyhedrons. Cryst. Growth Des. 2015, 15, 658–665. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisaki, I.; Miyano, N.; Tohnai, N.; Morimoto, K.; Sato, H.; Tsuzuki, S.; Miyata, M. Linkage control between molecular and supramolecular chirality in 21-helical hydrogen-bonded networks using achiral components. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.Y.; Salvo, F.D.; Teixidor, F.; Vinas, C.; Planas, J.G.; Choquesillo-Lazarte, D.; Vanthuyne, N. Is molecular chirality connected to supramolecular chirality? The particular case of chiral 2-pyridyl alcohols. Cryst. Growth Des. 2015, 15, 935–945. [Google Scholar] [CrossRef]
- Itoh, T.; Tachino, K.; Akira, N.; Uno, T.; Kubo, M.; Tohnai, N.; Miyata, M. Twofold helical polymerization: Thermal solid-state polymerization of 7-cyano-7-(2′-haloethoxycarbonyl)-1,4- benzoquinone methides. Macomolecules 2015, 48, 2935–2947. [Google Scholar] [CrossRef]
- Gardner, M. The New Ambidextrous Universe; W.H. Freeman & Company: New York, NY, USA, 1999. [Google Scholar]
- Hegstrom, R.A.; Kondepudi, D.K. The handedness of the universe. Sci. Am. 1990, 262, 108–115. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyata, M.; Tohnai, N.; Hisaki, I.; Sasaki, T. Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components. Symmetry 2015, 7, 1914-1928. https://doi.org/10.3390/sym7041914
Miyata M, Tohnai N, Hisaki I, Sasaki T. Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components. Symmetry. 2015; 7(4):1914-1928. https://doi.org/10.3390/sym7041914
Chicago/Turabian StyleMiyata, Mikiji, Norimitsu Tohnai, Ichiro Hisaki, and Toshiyuki Sasaki. 2015. "Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components" Symmetry 7, no. 4: 1914-1928. https://doi.org/10.3390/sym7041914
APA StyleMiyata, M., Tohnai, N., Hisaki, I., & Sasaki, T. (2015). Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components. Symmetry, 7(4), 1914-1928. https://doi.org/10.3390/sym7041914