Unveiling the Influence of the Menstrual Cycle on Mental Rotation Abilities: A Comparative Analysis of Three-Dimensional vs. Two-Dimensional Tasks
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measures
2.3. EEG Processing
3. Results
3.1. Accuracy and Speed of Solving the Mental Rotation Tasks as a Function of the Menstrual Cycle
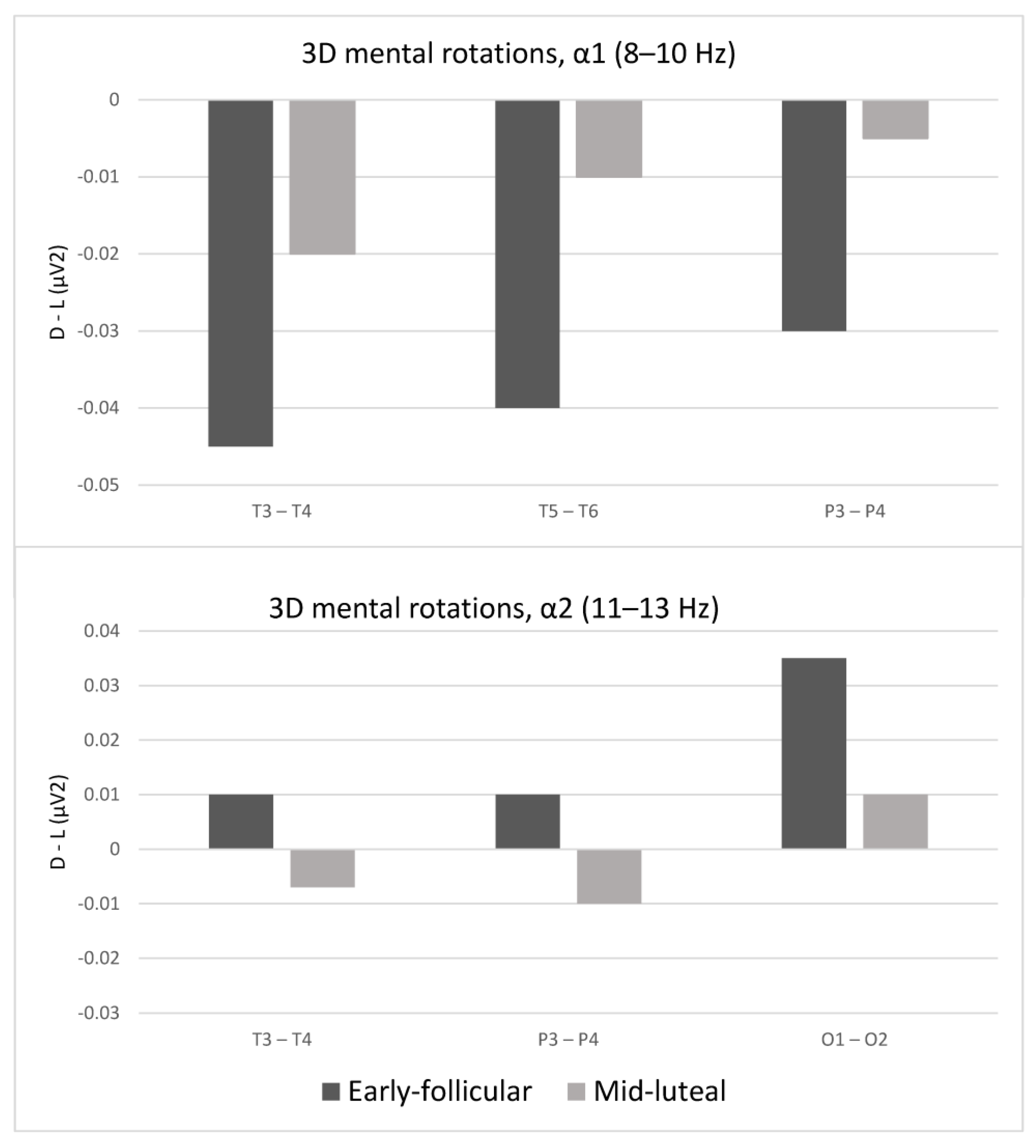
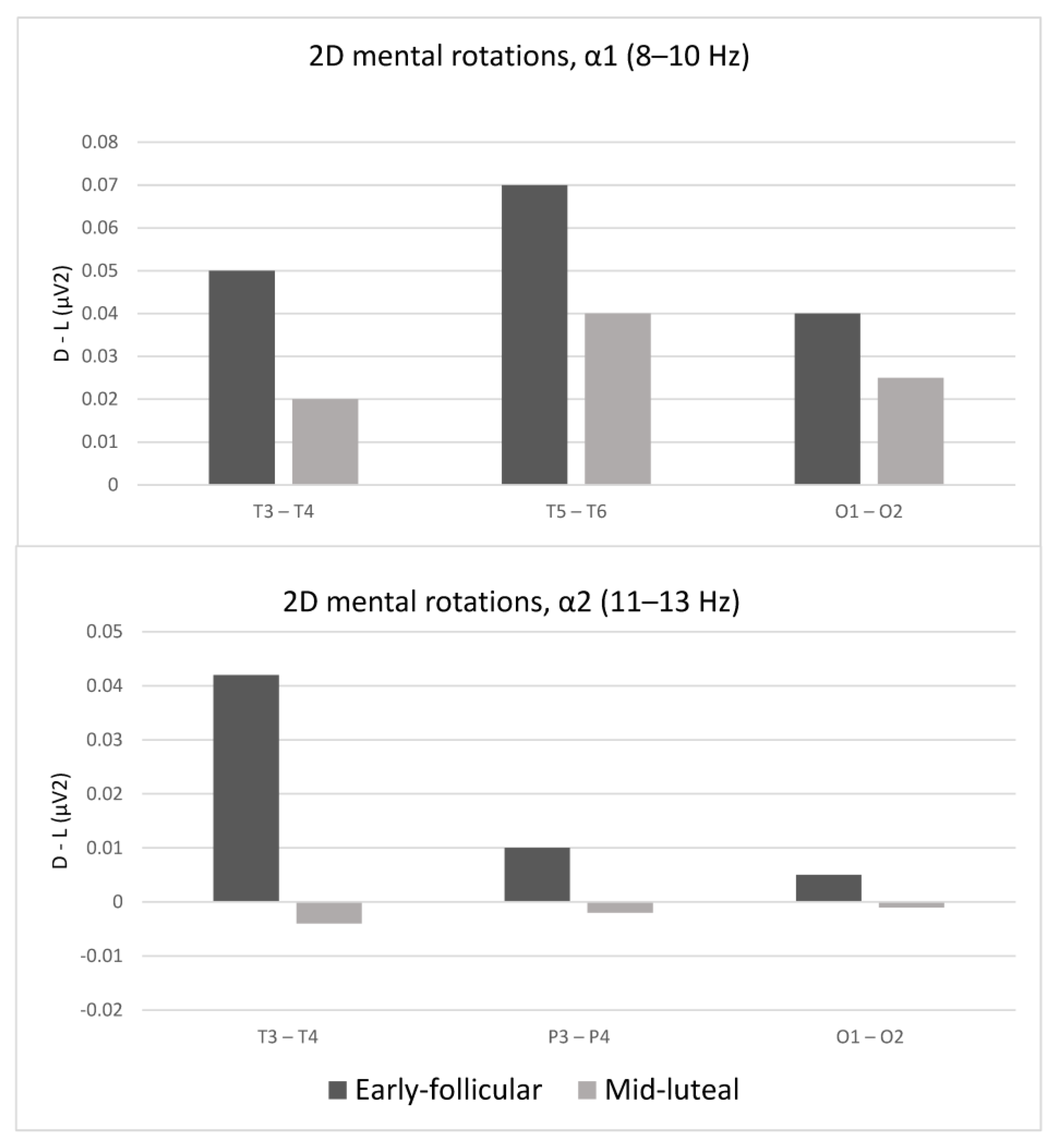
3.2. Laterality Indices While Solving the Mental Rotation Tasks as a Function of the Menstrual Cycle
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyer, M.M.; Phillips, L.L.; Neigh, G.N. Sex Differences in Synaptic Plasticity: Hormones and Beyond. Front. Mol. Neurosci. 2018, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, J.; McEwen, B.S. Sex in the brain: Hormones and sex differences. Dialog-Clin. Neurosci. 2016, 18, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; McCarthy, M.M. Steroid-induced sexual differentiation of the developing brain: Multiple pathways, one goal. J. Neurochem. 2008, 105, 1561–1572. [Google Scholar] [CrossRef]
- Juraska, J.M. Sex differences in “cognitive” regions of the rat brain. Psychoneuroendocrinology 1991, 16, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Meck, W.H. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 1991, 16, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Isgor, C.; Sengelaub, D.R. Prenatal Gonadal Steroids Affect Adult Spatial Behavior, CA1 and CA3 Pyramidal Cell Morphology in Rats. Horm. Behav. 1998, 34, 183–198. [Google Scholar] [CrossRef][Green Version]
- Bachevalier, J.; Hagger, C. Sex differences in the development of learning abilities in primates. Psychoneuroendocrinology 1991, 16, 177–188. [Google Scholar] [CrossRef]
- Reinisch, J.M.; Sanders, S.A. Effects of prenatal exposure to diethylstilbestrol (DES) on hemispheric laterality and spatial ability in human males. Horm. Behav. 1992, 26, 62–75. [Google Scholar] [CrossRef]
- Reinisch, J.M.; Ziemba-Davis, M.; Sanders, S.A. Hormonal contributions to sexually dimorphic behavioral development in humans. Psychoneuroendocrinology 1991, 16, 213–278. [Google Scholar] [CrossRef]
- Hines, M.; Sandberg, E.C. Sexual Differentiation of Cognitive Abilities in Women Exposed to Diethylstilbestrol (DES) Prenatally. Horm. Behav. 1996, 30, 354–363. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Maxey, J.; Herbst, A.L. Prenatal diethylstilbestrol exposure and performance on college entrance examinations. Horm. Behav. 1992, 26, 433–439. [Google Scholar] [CrossRef]
- Hampson, E. Spatial cognition in humans: Possible modulation by androgens and estrogens. J. Psychiatry Neurosci. 1995, 20, 397–404. [Google Scholar]
- Kimura, D. Sex and Cognition; The MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Hampson, E. Sexual Differentiation of Spatial Functions in Humans. In Sexual Differentiation of the Brain; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Hines, M.; Golombok, S.; Rust, J.; Johnston, K.J.; Golding, J. Avon Longitudinal Study of Parents and Children Study Team Testosterone during Pregnancy and Gender Role Behavior of Preschool Children: A Longitudinal, Population Study. Child Dev. 2002, 73, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Hines, M. Prenatal testosterone and gender-related behaviour. Eur. J. Endocrinol. 2006, 155, S115–S121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knickmeyer, R.C.; Wheelwright, S.; Taylor, K.; Raggatt, P.; Hackett, G.; Baron-Cohen, S. Gender-Typed Play and Amniotic Testosterone. Dev. Psychol. 2005, 41, 517–528. [Google Scholar] [CrossRef]
- Hampson, E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990, 14, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 1990, 15, 97–111. [Google Scholar] [CrossRef]
- Hampson, E.; Kimura, D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav. Neurosci. 1988, 102, 456–459. [Google Scholar] [CrossRef]
- Rosenberg, L.; Park, S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology 2002, 27, 835–841. [Google Scholar] [CrossRef]
- Saucier, D.M.; Kimura, D. Intrapersonal motor but not extrapersonal targeting skill is enhanced during the midluteal phase of the menstrual cycle. Dev. Neuropsychol. 1998, 14, 385–398. [Google Scholar] [CrossRef]
- Smith, C.A.; McCleary, C.A.; Murdock, G.A.; Wilshire, T.W.; Buckwalter, D.K.; Bretsky, P.; Marmol, L.; Gorsuch, R.L.; Buckwalter, J. Lifelong Estrogen Exposure and Cognitive Performance in Elderly Women. Brain Cogn. 1999, 39, 203–218. [Google Scholar] [CrossRef]
- Carlson, L.E.; Sherwin, B.B. Steroid Hormones, Memory and Mood in a Healthy Elderly Population. Psychoneuroendocrinology 1998, 23, 583–603. [Google Scholar] [CrossRef]
- Resnick, S.M.; Metter, E.J.; Zonderman, A.B. Estrogen replacement therapy and longitudinal decline in visual memory: A Possible Protective Effect? Neurology 1997, 49, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Rich, J.B.; Rosenbaum, R.S. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia 2001, 40, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and cognitive aging in women. Trends Pharmacol. Sci. 2002, 23, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.J.; Hampson, E. A Beneficial Effect of Estrogen on Working Memory in Postmenopausal Women Taking Hormone Replacement Therapy. Horm. Behav. 2000, 38, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Z.; Gilger, J.W.; Brink, T.M. Effects of Menstrual Cycle on Spatial Information-Processes. Percept. Mot. Ski. 1986, 63, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Silverman, I.; Phillips, K. Effects of estrogen changes during the menstrual cycle on spatial performance. Ethol. Sociobiol. 1993, 14, 257–269. [Google Scholar] [CrossRef]
- Phillips, K.; Silverman, I. Differences in the Relationship of Menstrual Cycle Phase to Spatial Performance on Two- and Three-Dimensional Tasks. Horm. Behav. 1997, 32, 167–175. [Google Scholar] [CrossRef]
- Galaburda, A.M. Anatomic Basis of Cerebral Dominance. In Brain Asymmetry; Davidson, R.J., Hugdahl, K., Eds.; The MIT Press: Cambridge, MA, USA, 1995; pp. 51–73. [Google Scholar]
- Zaidel, E.; Aboitiz, F.; Clarke, J.M.; Kaiser, D.; Matteson, R. Hemispheric Communication: Mechanisms & Models. In Sex Differences in Interhemispheric Relations for Language; Kitterle, U.F., Ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1995; pp. 85–175. [Google Scholar]
- Serrati, C.; Finocchi, C.; Calautti, C.; Bruzzone, G.L.; Colucci, M.; Gandolfo, C.; Del Sette, M.; Lantieri, P.B.; Favale, E. Absence of Hemispheric Dominance for Mental Rotation Ability: A Transcranial Doppler Study. Cortex 2000, 36, 415–425. [Google Scholar] [CrossRef]
- Richter, W.; Somorjai, R.; Summers, R.; Jarmasz, M.; Menon, R.S.; Gati, J.S.; Georgopoulos, A.P.; Tegeler, C.; Ugurbil, K.; Kim, S.-G. Motor Area Activity During Mental Rotation Studied by Time-Resolved Single-Trial fMRI. J. Cogn. Neurosci. 2000, 12, 310–320. [Google Scholar] [CrossRef]
- Cabeza, R.; Nyberg, L. Imaging Cognition II: An Empirical Review of 275 PET and fMRI Studies. J. Cogn. Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef]
- Jordan, K.; Heinze, H.-J.; Lutz, K.; Kanowski, M.; Jäncke, L. Cortical Activations during the Mental Rotation of Different Visual Objects. NeuroImage 2001, 13, 143–152. [Google Scholar] [CrossRef]
- Michel, C.M.; Thut, G.; Morand, S.; Khateb, A.; Pegna, A.J.; de Peralta, R.G.; Gonzalez, S.; Seeck, M.; Landis, T. Electric source imaging of human brain functions. Brain Res. Rev. 2001, 36, 108–118. [Google Scholar] [CrossRef]
- Levin, S.L.; Mohamed, F.B.; Platek, S.M. Common Ground for Spatial Cognition? A Behavioral and fMRI Study of Sex Differences in Mental Rotation and Spatial Working Memory. Evol. Psychol. 2005, 3, 147470490500300. [Google Scholar] [CrossRef]
- Luo, H.; Ni, J.-T.; Li, Z.-H.; Li, X.-O.; Zhang, D.-R.; Zeng, F.-G.; Chen, L. Opposite patterns of hemisphere dominance for early auditory processing of lexical tones and consonants. Proc. Natl. Acad. Sci. USA 2006, 103, 19558–19563. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.M.; Egan, G.F.; Sonkkila, C.; Tochon-Danguy, H.J.; Paxinos, G.; Watson, J.D.G. Selective right parietal lobe activation during mental rotation. Brain 2000, 123, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Chapman, J.P.; Chapman, L.J.; Henriques, J.B. Asymmetrical Brain Electrical Activity Discriminates between Psychometrically-Matched Verbal and Spatial Cognitive Tasks. Psychophysiology 1990, 27, 528–543. [Google Scholar] [CrossRef] [PubMed]
- Hoptman, M.J.; Davidson, R.J. Baseline eeg asymmetries and performance on neuropsychological tasks. Neuropsychologia 1998, 36, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.K.; Davidson, R.J. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia 2000, 38, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Hugdahl, K. Brain Asymmetry; MIT Press: Cambridge, MA, USA, 1995; Available online: https://mitpress.mit.edu/9780262540797/brain-asymmetry/ (accessed on 9 December 2023).
- Nettle, D. Hand laterality and cognitive ability: A multiple regression approach. Brain Cogn. 2003, 52, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Güntürkün, O. Steroid fluctuations modify functional cerebral asymmetries: The hypothesis of progesterone-mediated interhemispheric decoupling. Neuropsychologia 2000, 38, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M. Functional cerebral asymmetries during the menstrual cycle: A cross-sectional and longitudinal analysis. Neuropsychologia 2002, 40, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.J.; Costello, C.; Diepold, J. Interhemispheric integration during the menstrual cycle: Failure to confirm progesterone-mediated interhemispheric decoupling. Neuropsychologia 2004, 42, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Hausmann, M.; Stoffers, B.; Vohn, R.; Kellermann, T.; Sturm, W. Estradiol Modulates Functional Brain Organization during the Menstrual Cycle: An Analysis of Interhemispheric Inhibition. J. Neurosci. 2008, 28, 13401–13410. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.R.; Dixson, B.J.; O’Dean, S.M.; Denson, T.F. Standardized protocols for characterizing women’s fertility: A data-driven approach. Horm. Behav. 2016, 81, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gangestad, S.W.; Haselton, M.G.; Welling, L.L.; Gildersleeve, K.; Pillsworth, E.G.; Burriss, R.P.; Larson, C.M.; Puts, D.A. How valid are assessments of conception probability in ovulatory cycle research? Evaluations, recommendations, and theoretical implications. Evol. Hum. Behav. 2016, 37, 85–96. [Google Scholar] [CrossRef]
- Peters, M.; Laeng, B.; Latham, K.; Jackson, M.; Zaiyouna, R.; Richardson, C. A Redrawn Vandenberg and Kuse Mental Rotations Test—Different Versions and Factors That Affect Performance. Brain Cogn. 1995, 28, 39–58. [Google Scholar] [CrossRef]
- French, J.W.; Ekstrom, R.B.; Price, L.A. Manual for Kit of Reference Tests for Cognitive Factors (Revised 1963); Defense Technical Information Center: Fort Belvoir, VA, USA, 1963. [Google Scholar]
- Schneider, W.; Eschman, A.; Zuccolotto, A. E-Prime User’s Guide; Psychology Software Tools, Inc.: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Corsi-Cabrera, M.; Solís-Ortiz, S.; Guevara, M. Stability of EEG inter-and intrahemispheric correlation in women. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 248–255. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; Mcevoy, L.; Yu, D. High Resolution EEG Mapping of Cortical Activation Related to Working Memory: Effects of Task Difficulty, Type of Processing, and Practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef]
- Geary, D.C. Male, Female: The Evolution of Human Sex Differences, 3rd ed.; American Psychological Association: Washington, DC, USA, 2020. [Google Scholar]
- Roberts, J.E.; Bell, M.A. 2- and 3-dimensional mental rotation tasks lead to different parietal laterality for men and women. Int. J. Psychophysiol. 2003, 50, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Wüstenberg, T.; Heinze, H.-J.; Peters, M.; Jäncke, L. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia 2002, 40, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Voyer, D.; Voyer, S.; Bryden, M.P. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of some critical variables. Psychol. Bull. 1995, 117, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Fernández, G.; Weis, S.; Stoffel-Wagner, B.; Tendolkar, I.; Reuber, M.; Beyenburg, S.; Klaver, P.; Fell, J.; de Greiff, A.; Ruhlmann, J.; et al. Menstrual Cycle-Dependent Neural Plasticity in the Adult Human Brain Is Hormone, Task, and Region Specific. J. Neurosci. 2003, 23, 3790–3795. [Google Scholar] [CrossRef]
| Early Follicular Phase | Mid-Luteal Phase | |||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | ||
| 3D mental rotations | Accuracy | 11.20 | 1.1 | 8.3 | 1.2 | 10.08 *** |
| Speed (s) | 8.01 | 1.02 | 11.31 | 2.01 | 8.28 *** | |
| 2D mental rotations | Accuracy | 66.09 | 20.45 | 64.27 | 17.7 | 0.38 |
| Speed (s) | 3.54 | 1.35 | 3.69 | 1.40 | 0.44 | |
| 3D Mental Rotations | 2D Mental Rotations | |||
|---|---|---|---|---|
| Frequency Band | Frequency Band | |||
| α1 (8–10 Hz) | α2 (11–13 Hz) | α1 (8–10 Hz) | α2 (11–13 Hz) | |
| F | F | F | F | |
| Fp1–Fp2 | 0.12 | 0.27 | 0.76 | 0.15 |
| F3–F4 | 6.97 ** | 0.56 | 1.99 * | 0.01 |
| F7–F8 | 1.38 | 0.46 | 0.11 | 0.98 |
| C3–C4 | 2.38 * | 2.32 * | 0.19 | 0.01 |
| T3–T4 | 3.04 * | 4.38 * | 2.91 * | 2.12 * |
| T5–T6 | 2.56 * | 0.14 | 2.28 * | 0.80 |
| P3–P4 | 2.69 * | 2.86 * | 0.07 | 2.34 * |
| O1–O2 | 0.14 | 2.44 * | 2.03 * | 1.88 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromatko, I.; Tadinac, M. Unveiling the Influence of the Menstrual Cycle on Mental Rotation Abilities: A Comparative Analysis of Three-Dimensional vs. Two-Dimensional Tasks. Symmetry 2024, 16, 172. https://doi.org/10.3390/sym16020172
Hromatko I, Tadinac M. Unveiling the Influence of the Menstrual Cycle on Mental Rotation Abilities: A Comparative Analysis of Three-Dimensional vs. Two-Dimensional Tasks. Symmetry. 2024; 16(2):172. https://doi.org/10.3390/sym16020172
Chicago/Turabian StyleHromatko, Ivana, and Meri Tadinac. 2024. "Unveiling the Influence of the Menstrual Cycle on Mental Rotation Abilities: A Comparative Analysis of Three-Dimensional vs. Two-Dimensional Tasks" Symmetry 16, no. 2: 172. https://doi.org/10.3390/sym16020172
APA StyleHromatko, I., & Tadinac, M. (2024). Unveiling the Influence of the Menstrual Cycle on Mental Rotation Abilities: A Comparative Analysis of Three-Dimensional vs. Two-Dimensional Tasks. Symmetry, 16(2), 172. https://doi.org/10.3390/sym16020172






