How the Magnetization Angle of a Linear Halbach Array Influences Particle Steering in Magnetic Drug Targeting—A Systematic Evaluation and Optimization
Abstract
1. Introduction
- We systematically numerically investigate the impact of the magnetization angle in a reduced complexity 2D simulation in COMSOL Multiphysics® 6.1 regarding the steering performance of SPIONs in a background flow through a Y-shaped bifurcation.
- We further evaluate the magnetic force for the strong and weak side of the array for different magnetization angles at its strongest position. We additionally compare the magnitude of the magnetic forces with the hydrodynamic drag force.
- Since the calculation of the magnetic gradient leads to huge errors due to the discrete mesh in COMSOL, it was determined analytically using an exponential fitting function similar to [51]. By doing so, we further analyze if the magnetic flux density can be approximated with an exponential function for other magnetization angles too.
- Since we were not able to identify standardized evaluation parameters with our comprehensive analysis of the state of the art (compare Section 5.4), the significance of various evaluation parameters, such as the magnetic field, total applied magnetic energy, or maximum gradient in the vessel, is examined regarding their prediction of particle steering.
- Based on our investigations, recommendations for the design of a linear Halbach array for steering SPIONs in MDT are derived and discussed.
2. Fundamentals and Background
2.1. Superparamagnetic Iron Oxide Nanoparticles
2.2. Forces on SPIONs
2.3. Linear Halbach Array
2.4. Magnetic Flux Density of a Linear Halbach Array
3. Definition of Simulation Model and Data Evaluation
3.1. Model Definition
- (1)
- the “Magnetic Fields, No Currents (mfnc)” interface is used for generating the magnetic flux density;
- (2)
- the “Laminar Flow (spf)” interface is related to the velocity flow;
- (3)
- the “Particle Tracing for Fluid Flow (fpt)” interface is capable of mimicking particle trajectories in the velocity flow.
3.2. General Fluid Mechanics Model Considerations
3.3. Evaluation Procedure
3.3.1. Evaluation of the Magnetic Flux Density and Its Gradient
3.3.2. Evaluation of the SPION Distribution
3.3.3. Evaluation of the Magnetic and Hydrodynamic Drag Force
- (1)
- Evaluation of the (hydrodynamic) drag force :The drag force is calculated in the main branch using Equation (7). Thus, it can be assumed that the drag force only has a component in the x-direction. As the velocity is assumed to be laminar and constant in the main branch, it has a parabolic profile, which is provided bywith . y corresponds to the y-coordinate, and to the radius of the vessel.
- (2)
- Evaluation of the magnetic force :The magnetic force pulls the particles towards the magnet. Thus, in this paper, is assumed to have only a component in the y-direction as a consequence of the fitted exponential decay of in y-direction. This force is calculated according to Equation (14). Therefore, the particle’s magnetization M has to be known. It is determined by once reading out and the gradient grad(B) at a fixed location in the COMSOL simulation. Then, M is derived using Equation (14). For reasons of simplification, M is assumed to be constant. In the next step, is calculated using the fitted magnetic gradient for all .
4. Results
4.1. Evaluation of the Magnetic Flux Density
4.2. Evaluation of the Magnetic Gradient
4.3. Evaluation of the Fitting of the Magnetic Flux Density
4.4. Evaluation of the Parameter Study on the SPION Distribution
4.4.1. Influence of Weak vs. Strong Side of the Magnet Array
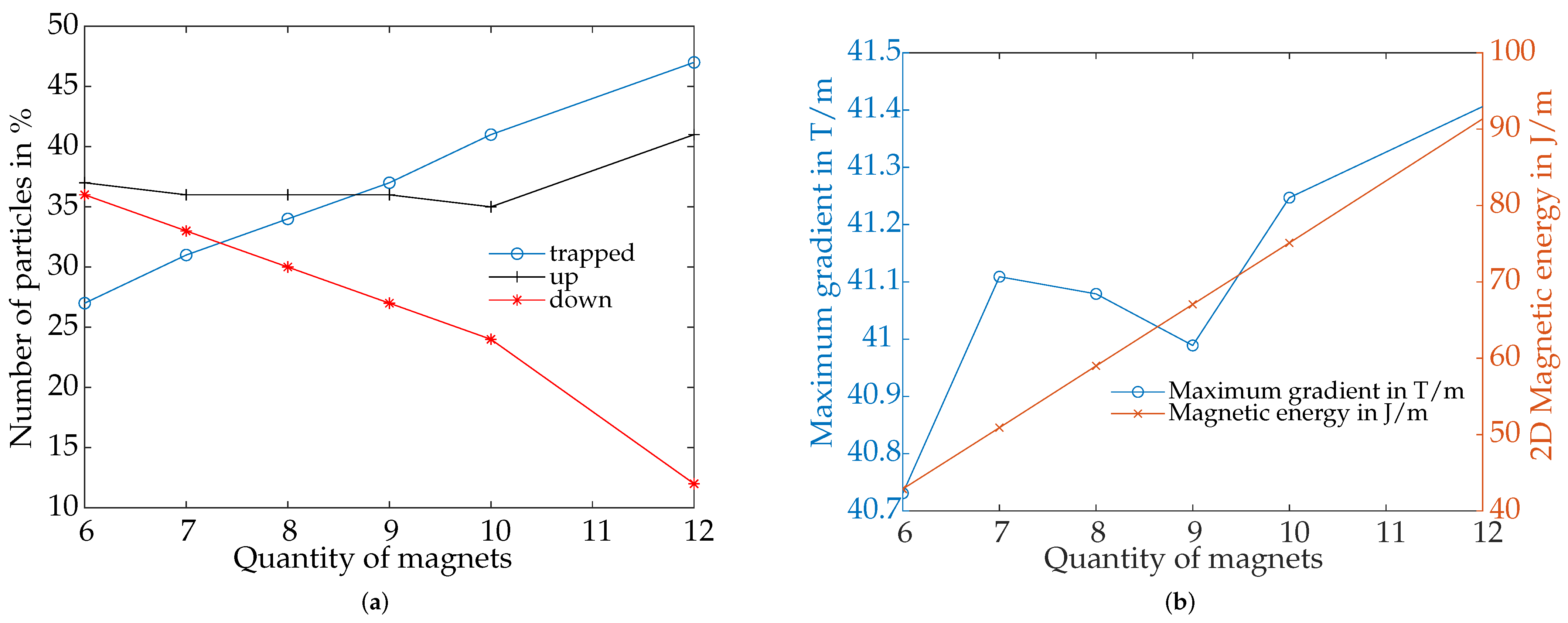
4.4.2. Influence by Quantity of Magnets
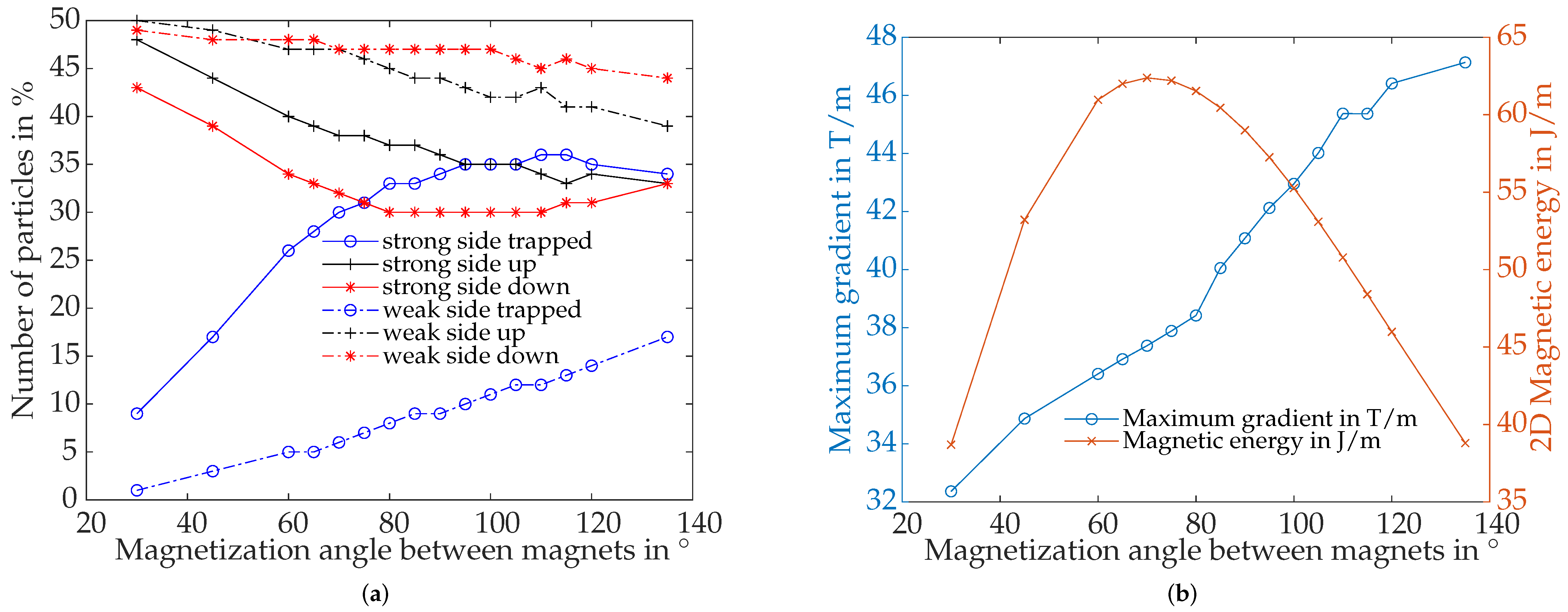
4.4.3. Influence of Magnetization Angle between Magnets
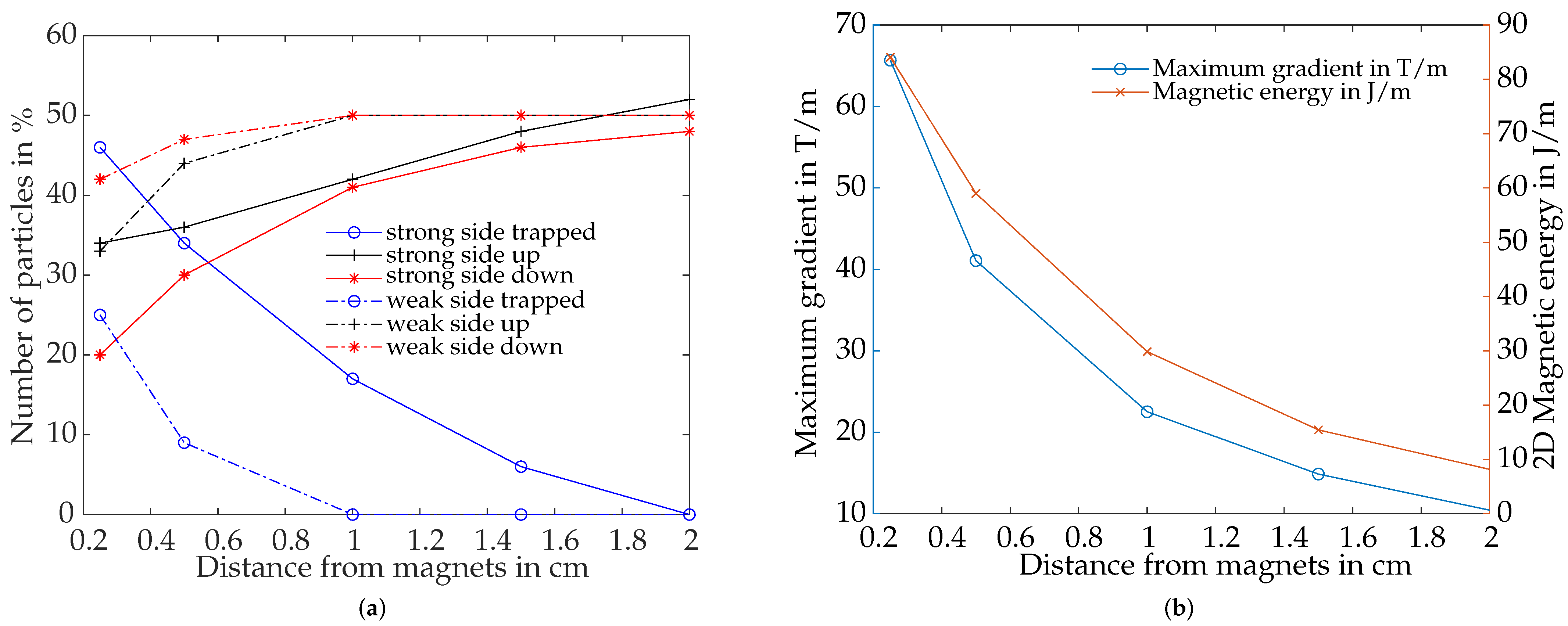
4.4.4. Influence of Distance between Magnetic Array and Vessel
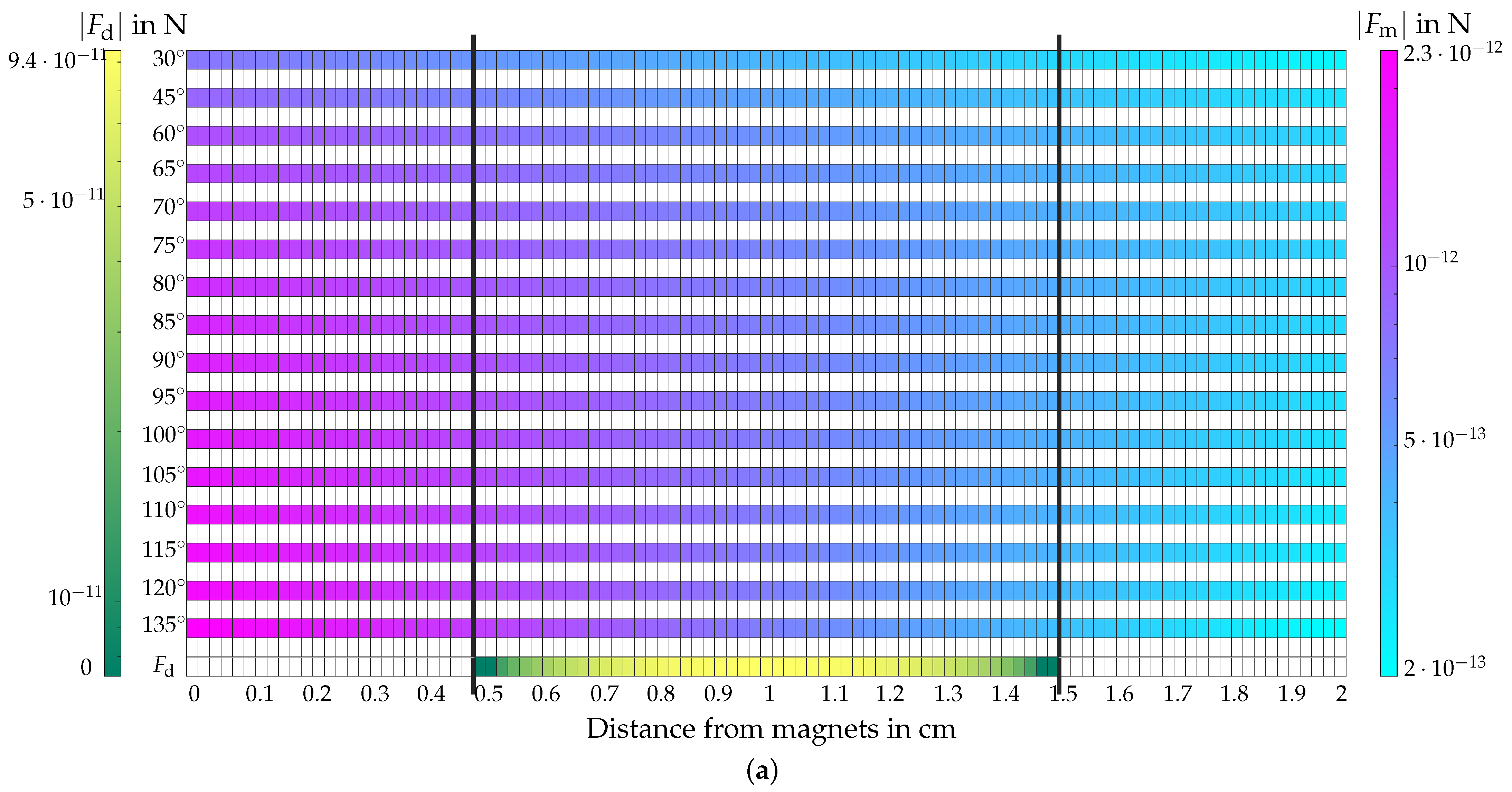
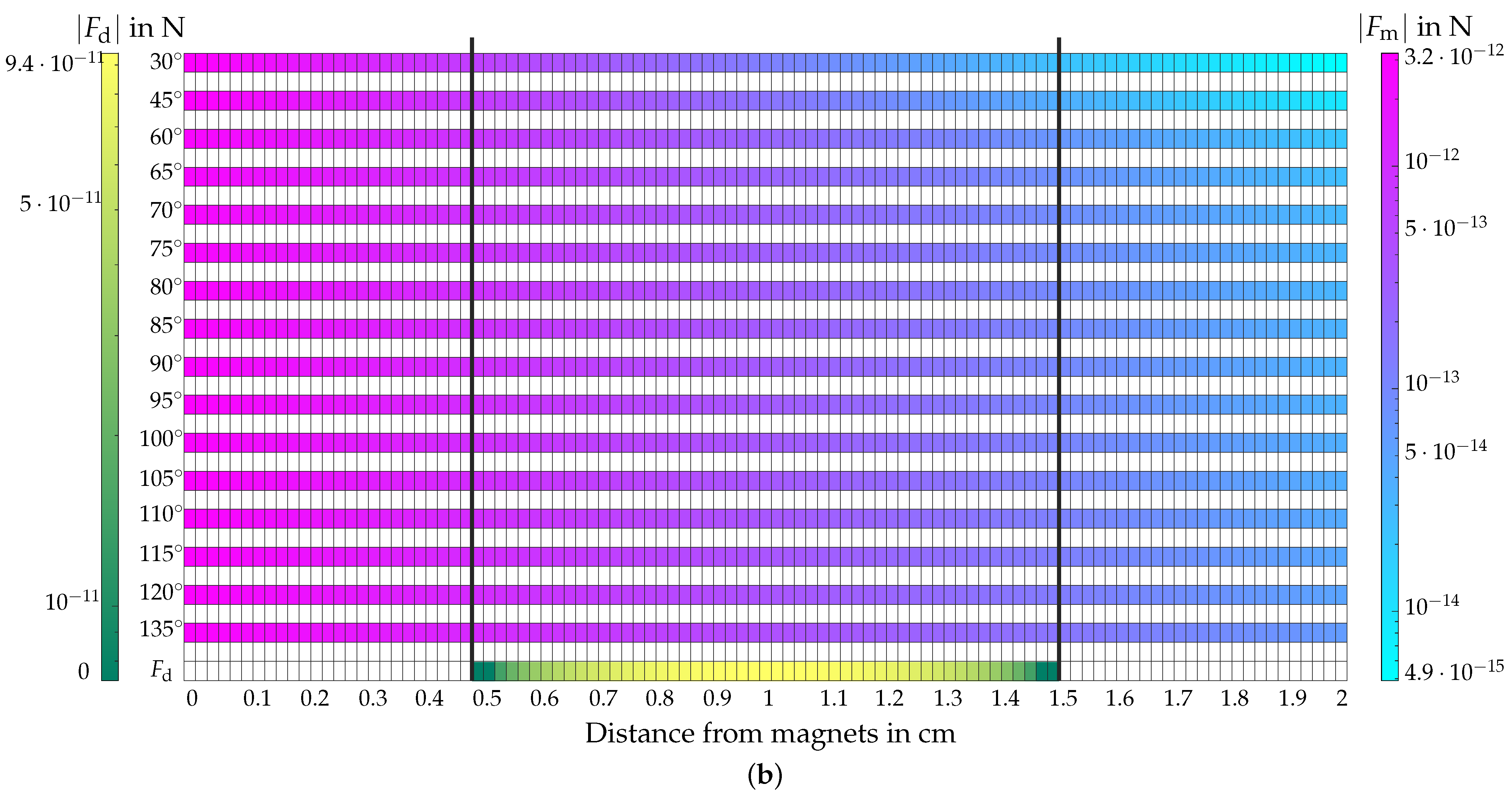
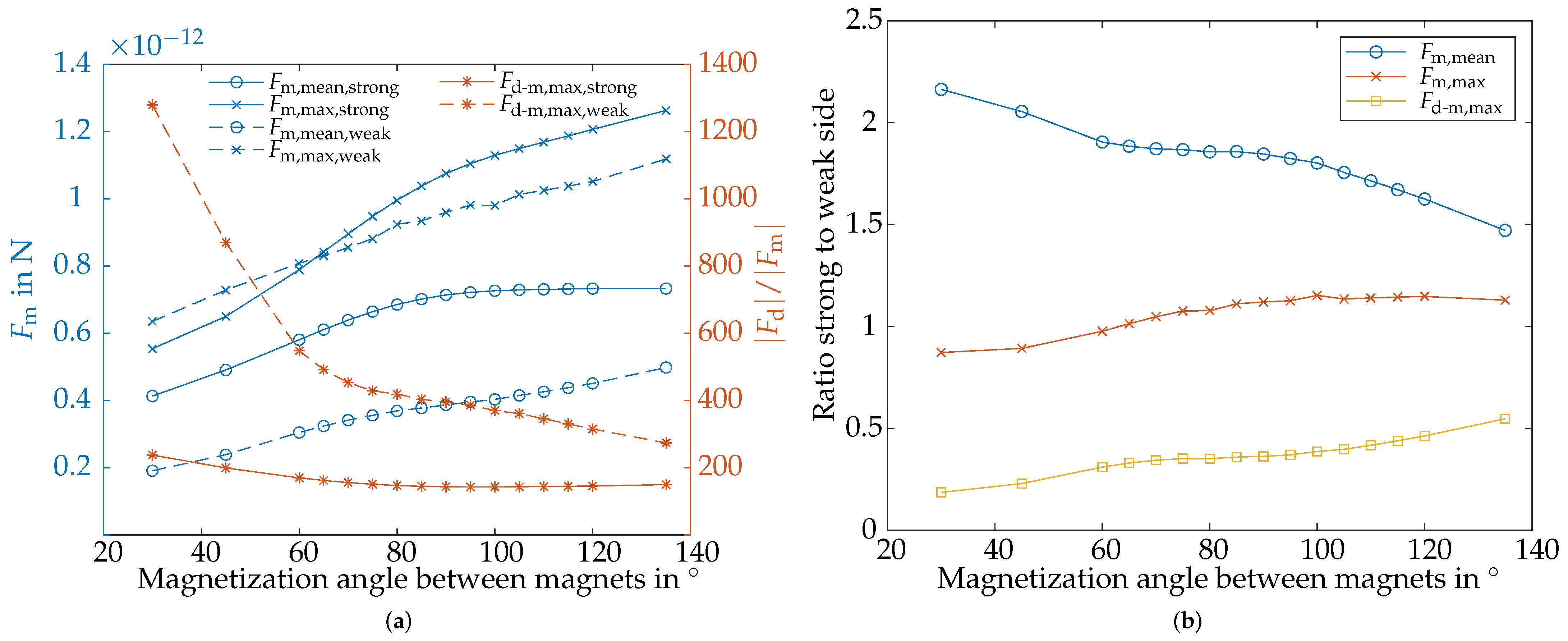
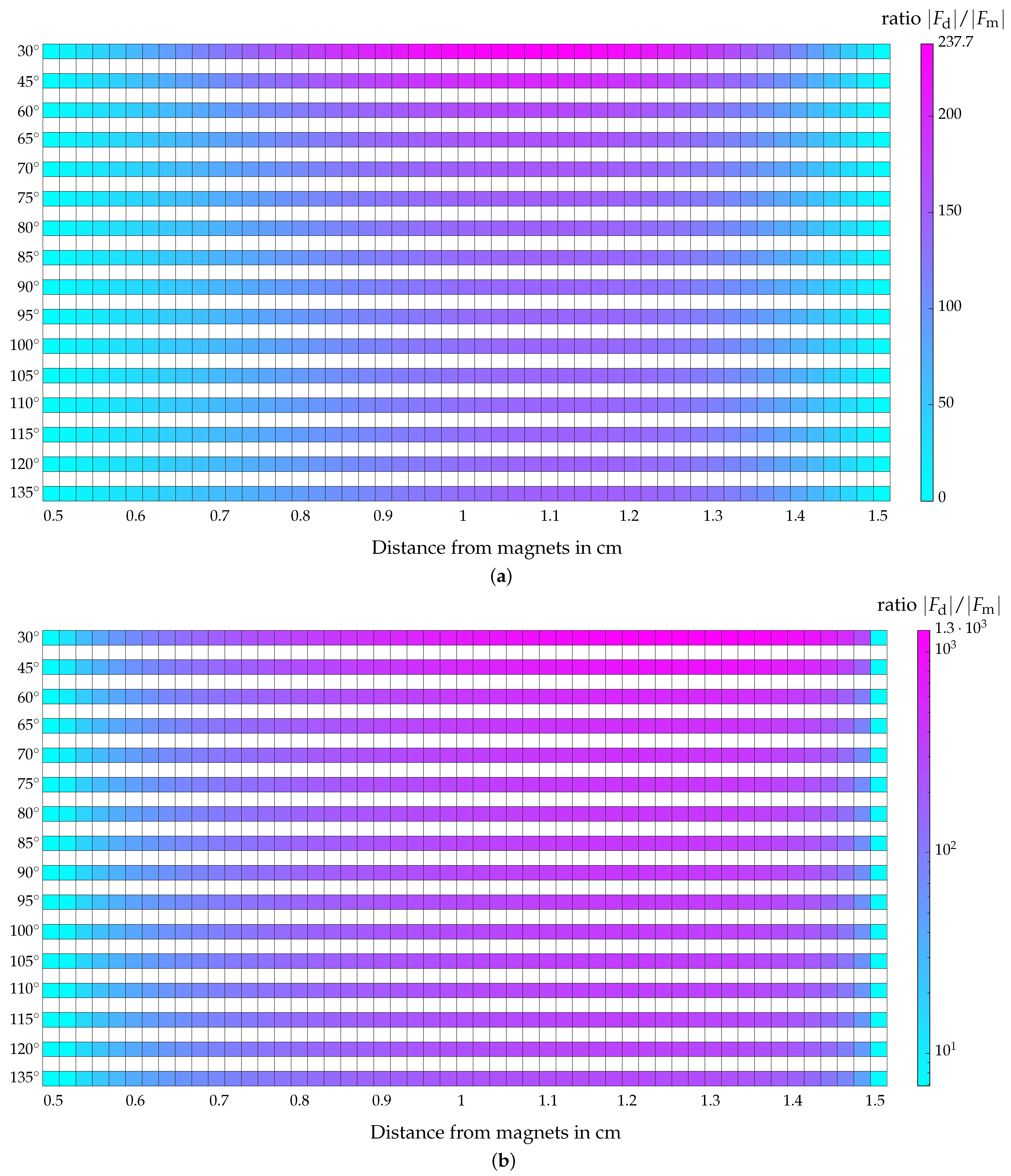
4.5. Evaluation of the Magnetic and Hydrodynamic Drag Force
4.5.1. Evaluation of
4.5.2. Evaluation of
4.5.3. Comparison of and
5. Discussion and Limitations of This Study
5.1. Discussion of the Magnetic Flux Density and Its Effect on Particle Steering
5.2. Discussion of the Fitting Results
5.2.1. Discussion of Fitting Parameter
5.2.2. Discussion of Fitting Parameter
5.3. Discussion of the Maximum Gradient and Magnetic Energy for Predicting Particle Steering
5.4. Comparison with the State-of-the-Art Research
5.5. Limitations of This Study
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EM | Electromagnet |
| LS | Least square |
| MDT | Magnetic Drug Targeting |
| MRI | Magnetic Resonance Imaging |
| PM | Permanent magnet |
| PML | Perfectly matched layer |
| RBC | Red blood cell |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| US | Ultrasound |
References
- Nacev, A.; Komaee, A.; Sarwar, A.; Probst, R.; Kim, S.H.; Emmert-Buck, M.; Shapiro, B. Towards Control of Magnetic Fluids in Patients: Directing Therapeutic Nanoparticles to Disease Locations. IEEE Control Syst. 2012, 32, 32–74. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, X. Magnetic Nanoparticles Mediated Thrombolysis—A Review. IEEE Open J. Nanotechnol. 2023, 4, 109–132. [Google Scholar] [CrossRef]
- Alnaimat, F.; Dagher, S.; Mathew, B.; Hilal-Alnqbi, A.; Khashan, S. Microfluidics Based Magnetophoresis: A Review. Chem. Rec. 2018, 18, 1596–1612. [Google Scholar] [CrossRef]
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Alexiou, C.; Diehl, D.; Henninger, P.; Iro, H.; Rockelein, R.; Schmidt, W.; Weber, H. A High Field Gradient Magnet for Magnetic Drug Targeting. IEEE Trans. Appl. Supercond. 2006, 16, 1527–1530. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Sabz, M.; Kamali, R.; Ahmadizade, S. Controlled Release of Magnetic Particles for Drug Delivery in the Human Lung. IEEE Trans. Magn. 2020, 56, 5400111. [Google Scholar] [CrossRef]
- Hajiaghajani, A.; Hashemi, S.; Abdolali, A. Adaptable setups for magnetic drug targeting in human muscular arteries: Design and implementation. J. Magn. Magn. Mater. 2017, 438, 173–180. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Klein, R.; Parak, F.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lübbe, A. Locoregional Cancer Treatment with Magnetic Drug Targeting1. Cancer Res. 2000, 60, 6641–6648. [Google Scholar] [PubMed]
- Arshadi, S.; Pishevar, A.R. Magnetic drug delivery effects on tumor growth. Inform. Med. Unlocked 2021, 27, 100789. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tuček, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Mues, B.; Bauer, B.; Roeth, A.A.; Ortega, J.; Buhl, E.M.; Radon, P.; Wiekhorst, F.; Gries, T.; Schmitz-Rode, T.; Slabu, I. Nanomagnetic Actuation of Hybrid Stents for Hyperthermia Treatment of Hollow Organ Tumors. Nanomaterials 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.; Saadat, M.; Mohammadi, M.; Kamali, R.; Shamsi, M.; Naseh, M.; Sanati Nezhad, A. Magnetic aerosol drug targeting in lung cancer therapy using permanent magnet. Drug Deliv. 2018, 26, 120–128. [Google Scholar] [CrossRef]
- Thalmayer, A.; Zeising, S.; Fischer, G.; Kirchner, J. Investigation of Particle Steering for Different Cylindrical Permanent Magnets in Magnetic Drug Targeting. In Proceedings of the 7th International Electronic Conference on Sensors and Applications, Basel, Switzerland, 15–30 November 2020. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Roy, S.; Paily, R. Stiction Mitigation of Nanoparticles in Magnetically Targeted Drug Delivery System. IEEE Trans. Magn. 2022, 58, 5500210. [Google Scholar] [CrossRef]
- Nguyen, T.; Go, G.; Zhen, J.; Hoang, C.; Kang, B.; Choi, E.; Park, J.O.; Kim, C.S. Locomotion and disaggregation control of paramagnetic nanoclusters using wireless electromagnetic fields for enhanced targeted drug delivery. Sci. Rep. 2021, 11, 15122. [Google Scholar] [CrossRef]
- Reinelt, M.; Ahlfs, J.; Stein, R.; Alexiou, C.; Bänsch, E.; Friedrich, R.; Lyer, S.; Neuss-Radu, M.; Neuss, N. Simulation and experimental validation of magnetic nanoparticle accumulation in a bloodstream mimicking flow system. J. Magn. Magn. Mater. 2023, 582, 170984. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, D.; Shang, P.; Yin, D.C. A review of magnet systems for targeted drug delivery. J. Control. Release Off. J. Control. Release Soc. 2019, 302, 90–104. [Google Scholar] [CrossRef]
- Felfoul, O.; Becker, A.; Fagogenis, G.; Dupont, P. Simultaneous steering and imaging of magnetic particles using MRI toward delivery of therapeutics. Sci. Rep. 2016, 6, 33567. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Lamura, G.; Peddis, D.; Canepa, F. Optimization of a NdFeB permanent magnet configuration for in-vivo drug delivery experiments. J. Magn. Magn. Mater. 2020, 522, 167491. [Google Scholar] [CrossRef]
- Kheirkhah, P.; Denyer, S.; Bhimani, A.; Arnone, G.; Esfahani, D.; Aguilar, T.; Zakrzewski, J.; Venugopal, I.; Habib, N.; Gallia, G.; et al. Magnetic Drug Targeting: A Novel Treatment for Intramedullary Spinal Cord Tumors. Sci. Rep. 2018, 8, 11417. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: Hoboken, NY, USA, 2009. [Google Scholar]
- Thalmayer, A.S.; Zeising, S.; Fischer, G. Reduced Steering Performance in Magnetic Drug Targeting Induced by Shielding Arising from Accumulation of Particles. Curr. Dir. Biomed. Eng. 2022, 8, 560–563. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Paily, R.P. Time-Varying Magnetic Field to Enhance the Navigation of Magnetic Microparticles in a Bifurcated Channel. IEEE Magn. Lett. 2022, 13, 3102705. [Google Scholar] [CrossRef]
- Park, M.; Le, T.A.; Yoon, J. Offline Programming Guidance for Swarm Steering of Micro-/Nano Magnetic Particles in a Dynamic Multichannel Vascular Model. IEEE Robot. Autom. Lett. 2022, 7, 3977–3984. [Google Scholar] [CrossRef]
- Tehrani, M.D.; Yoon, J.; Kim, M.O. A Novel Scheme for Nanoparticle Steering in Blood Vessels Using a Functionalized Magnetic Field. IEEE Trans. Biomed. Eng. 2015, 62, 303–313. [Google Scholar] [CrossRef]
- Durme, R.; Crevecoeur, G.; Dupré, L.; Coene, A. Improved magnetic drug targeting with maximized magnetic forces and limited particle spreading. Med. Phys. 2023, 50, 1715–1727. [Google Scholar] [CrossRef]
- Hamdipoor, V.; Afzal, M.R.; Le, T.A.; Yoon, J. Haptic-Based Manipulation Scheme of Magnetic Nanoparticles in a Multi-Branch Blood Vessel for Targeted Drug Delivery. Micromachines 2018, 9, 14. [Google Scholar] [CrossRef]
- Hoshiar, A.K.; Le, T.A.; Valdastri, P.; Yoon, J. Swarm of magnetic nanoparticles steering in multi-bifurcation vessels under fluid flow. J. Micro-Bio Robot. 2020, 53, 65. [Google Scholar] [CrossRef]
- Park, M.; Le, T.A.; Eizad, A.; Yoon, J. A Novel Shared Guidance Scheme for Intelligent Haptic Interaction Based Swarm Control of Magnetic Nanoparticles in Blood Vessels. IEEE Access 2020, 8, 106714–106725. [Google Scholar] [CrossRef]
- Le, T.A.; Bui, M.P.; Yoon, J. Electromagnetic Actuation System for Focused Capturing of Magnetic Particles with a Half of Static Saddle Potential Energy Configuration. IEEE Trans. Biomed. Eng. 2021, 68, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ren, Y. High-Gradient Magnetic Field for Magnetic Nanoparticles Drug Delivery System. IEEE Trans. Appl. Supercond. 2018, 28, 4402107. [Google Scholar] [CrossRef]
- Zahn, D.; Klein, K.; Radon, P.; Berkov, D.; Erokhin, S.; Nagel, E.; Eichhorn, M.; Wiekhorst, F.; Dutz, S. Investigation of magnetically driven passage of magnetic nanoparticles through eye tissues for magnetic drug targeting. Nanotechnology 2020, 31, 495101. [Google Scholar] [CrossRef]
- Gitter, K.; Odenbach, S. Investigations on a Branched Tube Model in Magnetic Drug Targeting—Systematic Measurements and Simulation. IEEE Trans. Magn. 2013, 49, 343–348. [Google Scholar] [CrossRef]
- Surpi, A.; Shelyakova, T.; Murgia, M.; Rivas, J.; piñeiro, Y.; Greco, P.; Fini, M.; Dediu, V. Versatile magnetic configuration for the control and manipulation of superparamagnetic nanoparticles. Sci. Rep. 2023, 13, 5301. [Google Scholar] [CrossRef]
- Baun, O.; Blümler, P. Permanent magnet system to guide superparamagnetic particles. J. Magn. Magn. Mater. 2017, 439, 294–304. [Google Scholar] [CrossRef]
- Wang, M.; Wu, T.; Liu, R.; Zhang, Z.; Liu, J. Selective and Independent Control of Microrobots in a Magnetic Field: A Review. Engineering 2023, 24, 21–38. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef]
- Blümler, P. Magnetic Guiding with Permanent Magnets: Concept, Realization and Applications to Nanoparticles and Cells. Cells 2021, 10, 2708. [Google Scholar] [CrossRef] [PubMed]
- Thalmayer, A.S.; Götz, K.; Zeising, S.; Fischer, G. Impact of Array Length on Particle Attraction in Magnetic Drug Targeting: Investigation Using an Exponential Approximation of the Magnetic Field. IEEE Magn. Lett. 2023, 14, 3100205. [Google Scholar] [CrossRef]
- Patel, P.; Alghamdi, A.; Shaw, G.; Legge, C.; Glover, M.; Freeman, D.; Hodgetts, H.; Wilson, E.; Nutter Howard, F.; Staniland, S.; et al. Development of a Personalised Device for Systemic Magnetic Drug Targeting to Brain Tumours. Nanotheranostics 2023, 7, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Thalmayer, A.S.; Zeising, S.; Fischer, G.; Kirchner, J. Steering Magnetic Nanoparticles by Utilizing an Adjustable Linear Halbach Array. In Proceedings of the 2021 Kleinheubach Conference, Miltengerg, Germany, 28–30 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Wong, Q.Y.; Liu, N.; Koh, C.G.; Li, H.Y.; Lew, W.S. Isolation of magnetically tagged cancer cells through an integrated magnetofluidic device. Microfluid. Nanofluid. 2016, 20, 139. [Google Scholar] [CrossRef]
- Liu, C.; Deng, S.; Zou, S.; Chen, P.; Liu, Y. Analysis and Design of a New Hybrid Array for Magnetic Drug Targeting. IEEE Trans. Magn. 2022, 58, 5700111. [Google Scholar] [CrossRef]
- Thalmayer, A.S.; Fischer, G. Innovative Hybrid Halbach Array for Steering Magnetic Nanoparticles Through a Bifurcation. Curr. Dir. Biomed. Eng. 2023, 9, 515–518. [Google Scholar] [CrossRef]
- Stevens, M.; Liu, P.; Niessink, T.; Mentink, A.; Abelmann, L.; Terstappen, L. Optimal Halbach Configuration for Flow-through Immunomagnetic CTC Enrichment. Diagnostics 2021, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, H.; Nakagawara, T. Optimization of rotation patterns of a mangle-type magnetic field source using covariance matrix adaptation evolution strategy. J. Magn. Magn. Mater. 2021, 527, 167752. [Google Scholar] [CrossRef]
- Kang, J.H.; Driscoll, H.; Super, M.; Ingber, D.E. Application of a Halbach magnetic array for long-range cell and particle separations in biological samples. Appl. Phys. Lett. 2016, 108, 213702. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Gilmour, K.; Zhou, A.; Lee, S.; Hayden, M.E. Modeling of magnetic bandages for drug targeting: Button vs. Halbach arrays. J. Magn. Magn. Mater. 2007, 311, 323–329. [Google Scholar] [CrossRef]
- Thalmayer, A.S.; Zeising, S.; Lübke, M.; Fischer, G. Towards Steering Magnetic Nanoparticles in Drug Targeting Using a Linear Halbach Array. Adv. Radio Sci. 2023, 20, 93–104. [Google Scholar] [CrossRef]
- Ijiri, Y.; Poudel, C.; Williams, P.S.; Moore, L.R.; Orita, T.; Zborowski, M. Inverted Linear Halbach Array for Separation of Magnetic Nanoparticles. IEEE Trans. Magn. 2013, 49, 3449–3452. [Google Scholar] [CrossRef][Green Version]
- Skiedraitė, I.; Dragašius, E.; Diliunas, S. Modelling of Halbach Array Based Targeting Part of a Magnetic Drug Delivery Device. Mechanics 2018, 23. [Google Scholar] [CrossRef][Green Version]
- Shiriny, A.; Bayareh, M. On magnetophoretic separation of blood cells using Halbach array of magnets. Meccanica 2020, 55, 1903–1916. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.G.; Cheng, H.; Liu, H.K. Analysis of the Planar Magnetic Field of Linear Permanent Magnet Halbach Array. Appl. Mech. Mater. 2011, 66–68, 1336–1341. [Google Scholar] [CrossRef]
- Di Gerlando, A.; Negri, S.; Ricca, C. A Novel Analytical Formulation of the Magnetic Field Generated by Halbach Permanent Magnet Arrays. Magnetism 2023, 3, 280–296. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Z.Q. General analytical model for calculating electromagnetic performance of permanent magnet brushless machines having segmented Halbach array. IET Electr. Syst. Transp. 2013, 3, 57–66. [Google Scholar] [CrossRef]
- Li, B.; Yang, B.; Xiang, F.; Guo, J. Optimal Design of a New Rotating Magnetic Beacon Structure Based on Halbach Array. Appl. Sci. 2022, 12, 506. [Google Scholar] [CrossRef]
- Hennig, T.L.; Unterweger, H.; Lyer, S.; Alexiou, C.; Cicha, I. Magnetic Accumulation of SPIONs under Arterial Flow Conditions: Effect of Serum and Red Blood Cells. Molecules 2019, 24, 2588. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Janko, C.; Unterweger, H.; Lyer, S.; Alexiou, C. SPIONs and magnetic hybrid materials: Synthesis, toxicology and biomedical applications. In Magnetic Hybrid-Materials—Multi-Scale Modelling, Synthesis, and Applications; Odenbach, S., Ed.; De Gruyter: Berlin, Germany, 2021; pp. 739–768. [Google Scholar] [CrossRef]
- Wetterau, L.; Abert, C.; Suess, D.; Albrecht, M.; Witzigmann, B. Extended micromagnetic model for the detection of superparamagnetic labels using a GMR vortex sensor. J. Phys. Commun. 2021, 5, 075017. [Google Scholar] [CrossRef]
- Lunnoo, T.; Puangmali, T. Capture Efficiency of Biocompatible Magnetic Nanoparticles in Arterial Flow: A Computer Simulation for Magnetic Drug Targeting. Nanoscale Res. Lett. 2015, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Kurgan, E.; Gas, P. Magnetophoretic placement of ferromagnetic nanoparticles in RF hyperthermia. In Proceedings of the 2017 Progress in Applied Electrical Engineering (PAEE), Koscielisko, Poland, 25–30 June 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Klohs, J.; Hirt, A.M. Investigation of the magnetic susceptibility properties of fresh and fixed mouse heart, liver, skeletal muscle and brain tissue. Phys. Medica 2021, 88, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.D.K.; Saadat, M.; Mohammadi, M.; Shamsi, M.; Dejam, M.; Kamali, R.; Nezhad, A.S. Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv. 2018, 25, 1963–1973. [Google Scholar] [CrossRef]
- Fanelli, C.; Kaouri, K.; Phillips, T.; Myers, T.; Font, F. Magnetic nanodrug delivery in non-Newtonian blood flows. Microfluid. Nanofluid. 2022, 26, 74. [Google Scholar] [CrossRef]
- Zafar, M.; Ullah, M.S.; Manzoor, T.; Ali, M.; Nazar, K.; Iqbal, S.; Manzoor, H.U.; Haider, R.; Kim, W.Y. Performance Analysis of Magnetic Nanoparticles during Targeted Drug Delivery: Application of OHAM. Comput. Model. Eng. Sci. 2022, 130, 723–749. [Google Scholar] [CrossRef]
- Furlani, E.P.; Sahoo, Y. Analytical model for the magnetic field and force in a magnetophoretic microsystem. J. Phys. D Appl. Phys. 2006, 39, 1724–1732. [Google Scholar] [CrossRef]
- Van Durme, R.; Crevecoeur, G.; Dupré, L.; Coene, A. Model-based optimized steering and focusing of local magnetic particle concentrations for targeted drug delivery. Drug Deliv. 2021, 28, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Munaz, N.; Shiddiky, M.J.A.; Nguyen, N.T. Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 2018, 12, 031501. [Google Scholar] [CrossRef] [PubMed]
- Thalmayer, A.S.; Ladebeck, A.; Zeising, S.; Fischer, G. Reducing Dispersion in Molecular Communications by Placing Decelerators in the Propagation Channel. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2023, 9, 334–339. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Kantorovich, S.S.; Reznikov, E.N.; Holm, C.; Pshenichnikov, A.F.; Lebedev, A.V.; Chremos, A.; Camp, P.J. Magnetic properties of polydisperse ferrofluids: A critical comparison between experiment, theory, and computer simulation. Phys. Rev. E 2007, 75, 061405. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Allia, P.; Tiberto, P. From spectral analysis to hysteresis loops: A breakthrough in the optimization of magnetic nanomaterials for bioapplications. J. Phys. Mater. 2023, 6, 035007. [Google Scholar] [CrossRef]
- Alnaimat, F.; Karam, S.; Mathew, B.; Mathew, B. Magnetophoresis and Microfluidics: A Great Union. IEEE Nanotechnol. Mag. 2020, 14, 24–41. [Google Scholar] [CrossRef]
- Mallinson, J. One-sided fluxes—A magnetic curiosity? IEEE Trans. Magn. 1973, 9, 678–682. [Google Scholar] [CrossRef]
- Halbach, K. Design of permanent multipole magnets with oriented rare earth cobalt material. Nucl. Instrum. Methods 1980, 169, 1–10. [Google Scholar] [CrossRef]
- Halbach, K. Application of permanent magnets in accelerators and electron storage rings. J. Appl. Phys. 1985, 57, 3605–3608. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Geng, W. Design and Optimization of Halbach-Array PM Rotor for High-Speed Axial-Flux Permanent Magnet Machine With Ironless Stator. IEEE Trans. Ind. Electron. 2020, 67, 7269–7279. [Google Scholar] [CrossRef]
- Huang, R.; Liu, C.; Song, Z.; Zhao, H. Design and Analysis of a Novel Axial-Radial Flux Permanent Magnet Machine with Halbach-Array Permanent Magnets. Energies 2021, 14, 3639. [Google Scholar] [CrossRef]
- Golovanov, D.; Gerada, C. An Analytical Subdomain Model for Dual-Rotor Permanent Magnet Motor With Halbach Array. IEEE Trans. Magn. 2019, 55, 1–16. [Google Scholar] [CrossRef]
- Zheng, J.; Cao, Z.; Han, C.; Wei, X.; Wang, L.; Wu, Z. A Hybrid Triboelectric-Electromagnetic Nanogenerator Based on Arm Swing Energy Harvesting. Nanoenergy Adv. 2023, 3, 126–137. [Google Scholar] [CrossRef]
- Aoyama, M.; Thimm, W.; Knoch, M.; Ose, L. Proposal and Challenge of Halbach Array Type Induction Coil for Cooktop Applications. IEEE Open J. Ind. Appl. 2021, 2, 168–177. [Google Scholar] [CrossRef]
- Jadhav, S.M.; Mahalingam, A.; Ugle, V.V.; Kamaraj, L. Increasing the waste heat absorption performance in the refrigeration system using electromagnetic effect. Int. J. Simul. Multidisci. Des. Optim. 2022, 13, 20. [Google Scholar] [CrossRef]
- O’Reilly, T.; Teeuwisse, W.M.; de Gans, D.; Koolstra, K.; Webb, A.G. In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn. Reson. Med. 2021, 85, 495–505. [Google Scholar] [CrossRef]
- Nishimura, K. Three-dimensional array of strong magnetic field by using cubic permanent magnets. Electr. Eng. Jpn. 2021, 214, 18–25. [Google Scholar] [CrossRef]
- Kararsiz, G.; Duygu, Y.C.; Wang, Z.; Rogowski, L.W.; Park, S.J.; Kim, M.J. Navigation and Control of Motion Modes with Soft Microrobots at Low Reynolds Numbers. Micromachines 2023, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.E.; McMurry, S.M. An adjustable linear Halbach array. J. Magn. Magn. Mater. 2012, 324, 2051–2056. [Google Scholar] [CrossRef]
- Bjørk, R.; Insinga, A.R. A topology optimized switchable permanent magnet system. J. Magn. Magn. Mater. 2018, 465, 106–113. [Google Scholar] [CrossRef]
- Schäfer, M.; Wicke, W.; Brand, L.; Rabenstein, R.; Schober, R. Transfer Function Models for Cylindrical MC Channels with Diffusion and Laminar Flow. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2021, 7, 271–287. [Google Scholar] [CrossRef]
- Jamali, V.; Ahmadzadeh, A.; Wicke, W.; Noel, A.; Schober, R. Channel Modeling for Diffusive Molecular Communication—A Tutorial Review. Proc. IEEE 2019, 107, 1256–1301. [Google Scholar] [CrossRef]
- Wicke, W.; Unterweger, H.; Kirchner, J.; Brand, L.; Ahmadzadeh, A.; Ahmed, D.; Jamali, V.; Alexiou, C.; Fischer, G.; Schober, R. Experimental System for Molecular Communication in Pipe Flow with Magnetic Nanoparticles. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2022, 8, 56–71. [Google Scholar] [CrossRef]
- Back, L.; Radbill, J.; Cho, Y.; Crawford, D. Measurement and prediction of flow through a replica segment of a mildly atherosclerotic coronary artery of man. J. Biomech. 1986, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, M.; Lee, C.; Chae, C.B.; Ji, F. A Two-Way Molecular Communication Assisted by an Impulsive Force. IEEE Trans. Ind. Inform. 2019, 15, 3048–3057. [Google Scholar] [CrossRef]
- Huysmans, M.; Dassargues, A. Review of the use of Péclet numbers to determine the relative importance of advection and diffusion in low permeability environments. Hydrogeol. J. 2003, 13, 894–895. [Google Scholar] [CrossRef]
- Barnsley, L.C.; Carugo, D.; Stride, E. Optimized shapes of magnetic arrays for drug targeting applications. J. Phys. D Appl. Phys. 2016, 49, 225501. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Z. Investigation of Magnetic Nanoparticle Motion under a Gradient Magnetic Field by an Electromagnet. J. Nanomater. 2018, 2018, 6246917. [Google Scholar] [CrossRef]
- Sharma, S.; Katiyar, V.K.; Singh, U. Mathematical modelling for trajectories of magnetic nanoparticles in a blood vessel under magnetic field. J. Magn. Magn. Mater. 2015, 379, 102–107. [Google Scholar] [CrossRef]
- Bernad, S.; Bernad, E. Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications. Micromachines 2022, 13, 1818. [Google Scholar] [CrossRef]
- Sarwar, A.; Nemirovski, A.; Shapiro, B. Optimal Halbach permanent magnet designs for maximally pulling and pushing nanoparticles. J. Magn. Magn. Mater. 2012, 324, 742–754. [Google Scholar] [CrossRef]
- Sim, M.S.; Ro, J.S. Semi-Analytical Modeling and Analysis of Halbach Array. Energies 2020, 13, 1252. [Google Scholar] [CrossRef]
- Abolfathi, K.; Yazdi, M.R.H.; Hoshiar, A.K. Studies of Different Swarm Modes for the MNPs Under the Rotating Magnetic Field. IEEE Trans. Nanotechnol. 2020, 19, 849–855. [Google Scholar] [CrossRef]
- Cai, Q.; Mai, X.; Miao, W.; Zhou, X.; Zhang, Y.; Liu, X.; Lu, W.; Zhang, J.; Gu, N.; Sun, J. Specific, Non-Invasive, and Magnetically Directed Targeting of Magnetic Erythrocytes in Blood Vessels of Mice. IEEE Trans. Biomed. Eng. 2020, 67, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.; Lee, H.; Park, S. Optimized Halbach array for focused magnetic drug targeting. J. Magn. Magn. Mater. 2020, 514. [Google Scholar] [CrossRef]
- Seyedmirzaei Sarraf, S.; Saeidfar, A.; Navidbakhsh, M.; Baheri Islami, S. Modeling and simulation of magnetic nanoparticles’ trajectories through a tumorous and healthy microvasculature. J. Magn. Magn. Mater. 2021, 537, 168178. [Google Scholar] [CrossRef]
- Hussain, S.; Mair, L.; Willis, A.; Papavasiliou, G.; Liu, B.; Weinberg, I.; Engelhard, H. Parallel Multichannel Assessment of Rotationally Manipulated Magnetic Nanoparticles. Nanotechnol. Sci. Appl. 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Camargo, L.; Rodriguez, D.; Benavides, J. Quantification of the efficiency of magnetic targeting of nanoparticles using finite element analysis. J. Nanoparticle Res. 2023, 25, 225. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, X.; Li, Y.; Yang, Z.; Chen, K. Cell migration-inspired stochastic steering strategy of magnetic particles in vascular networks. Phys. Fluids 2023, 35, 113320. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, T.; Huang, C.; Lai, Z.; Wu, X. Modeling and Closed-loop Control of Ferromagnetic Nanoparticles Microrobots. In Proceedings of the 2020 IEEE International Conference on E-health Networking, Application & Services (HEALTHCOM), Shenzhen, China, 1–2 March 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Riahi, N.; Komaee, A. Steering Magnetic Particles by Feedback Control of Permanent Magnet Manipulators. In Proceedings of the 2019 American Control Conference (ACC), Philadelphia, PA, USA, 10–12 July 2019. [Google Scholar] [CrossRef]
- Myrovali, E.; Papadopoulos, K.; Iglesias, I.; Spasova, M.; Farle, M.; Wiedwald, U.; Angelakeris, M. Long-Range Ordering Effects in Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 21602–21612. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Bai, J.; Wang, J.T.W.; Protti, A.; Southern, P.; Bogart, L.; Heidari, H.; Li, X.; Cakebread, A.; Asker, D.; et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016, 16, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Jurgons, R.; Schmid, R.; Bergemann, C.; Henke, J.; Erhardt, W.; Huenges, E.; Parak, F. Magnetic Drug Targeting—Biodistribution of the Magnetic Carrier and the Chemotherapeutic agent Mitoxantrone after Locoregional Cancer Treatment. J. Drug Target 2003, 11, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Thalmayer, A.S.; Xiao, K.; Lübke, M.; Borin, D.; Odenbach, S.; Unterweger, H.; Helmreich, K.; Fischer, G. A Simple and Low-Cost Technique to Measure the Magnetic Susceptibility of Ferrofluids. In Proceedings of the 2023 IEEE SENSORS, Vienna, Austria, 29 October–1 November 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Odenbach, S. Fluid mechanics aspects of magnetic drug targeting. Biomed. Tech. Biomed. Eng. 2015, 60, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, L.; Zarei, K.; Alizadeh, A.; Hosseini, O.; Aminian, S. Three-dimensional investigation of capturing particle considering particle-RBCs interaction under the magnetic field produced by an Halbach array. J. Drug Deliv. Sci. Technol. 2023, 79, 104046. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, L.; Hao, L.; Xiao, H.; Yao, Y.; Qi, L.; Yao, Y. A review on low-dimensional physics-based models of systemic arteries: Application to estimation of central aortic pressure. Biomed. Eng. Online 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; George, B.; Rupitsch, S.J.; Ermert, H.; Ullmann, I.; Vossiek, M.; Lyer, S. Ultrasound-Mediated Cavitation of Magnetic Nanoparticles for Drug Delivery Applications. Curr. Dir. Biomed. Eng. 2022, 8, 568–571. [Google Scholar] [CrossRef]
- Fink, M.; Rupitsch, S.J.; Lyer, S.; Ermert, H. Quantitative Determination of Local Density of Iron Oxide Nanoparticles Used for Drug Targeting Employing Inverse Magnetomotive Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2482–2495. [Google Scholar] [CrossRef] [PubMed]



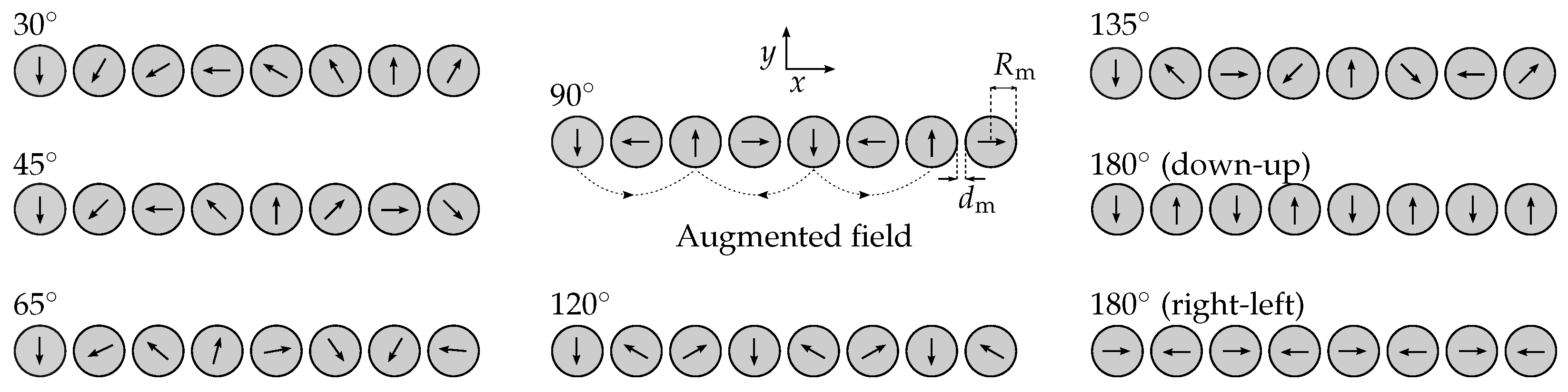




| Category | Symbol | Value | Unit | Label |
|---|---|---|---|---|
| 1.2 | T | Remanent flux density | ||
| N | 8 | 1 | Number of PMs in the array | |
| NdFeB | 1 | cm | Radius of one single magnet | |
| 0.25 | cm | Distance between single magnets | ||
| 90 | Angle between magnetization direction | |||
| 30 | cm | Length | ||
| 0.5 | cm | Radius | ||
| Vessel | d | 0.5 | cm | Distance between magnets and vessel wall |
| 1 | cm/s | Average background velocity | ||
| I | cm | Inlet range of SPIONs | ||
| 2200 | kg/m3 | Mass density | ||
| SPIONs | 100 | 1 | Number of simulated SPIONs | |
| 250 | nm | Radius of one single SPION | ||
| 10 | 1 | Relative permeability |
| Symbol | Value | Unit | Label |
|---|---|---|---|
| N | 1 | Number of PMs in the array | |
| Angle between magnetization direction | |||
| d | 0.25; 0.5; 1; 1.5; 2 | cm | Distance between magnets and vessel wall |
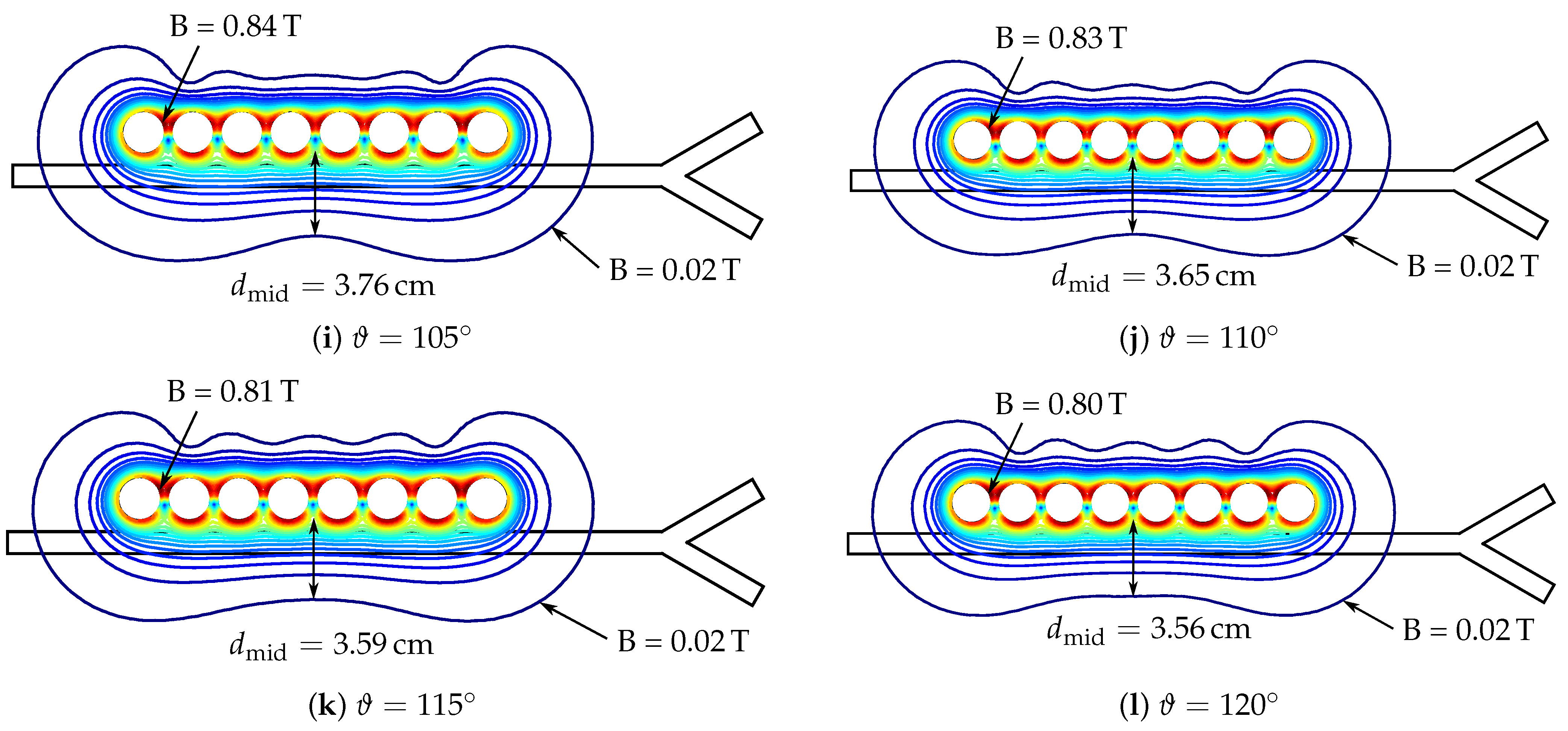
| 30 | 45 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 | 105 | 110 | 115 | 120 | 135 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| in T | 1.02 | 0.97 | 0.92 | 0.91 | 0.89 | 0.88 | 0.87 | 0.86 | 0.85 | 0.85 | 0.84 | 0.84 | 0.83 | 0.81 | 0.80 | 0.93 |
| in cm | 10.91 | 9.44 | 7.30 | 6.67 | 6.07 | 5.54 | 5.07 | 4.67 | 4.36 | 4.11 | 3.90 | 3.76 | 3.65 | 3.59 | 3.56 | 3.33 |
| in cm | 10.91 | 9.44 | 7.30 | 6.67 | 6.18 | 6.04 | 5.97 | 5.81 | 5.64 | 5.43 | 5.14 | 4.87 | 4.64 | 4.54 | 4.47 | 3.87 |
| Magnetization Angle | Side | ||||
|---|---|---|---|---|---|
| 30 | strong | 24.8 (9.4%) | 120.5 (22.2%) | 18.5 (7.3%) | 35.7 (12.5%) |
| weak | 5.0 (34.5%) | 17.3 (95.8%) | 2.5 (23.8%) | 7.6 (72.3%) | |
| 45 | strong | 21.5 (6.6%) | 118.0 (17.3%) | 16.1 (5.0%) | 30.6 (8.5%) |
| weak | 3.4 (27.5%) | 20.8 (122.3%) | 2.5 (26.2%) | 4.0 (122.3%) | |
| 60 | strong | 18.7 (5.4%) | 80.3 (13.7%) | 13.7 (3.9%) | 26.5 (6.4%) |
| weak | 2.1 (7.6%) | 7.8 (25.8%) | 1.6 (5.1%) | 2.9 (12.5%) | |
| 65 | strong | 18.2 (5.3%) | 80.5 (13.5%) | 13.2 (3.7%) | 25.9 (6.3%) |
| weak | 3.3 (12.8%) | 15.5 (49.6%) | 2.1 (6.9%) | 4.3 (21.3%) | |
| 70 | strong | 17.8 (5.2%) | 86.4 (13.5%) | 12.7 (3.6%) | 26.0 (6.0%) |
| weak | 4.4 (15.2%) | 28.2 (57.3%) | 2.7 (8.1%) | 5.5 (26.4%) | |
| 75 | strong | 17.5 (5.2%) | 88.5 (13.5%) | 12.2 (3.5%) | 26.1 (6.0%) |
| weak | 5.2 (16.2%) | 35.8 (59.2%) | 3.3 (8.9%) | 6.5 (28.5%) | |
| 80 | strong | 16.9 (5.2%) | 91.3 (13.5%) | 11.7 (3.5%) | 26.0 (5.9%) |
| weak | 6.9 (18.3%) | 25.5 (61.4%) | 4.5 (11.2%) | 7.7 (31.9%) | |
| 85 | strong | 16.2 (5.2%) | 86.4 (13.4%) | 11.1 (3.4%) | 25.0 (5.8%) |
| weak | 5.9 (16.1%) | 31.2 (56.6%) | 4.0 (9.6%) | 6.8 (28.1%) | |
| 90 | strong | 15.2 (5.1%) | 81.4 (13.2%) | 10.3 (3.3%) | 23.7 (6.0%) |
| weak | 5.9 (15.1%) | 25.0 (51.5%) | 4.0 (9.4%) | 6.5 (25.9%) | |
| 95 | strong | 14.0 (4.9%) | 81.7 (12.9%) | 9.5 (3.1%) | 21.6 (6.0%) |
| weak | 5.4 (13.3%) | 24.7 (43.8%) | 3.7 (8.6%) | 6.5 (22.6%) | |
| 100 | strong | 12.8 (4.7%) | 81.7 (12.6%) | 8.6 (2.9%) | 19.4 (5.9%) |
| weak | 3.5 (8.9%) | 10.4 (29.7%) | 2.4 (5.4%) | 5.1 (15.1%) | |
| 105 | strong | 11.6 (4.5%) | 75.0 (12.3%) | 7.7 (2.8%) | 17.2 (5.7%) |
| weak | 4.3 (9.1%) | 19.8 (26.5%) | 2.9 (6.2%) | 5.9 (15.0%) | |
| 110 | strong | 10.7 (4.3%) | 65.2 (12.1%) | 7.0 (2.6%) | 15.3 (5.6%) |
| weak | 3.7 (7.3%) | 16.0 (22.1%) | 2.4 (4.8%) | 5.1 (11.7%) | |
| 115 | strong | 10.1 (4.2%) | 52.7 (12.1%) | 6.5 (2.5%) | 14.2 (5.6%) |
| weak | 3.1 (6.0%) | 10.6 (0.4%) | 2.4 (3.7%) | 4.2 (9.3%) | |
| 120 | strong | 9.7 (4.3%) | 40.7 (12.4%) | 6.2 (2.5%) | 13.7 (5.6%) |
| weak | 2.7 (4.8%) | 8.7 (17.5%) | 1.9 (2.9%) | 3.4 (7.3%) | |
| 135 | strong | 1.6 (5.1%) | 53.6 (14.7%) | 6.3 (2.9%) | 15.1 (7.4%) |
| weak | 3.1 (4.4%) | 34.8 (12.7%) | 2.3 (3.4%) | 5.0 (8.1%) | |
| mean value | strong | 15.4 (5.3%) | 80.2 (13.1%) | 10.7 (3.5%) | 22.6 (6.6%) |
| weak | 4.2 (13.6%) | 20.7 (45.8%) | 2.8 (9.0%) | 5.4 (28.6%) |
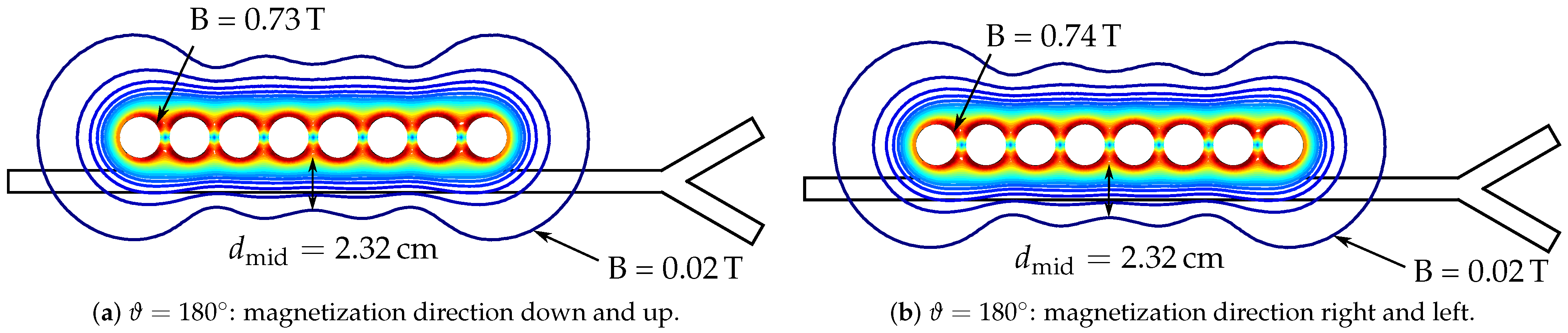
| Magnetization | Trapped | Upper Branch | Lower Branch | Maximum Gradient | 2D Magnetic Energy |
|---|---|---|---|---|---|
| Pattern | in % | in % | in % | in T/m | in J/m |
| Down–up | 27 | 34 | 39 | 45.761 | 20.983 |
| Right–left | 27 | 34 | 39 | 47.937 | 20.983 |
| Authors | Year | Study Type | Study Topology | Magnetic Field Source | Evaluation Parameters | Aim of Study, Optimization Parameter | |
|---|---|---|---|---|---|---|---|
| Sim. | Meas. | ||||||
| Abolfathi et al. [102] | 2020 | x | x | Sym. Y-vessel | 4 EMs on opposite sides | Dispersion changes in particles | Aggregating particles and reducing dispersion of particles |
| Cai et al. [103] | 2020 | x | x | Mice vessel | One EM | Particle attraction (capture rate), fluorescent imaging of mice w. particles | MDT efficiency in mice, optimizing capture rate |
| Hoshiaret et al. [30] | 2020 | x | Sym. vessel with mult. branches | 3 circular arranged EMs | Particle distribution | Algorithm for steering particles through mult. bifurcations at different velocities | |
| Kee et al. [104] | 2020 | x | x | Straight vessel | 3D Halbach array with 9 PMs, | , number of trapped particles, images of gray scale intensity | Trapping particles using 3D Halbach array |
| Park et al. [31] | 2020 | x | Sym. Y-vessel, brain vessel | 6 EMs on opposite sides | B, grad(B), particle distribution, target and sticking ratio, velocity profile | Algorithm for steering particles using a haptic device | |
| Shiriny et al. [54] | 2020 | x | Sym. vessel with 3 outlets | Halbach array with 3 PMs, | H, B, and along tube center line, particle distribution | Optimizing magnetophoretic separation, parameter study | |
| Le et al. [32] | 2021 | x | x | Sym. Y-vessel, 3D model vessel in human brain | 4 EMs on opposite sides | H, grad(H), , particle distribution and trajectory, images of vessel | Trapping and steering particles by shifting focal point |
| Nguyen et al. [17] | 2021 | (x) * | x | Sym. Y-vessel | 9 EMs focused on region of interest | B, grad(B), particle distribution, trapping rate | Design of magn. field for particle trapping, parameter study |
| Sarraf et al. [105] | 2021 | x | Sym. Y-vessel mult. branches, unsym. vessel mult. branches | 6 diff. array with each 5 PMs: linear and 3D Halbach arrays with | B, particle distribution | Comparing particle tracing through healthy and tumorous vessel structures | |
| Stevens et al. [47] | 2021 | x | x | Straight tube | Lin. Halbach arrays (diff. number of magnets), | B, grad(B), magn. recovery of particles, number of trapped particles via | Immunomagnetic enrichment process, magn. recovery of cells |
| Bernad et al. [99] | 2022 | (x) * | x | Unsym. vessel | Different single PMs (varying size, material) | at fixed pos. (all components), , camera images of tube cross-section, particles deposition and target efficiency, particle deposition length and thickness | Investigation of diff. PMs and PM positions for particle steering in MDT |
| Chakrabarty et al. [25] | 2022 | x | Sym. Y-vessel | 2 EMs on opposite side of vessel | H, grad(H), trajectory of one particle based on analytical calc. of and | Steering particles, avoiding trapping | |
| Chakrabarty et al. [16] | 2022 | x | x | Sym. Y-vessel | 2 double coils on opposite sides of vessel | H (diff. cross-sections), , images of vessel, particle trajectory | Steering particles and avoiding stiction using time dept. fields |
| Hussain et al. [106] | 2022 | x | Straight 8 parallel lanes | 2 EMs on opposite sides (Helmholtz coils) | B at fixed pos., velocity and translation velocity of particles, images of particles and fluorescence images along tubes | Controlling nanoparticle flow for simultaneous multichannel testing | |
| Liu et al. [45] | 2022 | (x) * | Hybrid array Straight tube | 6 EMs and 2 PMs | Magn. energy and in horiz. and vert. dir. at fixed pos. and distance; no particles evaluated | Generating in propagation dir. of particles for washing trapped particles out | |
| Camargo et al. [107] | 2023 | x | Topology of blood vessel | One rectangular PM | Trajectory of particles, particle distribution | Parameter study, varying distance magnet to vessel, varying nanoparticle size | |
| Durme et al. [28] | 2023 | x | Sym. Y-vessel mult. branches | 4 EMs on opposite sides | Ferrofluid concentration over cross-section, particle trajectory, particle distribution | Optimizing particle steering, parameter study | |
| Patel et al. [42] | 2023 | (x) * | x | In real mice | Structure with 4 PMs | B, , iron concentration at end of tube, fluorescent images of mice w. particles | MDT efficiency in tubes and mice, optimizing capture rate |
| Surpi et al. [36] | 2023 | (x) * | x | Straight tube | 2 PMs on opposite side of vessel | H, images of tube, velocity of particles, magn. energy, particle energy, magnetic moment of particles | Controlling particle velocity in a straight tube |
| Thalmayer et al. [46] | 2023 | x | Sym. Y-vessel | Lin. hybrid Halbach array with 3 EMs and 4 PMs, | B, and grad in vessel, particle distribution | Investigating hybrid Halbach array and strength of its current for particle steering | |
| Zhou et al. [108] | 2023 | x | Artificial vascular vessels, sym. Y-vessel mult. branches | Time-varying artificial magnetic field | Particle trajectory, particle distribution, particle speed | Steering particles using stochastic algorithms | |
| This work | 2024 | x | Sym. Y-vessel | Halbach arrays consisting of PMs, 6 to 12 magnets, | B, grad( at strongest pos., max. grad(B), magn. energy in vessel, , , SPION distribution | Steering SPIONs, parameter study, investigate potential of diff. parameters for particle steering incl. strength and range of | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalmayer, A.S.; Götz, K.; Fischer, G. How the Magnetization Angle of a Linear Halbach Array Influences Particle Steering in Magnetic Drug Targeting—A Systematic Evaluation and Optimization. Symmetry 2024, 16, 148. https://doi.org/10.3390/sym16020148
Thalmayer AS, Götz K, Fischer G. How the Magnetization Angle of a Linear Halbach Array Influences Particle Steering in Magnetic Drug Targeting—A Systematic Evaluation and Optimization. Symmetry. 2024; 16(2):148. https://doi.org/10.3390/sym16020148
Chicago/Turabian StyleThalmayer, Angelika S., Kilian Götz, and Georg Fischer. 2024. "How the Magnetization Angle of a Linear Halbach Array Influences Particle Steering in Magnetic Drug Targeting—A Systematic Evaluation and Optimization" Symmetry 16, no. 2: 148. https://doi.org/10.3390/sym16020148
APA StyleThalmayer, A. S., Götz, K., & Fischer, G. (2024). How the Magnetization Angle of a Linear Halbach Array Influences Particle Steering in Magnetic Drug Targeting—A Systematic Evaluation and Optimization. Symmetry, 16(2), 148. https://doi.org/10.3390/sym16020148








