Synthesis and Reactions of Dibenzo[a,e]pentalenes
Abstract
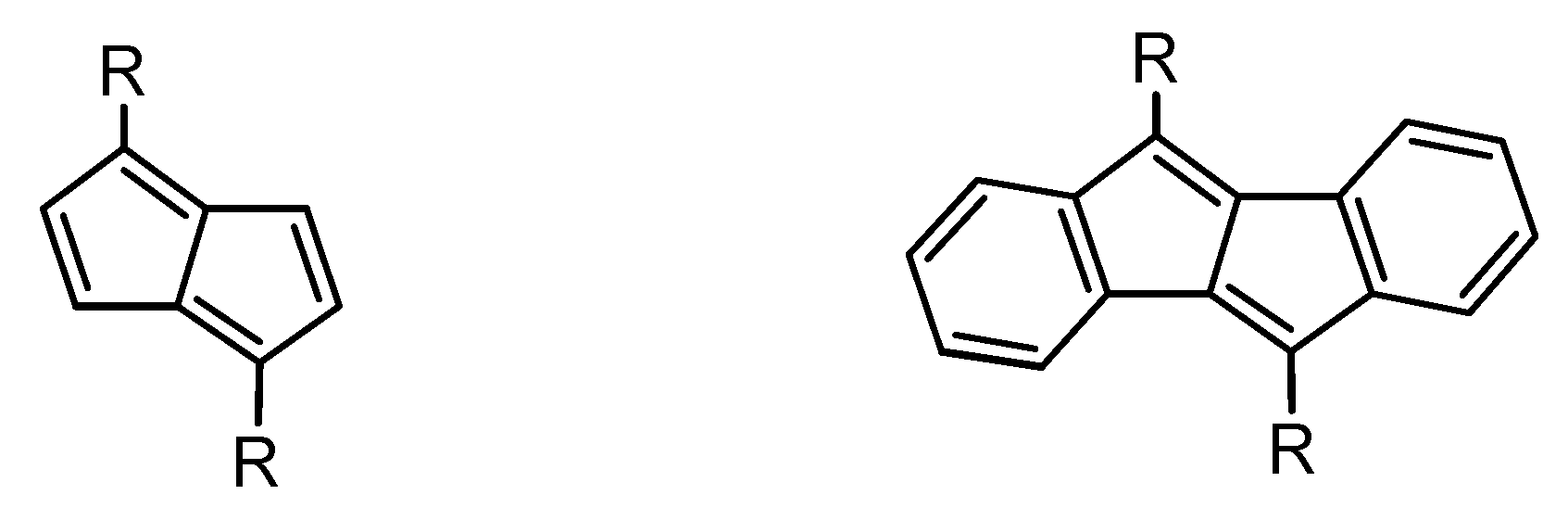
:1. Introduction
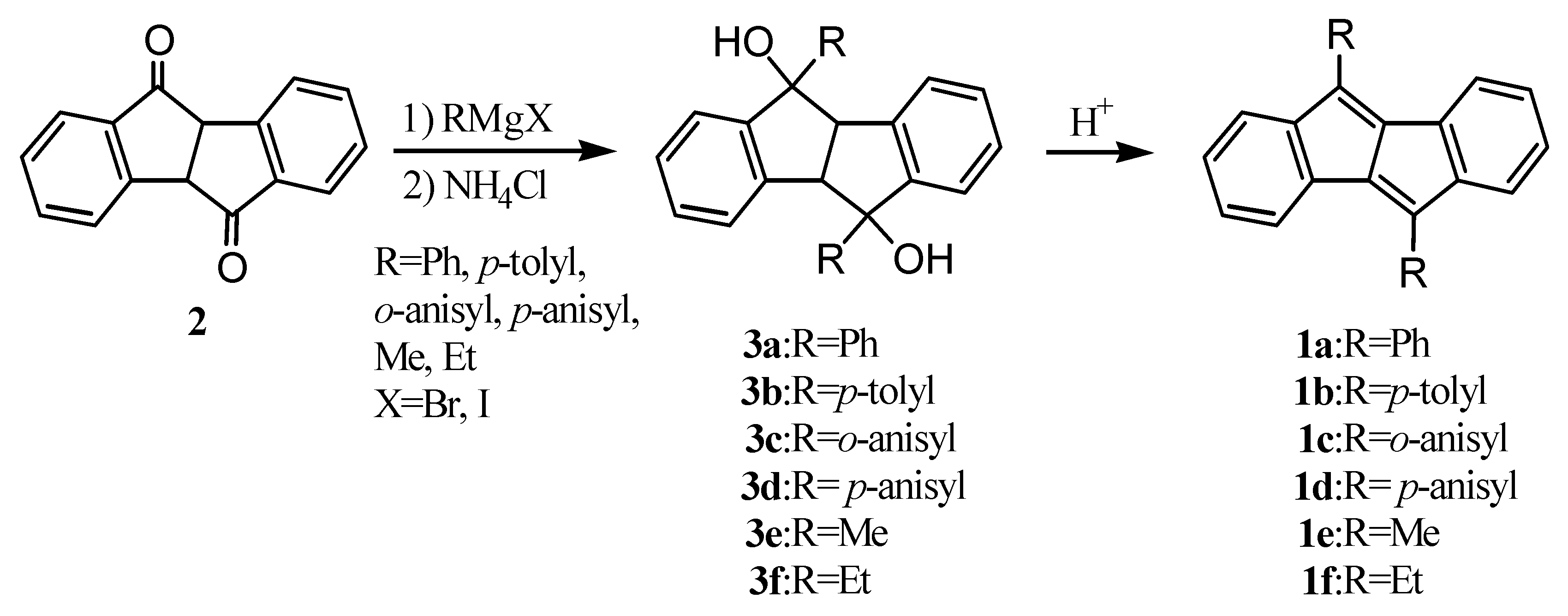
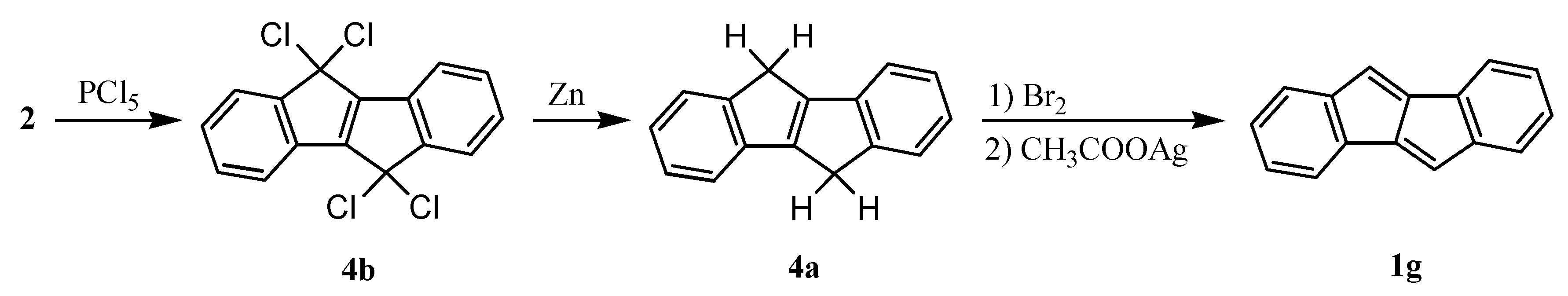
2. Early Studies on the Preparation of Dibenzopentalenes
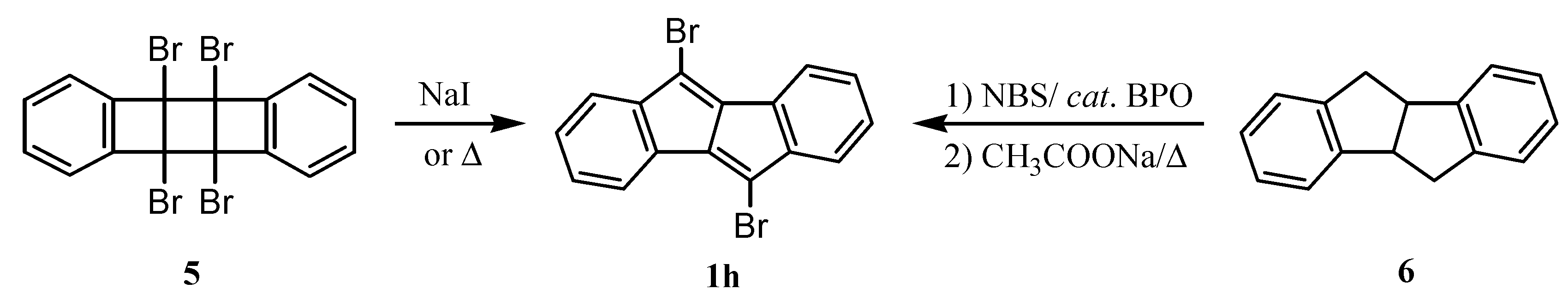
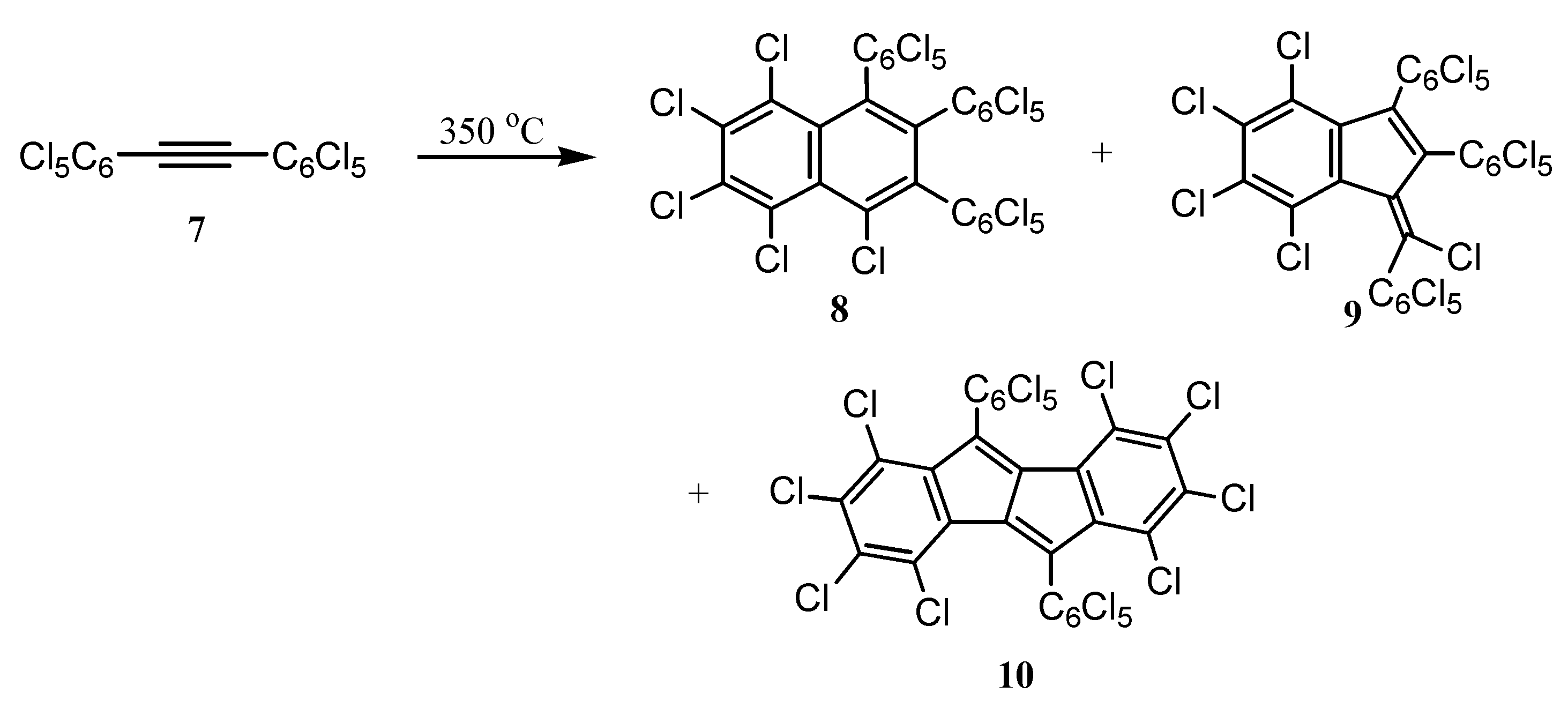
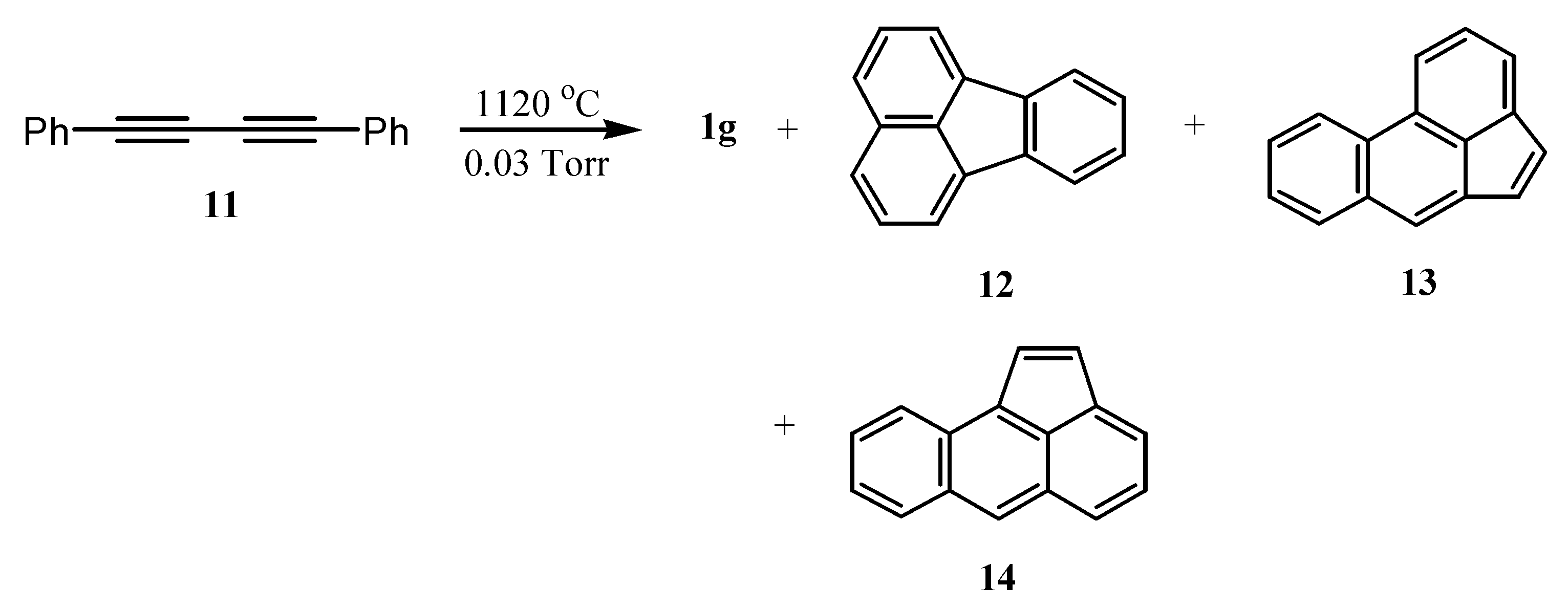
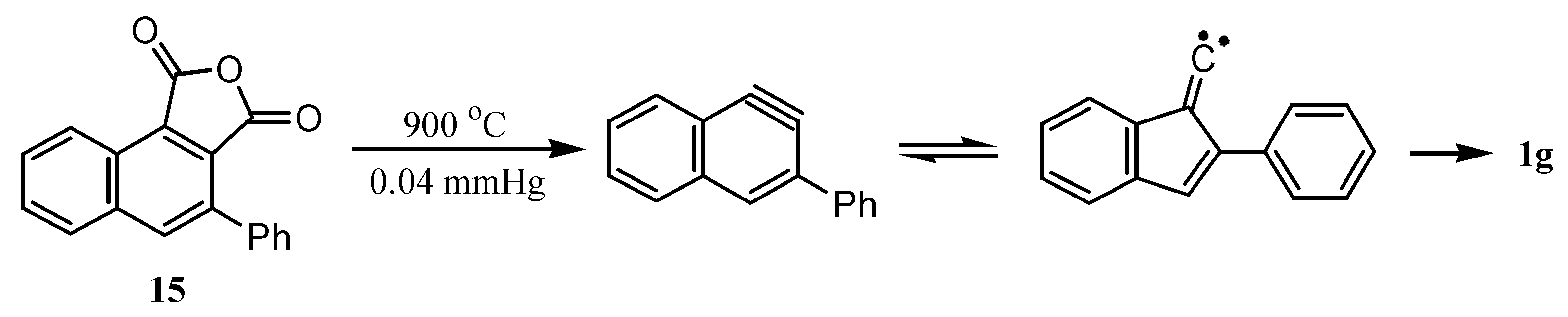
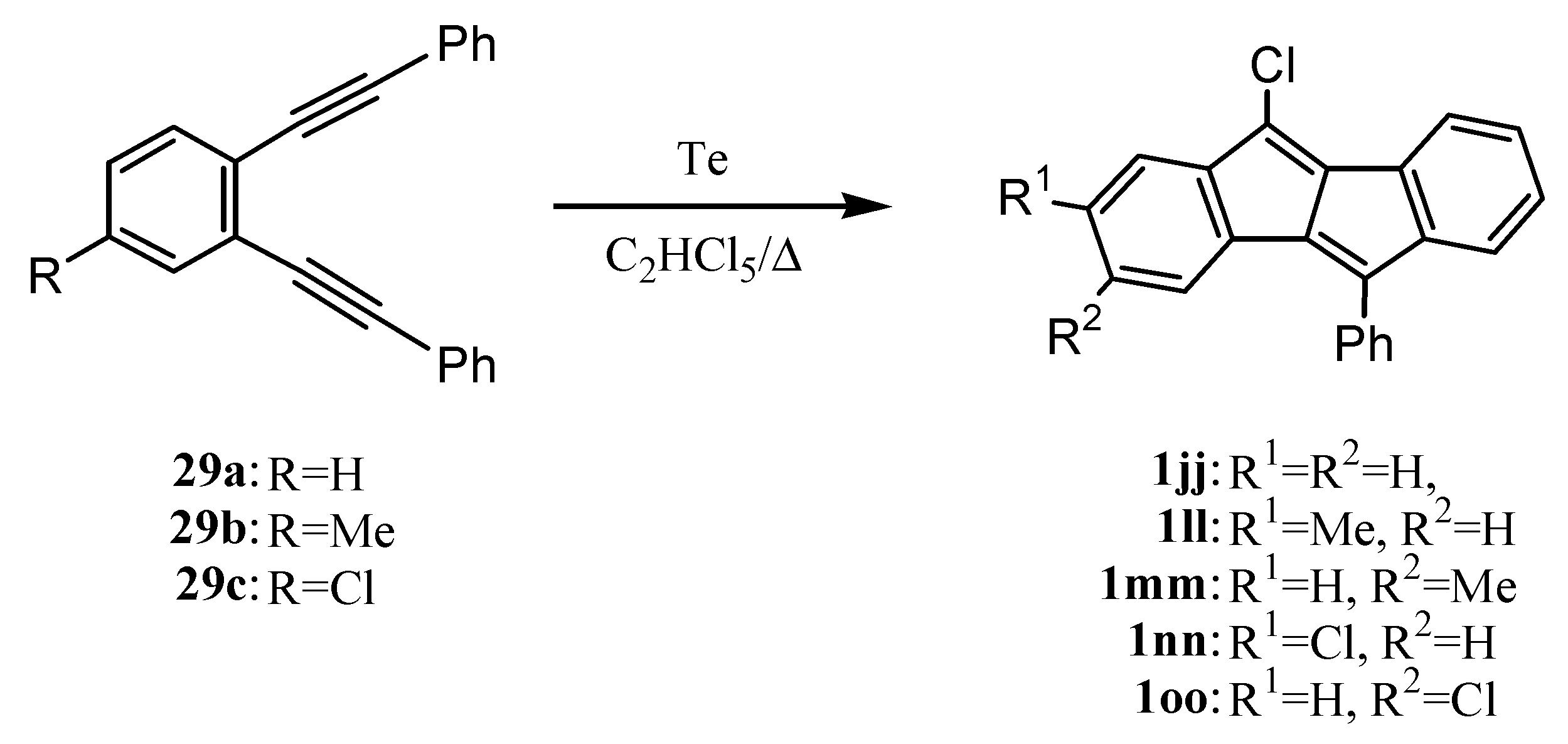
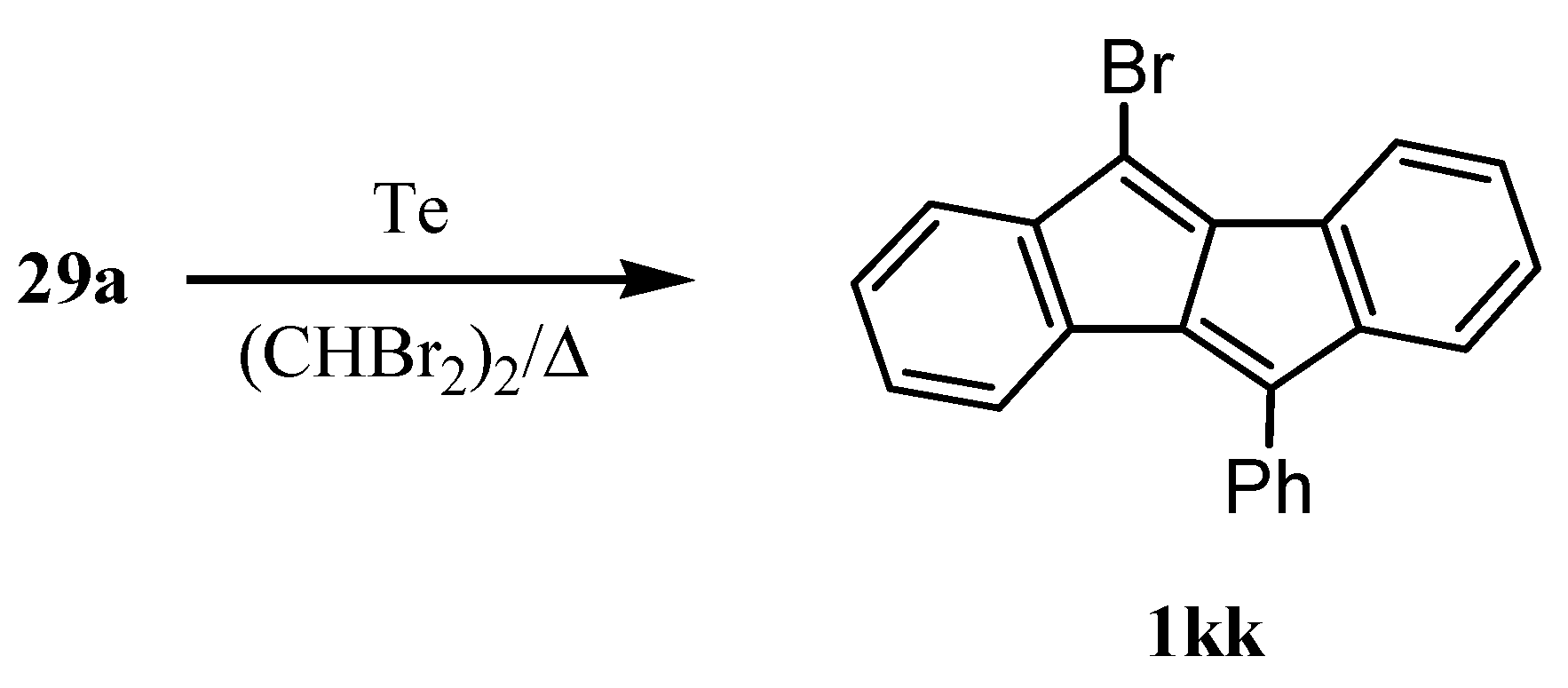
3. Preparation of Dibenzopentalenes by Thermolysis
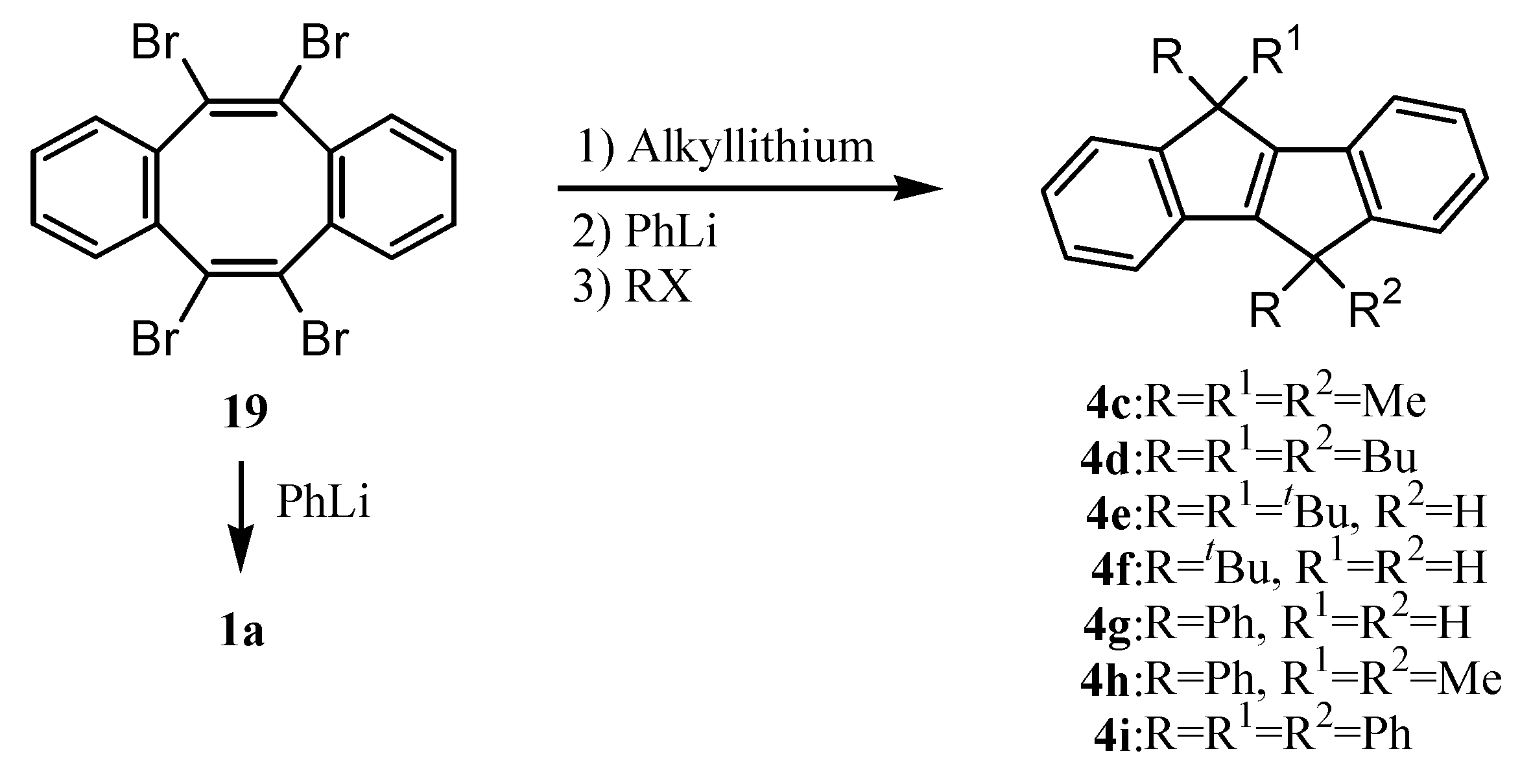
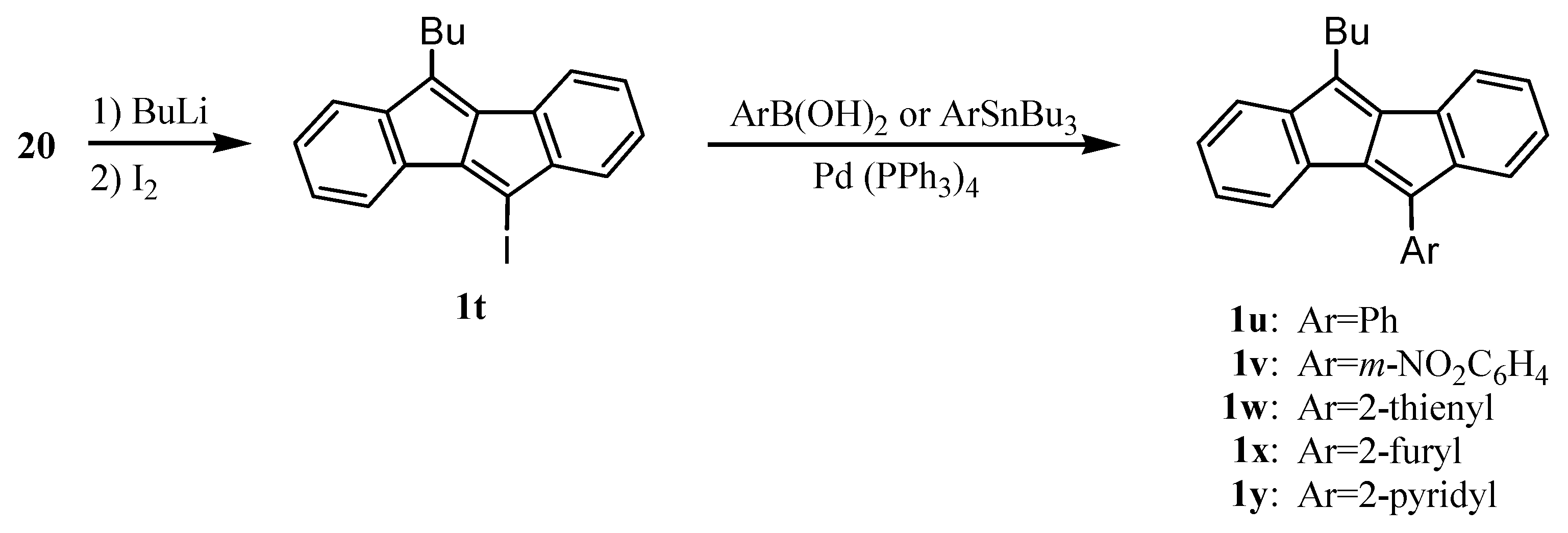
4. Preparation of Dibenzopentalenes from Dibenzocyclooctene Derivatives through Skeletal Rearrangement
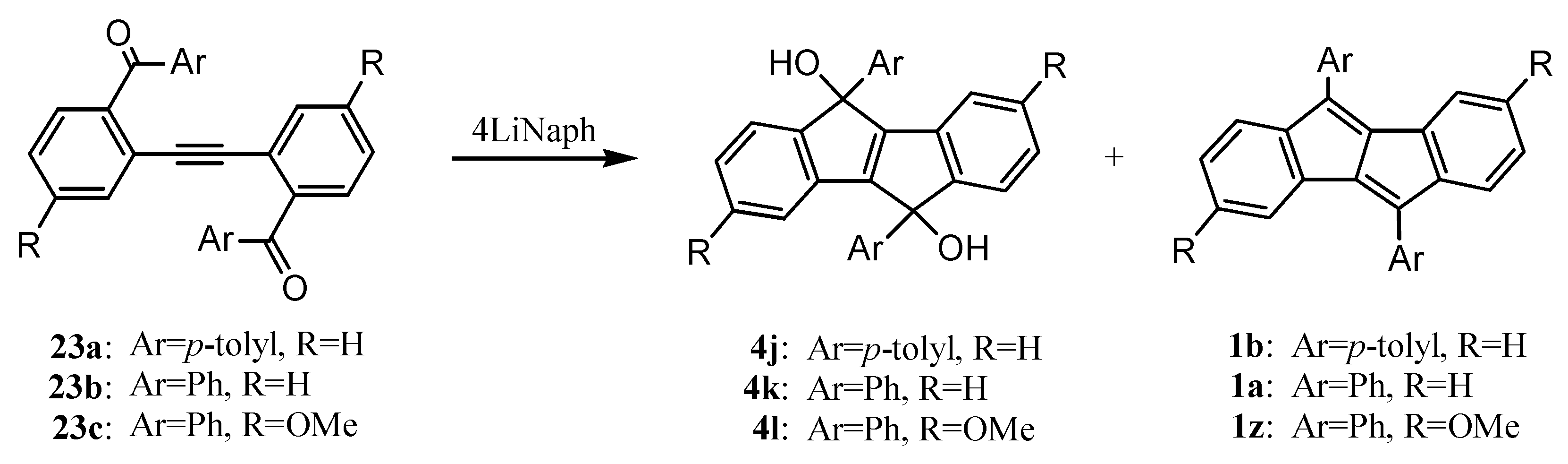
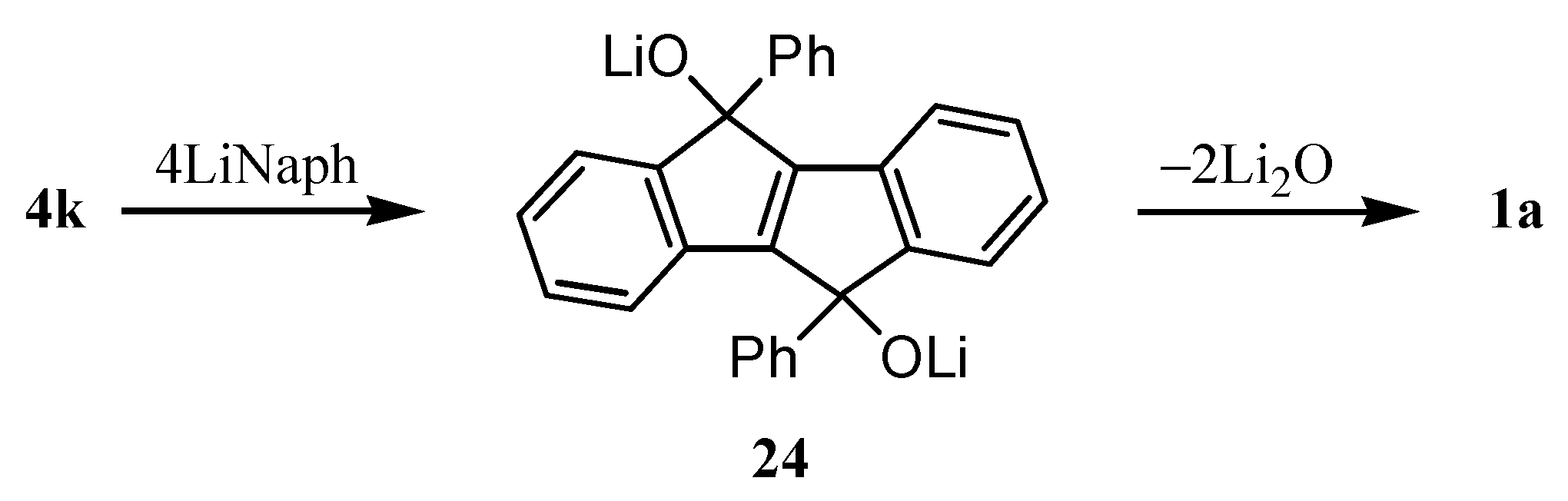
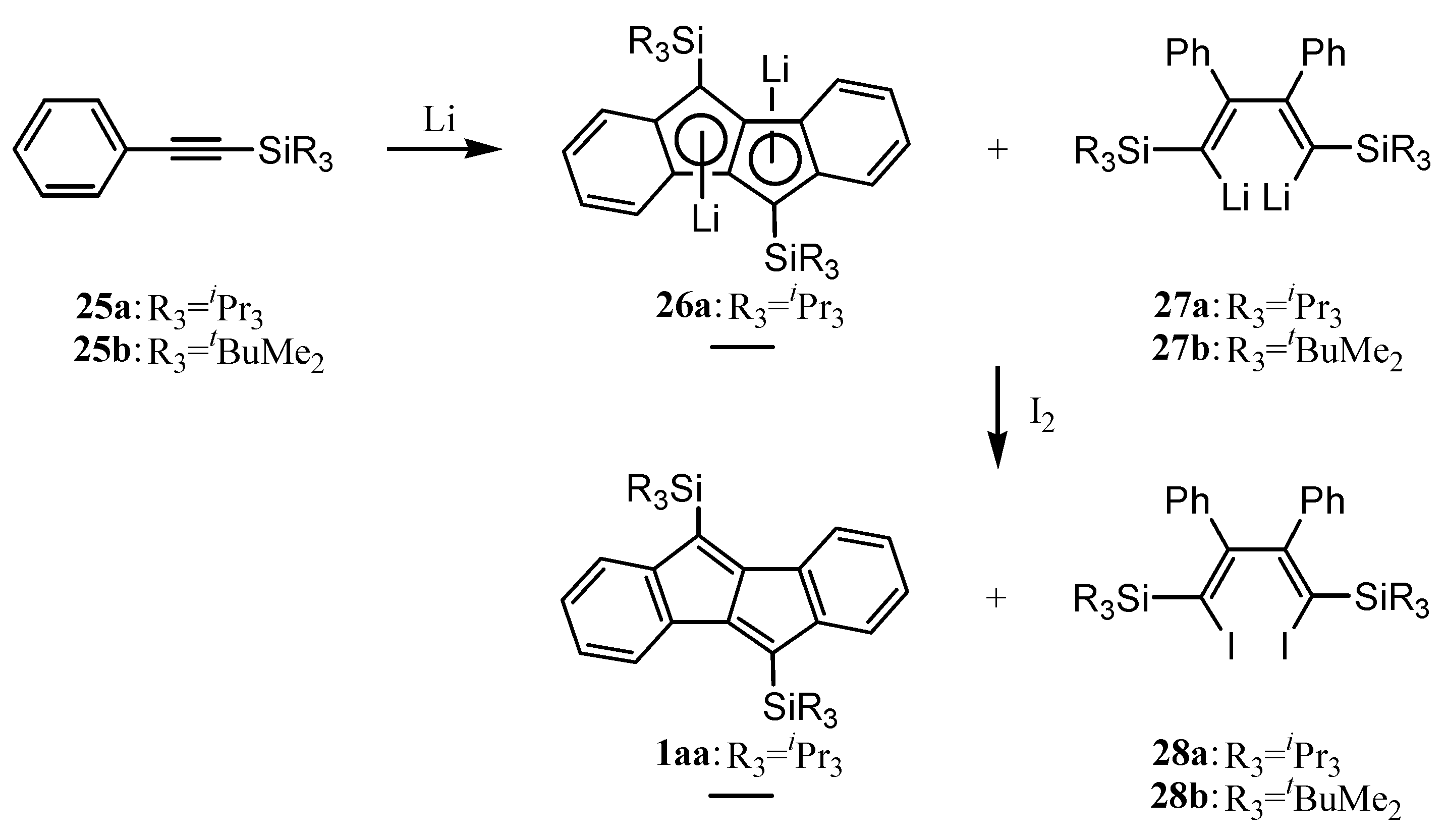
5. Preparation of Dibenzopentalenes through Reductive Cyclization
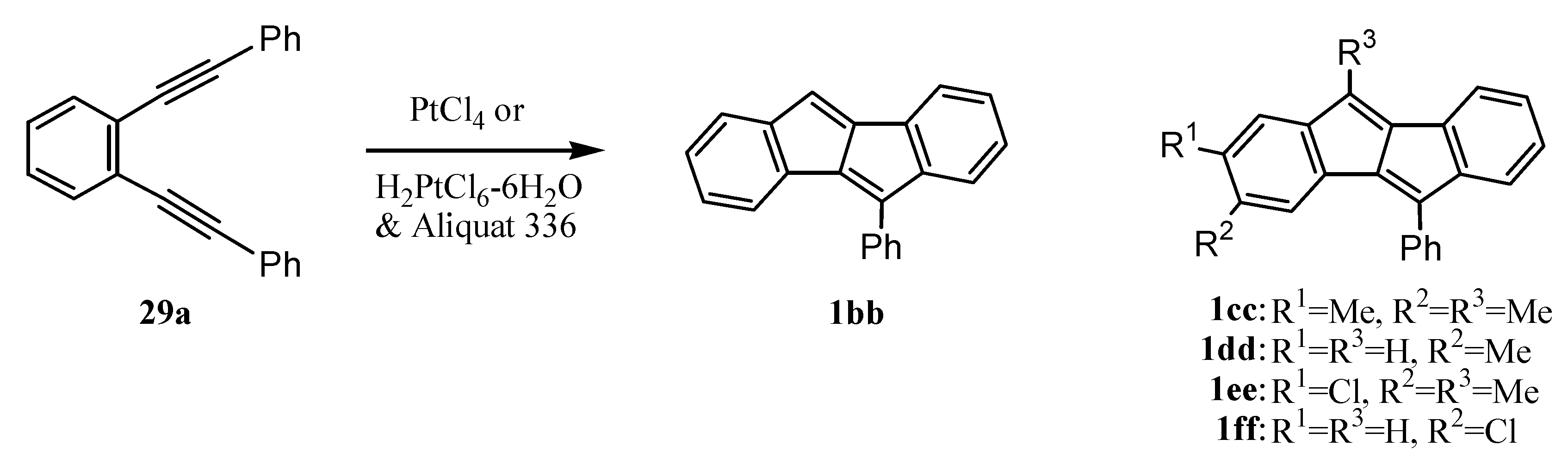
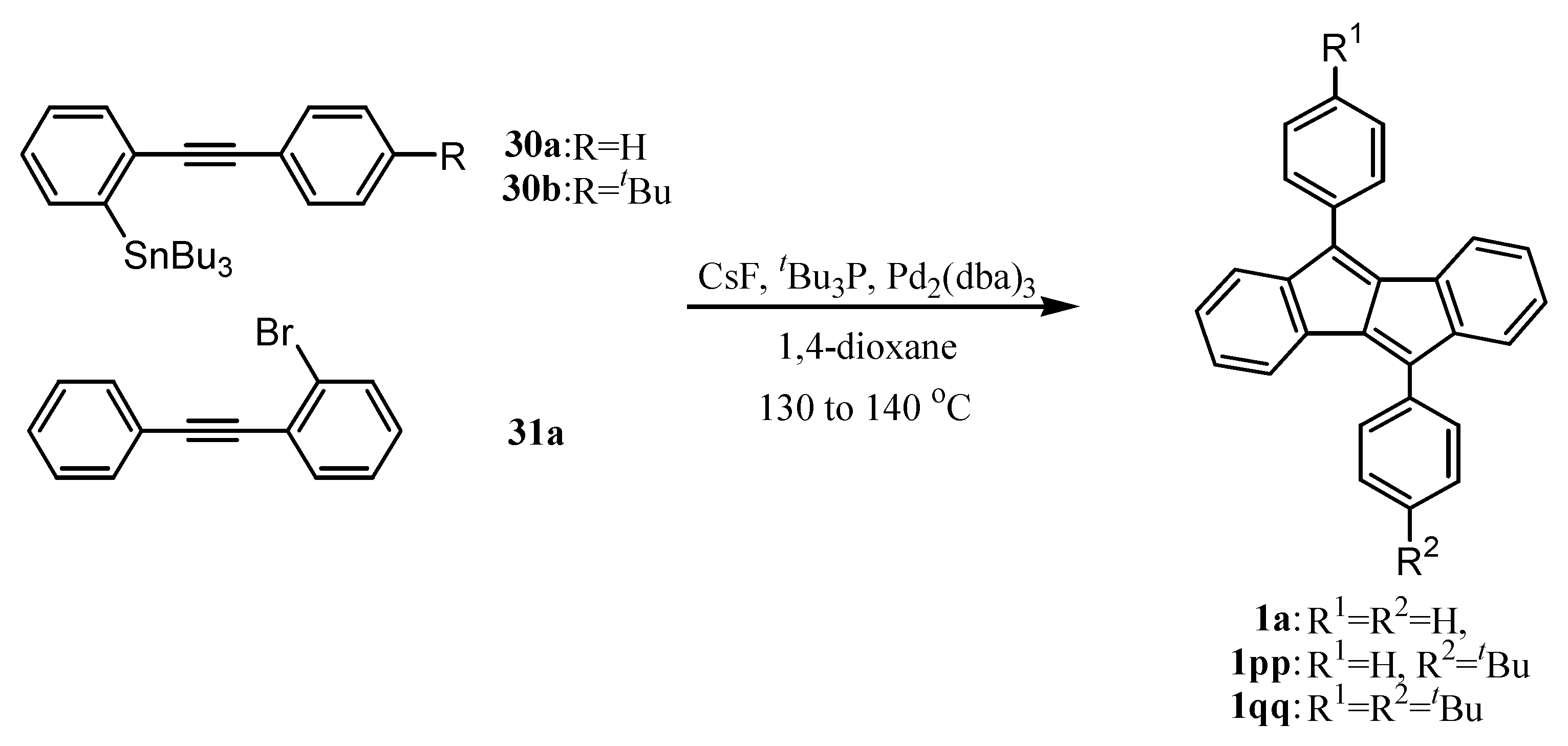
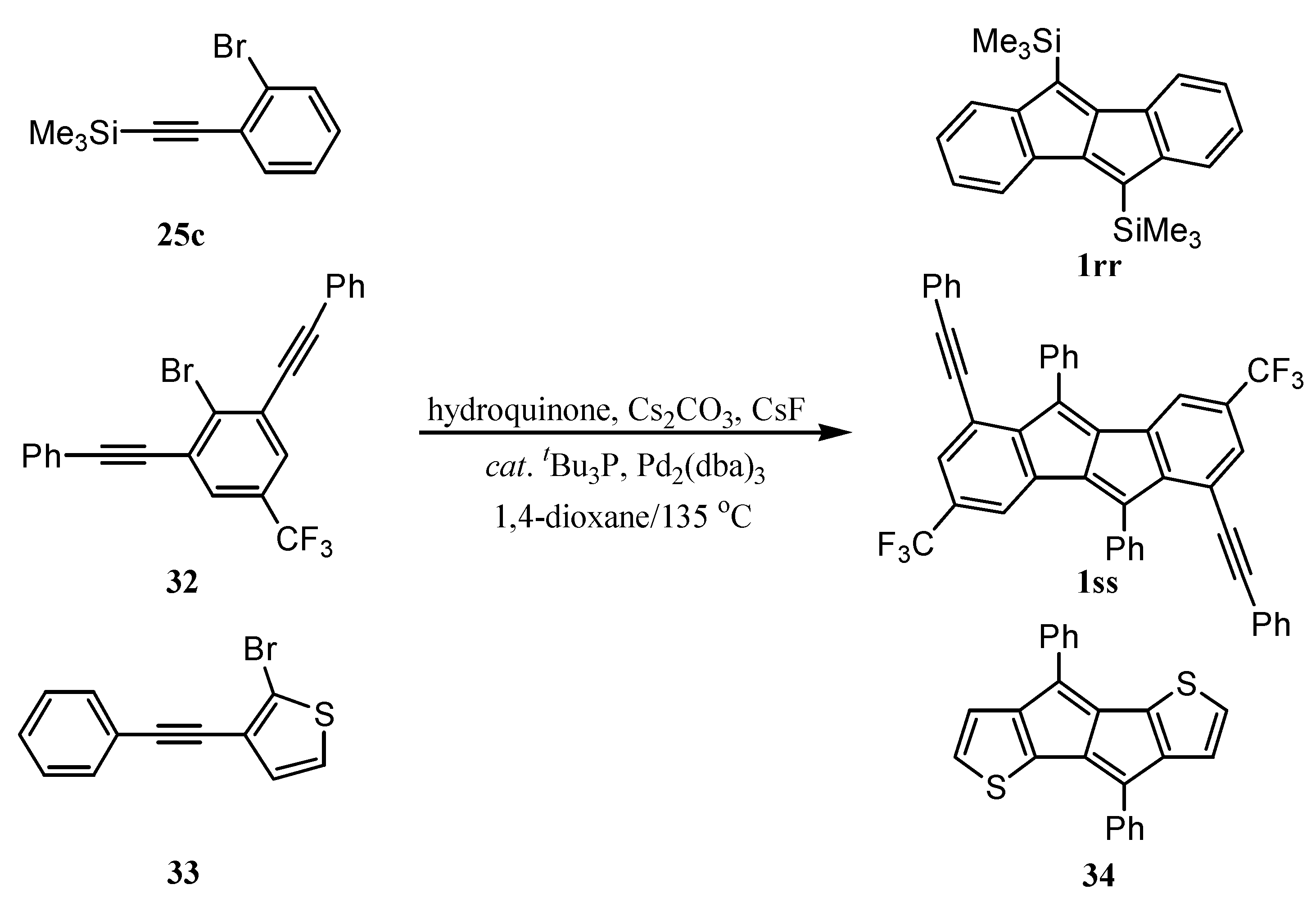
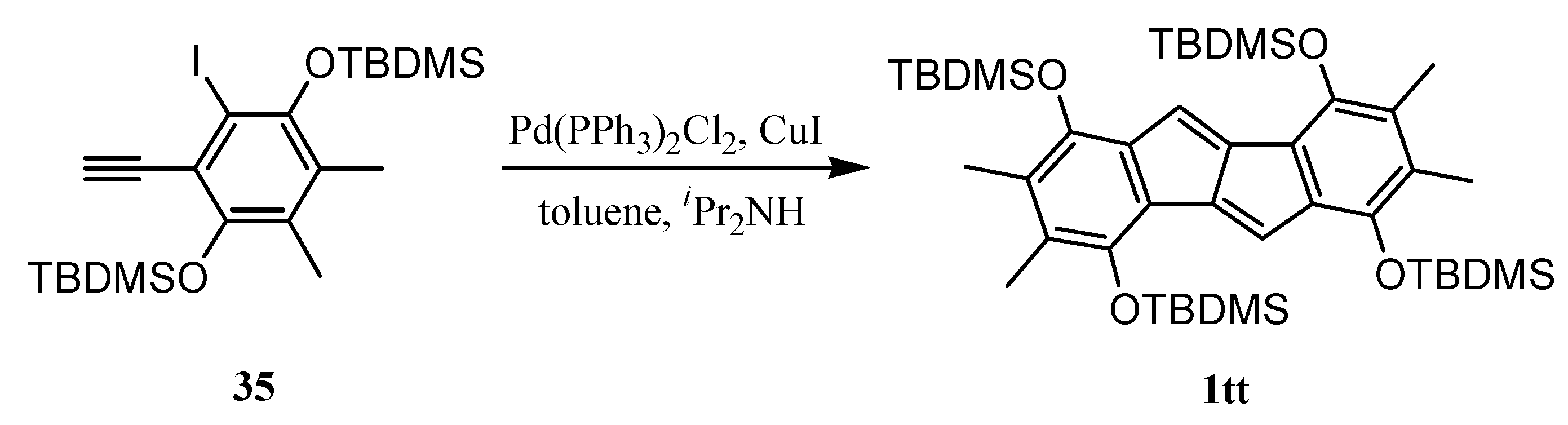
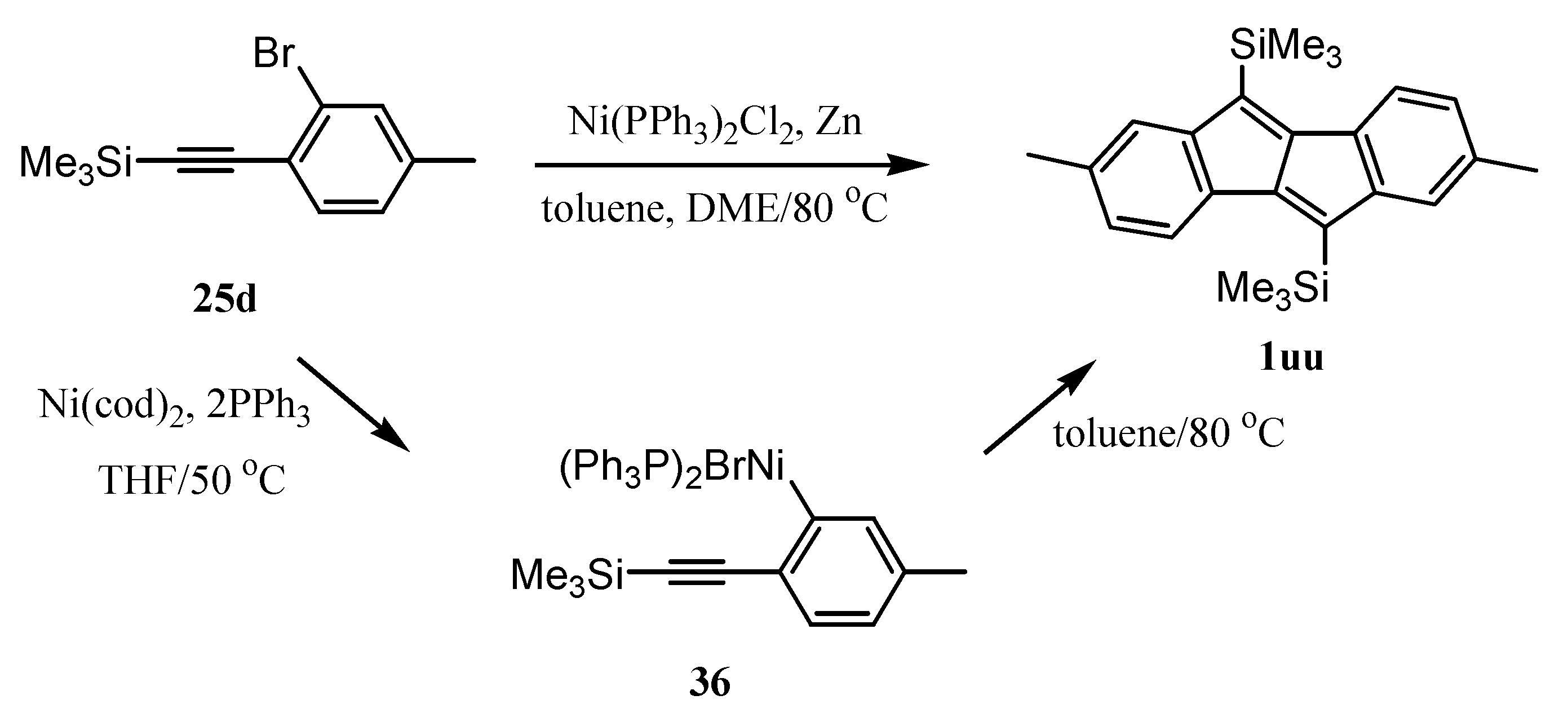
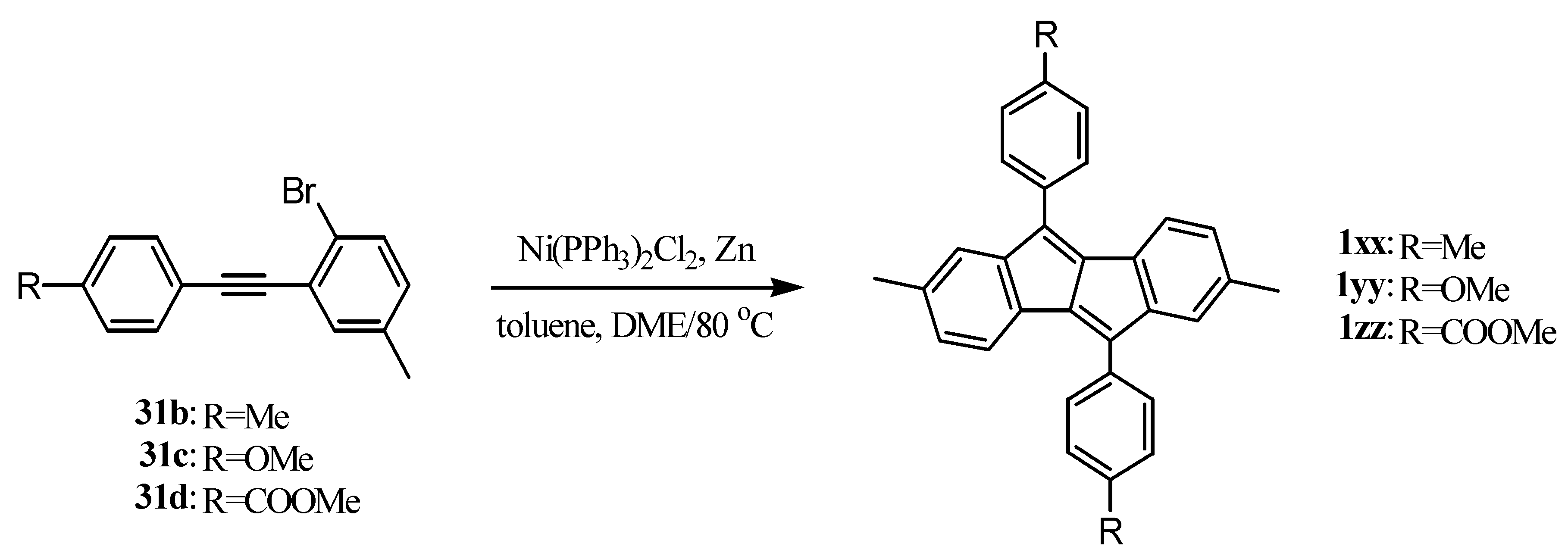
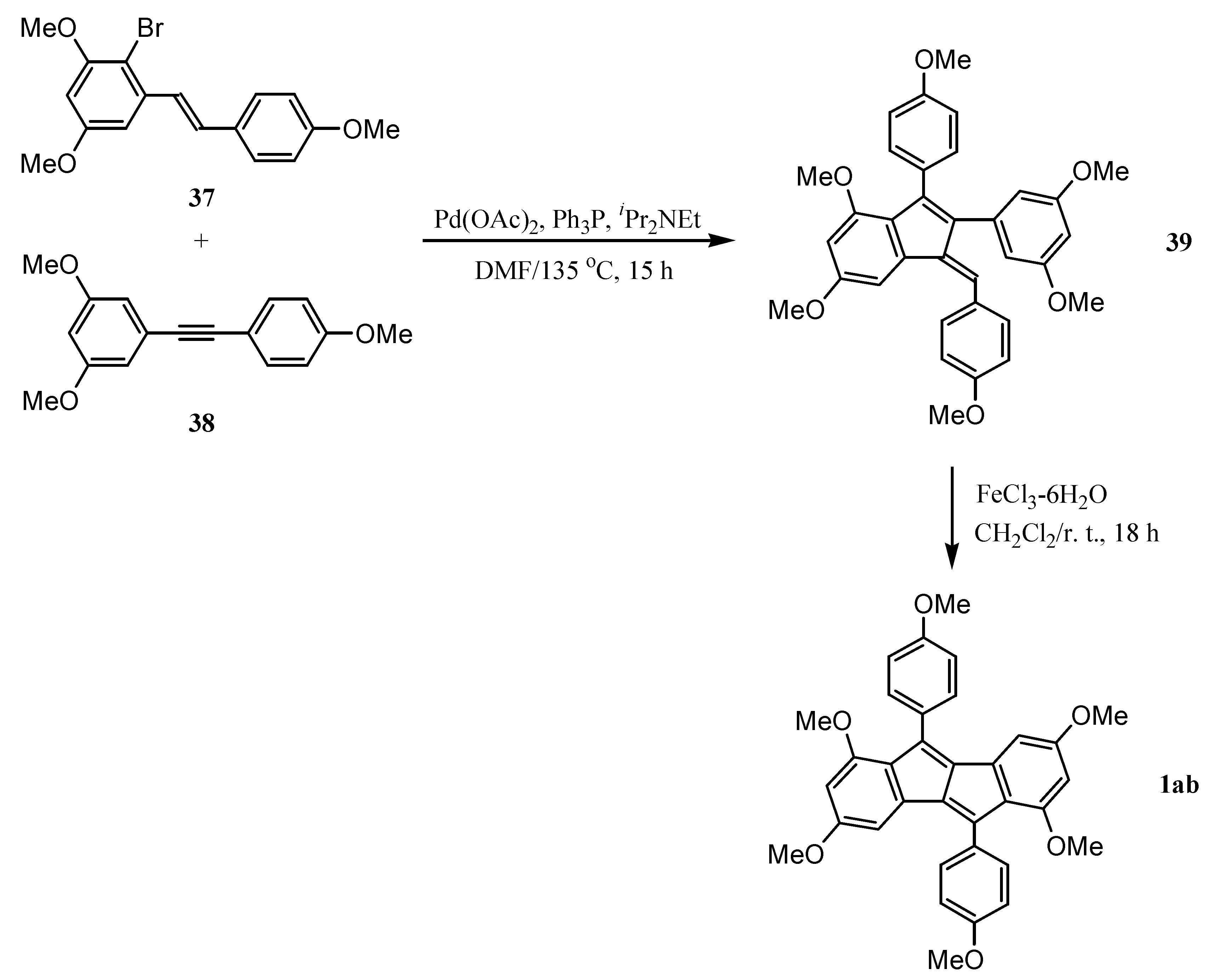
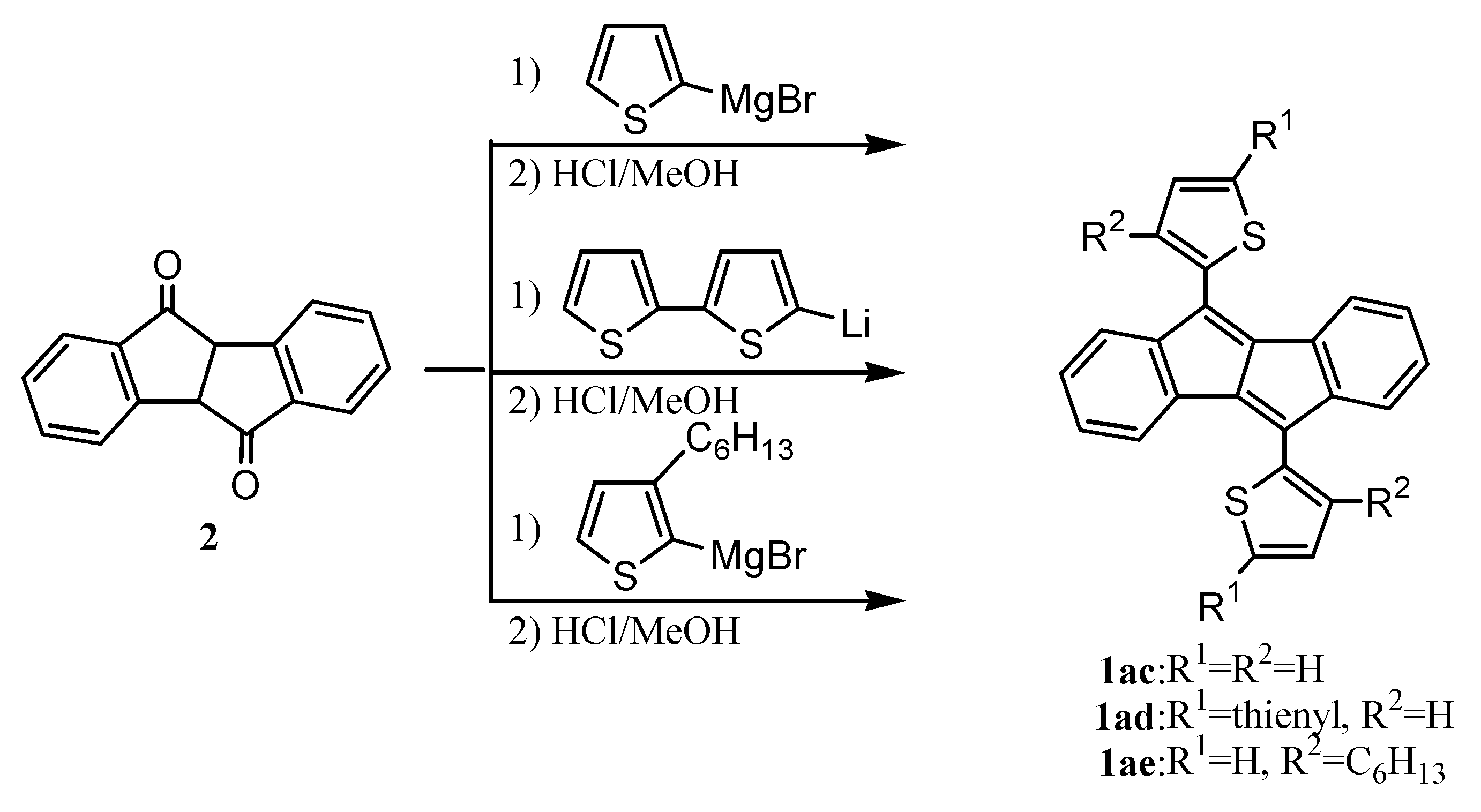
6. Preparation of Dibenzopentalenes in the Presence of Catalysts
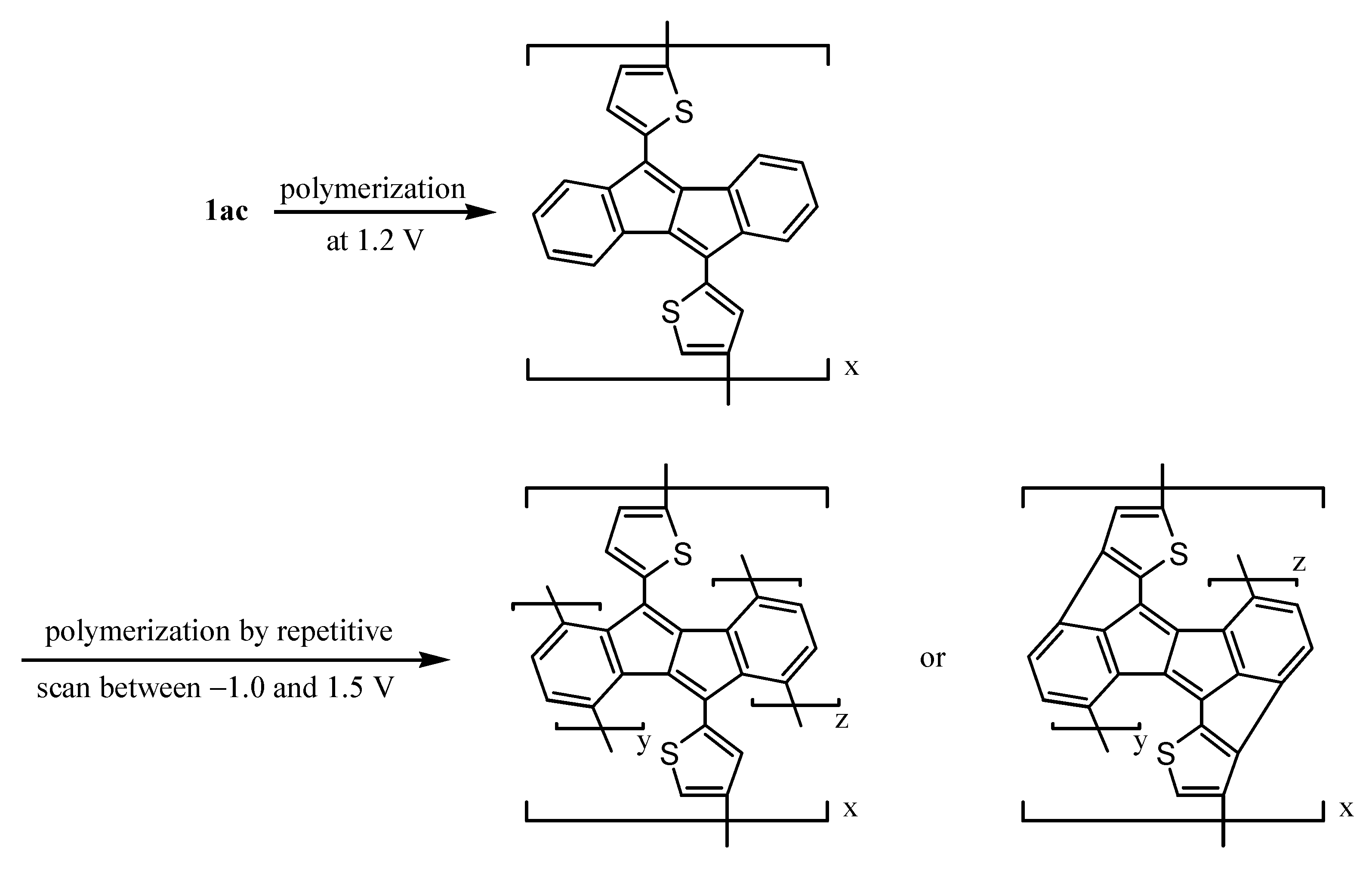
7. Preparation of Dibenzopentalene Polymer by Electrochemical Polymerization
8. Aromaticity of Dibenzopentalenes
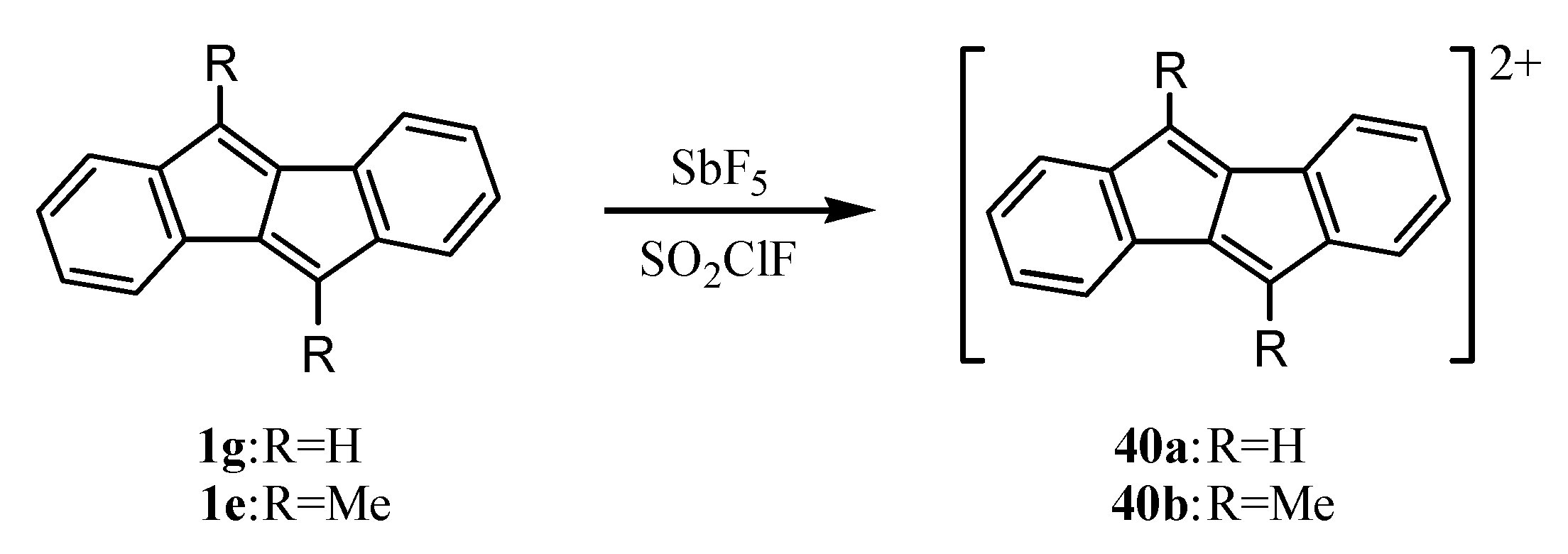
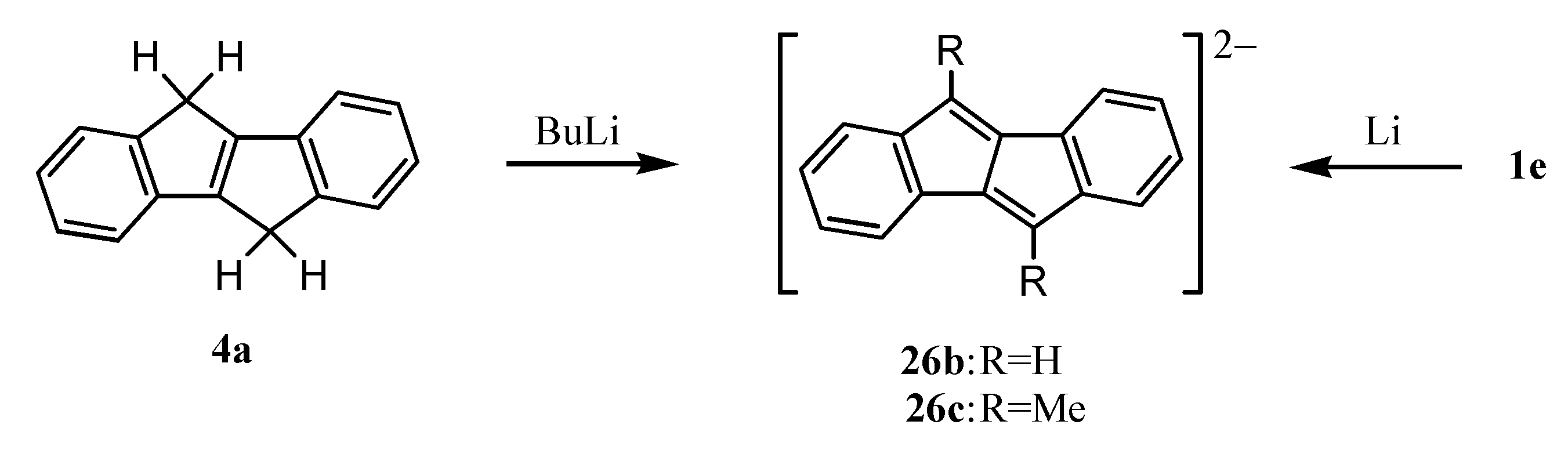
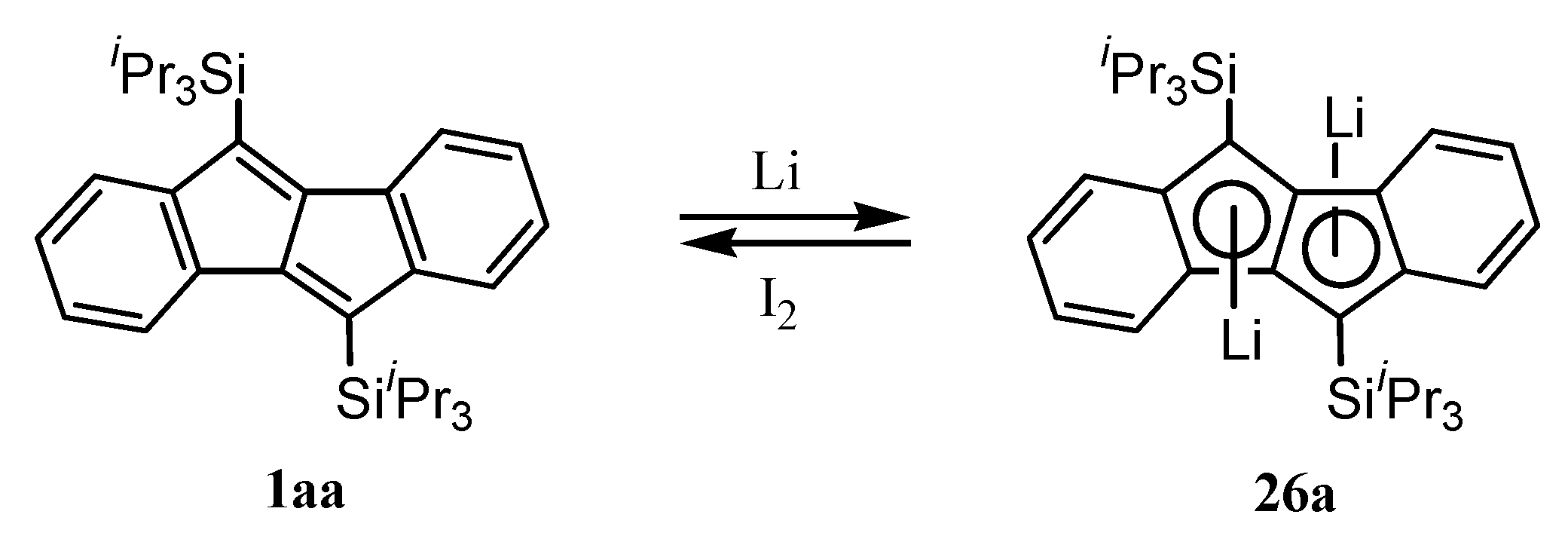
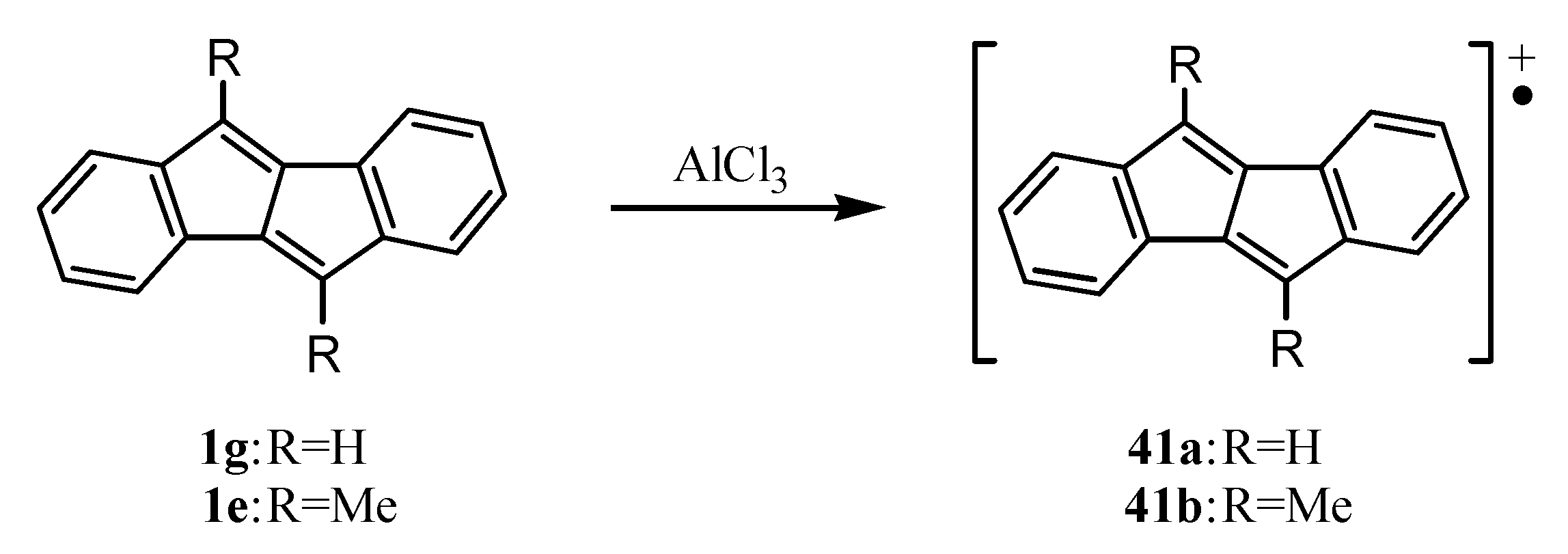
9. Ionic Species of Dibenzopentalenes
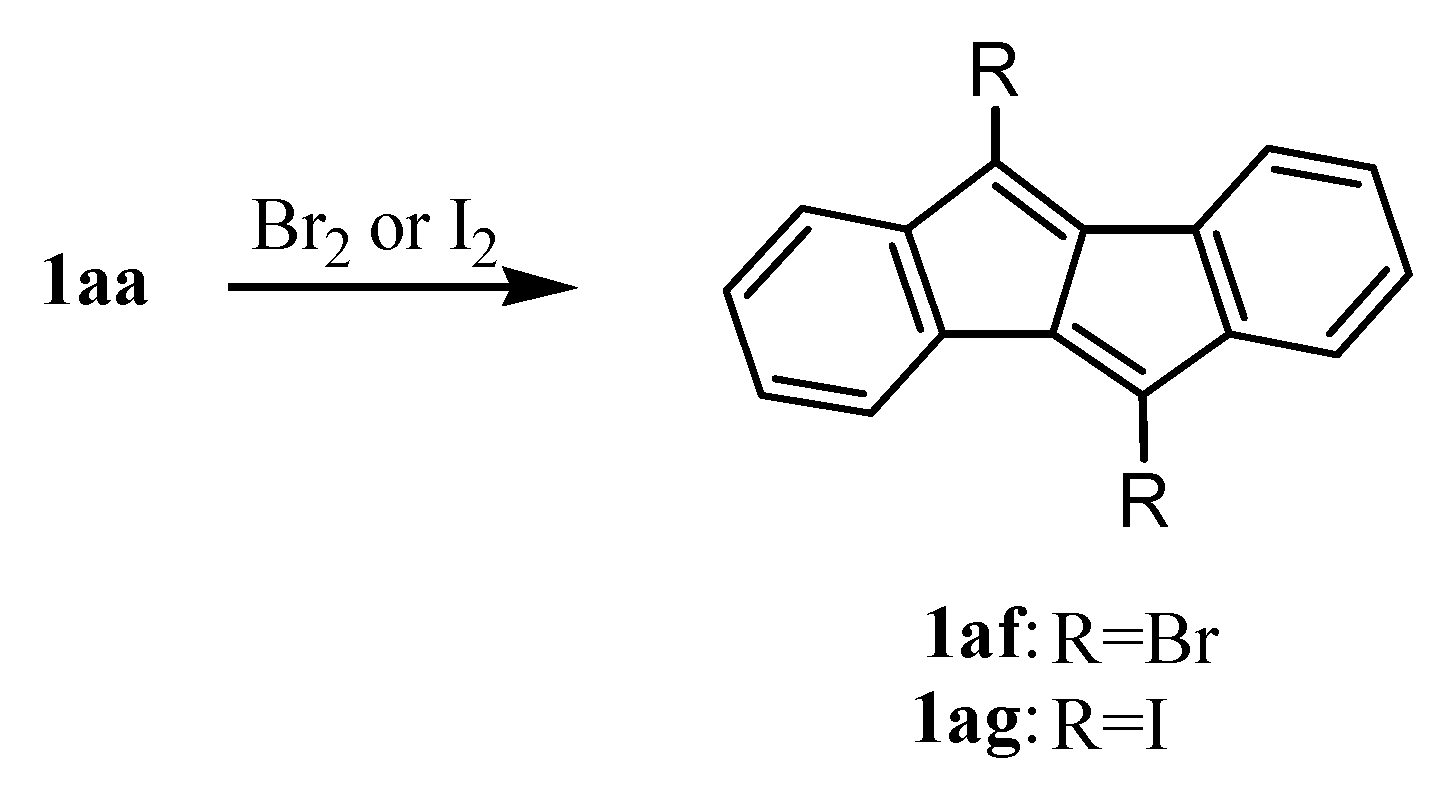
10. Reactions of Dibenzopentalenes
11. Summary and Outlook
Acknowledgments
References and Notes
- Hafner, K.; Dönges, R.; Goedecke, E.; Kaiser, R. Concerning Pentalene, 2-Methylpentalene, and 1,3-Dimethylpentalene. Angew. Chem. Int. Ed. Engl. 1973, 12, 337–339. [Google Scholar] [CrossRef]
- Dönges, R.; Hafner, K.; Lindner, H.J. Bildung und Isomerisierung des Dimeren Pentalens. Tetrahedron Lett. 1976, 17, 1345–1348. [Google Scholar] [CrossRef]
- Zywietz, T.K.; Jiao, H.; Schleyer, P.v.R.; de Meijere, A. Aromaticity and Antiaromaticity in Oligocyclic Annelated Five-Membered Ring Systems. J. Org. Chem. 1998, 63, 3417–3422. [Google Scholar] [CrossRef]
- Rosenberger, M.; Katz, T.J. The Pentalenyl Dianion. J. Am. Chem. Soc. 1962, 84, 865–866. [Google Scholar]
- Katz, T.J.; Rosenberger, M.; O’Hara, R.K. The Pentalenyl Dianion. J. Am. Chem. Soc. 1964, 86, 249–252. [Google Scholar] [CrossRef]
- Stezowski, J.J.; Hoier, H.; Wilhelm, D.; Clark, T.; Schleyer, P.v.R. The structure of an aromatic 10π electron ‘dianion’: Dilithium pentalenide. J. Chem. Soc. Chem. Commun. 1985, 1263–1264. [Google Scholar] [CrossRef]
- Aihara, J.-I. Bond-Length Equalization and Aromaticity in Charged π-Systems. Bull. Chem. Soc. Jpn. 2004, 77, 2179–2183. [Google Scholar] [CrossRef]
- Balazs, G.; Cloke, F.G.N.; Gagliardi, L.; Green, J.C.; Harrison, A.; Hitchcock, P.B.; Shahi, A.R.M.; Summerscales, O.T. A Dichromium(II) Bis(η8-pentalene) Double-Sandwich Complex with a Spin Equilibrium: Synthetic, Structural, Magnetic, and Theoretical Studies. Organometallics 2008, 27, 2013–2020. [Google Scholar] [CrossRef]
- Ashley, A.E.; Cooper, R.T.; Wildgoose, G.G.; Green, J.C.; O’Hare, D. Homoleptic Permethylpentalene Complexes: “Double Metallocenes” of the First-Row Transition Metals. J. Am. Chem. Soc. 2008, 130, 15662–15677. [Google Scholar] [CrossRef]
- Cloke, F.G.N. Organometallic Pentalene Complexes. Pure Appl. Chem. 2001, 73, 233–238. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Cloke, F.G.N. The Organometallic Chemistry of Pentalene. Coord. Chem. Rev. 2006, 250, 1122–1140. [Google Scholar] [CrossRef]
- Brand, K. Über Gefärbte Kohlenwasserstoffe der Diphensuccinden-Reihe. Ber. Detsch. Chem. Ges. 1912, 45, 3071–3077. [Google Scholar] [CrossRef]
- Roser, W. Einwirkung von concentrirter Schwefelsäure auf Diphenylbernsteinsäure: Diphensuccindon. Liebigs Ann. 1888, 247, 152–157. [Google Scholar] [CrossRef]
- Mittal, R.S.D.; Sothi, S.C. Azulenes and Related Substances—XV: Azuleno[2,1-a]azulene (part 1): Reaction of 3,6,7,8-Tetrahydrodibenzopentalene with Diazomethane; Synthesis of 11H-Indeno[2,1-a]azulene. Tetrahedron 1973, 29, 1321–1325. [Google Scholar] [CrossRef]
- Yang, J.; Lakshmikantham, M.V.; Cava, M.P.; Lorcy, D.; Bethelot, J.R. Synthesis and Characterization of 5,10-Bis(2-thienyl)indeno[2,1-a]indene Derivatives: The First Examples of Conducting Polymers Containing a Rigid Bis(thienyl)butadiene Core. J. Org. Chem. 2000, 65, 6739–6742. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.; Hoffmann, F.W. Über gefärbte Phenol-äther der Diphenylsuccinden-Reihe. Ber. Detsch. Chem. Ges. 1920, 53, 815–821. [Google Scholar] [CrossRef]
- Brand, K.; Schläger, F. Über farblose und farbige 9,12-Dialkyl-diphenylsuccindadiene-9,11. Ber. Detsch. Chem. Ges. 1923, 56, 2541–2545. [Google Scholar] [CrossRef]
- Blood, C.T.; Linstead, R.P. Fused Carbon Rings. Part XXI. Dibenzopentalene. J. Chem. Soc. 1952, 2263–2268. [Google Scholar] [CrossRef]
- Brand, K.; Müller, O. Über das 9.12-Dichlor-diphensuccindadien-9.11 und das Diphensuccinden-10. Ber. Detsch. Chem. Ges. 1922, 55, 601–608. [Google Scholar] [CrossRef]
- Wawzonek, S. An Attempt to Synthesize a Substituted Cycloöctatetraene. J. Am. Chem. Soc. 1940, 62, 745–749. [Google Scholar] [CrossRef]
- Jensen, F.R.; Coleman, W.E. Formation of 1,4-Dibromo-2,3-benzobiphenylene, 2,3-Benzobiphenylene, and 1,2,5,6-Tetrabromo-3,4:7,8-dibenzotricyclo[4,2,0,02,5]octadiene. Tetrahedron Lett. 1959, 1, 7–11. [Google Scholar] [CrossRef]
- Cava, M.P.; Pohlke, R.; Mitchell, M.J. Condensed Cyclobutane Aromatic Compounds. XXV. The Thermal Decomposition of 1,2,5,6-Tetrabromo-3,4,7,8-dibenzotricyclo[4.2.0.02,5]octadiene. J. Org. Chem. 1963, 28, 1861–1863. [Google Scholar] [CrossRef]
- Ballester, M.; Castañer, J.; Riera, J.; Armet, O. A New Synthesis, Chemical Behavior, and Spectra of Perchlorodiphenylacetylene. J. Org. Chem. 1986, 51, 1100–1106. [Google Scholar] [CrossRef]
- Brown, R.F.C.; Eastwood, F.W.; Wong, N.R. The Ethyne-Ethylidene Rearrangement: Formation of Indeno[2,1-a]indene and Fluoranthene on Flash Vacuum Pyrolysis of 1,4-Diphenylbutadiyne. Tetrahedron Lett. 1993, 34, 3607–3608. [Google Scholar] [CrossRef]
- Anderson, M.R.; Brown, R.F.C.; Coulston, K.J.; Eastwood, F.W.; Ward, A. The Pyrolysis of Phenylnaphthalenedicarboxylic Anhydrides: Products of Ring Contraction and of Radical Cyclization. Aust. J. Chem. 1990, 43, 1137–1150. [Google Scholar] [CrossRef]
- Kendall, J.K.; Shechter, H. Intramolecular Behaviors of Anthryldicarbenic Systems: Dibenzo[b,f]pentalene and 1H,5H-Dicyclobuta[de,kl]anthracene. J. Org. Chem. 2001, 66, 6643–6649. [Google Scholar] [CrossRef]
- Preda, D.V.; Scott, L.T. Phenyl Migrations in Dehydroaromatic Compounds. A New Mechanistic Link between Alternant and Nonalternant Hydrocarbons at High Temperatures. Org. Lett. 2000, 2, 1489–1492. [Google Scholar] [CrossRef]
- Hellwinkel, D.; Hasselbach, H.-J.; Lämmerzahl, F. Carbanion-Induced Skeletal Rearrangements: From the Dibenzo[a,e]cyclooctene to the Indeno[2,1-a]indene Framework. Angew. Chem. Int. Ed. Engl. 1984, 23, 705–706. [Google Scholar] [CrossRef]
- Babu, G.; Orita, A.; Otera, J. Facile Carbolithiation of Bent Alkyne without Catalyst. Tandem Route to Dibenzo[b,f]pentalenes from Dibenzocyclooctadiyne. Chem. Lett. 2008, 37, 1296–1297. [Google Scholar] [CrossRef]
- Zeni, G.; Larock, R.C. Synthesis of Heterocycles via Palladium-Catalyzed Oxidative Addition. Chem. Rev. 2006, 106, 4644–4680. [Google Scholar] [CrossRef]
- Patil, N.P.; Yamamoto, Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Karasawa, T.; Tamada, H.; Wakamiya, A.; Yamaguchi, S. Intramolecular Reductive Double Cyclization of o,o’-Bis(arylcarbonyl)diphenylacetylenes: Synthesis of Ladder π-Conjugated Skeletons. Org. Lett. 2009, 11, 3076–3079. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Nakamura, M.; Tajima, T.; Yoshioka, M. Reduction of Phenyl Silyl Acetylene with Lithium: Unexpected Formation of a Dilithium Dibenzopentalenide. Angew. Chem. Int. Ed. 2007, 46, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; Evans, J.C.; Emes, P.J.; Phelan, T.J. Reactions of Radical Anions. Part IX. The Radical Anion of 1-Phenyl-2-trimethylsilylacetylene. J. Chem. Soc. B 1971, 315–318. [Google Scholar] [CrossRef]
- Ashe, A.J., III; Kampf, J.W.; Savla, P.M. The Structure of (1Z,3Z)-1,4-Bis(trimethylsilyl)-1,4-bis(lithiotetramethylethylenediamine)-2,3-dimethyl-1,3-butadiene. A Double Bridged Dilithium Compound. Organometallics 1993, 12, 3350–3353. [Google Scholar] [CrossRef]
- Müller, V.E.; Munk, K.; Ziemek, P.; Sauerbier, M. Palladium(II)- und Platin(IV)-Komplexe von 1,2-Bis-phenyläthinyl-benzol. Liebigs Ann. Chem. 1968, 713, 40–48. [Google Scholar] [CrossRef]
- Müller, V.E.; Munk, K.; Fritz, H.-G.; Sauerbier, M. Indeno-indene aus Schwermetallkomplexen von 1,2-Bis-phenyläthinyl-benzol. Liebigs Ann. Chem. 1969, 723, 76–82. [Google Scholar] [CrossRef]
- Blum, J.; Baidossi, W.; Badrieb, Y.; Hoffman, R.E. Tellurium-Mediated Halogen Transfer from Polyhaloalkanes to Diyne Acceptors. J. Org. Chem. 1995, 60, 4738–4742. [Google Scholar] [CrossRef]
- Badrieh, Y.; Blum, J.; Amer, I.; Vollhardt, K.P.C. Cyclo-oligomerization and Rearrangement of Some Phenylated Diynes by the RhCl3-Aliquat 336 and by the H2PtCl6-Aliquat 336 Catalysts under Phase Transfer Conditions. J. Mol. Catal. 1991, 66, 295–312. [Google Scholar] [CrossRef]
- Badrieh, Y.; Greenwald, A.; Schumann, H.; Blum, J. Some Unusual Reactions of 1,2-Bis(phenylethynyl)benzene with Sulfur, Carbon Monoxide and Alkyl Acetylenedicarboxylates. Chem. Ber. 1992, 125, 667–674. [Google Scholar] [CrossRef]
- Levi, Z.U.; Tilley, T.D. Versatile Synthesis of Pentalene Derivatives via the Pd-Catalyzed Homocoupling of Haloenynes. J. Am. Chem. Soc. 2009, 131, 2796–2797. [Google Scholar] [CrossRef] [PubMed]
- Chakrabirty, M.; Tessier, C.A.; Youngs, W.J. Unusual Formation of a Cyclyne Dimer and an Indenoindene Derivative. J. Org. Chem. 1999, 64, 2947–2949. [Google Scholar] [CrossRef]
- Kawase, T.; Konishi, A.; Hirao, Y.; Matsumoto, K.; Kurata, H.; Kubo, T. An Extremely Simple Dibenzopentalene Synthesis from 2-Bromoethynylbenzenes Using Nickel(0) Complexes: Construction of Its Derivatives with Various Functionalities. Chem. Eur. J. 2009, 15, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Zografos, A.L.; Lin, Y. Total Synthesis of Resveratrol-Based Natural Products: A Chemoselective Solution. Angew. Chem. Int. Ed. 2007, 46, 8186–8191. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.L.; Sarpong, R. An Approach to the Synthesis of Dimeric Resveratrol Natural Products via a Palladium-catalyzed Domino Reaction. Tetrahedron Lett. 2009, 50, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.W.; Steiner, E.; Havenith, R.W.A.; Jenneskens, L.W. Current Density, Chemical Shifts and Aromaticity. Magn. Reson. Chem. 2004, 42, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.V.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Willner, I.; Rabinovitz, M. 1,9-Dimethyldibenzo[b,f]pentalene Dication and Dianion. New 14π and 18π Aromatic Systems. J. Am. Chem. Soc. 1978, 100, 337–338. [Google Scholar] [CrossRef]
- Willner, I.; Becker, J.Y.; Rabinovitz, M. Manifestation of Dual Aromaticity in Doubly Charged Annelated Pentalenes. J. Am. Chem. Soc. 1979, 101, 395–401. [Google Scholar] [CrossRef]
- Jiao, H.; Schleyer, P.v.R.; Mo, Y.; McAllister, M.A.; Tidwell, T.T. Magnetic Evidence for the Aromaticity and Antiaromaticity of Charged Fluorenyl, Indenyl, and Cyclopentadienyl Systems. J. Am. Chem. Soc. 1997, 119, 7075–7083. [Google Scholar] [CrossRef]
- Choi, S.-B.; Boudjouk, P.; Wei, P. Aromatic Benzannulated Silole Dianions. The Dilithio and Disodio Salts of a Silaindenyl Dianion. J. Am. Chem. Soc. 1998, 120, 5814–5815. [Google Scholar] [CrossRef]
- Choi, S.-B.; Boudjouk, P.; Qin, K. Aromatic Benzannulated Germole Dianions. The Dilithio and Disodio Salts of a Germaindenyl Dianion. Organometallics 2000, 19, 1806–1809. [Google Scholar] [CrossRef]
- Liu, Y.; Stringfellow, T.C.; Ballweg, D.; Guzei, I.A.; West, R. Structure and Chemistry of 1-Silafluorenyl Dianion, Its Derivatives, and an Organosilicon Diradical Dianion. J. Am. Chem. Soc. 2002, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ballweg, D.; Müller, T.; Guzei, I.A.; Clark, R.W.; West, R. Chemistry of the Aromatic 9-Germafluorenyl Dianion and Some Related Silicon and Carbon Species. J. Am. Chem. Soc. 2002, 124, 12174–12181. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Shimosawa, M.; Yoshioka, M.; Ishimura, K.; Nagase, S. Synthesis of Stannaindenyl Anions and a Dianion. Organometallics 2006, 25, 2967–2971. [Google Scholar] [CrossRef]
- Saito, M.; Shimosawa, M.; Yoshioka, M.; Ishimura, K.; Nagase, S. Synthesis and Characterization of Dimetallostannafluorenes. Chem. Lett. 2006, 35, 940–941. [Google Scholar] [CrossRef]
- Saito, M.; Nakamura, M.; Tajima, T. New Reactions of a Dibenzo[a,e]pentalene. Chem. Eur. J. 2008, 14, 6062–6068. [Google Scholar] [CrossRef]
- Fürderer, P.; Gerson, F.; Rabinovitz, M.; Willner, I. Radical Ions in the Pentalene Series [1]. Part II. Dibenzo[b,f]pentalene and its 5,10-Dimethyl Derivative. Helv. Chim. Acta 1978, 61, 2981–2988. [Google Scholar] [CrossRef]
- Atormyan, L.; Mkoyan, S.; Urazowski, I.; Broussier, R.; Ninoreille, S.; Perron, P.; Gautheson, B. Novel Chiral ansa-Metallocene Complexes of Titanium and Zirconium with a Semirigid Bridge. Organometallics 1995, 14, 2601–2604. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Manoharan, M.; Wang, Z.-X.; Kiran, B.; Jiao, H.; Puchta, R.; Hommes, N.J.R.v.E. Dissected Nucleus-Independent Chemical Shift Analysis of π-Aromaticity and Antiaromaticity. Org. Lett. 2001, 3, 2465–2468. [Google Scholar] [CrossRef]
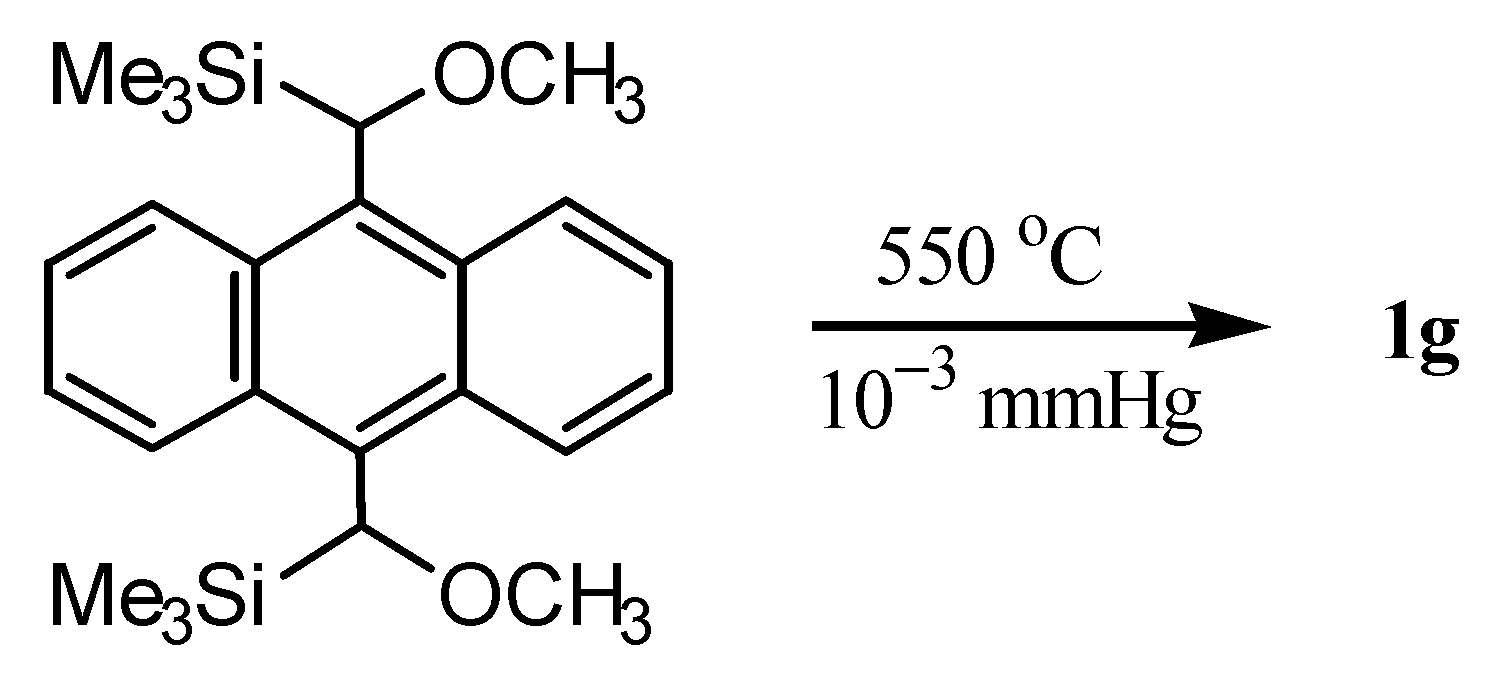



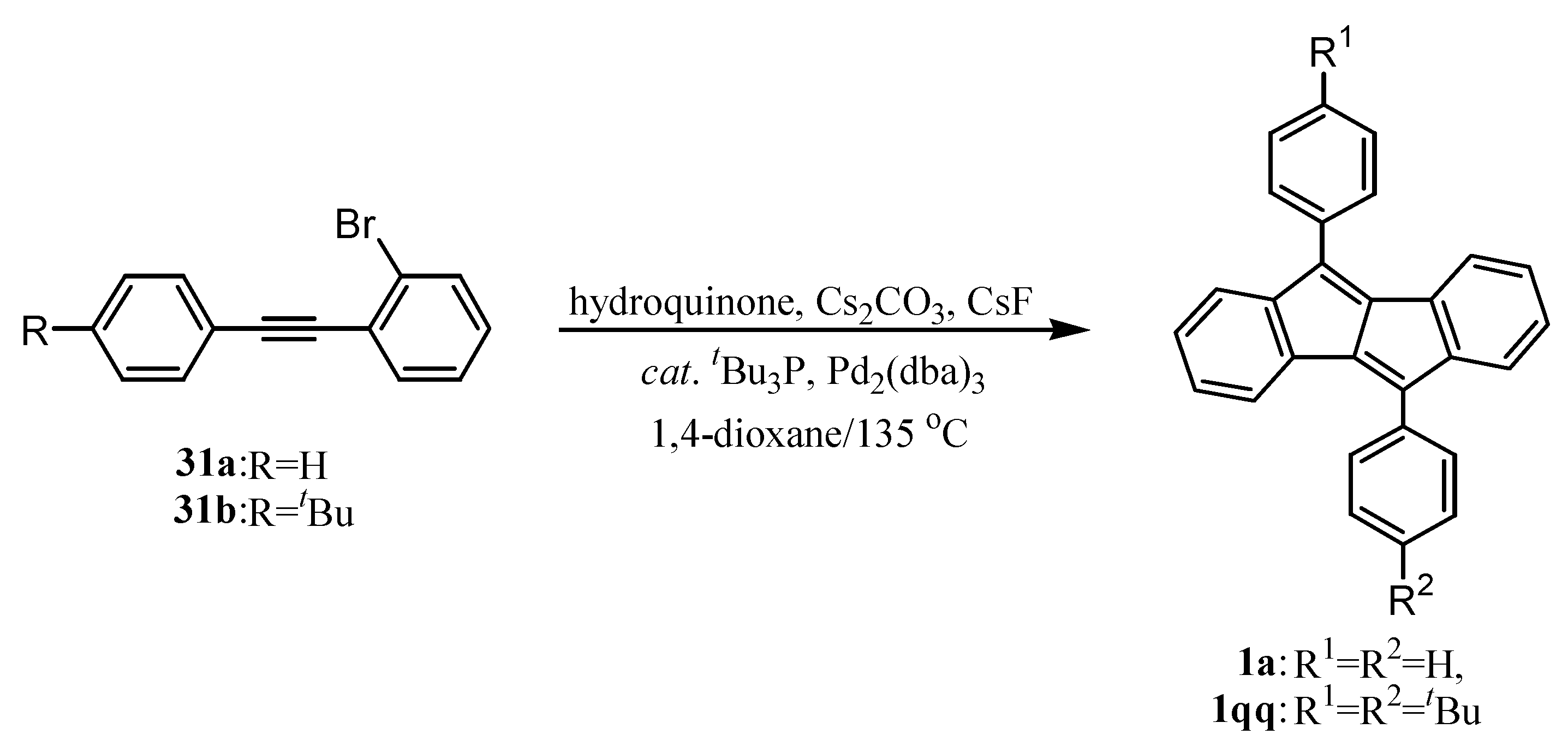
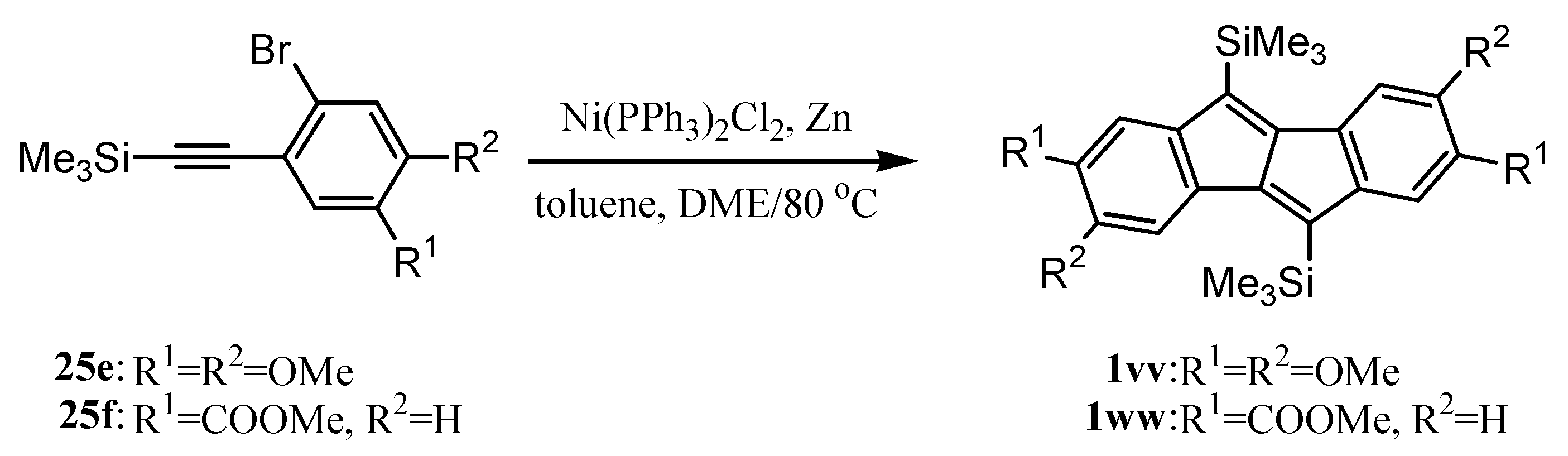


© 2010 by the authors; Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saito, M. Synthesis and Reactions of Dibenzo[a,e]pentalenes. Symmetry 2010, 2, 950-969. https://doi.org/10.3390/sym2020950
Saito M. Synthesis and Reactions of Dibenzo[a,e]pentalenes. Symmetry. 2010; 2(2):950-969. https://doi.org/10.3390/sym2020950
Chicago/Turabian StyleSaito, Masaichi. 2010. "Synthesis and Reactions of Dibenzo[a,e]pentalenes" Symmetry 2, no. 2: 950-969. https://doi.org/10.3390/sym2020950
APA StyleSaito, M. (2010). Synthesis and Reactions of Dibenzo[a,e]pentalenes. Symmetry, 2(2), 950-969. https://doi.org/10.3390/sym2020950



