Positional Effects of a Fly’s Wing Vein in the Asymmetric Distribution of Hydraulic Resistances
Abstract
:1. Introduction
2. Materials and Methods
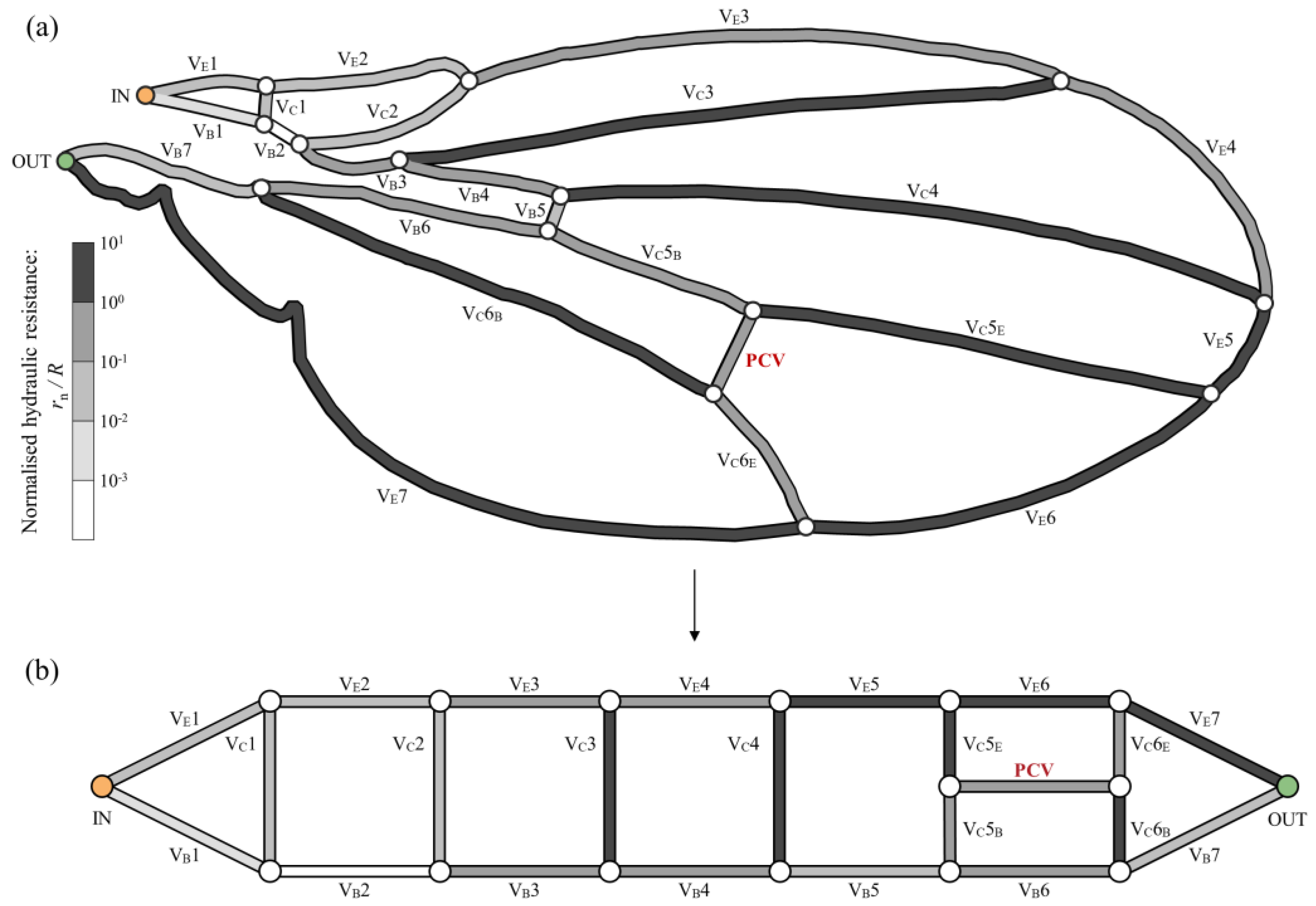
2.1. Network Modelling
2.2. Estimation of Haemolymph Flow Rate and Pressure Loss
3. Results and Discussion
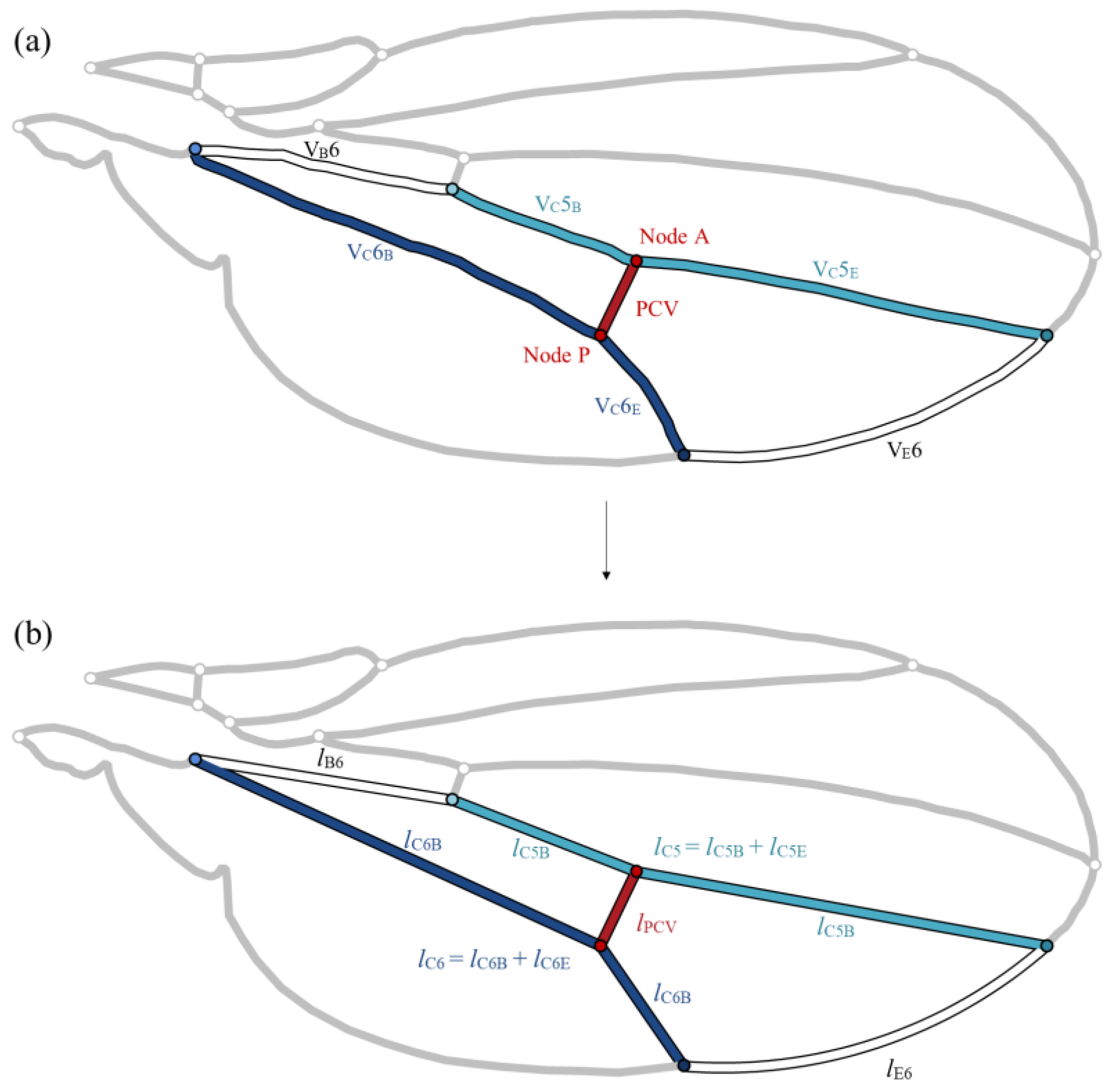
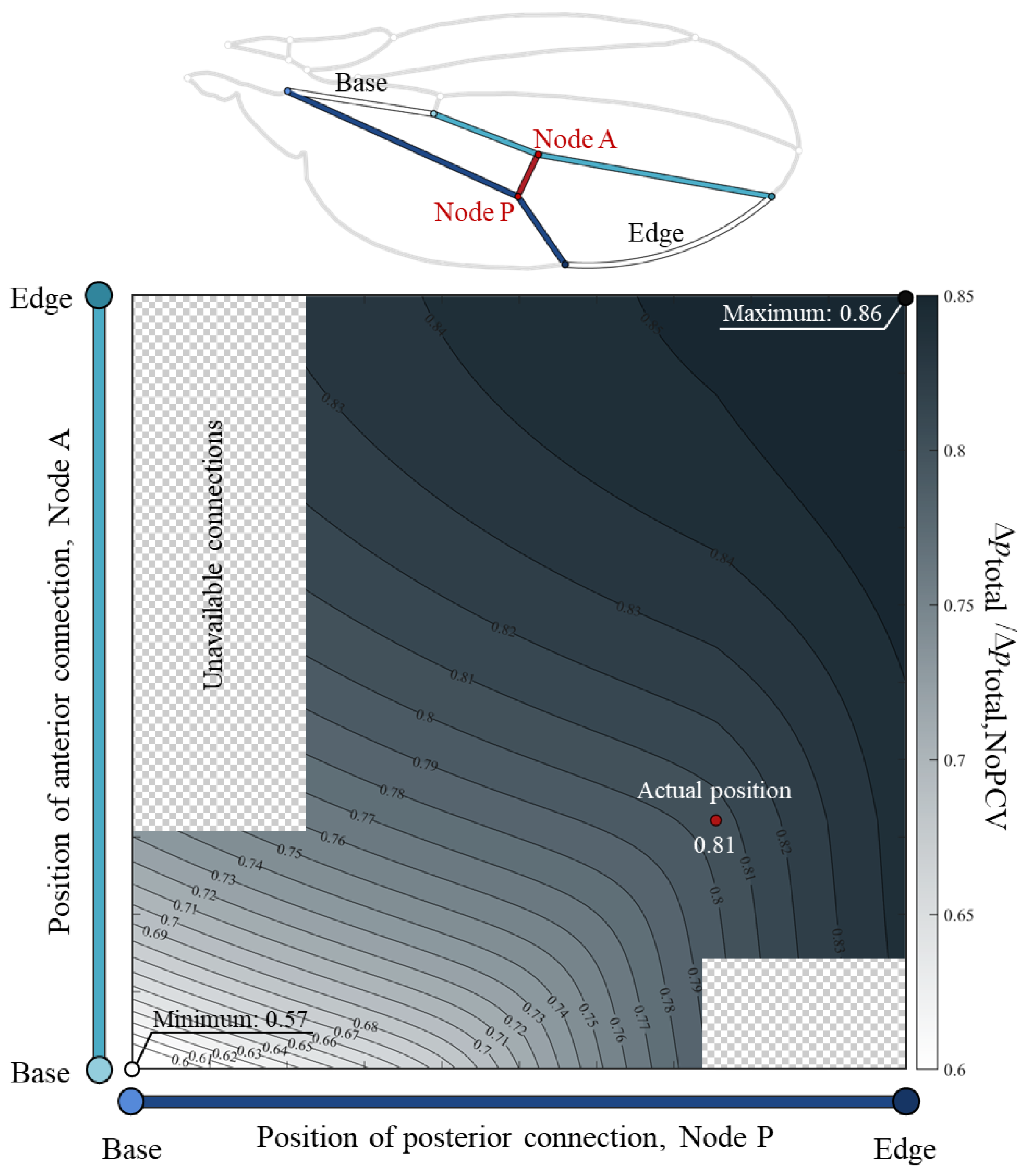
3.1. Total Pressure Loss Variation with PCV Position
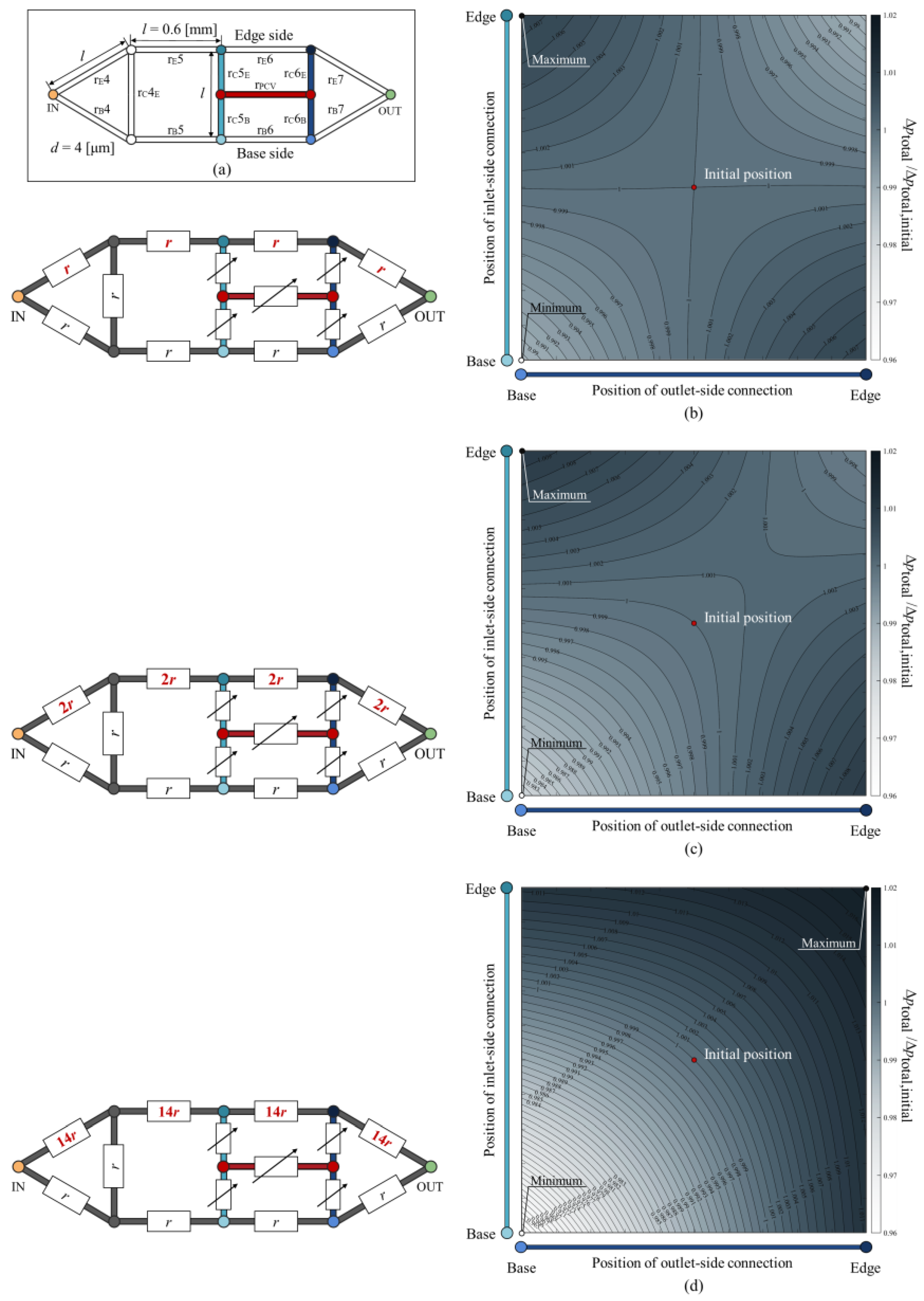
3.2. Total Pressure Loss in Idealised Circuit with Rearranged PCV
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Geometries of Wing Veins in the Simplified Venous Network Model of D. melanogaster
| Vein | Length: ln [µm] | Inner Diameter: dn [µm] |
|---|---|---|
| VE1 | 3.0 × 102 | 5.6 × 100 |
| VE2 | 4.2 × 102 | 4.4 × 100 |
| VE3 | 1.2 × 103 | 4.5 × 100 |
| VE4 | 6.4 × 102 | 3.1 × 100 |
| VE5 | 2.2 × 102 | 1.9 × 100 |
| VE6 | 8.7 × 102 | 1.7 × 100 |
| VE7 | 1.9 × 103 | 2.2 × 100 |
| VB1 | 3.1 × 102 | 1.2 × 101 |
| VB2 | 8.7 × 101 | 9.9 × 100 |
| VB3 | 2.2 × 102 | 3.4 × 100 |
| VB4 | 3.3 × 102 | 3.4 × 100 |
| VB5 | 6.9 × 101 | 3.4 × 100 |
| VB6 | 5.9 × 102 | 2.7 × 100 |
| VB7 | 4.0 × 102 | 5.7 × 100 |
| VC1 | 7.6 × 101 | 3.9 × 100 |
| VC2 | 3.7 × 102 | 5.8 × 100 |
| VC3 | 1.3 × 103 | 2.6 × 100 |
| VC4 | 1.4 × 103 | 3.1 × 100 |
| VC5E | 9.3 × 102 | 2.5 × 100 |
| VC5B | 4.4 × 102 | 2.8 × 100 |
| VC6E | 3.3 × 102 | 2.5 × 100 |
| VC6B | 1.0 × 103 | 2.7 × 100 |
| PCV | 1.8 × 102 | 3.1 × 100 |
References
- Arnold, J.W. Blood circulation in insect wings. Mem. Entomol. Soc. Can. 1964, 96, 5–60. [Google Scholar] [CrossRef]
- Chintapalli, R.T.V.; Hillyer, J.F. Hemolymph circulation in insect flight appendages: Physiology of the wing heart and circulatory flow in the wings of the mosquito Anopheles gambiae. J. Exp. Biol. 2016, 219, 3945–3951. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, M.K.; Jun, B.H.; Socha, J.J.; Pierce, N.E.; Vlachos, P.P.; Combes, S.A. Complex hemolymph circulation patterns in grasshopper wings. Commun. Biol. 2023, 6, 313. [Google Scholar] [CrossRef] [PubMed]
- Pass, G.; Tögel, M.; Krenn, H.; Paululat, A. The circulatory organs of insect wings: Prime examples for the origin of evolutionary novelties. Zool. Anz. J. Comp. Zool. 2015, 256, 82–95. [Google Scholar] [CrossRef]
- Munson, B.R. Fundamentals of Fluid Mechanics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rousset, N.; Lohasz, C.; Boos, J.A.; Misun, P.M.; Cardes, F.; Hierlemann, A. Circuit-based design of microfluidic drop networks. Micromachines 2022, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Schachat, S.R.; Boyce, C.K.; Payne, J.L.; Lentink, D. Lepidoptera demonstrate the relevance of Murray’s law to circulatory systems with tidal flow. BMC Biol. 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kubota, Y.; Mochizuki, O. Circuit analogy unveiled the haemodynamic effects of the posterior cross vein in the wing vein networks. PLoS ONE 2024, 19, e0301030. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Huizar, F.J.; Farfán-Pira, K.J.; Brodskiy, P.A.; Soundarrajan, D.K.; Nahmad, M.; Zartman, J.J. MAPPER: An open-source, high-dimensional image analysis pipeline unmasks differential regulation of drosophila wing features. Front. Genet. 2022, 13, 869719. [Google Scholar] [CrossRef] [PubMed]
- Brasovs, A.; Palaoro, A.V.; Aprelev, P.; Beard, C.E.; Adler, P.H.; Kornev, K.G. Haemolymph viscosity in hawkmoths and its implications for hovering flight. Proc. Biol. Sci. 2023, 290, 20222185. [Google Scholar] [CrossRef] [PubMed]
- Zabihihesari, A.; Parand, S.; Rezai, P. PDMS-Based Microfluidic Capillary Pressure-Driven Viscometry and Application to Drosophila melanogaster’s Hemolymph. Microfluid. Nanofluid. 2023, 27, 8. [Google Scholar] [CrossRef]
- Chatzarakis, G.E.; Tortoreli, M.D.; Tziolas, A.D. Fundamental loop-current method using “virtual voltage sources” technique for special cases. Int. J. Electr. Eng. Educ. 2003, 40, 188–207. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Gompper, G.; Fedosov, D.A. Modeling microcirculatory blood flow: Current state and future perspectives. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kubota, Y.; Mochizuki, O. Network topology of wing veins in Hawaiian flies mitigates allometric dilemma. Biomimetics 2024, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Muijres, F.T.; Elzinga, M.J.; Melis, J.M.; Dickinson, M.H. Flies evade looming targets by executing rapid visually directed banked turns. Science 2014, 344, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Farisenkov, S.E.; Kolomenskiy, D.; Petrov, P.N.; Engels, T.; Lapina, N.A.; Lehmann, F.-O.; Onishi, R.; Liu, H.; Polilov, A.A. Novel flight style and light wings boost flight performance of tiny beetles. Nature 2022, 602, 96–100. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, K.; Kubota, Y.; Mochizuki, O. Positional Effects of a Fly’s Wing Vein in the Asymmetric Distribution of Hydraulic Resistances. Symmetry 2024, 16, 1212. https://doi.org/10.3390/sym16091212
Sugiyama K, Kubota Y, Mochizuki O. Positional Effects of a Fly’s Wing Vein in the Asymmetric Distribution of Hydraulic Resistances. Symmetry. 2024; 16(9):1212. https://doi.org/10.3390/sym16091212
Chicago/Turabian StyleSugiyama, Kazuki, Yoshihiro Kubota, and Osamu Mochizuki. 2024. "Positional Effects of a Fly’s Wing Vein in the Asymmetric Distribution of Hydraulic Resistances" Symmetry 16, no. 9: 1212. https://doi.org/10.3390/sym16091212
APA StyleSugiyama, K., Kubota, Y., & Mochizuki, O. (2024). Positional Effects of a Fly’s Wing Vein in the Asymmetric Distribution of Hydraulic Resistances. Symmetry, 16(9), 1212. https://doi.org/10.3390/sym16091212







