Abstract
Schiff bases are organic compounds that are often used as ligands in metal complexes. In addition to the C=N double bond, which is characteristic of Schiff bases, intermolecular hydrogen bonds are frequently observed in both the twisting of planar substituents in organic compounds and the geometric structure of the coordination environment in metal complexes. The results of the crystal structure analyses are stored in databases, which can be used to assess three-dimensional structures. To examine the important structural aspects for novel molecular and material designs, this review examines the important discussion of crystal structure “features” from various viewpoints based on papers on Schiff bases and Schiff base metal complexes from recent years.
1. Introduction
In recent years, with the enrichment of crystal structure data and the spread of data science and artificial intelligence (AI), hot topics in crystal chemistry (the types of structural features and analysis data discussed in the main text, namely potential “features” in AI terms) have changed gradually. The long data previously included in the paper are now (CIF) files of supporting information, and only the important arguments are included in research papers.
For example, for Schiff base compounds, there has been an obvious trend change in Acta Crystallographica reported in the 1970s–2020s. The continuous bridge Pd complex C24H28N2O2Pd2 was reported in 1974 [1]. Measurement of intensity was mentioned; however, for the use of a diffractometer, there is no list of reflection intensities as in the days of photography method before it. Selected intramolecular distances and angles (including coordination environment and C=N and other covalent bonds) as well as atomic fractional coordinates with thermal parameters were tabulated. The deviation of atoms from the least square atomic planes is discussed in the text. There is an ORTEP drawing and packing diagram, but no intermolecular hydrogen bonds are depicted. A bridged feature of the Cu complex C24H42Cu3N2O21 was closely reported in 1984 [2]. Interatomic distances and angles include many covalent bonds such as C=N bond distances. The atomic coordinates and anisotropic temperature factor coefficients were tabulated. ORTEP of molecules and crystal packing (without intermolecular hydrogen bonds) were provided. In 1994, the Cu complex [Cu(C21H24N4S3)][BF4] was reported [3]. Coordination bond distances and angles were mentioned in the text. Fractional atomic coordinates, simply, “isotropic” displacement parameters, and selected geometric parameters (without C=N bonds) were tabulated. Although ORTEP for the molecule is provided, there is no mention of the packing diagram or intermolecular interactions. A mononuclear Ni(II) complex [Ni(C26H17ClN2O3)] 2CH4O with asymmetric Schiff base ligands was reported in 2004 [4]. Not only the coordination geometry around and its deviation from the average plane, but also hydrogen bonds and π-π interactions were described in the text. The tables included selected geometric parameters, e.g., coordination environment (but did not refer to C=N or covalent bonds) and hydrogen bonds. However, there is currently no table of atomic coordinates. There is an ORTEP for the molecule, but no packing diagram for the crystal. In 2014, [Zn(C17H15NO4)] and [Co(C17H15NO4)] were reported [5], which have bridging ligands with a Schiff base moiety and bidentate chelating -COO− groups. There is an ORTEP diagram of the metal center and a packing diagram of the bridged structure. The text mentions the coordination number, coordination bond distance, and bridged style (dimensionality of the framework). However, there is no mention of other bond distances (no mention of C=N), valence or torsion angles, or hydrogen bonds. There is no table of hydrogen bonding geometry. In 2024, an organic Schiff base compound C18H19BrN2O3S was reported simply about space groups, angles between planar moieties, hydrogen bonds, and non-covalent “sites” of Hirshfeld surface analysis. There is a table of hydrogen bond geometry, but there is no mention of covalent bond distances [6].
Schiff bases and their metal complexes are an important and useful group of compounds, and we focus on them in this review. Many syntheses and single-crystal X-ray structure analyses of Schiff base compounds have been reported for a long time. It was first reported by H. Schiff: Schiff bases are organic compounds containing imine or azomethine (RR′C=NR″) groups obtained from the dehydration condensation of aldehydes and primary amines. Schiff bases have long been known as both compounds and typical ligands for metal complexes, as they are Lewis bases that can donate lone pairs of electrons to nitrogen atoms. Although Schiff bases can be said to be classic and typical, they can also be said to be evergreen, with new research results still being actively reported in many fields [7]. For example, this review contains many chapters that introduce the potentially novel structural aspects of Schiff bases.
The synthetic and structural features that are believed to be of chemical importance are described here. Schiff bases are characterized by a C=N imine bond formed by the condensation of an aldehyde and an amine. In the field of coordination chemistry, Schiff bases are classical and representative organic ligands, representing a major group of compounds in both the literature and many databases. Salen ligands are one such example, and their (chiral) catalytic [8,9,10] and physical (intermolecular forces, magnetic, optical, or redox, if possible) properties [11,12,13] have been widely studied. Furthermore, there are many reported examples of Schiff base and Schiff base metal complex crystal structure analyses; thus, many examples with similar characteristics can be obtained from the literature and databases, which is a great advantage. For this reason, in addition to the conventional use of crystal structure databases, new ways of using big data and data science are becoming widespread [14,15], in addition to standard ranges of geometries. A systematic but classical study of “hydrogen bonding” is famous because of the statistical evidence extracted from crystal structure databases [16,17]. While can often be classified by the types of donor atoms (e.g., C–H, O–H, and so on), acceptor atoms (e.g., H···N, H···O, H···Cl, and so on), or the number of branches (e.g., besides one N–H···one O, two N–H···one O, one N–H···two O), the trends derived from the statistics of the distance and angle distributions provide important indicators for structural (organic or organometallic) chemistry. In contrast, proteins are treated separately in the Protein Data Bank (PDB). However, a large amount of data has been accumulated since the first crystal data of (inorganic) compounds.
It can be said that technological advances in crystal structure analysis have brought about a shift (to measure easily) in the status of structure analysis to be treated relatively “lightly”. With the advent of two-dimensional highly sensitive detectors and powerful X-ray sources, faster computers, and standardized GUI programs, small molecule crystal analysis has now become a rapid method. As a result, the number of reports rapidly increased (information distribution formats such as CIF and data quality inspection systems were established and automated), and data science was also applied using a crystal structure database that exceeded one million records a few years ago. As a result, structural chemistry has entered a new phase. Nearly 50 years ago, we reported individual reflections in papers; we reported all covalent bond distances, angles, and hydrogen bonds 30 years ago [16,17], and the researchers reported statistically 20 years ago. The position of the crystal structure analysis results has changed, with rare or unusual structural features no longer being reported and some journals no longer including crystallographic data tables in the text of the paper, as they do now.
Advances in crystal structure databases and formatting CIF have led to changes in formatting rules in special field journals, such as Acta Crystallographica, published by IUCr. Among specialized academic journals that report on crystal structure analysis of small molecules, there is no doubt that IUCr’s Acta Cryst. C, Acta Cryst. E, and IUCr Data has a reputation for tradition and high quality (typically, the items in the main text are chemical context, structural commentary (mentioned only important geometries or facts, not necessarily selected/all bond distances and angles), supramolecular features (Table of hydrogen bonds, packing and if any Hirshfeld surface analysis), database survey (previous similar examples), synthesis or crystallization and refinement (crystallographic data)). The CIF format provides explanations for standardized measurements and data analysis, and the stylized main text items contain only the right amount of information and noteworthy original content. Undoubtedly, it is natural to regard this as the de facto standard format. However, as it has become easy to obtain crystal structures in recent years, in papers published in other regular format chemistry journals, the only content that needs to be reported in the main text (besides submitting a CIF) is based on the author’s interest. It should be limited to items of such importance. However, statistically abnormal (rare) structural features become more difficult to identify as the amount of data increases.
If we were to conduct research on the crystal structure of Schiff bases based on today’s big data, which “moiety” of the Schiff base compound should be selected as the characteristic? Information provided by a crystal structure can be organized hierarchically, including the crystal system, space group, point group (the existence of chirality), hydrogen bonding or other intermolecular interactions, intramolecular non-covalent interactions, and the presence of covalent bonds (and, if so, bond distances, bond angles, dihedral angles, torsion of planar parts, etc.). Characteristics specific to metal complexes include cluster formation, number of metal ions, and coordination bonds around the metal (and thus coordination number, coordination geometry, bond distance, angle, and degree of distortion).
Of course, it goes without saying that the viewpoints to be discussed depend on the purpose of each study. For example, when one of the authors (T.A.) considered the crystal structure and three-dimensional structure of organic compounds and their metal complexes from the perspective of biological activity, he manually (or by human thought) considered the viewpoints shown in Figure 1. The purpose of Figure 1 is to schematically use Schiff base compounds to explain the importance of structural chemistry. In other words, the structural features near the heteroatoms, possible interaction sites, and molecular design intentions are shown. In this review, various recent papers that reported crystal structure analyses for diverse research reasons were selected. From these, we assess the viewpoints from which Schiff bases and Schiff base metal complexes are discussed in terms of structural chemistry or chemical crystallography.
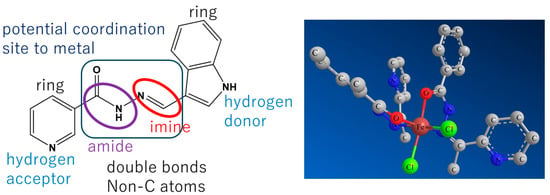
Figure 1.
(Left) A Schiff base compound with structurally integrated aspects. Two-ring moieties can rotate around the binding single bonds. (Right) Model of a distorted octahedral Fe(II) complex incorporating Schiff base ligands acting as a bidentate chelate and two Cl- ligands in cis-sites.
In this way, the purpose of this review is to collect crystal structure papers on Schiff base compounds, focusing on very recent papers (mainly in 2023), and to introduce the “structural features” that are currently being openly discussed in the text. This is not a long-term, exhaustive review of the crystal structures or chemistry of Schiff base compounds.
2. Organic Schiff Base
Section 2, Section 3, Section 4 and Section 5 provide examples of specific papers and introduce the structural elements. The general policy is to introduce the most typical and important content, in that order. Therefore, please note that the order does not necessarily reflect the classification of the compounds.
There is a hierarchy of information on Schiff base compounds and their metal complexes as well as their crystal structures. Here, we will look at the viewpoints used to describe and discuss crystal structures in the order of simplicity (from Schiff base organic compounds: crystal lattice, intramolecular, intermolecular, etc.). The C=N imine bond distance (generally shorter than a C-N single bond), which characterizes Schiff bases, is not necessarily pointed out, but the stereochemistry of the double bond is sometimes mentioned. The mainstream approach is to explain the overall shape of organic molecules and intermolecular interactions in crystal packings based on facts. A noteworthy recent trend is that Hirshfeld surface analysis is increasingly being used to quantify and visualize intermolecular interactions.
Murugan (2023) [18] reported a Schiff base compound (2,4-dichlorobenzylidene)[2-(1H-indol-3-yl)ethyl] amine (C17H14Cl2N2), aiming at the pharmaceutical and biological functions of indole. According to the journal’s rule (IUCr Data), which is highly systematic, numerical data are reported in tables and the essential structural description in the text. As regards the molecule, the imine C=N bond length (1.256(3) Å) and dihedral angles between planar moieties (phenyl group) were reported, and the E-configuration around the double bond was highlighted, as shown in Figure 2. Intermolecular hydrogen and π-π interactions were also mentioned in the text.
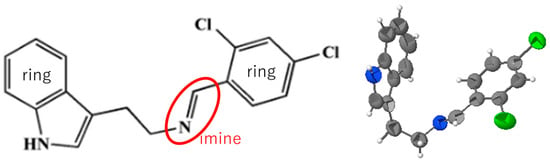
Figure 2.
(Left) Molecular structure of a Schiff base compound [18], denoted by an imine C=N bond and two planar ring moieties. (Right) The corresponding ORTEP drawing.
Khan et al. (2023) [19] reported a dithiocarbazate Schiff base ligand (C17H26N2S2) and its four-coordinated square-planar Ni(II) complex for biological activities, in which the ligand acted as an S,N,N,S-tetradentate chelate. Single and double bonds involving N and S atoms (Figure 3) were mentioned in the text, namely, the N=C bond of 1.340(4) Å in the organic ligand and 1.293(3) and 1.290(2) Å in the complex. It should be noted that the compound has a long alkyl chain (in other words, the three-dimensional conformation of the covalent bond, which cannot be determined without actual measurement), and its crystal conformation was depicted in terms of the molecular structure and crystal-packing figures from the original paper.
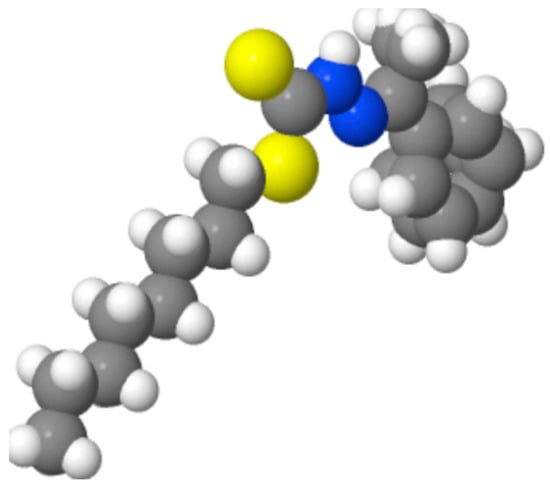
Figure 3.
Blue and yellow atoms in C17H26N2S2 [19] with a long alkyl chain denote N and S atoms, respectively.
Shankar et al. (2023) [20] reported two 4-amino antipyrine Schiff base compounds in the systematic format of Acta Cryst. E, aiming at the antioxidant, anti-putrefactive, and optical functions of antipyrine. Important covalent bond lengths (1.291(2) Å, mainly the imine C=N double bond of E-configuration) and bond angles (sum of three = 350.47(9)° < 360°) or dihedral (–177.11(11)°) angles were mentioned and listed. There are three (five- and six-membered) planar ring moieties in the molecules, and the dihedral angles between planes (e.g., 54.87(7)°) were mentioned in the text. Intermolecular hydrogen bonds, including halogen atoms, were also mentioned in the text. As shown in Figure 4 (C22H26N4O), Hirshfeld surface analysis was also employed to discuss the intermolecular interactions (see later section on intermolecular interactions).
Figure 4.
Crystal packing of a Schiff base compound [20] from Hirshfeld surface analysis for one molecule only.
Halz et al. (2003) [21] reported three Schiff bases derived from 3-formylacetylacetone and three amines in Acta Cryst E from the interest of organic synthesis. Only one (C14H17NO2) of the three formed chiral amines (due to asymmetric carbon) crystallized in the space group P21, as depicted in Figure 5. Double bond (enamine C=C and C=N) distances and torsion angles of C-C-N-C bonds (e.g., −134.31 (11)° for achiral bonds) were reported in detail for three compounds. Intramolecular and intermolecular hydrogen bonds were described and analyzed using Hirshfeld surface analysis.
Figure 5.
(Left) Part of the Schiff base moiety discussed is its double bond distances (denoted as C=N) and torsion angles of the C-C-N-C bonds [21]. (Right) Crystal packing of the compound (C14H17NO2) in the space group P21.
Yahiaoui et al. (2023) [22] reported an organic Schiff base, namely, (E)-2-{[(4-methoxynaphthalen-1-yl)methylidene] amino}-4-methylphenol (C19H17NO2) aiming at inhibiting corrosion on metals and alloys. Important structural data were reported in the Acta Cryst C format. The arrangement (dihedral angle = 6.08 (12)°) of two planar rings (atoms C1–C10; r.m.s. deviation 0.015 Å) forming an S(5) ring motif (naphthyl and phenyl binding to the N=C imine bond connected via an intramolecular hydrogen bond, as shown in Figure 6) were one of the most interesting structural features. Intermolecular hydrogen bonds were mentioned in the text alongside the Hirshfeld surface analysis.
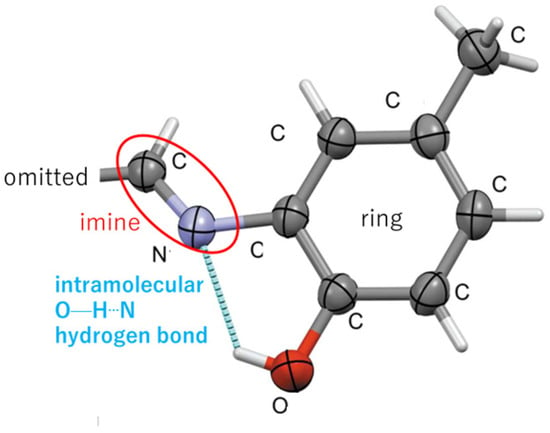
Figure 6.
Intramolecular hydrogen bond of a Schiff base compound [22] (part of the molecule is omitted for clarity).
Matsumoto (2023) [23] reported a macrocycle Schiff base compound (C42H30N6·0.5THF(C4H8O)·3H2O) derived from hexakis(μ-phenyleneimine) as a functional material. The figures depict both the molecular structures and crystal-packing structures. However, it should be noted that detailed numerical data were only reported in the supplementary data of geometry. The important fact (overall molecular shape and size) is that it adopts an outer-rim configuration with respect to its six N atoms and has a chair conformation. According to the top view, the THF molecules were located at the center of the pore over the ring. According to the side view, the ring molecule slightly deviates from a genuine planar shape, forming stacked columns in the crystal (Figure 7).
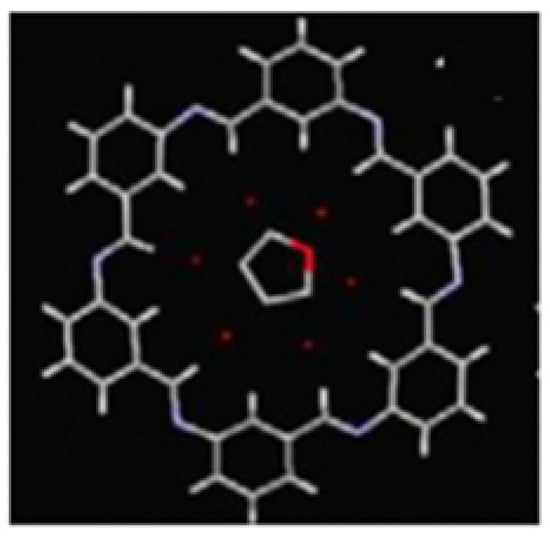
Figure 7.
Top view of the compound [23].
Mustufa et al. (2023) reported [24] a fluorescent Schiff base chemosensor (C18H17N3) for metal ion detection. In the text, the infrequent space group (Pca21), bond length of the imine C=N double bond, and geometry of the ring moieties (the distance of the C-C(ring) and dihedral angles of the two planar moieties) were also mentioned.
Aslam et al. (2023) [25] reported an N,N-donor Schiff base ligand (C18H20N2O2) without the crystal structures of its metal complexes for biological functions. The molecular structure (ORTEP) and crystal-packing (without hydrogen bonds) figures were depicted graphically. As regards the covalent bonds, the distance of the azomethine double bond (adopting an E-configuration) and the torsion angle around it (involving planar phenyl groups) were reported. No noteworthy bond distances or bond angles were reported in the main text.
3. Coordination Complexes
Some organic compounds have also been reported as (potential) ligands for metal complexes, and it may not be possible to classify them strictly based on the presence or absence of a coordination environment. Contrary to organic Schiff base compounds, in the case of metal complexes, the authors of the original paper were less interested in weak intermolecular interactions (van der Waals or aromatic stacking, etc.), of which only a few examples have been described, and were more concerned with the surrounding coordination environments (which were also requested by this reviewer). There is a clear tendency to focus on the interest around the central metals in metal complexes.
We will explain them roughly in the order of central elements, coordination environment, coordination geometries, and other structural parameters. We would like to highlight and introduce them in the order of basic importance, namely isomers and chirality, clusters, and so on.
Al-Zeudaneen et al. (2023) [26] reported a redox-active and a distorted square-planar Ni(II) complex ([Ni(C19H11N3OS2)]) with an unsymmetrical tetra dentate-chelating Schiff base ligand. The most important fact reported in this study was the unsymmetrical ligand, as shown in Figure 8. Regarding information selection, the crystal structure and data of this compound were reported according to Acta Cryst. E format, i.e., important bond lengths and angles were listed, and additional C=O bond lengths (1.229(4) Å) were mentioned in the text. The distortion of the four-coordinate [NiN3S] from a regular square planar one was described as a deviation from the mean plane (0.0867 Å). Intermolecular C–H···S hydrogen bonds were also highlighted in the text.
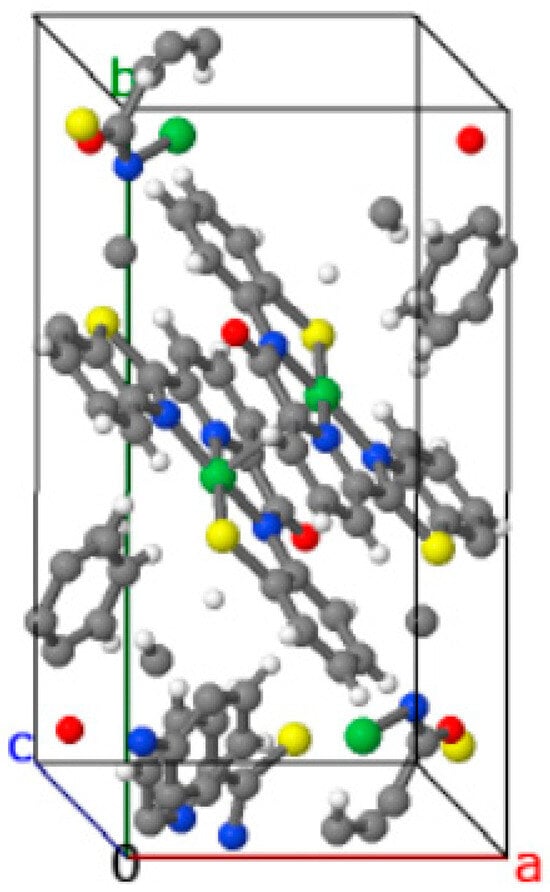
Figure 8.
Crystal packing of the Ni(II) complex with an unsymmetrical ligand (Ni···Ni = 3.3305(9) Å). White—H; black—C; green—Ni; red—O; blue—O; yellow—S [26].
Gholivand et al. (2023) [27] reported two Cu(I) complexes with a Schiff base ligand adopting a bidentate chelating mode that was prepared for a “click” reaction catalyst (C38H35ClCuN2P and C38H35ClCuN2P). As shown in the molecular structure and crystal-packing figures, a four-coordinated triangular pyramidal coordination geometry (angular index, τ4 values = 0.82–0.83; For an ideal square-planar structure, τ4 = 0, and for an ideal tetrahedral, τ4 = 1.) was observed, and coordination bond lengths and angles, in addition to hydrogen bonds were also listed. In addition, no numerical explanations were obtained from the crystal structure analysis, and only the presence of the prepared metal complexes was confirmed.
Haldar et al. (2023) [28] reported Sn(III) and Bi(III) complexes of heavier pnictogens with a redox-active Schiff base ligand, bis(α-iminopyridine) (C17H18Cl2F3N4O3SSb). The space groups and coordination geometries (M-L bond lengths (Sb1-N2 = 2.249(2) and Sb1-N3 = 2.293(2) Å) and L-M-L bond angles (Cl1-Sb1-Cl2 angle of 162.076(19)°)) were reported in the text. This Schiff base ligand acts as a tetradentate ligand, and the geometry of the apical monodentate ligands can vary in the crystal. A comparison of the sum of van der Waal’s radii (ΣBi-O = 3.59 Å) for the coordination compounds, including typical elements, was novel in this paper.
Böhme et al. (2023) [29] reported a Si(IV) complex with an O,N,O-tridentate Schiff base ligand (the E-configuration of the C=N imine bond) and two phenyl groups directly binding to the Si atom (C28H23NO4Si). The five-coordinate coordination environment of this novel example of a Si complex was described using tau parameters (τ = (β − α)/60° = 0.79; if τ = 0, it is a perfect square pyramid, while τ = 1 indicates a perfect trigonal bipyramid.) in the text, and Si-N and Si-O bond lengths and the related angles were mentioned in the tables (using the format of a specific journal: IUCrData). The Si-C(phenyl) coordination bond distances (1.8817(13) and 1.8940(13) Å) were similar to the reported values.
Faye et al. (2023) [30] reported a Schiff base dinuclear Y(III) complex (0.25(C34H40N8O12Y2)) bridged by two oxo ligands from the interest of organic synthesis. The tridentate Schiff base ligand (N’– (1–(pyridin-2–yl)ethylidene) nicotinohydrazide) and other ligands formed a nine-coordinated coordination geometry, whose lanthanide complex M-L bond distances were assessed in detail (they are relatively longer than those of d-block transition metal complexes because of their large ion radii and high coordination numbers). Furthermore, the coordination fashion of the bridging ligands (e.g., η2-) was classified and mentioned in the text closely.
Li et al. (2023) [31] reported homo-dinuclear and hetero-dinuclear Nd(III) complexes (C50H56Nd2N6O16, C68H76Nd2N4Na2O16, C68H76Ce2N4Na2O16) with tetradentate Schiff base ligands. The coordination environment (including the dinuclear moiety) was represented in an ORTEP drawing with metal and bridged O atoms only, and the coordination geometry parameters (mainly long Ln-L bond distances due to lanthanide contraction at high coordination numbers) were also listed in the tables.
Barbosa et al. (2023) [32] reported a Schiff base U(VI)=O (uranyl) complex with a N2O2-Schiff base ligand and water ligands ([UO2(C22H24N2O4)(H2O)]) for application for direct ethanol fuel cells. The structural discussion of the coordination environment focused on the five-coordinate distorted UN2O3 basal plane with U-L lengths (U1–N1 = 2.602(5) Å; U1–N2 = 2.524(5) Å), L-U-L angles, and deviation of an atom from the mean plane. Intermolecular hydrogen bonds via water molecules formed a dimeric complex, maintaining a near C1 symmetry, although numerical data were not included in the main text.
Kumar et al. (2023) [33] reported two four-coordinate Ni(II) complexes with Schiff base complexes to study ligand-protein docking using a potential effect against SARS-CoV-2 (C38H32ClN2NiO3P and C24H18N2NiO2). One of the tetradentate ligands exhibits a typical salen-like square-planar coordination, and the other has a tridentate Schiff base binding to the fourth phosphine coordinating site. To describe the coordination environment, the M-L bond distances and L-M-L bond angles were listed, while the geometrical parameters of the covalent bonds in the ligands were not mentioned. Intermolecular hydrogen bonds and Ni···Ni non-bonding distances in the linearly stacked structure of Salen-type coordination were also listed numerically, as well as in the Hirshfeld surface and fingerprint plot analysis. Nevertheless, the most important information in this study may be the strength of the interaction and the 3D topology of the crystal packing.
Mamindla et al. (2023) [34] reported a mixed-ligand Cu(II) complex incorporating a tridentate Schiff base ligand, rigid bidentate ligand, and monodentate water ([Cu(C11H15N2O)(C12H8N2)]ClO4) in Acta Cryst. E. The design and synthesis of mixed-ligand copper(II) complexes for biological activity is the driving force behind this study. The space group was mentioned to explain the overall centro-symmetry (the asymmetric unit contains two crystallographically independent molecules.). The trigonality index (τ = (β − α)/60°) was used to evaluate the square pyramidal and trigonal bipyramidal geometries in a five-coordinate coordination environment. In addition to this index, the Cu-L bond lengths and L-Cu-L bond angles were used to describe the degree of distortion. However, the covalent bonds of these ligands (sp3 and sp2 hybridizations of the amine) were barely mentioned in the text. The supramolecular interaction (conventional hydrogen bonds and contacts including π-moieties) was described in an independent section of the text according to the journal’s rule.
Sane et al. (2023) [35] reported a six-coordinate octahedral Ni(II) complex (C10H14N10Cl2NiS2) with two tridentate Schiff base ligands for antitumor functions. The coordination geometries (Ni-L and L-Ni-L) and space group (P21/c) were mentioned in the text and tables to show a deviation from a regular octahedron. The dihedral angles of the two planar moieties in the two ligands were stated (within the normal ranges). The overall shape was depicted in the figures. Intermolecular hydrogen bonds were mentioned in the text and tables, which were supported by packing figures from several angles.
Majumder et al. (2023) [36] reported six-coordinated oxidovanadium (the V=O bond length was mentioned) complexes (C35H26N4O4V and C34H23N3O5V) containing coumarin and a naphthalene Schiff base ligand for DNA/BSA (bovine serum albumin) binding studies. The three ligands have one (=O), two (1,10-phenanthroline), and three (NON-Schiff base) coordination sites, respectively. Coordination geometries (namely V-L and L-V-L data) from X-ray analysis were listed and compared with DFT-optimized structures.
Rekha et al. (2022) [37] reported a dimeric form of a five-coordinated Mn (Salen) complex (C16H12N2O2Cl3Mn), a typical Schiff base ligand (C16H14Cl2N2O2) used for catalytic application, and a non-metal–organic ligand. As regards the coordination geometry around the Mn atom, the Mn-L bond distances, L-Mn-L bond angles (in the text and tables), and deviation of the apical L from the MnN2O2 mean plane were used to represent the distorted square pyramidal environment (parameter, τ = 0.084). No description could be found for the organic ligands, namely, covalent bond geometries. Dimeric forms and intermolecular hydrogen bonds (also including Cl-atom) were mentioned in the text.
Ghasemi et al. (2023) [38] reported two crystal structures of Cu(II) complexes with tri-dentate unsymmetrical Schiff base ligands for molecular docking against SARS-CoV-2 (C29H27CuN4OS2ClO4 and C34H33CuN5O2S3ClO4). Therefore, the overall shape of the molecules may be important. As regards the organic ligand (the tridentate NN′O type), the differences between the two amino groups in the reactants were confirmed, i.e., reacted or not, meso-configuration when reacted. Concerning the metal complex, it was reported as being penta-coordinated with a distorted square-based pyramidal geometry with L-M-L bond angles. The tau five index (τ5) was also employed to evaluate the magnitude of the square-based pyramidal geometry or trigonal bipyramidal geometry. A numerical discussion of the bond lengths and angles was not published.
Mandal et al. (2023) [39] reported a pyrazole-based heterocyclic Schiff base compound (C10H11N3O) and its monodentate metal complexes (C20H30Cl2N6NiO6 and C30H33Cl2N9NiO11) to investigate catalytic and anticancer activities. As regards the metal complexes, the possibility of forming two types of metal complexes, i.e., so-called coordination fashions, was highlighted, and the chelate ring atoms in the octahedral 6-coordinated Ni(II) complexes were described. Conventional intramolecular hydrogen bonds and weak π···π stacking interactions were also described in detail.
Ghasemi et al. (2023) [40] reported mixed-ligand Cu(II) complexes with a tridentate NN’O-type unsymmetrical Schiff base main ligand (C21H23CuN4O2,ClO4). X-ray crystallography revealed penta-coordinated complexes with square-based pyramidal coordination geometry, as determined from the geometry index (τ5), which was calculated as follows: τ5 = (β − α)/60°. In addition, a deviation of the mean plane was observed for certain atoms in a coordination environment. The final purpose of this study was to investigate Schiff base complexes in human colorectal, lung, and breast carcinoma cell lines. A docking study was carried out; however, the complex’s intermolecular interactions were not addressed in detail, except for conventional intermolecular hydrogen bonds and face-to-face π-π stacking.
Alwi et al. (2023) [41] reported the use of Schiff base metal complexes derived from 2-acetylpyridine for antibacterial applications. The Ni(II) complex (C19H20N6S2Ni) produces a distorted octahedral environment; the distortion between a trigonal bipyramid and a square pyramid was discussed, with a geometry parameter of τ = (β − α)/60° and coordination bond angles and lengths. In addition, the Cu complex (C19H20N6S2Cu) has a complex environment, falling between square pyramidal and trigonal bipyramidal, with a terminal NCS ligand. The ring formed by the metal and ligand adopts a half-chair conformation, forming conventional C–H···S interactions. As for the purpose, the overall shapes of the molecules may be important.
As for topics about isomers and chirality, confirming the molecular shape may be essential. Hollandsworth et al. (2023) [42] reported Fe(II) complexes (C25H36F12FeN6P2Cl2 and C31H49F12FeN7OP2) with tridentate mixed amine/imine Schiff base ligands (C18H15N2). The distortion of the coordination environment (deviation from regular octahedron) was visually described by the N-Fe-N bond angles. In addition, the C=N bond distances, chiral (S- or R-) stereocenters, and cis- or trans- geometries of the cyclohexane rings in the organic ligands were also analyzed.
Akiyama et al. (2023) [43] reported a Cu(II) complex with a (chiral) tridentate amino acid Schiff base ligand ([Cu(C12H13N2O3)(H2O)2]-[Cu(C12H13N2O3)(H2O)]). In the asymmetric unit, there are two crystallographic independent molecules: one is four-coordinated (one water ligand), and the other is five-coordinated (two water ligands). The coordination geometries were explained by the Cu-L bond lengths and L-Cu-L bond angles, which cannot be understood without further analysis. The imine C=N bond distance was determined for both the molecules. Hirshfeld surface analysis and supramolecular hydrogen bonds were independently published in the text, figures, and tables.
Suzuki et al. (2023) [44] reported an analogous Cu(II) complex with a (chiral) tridentate amino acid Schiff base ligand ([Cu(C16H13NO4)(C3H4N2)]). In this case (Figure 9), the additional monodentate ligand is not water but imidazole, which may generally lead to four-coordinated complexes. The coordination bond lengths and angles and the bond length of the imine C=N double bond were described in the text. Intermolecular hydrogen bonds were described numerically in the text, tables, and Hirshfeld surface analysis.
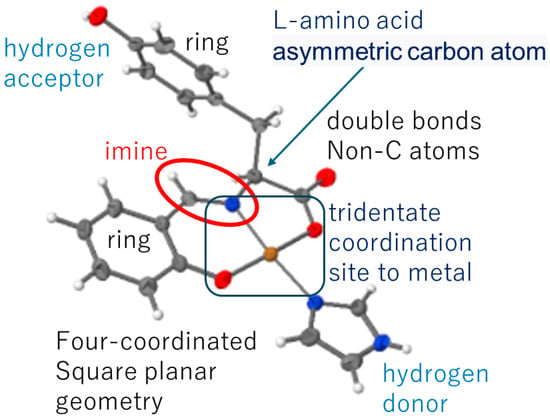
Figure 9.
Cu(II) complex with a tridentate amino acid Schiff base ligand [44] with various structural aspects mentioned in Figure 1 marked.
They (T.A. and D.N.’s group) also referenced a new paper on the imidazole-bound (chiral) complex by Kaneda et al. (2024) [45], in which the E-configuration of azobenzene N=N and imine C=N bonds were not mentioned at all. However, we will refrain from stating what we focused on in these papers [43,44,45], except for Figure 9, as this would reflect the author’s subjective opinion.
Boudraa et al. (2023) [46] reported a Ni(II) complex (C20H22N2NiO2.0.5(H2O)) derived from a N2O2 tetradentate Schiff base for biological studies. Square pyramidal (numerically represented by the τ parameter) Ni(II) complexes are bridged via a solvent water molecule in the center of symmetry (with a 2-fold axis), resulting in eight molecules per unit cell (Pbcn, Z = 8). The Ni-O or Ni-N coordination bond lengths are mentioned in the text in a normal way. The conformation of the benzene rings in the two complex molecules was also compared in a similar way to that of organic compounds. Moreover, Hirshfeld surface analysis was used to assess the conventional intermolecular C–H···O hydrogen bonding.
Maity et al. (2023) [47] reported a Schiff base mononuclear Ni(II) complex (C28H30Br2NiN4O5) for anti-fungal applications. The tridentate ligands formed a six-coordinate octahedral coordination environment with Ni-L, which was mentioned in the text. In the situation around the central Ni atom, the N coordination site distances of the ligands were represented numerically. The two phenyl groups in the ligands were manipulated intramolecularly to form face-to-face arrangements with an interplanar distance of 3.784 Å. Moreover, conventional hydrogen bonds were mentioned to explain intermolecular interactions and crystal packings.
Valentova et al. (2023) [48] reported chiral Schiff base ligand (from DL-α-alanine, β-alanine, taurine, and γ-aminobutanoic acid)-induced E/Z-isomerism in Cu(II) complexes (C12H13CuNO6) aiming at urease inhibitory activities. The coordination features of the tetradentate chelate ligands and the resulting distorted square-based pyramidal coordination geometry around the central Cu atom (containing the apical water ligands) were confirmed. Moreover, stereochemistry involving optical isomerism (chirality; R/S) and E/Z-isomerism was investigated based on the crystal structures (Figure 10). However, the Flack parameters were reported only in the supplementary information (not in the table of crystallographic data in the main body).
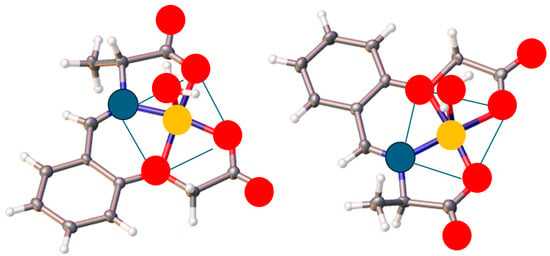
Figure 10.
(Left) E-isomer and (Right) Z-isomer of Cu(II) complexes with a tridentate Schiff base ligand [48]. The basal planes of the distorted square-based pyramidal coordination are also indicated (Yellow—Cu, Bule—N, and Red—O atoms).
As for clusters or aggregated metal complexes, polynuclear metal complexes can be considered assemblies of the aforementioned chelating or bridging ligands and mononuclear metal complexes. Thus, from individual viewpoints and intermolecular interactions, they are essentially similar. However, the inclusion of multiple metal ions introduces new issues regarding their positional relationships (metal-metal distances and metal-bridging atom-metal angles) and bridging modes, which are symbolically defined in the nomenclature. In addition to structural chemistry, metal-metal interactions, such as magnetic interactions, tend to be important. These are not necessarily listed in a table, as are the coordination structures (metal-coordinating atoms).
Hou et al. (2023) [49] reported three metal complexes with Schiff base ligands, namely 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl) benzimidazole derivatives (C24H26ClCuN3O4, C56H62N6Ni4O16, and C40H27CoN6O4) for anticancer drugs. Although similar ligands were used, the number of metal ions in the complexes was different. The Cu(II), Ni(II), and Co(II) complexes are mononuclear, dinuclear, and trinuclear, respectively. For the three types of octahedral coordination geometries, cis- and trans- L-M-L bond angles were used to describe the distortion of the environment. However, most of the bond distances are in the normal range. Intramolecular and intermolecular hydrogen bonding were also depicted in the figures of the original paper to represent the molecule being focused on.
Paliwal et al. (2023) [50] reported a Cu(II) complex (C32H26Cu2N6O6S2) with a thiolate Schiff base and imidazole ligands, which was prepared via the oxidation of an S-coordination atom after coordination to a Cu(II) ion. Dinuclear structures were formed, which may exhibit DNA/HAS (human serum albumin) interactions for anticancer activity. The square pyramidal penta-coordinated environment of each Cu(II) atom was described using the τ parameter and coordination bond lengths and angles in the tables in the original paper. Intermolecular hydrogen bonds were numerically described using both distances and angles.
Ranaweera et al. (2023) [51] reported a dinuclear metal complex with a cyclic Schiff base ligand, including six sites (atoms) ready for the coordination of two metal ions ([Cu2(C16H20N2O4)(C5H5N)]) (Figure 11). The coordination geometries (bond distances and angles) around the two metal centers, including the displacement of the apical ligand from the mean basal plane, crystal packing, and intermolecular hydrogen bond interactions, were mentioned. It should be noted that Bond Valance Sum (BVS) calculations were used to investigate the oxidation states of the metal ions based on their bond distances. The BVS calculation was performed to investigate the oxidation states of the two copper ions separately based on the Cu-L lengths, which may be the basis for the preparation of catalytic precursors.
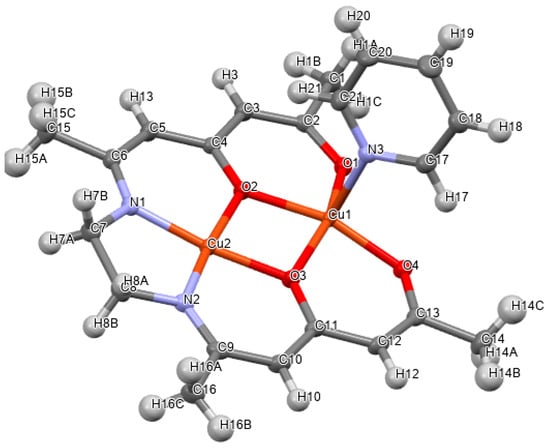
Figure 11.
Crystal structure of a dinuclear copper(II) complex [51].
Yilmaz et al. (2023) [52] reported multi-nuclear Ni(II) complexes of a distorted pyramidal geometry with Schiff base ligands (C56H62N6Ni4O16) for the investigation of non-covalent interactions. The molecular structures were not explained in the text, although the intermolecular hydrogen bond networks in the crystal packing were depicted clearly. Non-covalent interactions, primarily H-bonding and π···π stacking, were the main focus of this study. Intermolecular interactions were also investigated using Hirshfeld surface analysis.
Aprajita et al. (2023) [53] reported three cubane-type Cu(II) complexes (C39H61Cl2Cu3N9O12, C32H34Br2Cu2N4O8, and C34H24Br2Cl2Cu2N4O12) with tridentate Schiff base ligands with ONO donor sites (without abnormal bond geometries), focusing on the structural features, magnetic, thermal, and redox properties, and a theoretical interpretation. As the main point, the coordination geometries of the phenoxy-bridged Cu(II) complexes were µ3-fashion for one of them and µ2-fashion for two of them, which resulted in centro-symmetric dimers being formed. Intermolecular C–H···π and π···π interactions (π-conjugates) and the C–H···Br interaction (halogen) were analyzed using curvature Hirshfeld surfaces.
Ressler et al. (2023) [54] reported various metal complexes of a chromene-based Schiff base ligand for DNA interaction studies. The Zn(II) cluster of the chiral P21 space group (C80H76N4O14Zn4) and Ni(II) clusters have similar structures with a defective di-cubane core (one metal ion through three donor atoms). The Fe complex forms a tetranuclear star-shaped [Fe4L6] cluster, which possesses a 2-fold rotation axis from the [FeO6] units, whose M-O coordination bond distances were mentioned, forming the corners of an equilateral triangle. Although docking to DNA was investigated, the intermolecular interactions of the metal complexes in the crystals were not analyzed in detail.
Lin et al. (2023) [55] reported chiral self-assembly of Schiff base Zn(II) (C46H42N10O10Zn2), Cd(II) (C22H22CdCl2N6O2, C38H30Cd2I4N10O2, and C40H42CdN12O12), or Hg(II) (C44H44Cl4Hg2N12O4) complexes obtained from chiral Schiff base ligands (C19H17N5O2). The space groups and arrangements of the dimeric forms in the crystals were used to confirm the chirality of the compounds. They were expected to have potential applications in the removal of pollutant iodine. Coordination numbers, geometrical distortion represented by parameters, and coordination bond lengths (normal ranges) were mentioned in the text. The pore sizes with intermolecular hydrogen bonds were mainly stated using interatomic distances; however, there were no details about covalent bonds in ligands (besides coordination fashions) (Figure 12).
Figure 12.
Simple crystal packing of chiral self-assembly of Schiff base compounds (not as continuous porous structures) [55].
Sepehrfar et al. (2023) [56] reported Schiff base Cu(II), Mn(II), and Mn(III) complexes with non-covalent interactions for a simulation investigation of SARS-CoV-2. The 6-coordinated Cu(II) complex (C48H60Cu4N4O12·4(C2H6OS)) (in a tetragonal crystal system) formed an S4-symmetrical tetrameric cage with square-planar coordinated Cu and bridging O atoms at the vertexes of the approximate cube. Fashion was important, while the detailed values were not important. This results in large channels along the c-axis between the tetramers, which are formed by intermolecular hydrogen bonds. In addition, the Mn mixed-valence complex (monoclinic, P21/c) consisted of two Mn(III) and one Mn(II) ions, two ligands, four acetates, and two DMSO molecules. It should be noted that it is a Ci-symmetrical trimer (the Mn ions lie across the inversion center).
Severin et al. (2023) [57] reported tetra- and hexanuclear Zn(II) complexes (C74H90N7O20.5Zn6 and C108H123.5Cl2N8.5O17Zn4) prepared from tridentate Schiff base ligands (C26H28N2O). The crystal structures of both the metal complexes and the organic ligand were reported. The overall characteristics (such as the C2 symmetry), the μ1,3-bridging features of the O atoms to form multi-nuclear complexes, and the coordination geometries (Zn-L and L-Zn-L) were reported in lists for the metal complexes. In addition to the geometrical parameters or coordination polyhedra, the SHAPE symmetry factors were also used to describe the coordination environment based on X-ray crystallography. Moreover, the C-N and C=N bond lengths, imine groups of the E-configuration, stacking interactions, interplanar distance, and hydrogen bonds were analyzed for the ligand.
Karakousi et al. (2023) [58] reported 2D and 3D Zn(II) complexes as coordination polymers with Schiff base amino acid-based ligands (C15H13NO3Zn and C13.33H10.33NO3.33Zn). These space groups were determined to be achiral in nature. Besides the distorted (τ = 0.46) square pyramidal ZnO4N environment, intramolecular information was limited to the coordination fashion, such as “the ligand adopts a syn, anti-1:1:coordination mode”. Coordination polymers were represented as figures for the most part. Furthermore, structural information for nano-sized pores (Zn···Zn distances, etc.) and intermolecular C–H···π interactions were assessed, which was also supported by numerical data (the solvent-accessible volume and corresponding ratios concerning the unit cell volume).
4. Supramolecular Interactions
In this section, we briefly explain the expression of intermolecular interactions (increase or decrease in interatomic distance due to repulsive or attractive forces) in Hirshfeld surface analysis. The Hirshfeld surface encompasses the region of the space surrounding a particular molecule in a crystal, where the electron distribution of the molecule exceeds that of other molecules. In practice, a typical Hirshfeld surface is represented by tens of thousands of surface points obtained by triangulation, and two parameters (di and de) convey information about the contact distance from each point. Here, di is defined as the distance from the surface to the atom closest to the surface, and de is defined as the distance from the surface to the atom outside the surface. Plotting di and de at each point on the molecular surface results in a two-dimensional fingerprint diagram.
Hoque et al. (2023) [59] reported an amido Schiff base compound (C39H35N5O6) for a selective fluorescent chemosensor for Al(III) ions. The space group and overall shape (described by the dihedral angles of the planar moieties) were mentioned. For the amide bond, more importance was placed on the C=O bond distance than on the adjacent imine C=N bond geometry. Intermolecular hydrogen bonding and π-interactions were numerically investigated. For example, hydrogen bond O1⋯N1 and O4⋯N4 distances are 2.554(2) and 2.568(2) Å, respectively, and most of them are within a normal range, which may be established by previous statistical studies of databases as mentioned in the introduction (Figure 13).
Figure 13.
Crystal packing of the compound showing predominant hydrogen bonds as light blue lines [59].
Jiang et al. (2023) [60] reported 2-nitrobenzaldehyde amino acid Schiff base reductive amination compounds (Figure 14) with characteristic nitro-groups (C9H10N2O4). Note that there are several typical sites where hydrogen bonds can occur. The space group and molecular structure (mainly the geometries of the hetero atoms, including nitro-groups) were mentioned in the text and a table. In particular, the dihedral angles of the planar moieties, sp2 and sp3 hybrid orbitals of the C and N atoms, and π-electron delocalized systems involving ring or double bonds were observed based on the crystallographic results. In addition, intermolecular hydrogen bonds and crystal-packing figures were highlighted in the text.
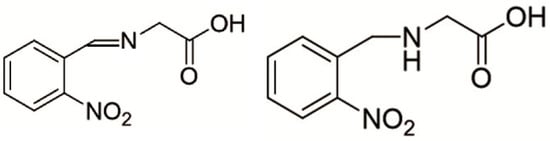
Figure 14.
(Left) Schiff base with CH=N imine, (Right) “reduced” Schiff base with a CH2–NH moiety; both are potentially ready for hydrogen bonds.
Break et al. (2023) [61] reported macroacyclic dithiocarbazate Schiff bases (C20H22N4S4) and their corresponding Cu(II) complexes. Concerning organic ligands, bond lengths, bond angles, and torsion angles involving S atoms were mentioned, as was a comparison of the C–N and C=N (imine) bond distances. The dihedral angles of the planar moieties were assessed in the whole-molecule figures and text. The N–H···S hydrogen bonds linked the molecules to form R22(8) ring motifs (Figure 15). It should be noted that there are various topological methods for representing crystal structures and their elements. As regards the Cu(II) complex, coordination bond lengths and angles and the resulting metal and ligand rings were mentioned, with a focus on S atoms.
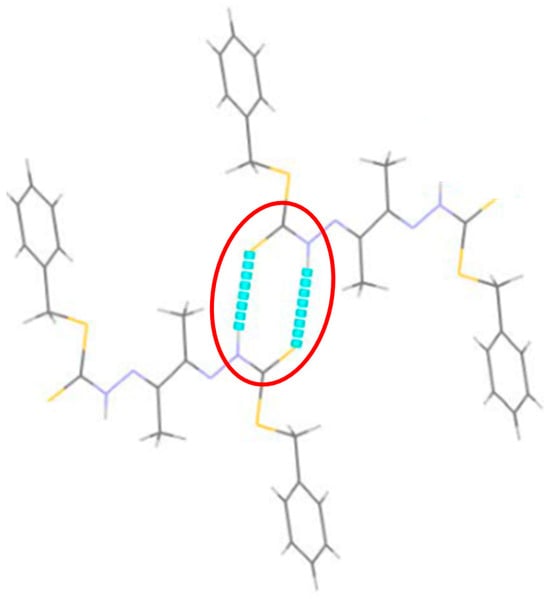
Figure 15.
The red circle highlights the R22(8) ring motifs formed by two N–H···S hydrogen bonds.
Aazam et al. (2023) [62] reported a Schiff base derived from amino pyrene (C25H19NO2). “Tautomerism” in the solid state was confirmed to be of a phenol-imine form (Figure 16). The crystal structure was reported to be the phenol-imine (N···H–O hydrogen bond) form. The C=N and C–N bond distances (in the form of common C=N values found for other Schiff bases), dihedral angles of the planar moieties (pyrene was a large example), and intramolecular and intermolecular hydrogen bonds were mentioned.
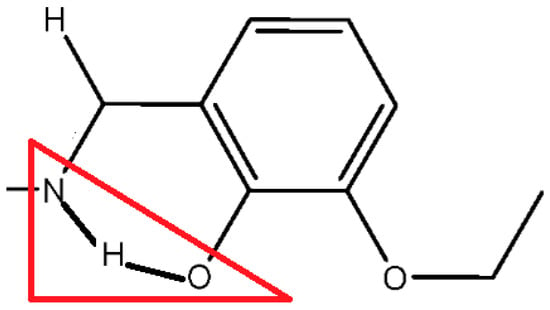
Figure 16.
The red triangle highlights the potential tautomerism between the keto-amine form (N–H···O hydrogen bond) and phenol-imine (N···H–O hydrogen bond) form [62]. The pyrene moiety was omitted for clarity.
Bennour et al. (2023) [63] reported a triazine ring Schiff base compound (C18H17N3O2) that could potentially act as a mixed-ligand metal complex. The planar part of the molecule has a curved shape, but it is only depicted in the diagram, and the geometry has not been reported numerically. Covalent bond distances and angles were listed in tables, while H···H, O···H, H···C, C···C, C···N, C···O, and N···H hydrogen interactions were mentioned in the text (most are within common ranges) and a Hirshfeld surface analysis. In particular, the short distance between the donor and acceptor atoms in the O–H⋯N intramolecular hydrogen bond was highlighted.
Ngoudjou et al. (2023) [64] reported a Schiff base (n 2-(4-dimethylamino) benzylidene) hydrazine-1-carbothioamide) compound (C10H14N4S) for antioxidant studies. The overall centro-symmetric (due to the space group) V-shaped structure of its dimers, long C–S bond distance (thiol tautomeric form), comparable C–N bond distance (trans-configuration), and short C(phenyl)–C(keto) bond distance were mentioned. The results of the Hirshfeld surface analysis also revealed the presence of π···π stacking and N–H···π interactions (Figure 17). Hirshfeld surface analysis, which is presently important for describing intermolecular interactions, was explained at the beginning of this section briefly.
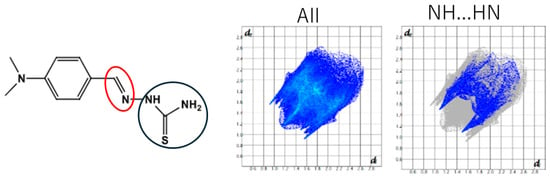
Figure 17.
(Left) Molecular structure of C10H14N4S with imine C=N bond (red circle) and potentially tautomeric moiety (blue circle); (Right) two-dimensional fingerprint plots (all interactions and N–H···H–N interactions) of the compounds [25].
Majumdar et al. (2023) [65] reported an adamantan-1-amine-based Schiff base compound with antibacterial and fungal activities. In the crystal structure description section, the space group P21/c (C21H23NO) was stated because another non-Schiff base compound was crystallized in the chiral space group P212121 (C10H18N2O3). A naphthalene ring and imine (C=N) groups that are almost in the same plane but with a slight deviation were pointed out. It should also be noted that this Schiff base compound adopts a keto-imine-type tautomeric form associated with hydrogen bonds, which was observed as a solid-state structure using X-ray crystallography.
Esfahani et al. (2023) [66] reported polycyclic aromatic pyrazolone-based Schiff bases for docking against SARS-COVID-19 (C18H18N4O and C19H20N4O). The space group and covalent bond lengths and angles (which were common to the two compounds and were compared with each other) were listed in the tables and described in the text. For example, C=O was within the normal range for known C-O bond lengths. Intramolecular hydrogen bonds involving the C=O group were mentioned; however, intermolecular hydrogen bonds were not mentioned in the text.
The potential ligands of metal ions through coordination bonds, as well as traditional intermolecular hydrogen bonds are important for non-covalent supramolecular chemistry and its structural chemistry. Crystals of metal complexes with intermolecular hydrogen bonds formed by ligands can essentially form supramolecular complexes. Such examples are often repeats of what has gone before; therefore, we will only present the most interesting examples.
Aprajita et al. (2022) [67] reported a Schiff base compound (C12H11N3OS) potentially acting as a ligand of Cu(II) and Ni(II) complexes in a docking study on SARS-CoV-2 and HIV virus. Only the crystal structure of the organic ligand was determined. The overall molecular structure and crystal packing shown in the original paper were the E-configuration of the double bond, and the transposition of the C=O atom and planarity around the C-N bond were mentioned in the text. Intermolecular hydrogen bonds (of the oxygen acceptors) were also reported.
Singh et al. (2023) [68] reported various Schiff base compounds (C20H15NO, 2(C22H19NO2), and C21H17NO2) designed for the selective detection of Zr(IV) ions and biological activity (protein inhibitor). By comparing these compounds, the Schiff base C=N bond lengths and the adjacent terminal alkyne moiety (C–C–H) were determined in terms of the molecular structure. The rotation of the C-N bond, which connects to the naphthalene moiety, was only indicated in the figures. In addition, intermolecular interactions (N···H–C and O···H–C) were mentioned, with crystal-packing figures showing sheet structures along the crystallographic axis.
Neela et al. (2023) [69] reported a Schiff base organic compound, 1-((E)-(4-chlorophenylimino)methyl) naphthalen-2-ol (C17H12ClNO). They investigated the non-linear optical properties of this compound using DFT-based computation. Its non-chiral space group was P21/c. This imine C=N bond moiety adopts an E-configuration. The bond lengths and dihedral angles of the molecular structure were listed as supplemental information. The results of the Hirshfeld surface analysis and the D···A distances and D–H···A angles of the intermolecular hydrogen bonds were discussed in detail.
Zhang et al. (2024) [70] reported that an organic Schiff base compound (2(C21H17NO3)) exhibits emissions for VOC sensors. The bond lengths and bond angles of covalent bonds were not mentioned in the main text but in the supplementary information. The torsion angles of the three aromatic units were described not only in terms of molecular structures but also in terms of the features of a molecular dimer with different conformations. With the aid of packing figures with the interlayer distances of the molecules in the crystal, intermolecular interactions, including C–H···π, C=O···H–C, and C–H···C=O, were mentioned in the text.
Ati et al. (2023) [71] reported new Schiff base compounds (C21H23N5O and C20H20N4O2⋅0.5(H2O)) designed to detect biological activity. The crystallographic study results mainly showed the crystal packing of 1D and 2D supramolecular architectures via various hydrogen bonding interactions. Intermolecular hydrogen bonds (N–H···N and C–H···O) involving phenyl groups (C–H···Cg (center of the ring)) were mentioned and were also represented using Hirshfeld surface analyses. Figures of the molecular structures were also depicted, with characteristic C–C–C–C torsion angles. It should be noted that the details of the C–H and N–H bond refinement were described.
Singh et al. (2023) [72] reported a planar Schiff base organic compound (C14H10N4O) as a potential probe to detect Co(III) ions. Due to the terminal alkyne group, carbon–carbon bond distances and the related bond angles were the main focus. The Schiff base’s imine group belonged to the conjugated atoms, although the structural characteristics were not mentioned in detail. Intermolecular hydrogen bonds (via amino groups) were also mentioned and described in the crystal-packing schemes.
Misigo et al. (2023) [73] reported thio-semicarbazones, a typical N, S-donor Schiff base ligand (C14H15N3S), and their Pd(II) complexes without crystal structures for anticancer studies. They mentioned that the compound possesses an E-configuration and described the C=N and C=S double bond distances, S-C-N-N and N-N-C-C torsion angles, intra/intermolecular hydrogen bonds (the D–H···A angle was almost 180 degrees), and contact sites via van der Waals interactions. The molecular structure and crystal packing were mentioned in the original paper.
Shaheen et al. (2023) [74] reported a nitro-terminated azo-Schiff base compound (showing photo-isomerization) that was used to investigate DNA binding, antioxidant activity, and enzyme inhibition. This organic azo compound (C25H18N4O4) was reported to have N=N bond and azomethine C=N double bond distances. Besides the C–H···π interactions, it exhibited only one intermolecular C–H···N hydrogen bonding interaction, with donor and acceptor distances being described as forming the elegant 3D network of the structure, namely, the S6 ring of the graph representation, in the crystal.
Shruthi et al. (2023) [75] reported the use of an organic Schiff base compound (C14H11BrClNO) containing Br- and Cl- groups as a fluorescent and colorimetric sensor to detect Zn(II) ions. Between the imine and phenolate groups, an intramolecular hydrogen bond was formed. The authors stated that this compound crystallizes in the triclinic system with the space group P-1.
Behera et al. (2023) [76] reported a halochromic hydrazine-bridged Schiff base compound that could be regarded as a B(III) complex (C42H32B2F4N8), although the C=N sites were not coordination sites. It was investigated as a light-emitting fluorophore. The overall structure was in reasonable agreement with the space group and molecular symmetry of the C2v point group. The B-N bond distances and F-B-N bond angles were mentioned because B and F were important for this compound’s functions. The imine C=N bond distances and angles between the planar moieties were also mentioned in the text.
In this category, Hirshfeld surface analysis may now appear to be an important presence. Khaidir et al. (2023) [77] reported the synthesis, structure, and Hirshfeld surface analysis of dinuclear Schiff base complexes with cyclic Schiff base ligands, including ONNO donor atoms (C50H48N4O8Zn2). The distorted tetrahedral geometry of the Zn(II) complex was emphasized among the centro-symmetric complexes. The Schiff base ligands’ C=N bond lengths and C-N-C-C torsion angles were mentioned. Hirshfeld surface analysis revealed that the H⋅⋅⋅H interaction was predominant, and conventional C–H···O hydrogen bonds, as well as weak π···π stacking interactions, were also highlighted.
Sarkar et al. (2023) [78] reported binuclear mono-oxo Mo(VI) complexes with a distorted octahedral environment (C31H27Cl3N4O7S4Mo2, C60H48N8O12S8Mo4, and C30H22Br2N4O6S4Mo2), exhibiting a metal–organic supramolecular architecture (expanded via symmetry operations). The coordination bond lengths and bond angles were mentioned in the text. There are two diprotic tridentate ONS donor Schiff base ligands. The authors reported not only C–H···O, O–H···O, O–H···N, C–X···π (X=Br), but also weak π···π stacking interactions in the crystal packing of these complexes with lists of geometrical parameters. Hirshfeld surface analysis was also performed to represent all types of intermolecular interactions.
5. Functional Schiff Base Complexes for Material and Biological Systems
The final section describes examples of research oriented toward functions and applications. A defining feature of such research is that it is not just about synthesizing a single molecule but is intended to combine or react with other molecules. As a result, the identification and structure of the target molecule are not necessarily clarified through structural analysis using single crystals; therefore, it enters a field in which other techniques are used (beyond the scope of this review). The increase in such situations has also been a noteworthy research trend in recent years.
Kong et al. (2023) [79] investigated Ru (II) carbonyl complexes involving Schiff base ligands in terms of their catalytic activities for benzylic C–H oxidation (C39H30Cl2N2O6Ru, C38H22F6N2O4Ru, and C36H22Cl2N2O4Ru). The space groups, Ru–N or Ru–O coordination bond lengths, and C-Ru-C bond angles were mentioned in the text. The most important information may be that the dissociated octahedral Ru metal center is surrounded by two N,O-bidentate ligands. Based on their steric molecular structures, the open space for binding substrates was also highlighted from the perspective of catalytic applications.
Muddassir et al. (2023) [80] reported a multifunctional Zn(II) coordination polymer that could potentially be applied as a selective fluorescent sensor (C28H20N2O8Zn). Basically, the space group and asymmetric unit contents (infinitely linking metal–organic framework structures in a crystal) were mentioned, and the coordination geometries (Zn-L and L-Zn-L) were compared with those of related discrete Zn(II) complexes. With regard to crystal packing, intermolecular (and intramolecular for metal–organic frameworks) hydrogen bonds within the framework structures were described as the overall stacking features linked via hydrogen bonds. In addition, Hirshfeld surface analysis was performed.
Ganczar et al. (2023) [81] analyzed the crystal structures of a Cu(I) coordination compound with a triazolamine Schiff base, including different guest molecules (C36H28N16Br4Cu22CF3SO3- 2.41CH3CN, C36H28N16Br4Cu22CF3SO3- 1.07CH3CN, and C36H28N16Br4Cu22CF3SO3- 0.8C6H140.5CH3CN). The space groups and special comments (disorder or occupancies) were assessed using overall structural features ( chair conformation of the ring moiety) without numerical data in the text. Forming host lattices in the crystal, intermolecular interactions, such as halogen (F) or normal (O) hydrogen bonds, halogen–halogen contact, and the resulting structural changes (overlay figures), were used to explain the host–guest inclusion in the metal–organic framework (Figure 18).
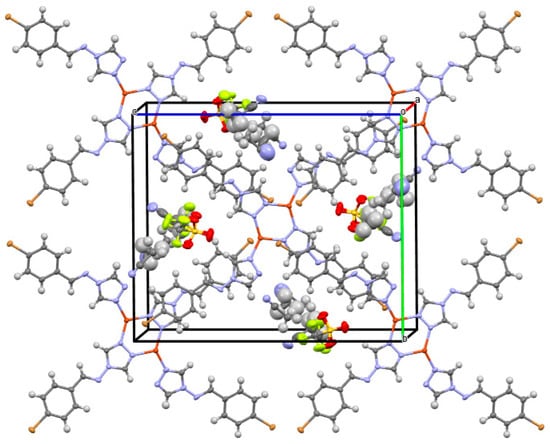
Figure 18.
Crystal packing of host–guest inclusion in the metal–organic framework [81].
Wang et al. (2023) [82] reported octanuclear Gd(III) (C112H128Cl4Gd8N12O42), Dy(III) (C112H128Cl4Dy8N12O42), and Ho(III) (C112H128Cl4Ho8N12O42) clusters assembled by a polydentate Schiff base ligand, which was depicted in a figure, for magnetic materials and catalytic compounds. The coordination modes of the O atoms (expressed in a figure that omitted organic ligands) were the focus of this paper. In addition, the bond lengths (Ln-O or Ln-N bond lengths) and the point group symmetry of the coordination environment were assessed.
Iorungwa et al. (2023) [83] reported Fe(II) and Cu(II) complexes with Schiff base ligands derived from N-N1-diphenyl-O-pyrol-6 methyleneacetate. In this study, powder XRD was employed for structural characterization (without steric structure determination), and only the crystal systems, lattice constants, the unit cell volume, crystallite size, and d-spacing were listed in the tables. From the viewpoint of material science, crystal grain size is more important than 3D molecular structures.
Kummar et al. (2023) [84] reported an in silico study of a typical Schiff base ligand (Salen-type) of Ni(II) complexes as drug candidates. The crystal structures of the proteins obtained from PDB were used for the docking computation, and the steric structures of Ni(II) complexes were obtained based on the optimized DFT structure calculations. After the docking simulation, the conformation around the peptide bonds of the peptide chains was described using Ramachandran plots.
6. Conclusions
In 2019, the number of entries in the Cambridge Crystal Structure Database exceeded one million. Moreover, the application of artificial intelligence to big data is attracting attention. Artificial intelligence methodologies [85,86], in particular, deep learning, Schiff base compounds, and metal complex crystal structure data, can be used to predict functions such as magnetism [87] and reactivity [88]. However, since it is difficult to select descriptors suitable for machine learning, research on extracting “feature values” of chemical properties has become an important issue for chemists. For example, if we take statistics on the molecular skeletal structure of a Salen-type complex, we see a correlation between the distance between the metal nucleus and nitrogen and the torsion angle. However, this correlation may be primarily due to changes in the bite angle of the chelating ligand because of the radius of the metal ion. Thus, finding structural information that represents candidates for feature quantities and discussing the trends observed in Schiff base ligands and the metal complexes containing them are of great significance. In addition, the question of whether there is any difference between discussing experimental results empirically (based on chemical knowledge) and assessing results selected via machine learning is of great research value.
From the above investigation, the viewpoints in the report on the crystal structures of Schiff bases and their metal complexes can be summarized as follows:
@ Organic Schiff base (Section 2)
Crystallographic journals’ format and basic information
-Crystal systems
-Space groups (rare, chiral, chiral crystallization from achiral components).
Molecular symmetry
-Molecular point groups (Ci, C2,, etc.)
-Centro-symmetry (2-fold rotation axis, etc.)
Structure and geometry of covalent bonds
-Imine C=N double bond length and E- (trans-) configuration
-C=C double bond length
-C=O bond length
-N=N (azo) double bonds and E-configuration
-C-N single bond length
-C-(S, Cl, Br, Bi) bond lengths
-Tautomerism (keto-enol) about NHCO, NHCS)
-Delocalization in π-conjugated system
-Hybridization (sp2 or sp3) of C atom
Substitution groups and their rotation or conformation
-Ring moieties (and graph set or ring motifs)
-Deviation from mean plane of ring
-Dihedral angles between planar moieties
-Conformation of long alkyl chain
-Terminal substituted group (alkyne)
Potentially as organic ligands
-Chelating site numbers and coordination atoms of ligands
-Chirality (R/S) due to organic ligands
@ Coordination complexes (Section 3)
Coordination environment
-Central atoms (transition or main group metals, lanthanides and U)
-Coordination numbers
-Coordination geometries (polyhedral, etc.)
M-L bond length
M=O bond (such as V=O and U=O)
L-M-L (cis and trans) bond angles
Deviation from mean the plane
-Distortion from regular polyhedra
(Trigonality index, BVS calculations, SHAPE factor, etc.)
-Cis/trans-isomers
Whole molecular structure (shape) of complex
-Multi-nuclear complex
-Bridging mode ligands
-Mixed ligand, unsymmetric ligand
-Shapes of the complex (curved, V-shape, etc.).
@ Supramolecular interactions (Section 4, partly 5)
Non-covalent interactions
-Intramolecular hydrogen bond
-Intermolecular hydrogen bond (linear or bent)
-Halogen hydrogen acceptor
-Sulfur hydrogen acceptor
-C–H···π interaction
-π···π interactions
-Hirshfeld surface analysis and 2D fingerprint plots
Crystal packing
-Metal–organic frameworks
-Stacking of planar molecules
-1D and 2D supramolecular architecture
-Alignment to the crystallographic axes
Beyond sole component
-Powder XRD parameters (materials chemistry)
-Ramachandran plots (protein crystallography)
In any case, the chemical properties of metal complexes depend on the geometry of the organic ligands and their coordination with the metal center. Machine learning-based approaches, such as the MetalHawk program [89], have been reported. It has been reported that this output can be considered a surrogate indicator of metal site distortion and interpreted as a low-dimensional representation of the subtle geometric features present in the metal site structure. Thus, discussing the correlation between structures, important functions, and physical properties using “sophisticated” indicators and expressions from a structural database is consistent with previous experimental crystal structures and electronic states.
We hope that reasonable indicators will continue to be developed in the future. Because we live in an era in which sufficient statistics such as simple bond distances and bond angles have been accumulated, descriptors may begin to take on a life of their own. We have listed various focuses of structural and chemical significance as a warning. The significance and merits of this review are summarized as follows:
(1) The paper successfully incorporates the recent literature, providing a thorough overview of the current state of research on Schiff bases and their metal complexes;
(2) It effectively highlights important structural features, such as C=N double bonds, intermolecular hydrogen bonds, and the geometric structures of these compounds;
(3) A forward-thinking and relevant discussion on the application of big data and machine learning in the analysis of crystal structures is presented.
The use of deep learning in the study of crystal structures produced no correlation with the physical properties (instability with explosive properties) of the Schiff base cation complex. Thus, we concluded that they originated from a counter anion [83]. Because big data are complex to discuss, we hope that this review emphasizes the structural viewpoint, which is a necessary discussion in terms of chemical significance.
It is expected that as the amount of data increases, the statistics of bond distances and angles obtained because of structural analysis will approach a normal distribution, although examples of deviations from the average value will appear. Therefore, the number of “normal” structures within the range of experience will increase, and the traditional discussion of quantitative numerical data will become less popular. In such a situation, what each author finds relevant, what they judge to be important, and what to report or discuss depend on the qualitative content of the discussion (not necessarily valuable as a statistical value but not reusable). It will not be easy.
However, statistics on space group distributions, of which there are only 230, have been reported for many years, changing the target materials. Two of the authors (T.A. and D.N.) also recently published a mini-review article on this topic [90]. In fact, it is appropriate for the era of data science, and there was a response from statistical mathematicians. In this way, chemists who spend all their time acquiring and reporting experimental facts may not be interested in it, but they are sensitive to the new era of data science and think about what to discuss from the rich information of crystal structure data. We have written this review with the hope that it will serve as a useful guide for researchers.
Looking ahead, it will become increasingly important to expand from single molecules to complex systems and to extract and discuss meaningful structural features that take into account the correlation between structure and function. As the amount of crystal structure data accumulated over the years increases, there tend to be fewer examples of characteristic bond lengths and angles that deviate from statistical values. Organic compounds and metal complexes differ in that the latter discuss the distances and angles of coordinate bonds, but there were a few notable examples of distances and angles of covalent bonds or intermolecular interactions that were pointed out in the text of each paper.
Author Contributions
Conceptualization, T.A.; writing—original draft preparation, T.A.; writing—review and editing, D.N. and B.M.; data (literature) curation, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Sonia Naz for her help in remaining motivated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gatehouse, B.M.; Reichert, B.E.; West, B.O. The crystal and molecular structure of μ-N,N′-ethylene-bis(salicylaldiminato)bis-[2-methylallylpalladium(II)]. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 2451–2454. [Google Scholar] [CrossRef]
- Korhonen, K.; Hämäläinen, R.; Turpeinen, U. Structure of catena-tetraaqua-di-μ3-(N-salicylidene-DL-glutamato)-tricopper(II) heptahydrate [Cu3(C12H10NO5)2(H2O)4].7H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, 40, 1175–1177. [Google Scholar] [CrossRef]
- Gallagher, J.F.; Alyea, E.C.; Ferguson, G.; Xu, Z. Structure of the Copper Tripodal Schiff Base Complex {Tris[4-(2-thienyl)-3-azatcN-3-butenyl]amine-tcN}copper(I) Tetrafluoroborate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 16–18. [Google Scholar] [CrossRef]
- Zhu, B.; Ruang, W.; Zhu, Z. A nickel(II) complex of an unsymmetrical tetradentate Schiff base. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m634–m636. [Google Scholar] [CrossRef]
- Ying, S.-M.; Huang, X.-H.; Luo, W.-K.; Xiao, Y.-C. Synthesis, crystal structures and characterizations of two homochiral coordination polymers based on a chiral reduced Schiff base ligand. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 375–378. [Google Scholar] [CrossRef]
- Kolade, S.O.; Aina, O.S.; Gordon, A.T.; Hosten, E.C.; Olasupo, I.A.; Ogunlaja, A.S.; Asekuna, O.T.; Familonia, O.B. Synthesis, crystal structure and in-silico evaluation of arylsulfonamide Schiff bases for potential activity against colon cancer. Acta Crystallogr. Sect. C Struct. Chem. 2024, 80, 129–142. [Google Scholar] [CrossRef]
- Akitsu, T. (Ed.) Schiff Base in Organic, Inorganic and Physical Chemistry; InTech: Rijeka, Croatia, 2023. [Google Scholar]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Salan vs. salen metal complexes in catalysis and medicinal applications: Virtues and pitfalls. Coord. Chem. Rev. 2019, 388, 227–247. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic assemblies based on Mn(III) salen analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Chen, Z.-Q.; Bian, Z.-Q.; Huang, C.H. Sensitized luminescence from lanthanides in d–f bimetallic complexes. Coord. Chem. Rev. 2010, 254, 991–1010. [Google Scholar] [CrossRef]
- Allen, F.H.; Taylor, R. Research applications of the Cambridge Structural Database (CSD). Chem. Soc. Rev. 2004, 33, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Taylor, R. Systematic Analysis of Structural Data as a Research Tequnique in Organic Chemistry. Acc. Chem. Res. 1983, 16, 146–153. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Hydrogen-Bond Geometry in Organic Crystals, Acc. Chem. Res. 1983, 17, 320–326. [Google Scholar] [CrossRef]
- Murugan, S.; Paul, A.C.; Khamrang, T.; Kavitha, S.J.; Rajakannan, V.; Hemamalini, M. (2,4-Dichlorobenzylidene)[2-(1H-indol-3-yl)ethyl] amine. IUCrData 2023, 8, x230780. [Google Scholar] [CrossRef]
- Khan, S.S.; Howlader, M.B.H.; Sheikh, M.C.; Miyatake, R.; Zangrando, E.; Ansary, M.R.H. Crystal structure of S-n-octyl 3-(1-phenylethyl idene)dithiocarbazate and of its bis-chelated nickel(II) complex. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 1137–1141. [Google Scholar] [CrossRef]
- Shankar, M.G.; Kumaravel, R.; Subashini, A.; Ramamurthi, K.; Kučeráková, M.; Dušek, M.; Stoeckli-Evans, H. Syntheses, crystal structures, Hirshfeld surface analyses and energy frameworks of two 4-amino antipyrine Schiff base compounds: (E)-4-{[4-(di ethylamino)benzylidene]amino}-1,5-dimethyl-2 phenyl-1H-pyrazol-3(2H)-one and (E)-4-[(4-fluoro benzylidene)amino]-1,5-dimethyl-2-phenyl-1H pyrazol-3(2H)-one. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 538–544. [Google Scholar] [CrossRef]
- Halz, J.H.; Hentschi, A.; Wagner, C.; Merzweiler, K. Synthesis and crystal structures of three Schiff bases derived from 3-formylacetylacetone and benzyl-, tert-butyl- and (S)-methylbenzylamine. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 707–713. [Google Scholar] [CrossRef]
- Yahiaoui, A.A.; Ghichi, N.; Hannachi, D.; Mezhoud, B.; Djedouani, A.; Kraim, K.; Crochet, A.; Stoeckli-Evans, H. Thesynthesisandcrystal structureof (E)-2-{[(4-methoxynaphthalen-1-yl)methylidene] amino}-4-methylphenol:Hirshfeldsurfaceanalysis, DFTcalculationsandanticorrosionstudie. Acta Crystallographica Sect. C Struct. Chem. 2023, 79, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T. Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry. Polymers 2023, 15, 3542. [Google Scholar] [CrossRef] [PubMed]
- Mustufa, M.; Abbasi, A.; Hanif, S.; Bhat, Z.U.; Kabeer, H.; Alam, M.J.; Ahmad, M.; Shakir, M. A combined theoretical and experimental study of ratiometric fluorescent Schiff base chemosensor for detection of Fe3þ ion and its anticancer activity. Chem. Inorg. Mater. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Aslam, M.; Anis, I.; Noreen, Z.; Chaudhary, A.; Hussain, A.; Lateel, M.; Mansha, M.; Ali, A. Synthesis, single crystal X-ray and biological study of transition metal complexes of N, N-donor schiff base ligand: N1, N2-BIS[(4-methoxyphenyl) methylidene]-1,2-ethanediamine. J. Nat. Appl. Sci. Pak. 2023, 5, 1222–1231. [Google Scholar]
- Al-Zeudaneen, F.K.; Anson, C.E.; Powell, A.K. A nickel(II) complex with an unsymmetrical tetra dentate chelating ligand derived from pyridine-2,6-dicarbaldehyde and 2-aminothiophenol. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 791–794. [Google Scholar] [CrossRef]
- Gholivand, K.; Faraghi, M.; Malekshah, R.E.; Jafari, M.; Akbarzadeh, A.R.; Latifi, R.; Fadaei-Tirani, F.; Fallah, N.; Farshadfar, K. NewCu(I) complexes as catalyst for “click” reaction: An experimental and computational study. Appl. Organomet. Chem. 2023, 37, e6924. [Google Scholar] [CrossRef]
- Haldar, H.; Yildiz, C.B.; Majumdar, M. Coordination Chemistry of the Antimony(III) and Bismuth(III) Cations using Bis(α-iminopyridine) as Ligand. ChemPlusChem 2023, 88, e202300211. [Google Scholar] [CrossRef]
- Böhme, U.; Fels, S. {N-[(4-Methoxy-2-oxidophenyl)(phenyl)methyl idene]glycinato}diphenylsilicon(IV). IUCrData 2023, 8, x230306. [Google Scholar] [CrossRef]
- Faye, M.; Gueye, M.N.; Dieng, M.; Tamboura, F.B.; Gaye, M. Crystal structure of Y(III) Complex with the Tridentate Schiff Base Ligand N′–(1–(pyridin 2–yl)ethylidene)nicotinohydrazide. Int. J. Nov. Res. Phys. Chem. Math. 2023, 10, 60–66. [Google Scholar] [CrossRef]
- Li, L.; Yuan, F. Homodinuclear and heterotetranuclear lanthanide complexes built by 2-bis (salicylidieneamino)methylphenol via alkaline regulation. J. Struct. Chem. 2023, 64, 474–485. [Google Scholar] [CrossRef]
- Barbosa, E.M.; Soares, K.S.; Cruz, J.F.; Doring, T.H.; de França, I.V.; Mello, L.D.S.; Nagurniak, G.R.; Parreira, R.L.; Alvarenga, M.E.; Martins, F.T.; et al. Uranyl N2O2-Schiff base complex as co-catalyst in ethanol electro-oxidation: Synthesis, crystallographic, spectroscopic, electrochemical, and DFT characterization, and catalytic investigation. New J. Chem. 2023, 47, 17393–17405. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, M. New nickel(II) Schiff base complexes as potential tools against SARS-CoV-2 Omicron target proteins: An in silico approach. New J. Chem. 2023, 47, 2350–2371. [Google Scholar] [CrossRef]
- Mamindla, A.; Varahdan, M.; Velusamy, M.; Ulaganathan, V.; Rajendiran, V. Crystal structure of [2-({[2-(dimethylamino-jN) ethyl]imino-jN}methyl)phenolato-jO](1,10-phen anthroline-j2N,N00 0)copper(II) perchlorate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.; Gueye, M.N.; Seck, G.A.; Thiam, I.E.; Diouf, O.; Gaye, M.; Retailleau, P. Synthesis And Characterization Of A Ni(II) Complex Derived From The Schiff Base (E)-2-((1H-Imidazol-4 Yl)Methylene)Hydrazine-1-Carbothioamide. IOSR J. Appl. Chem. 2023, 16, 10–18. [Google Scholar]
- Majumder, M.; Das, T.; Rajak, K.K. Synthesis of oxidovanadium complexes containing coumarin and naphthalene: Bromoperoxidase activity and DNA/BSA binding. J. Coord. Chem. 2023, 76, 749–770. [Google Scholar] [CrossRef]
- Rekha, R.; Jana, T.S.; Maji, S.K.; Sarkar, S. Catecholase Activity of a Mn(III) Complex: An Approach through H-NMR spectroscopy. ChemistrySelect 2022, 7, e202203205. [Google Scholar] [CrossRef]
- Ghasemi, L.; Behzad, M.; Abedi, A.; Khaleghian, A. The effect of structural variations of heteroleptic Cu(II) complexes of tri-dentate unsymmetrical Schiff-base main ligands with pyridine or bithiazole co-ligands on molecular docking against SARS-CoV-2 and its Omicron variant main proteases. Appl. Organomet. Chem. 2023, 37, e7256. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, M.; Denrah, S.; Bagchi, A.; Biswas, A.; Cordes, D.B.; Slawin, A.M.Z.; Saha, N.C. Catalytic and anticancer activity of two new Ni(II) complexes with a pyrazole based heterocyclic Schiff-base ligand: Synthesis, spectroscopy and X-ray crystallography. J. Mol. Struct. 2023, 1287, 135648. [Google Scholar] [CrossRef]
- Ghasemi, L.; Esfahani, M.H.; Sahebi, U.; Dicsalar, A.; Abbasi, A.; Behzad, M. Experimental and molecular docking investigation of anticancer activity of new mixed-ligand Schiff base complexes against human colorectal (HCT116), lung (A549) and breast (MCF7) carcinoma cell lines. J. Mol. Struct. 2023, 1294, 136568. [Google Scholar] [CrossRef]
- Alwi, N.S.M.; Hamali, M.A.; Rosnizam, A.N.; Low, A.L.M.; Hassane, A.E.; Nassar, A.A.; Tajuddin, A.M. Crystallographic, antibacterial, and in silico studies of Ni(II) and Cu(II) Schiff base complexes derived from 2-acetylpyridine. Inorg. Chim. Acta 2023, 558, 121742. [Google Scholar] [CrossRef]
- Hollandsworth, C.B.; Griffin, B.M.; Pruden, J.R.; Gerasimchuk, N.N.; Nevonen, D.E.; Nickel, R.R.; van Lierop, J.; Nemykin, V.N. Synthesis and characterization of iron(II) Schiff-base complexes of tridentate mixed amine/imine ligands with cis- and trans-1,2-diaminocyclohexane backbones. Polyhedro 2023, 246, 116669. [Google Scholar] [CrossRef]
- Akiyama, Y.; Suzuki, S.; Suda, S.; Takiguchi, Y.; Nakane, D.; Akitsu, T. Crystal structure and Hirshfeld surface analysis of mono/bis(aqua-jO)[N-(2-oxidobenzylidene) valinato-j3O,N,O00 0]copper(II): Dimeric Schiff base copper(II) complexes having different numbers of coordinated water molecules. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Akiyama, Y.; Nakane, D.; Akitsu, T. Crystal structure and Hirshfeld surface analysis of (1H-imidazole-jN3)[N-(2-oxidobenzylidene) tyrosinato-j3O,N,O]copper(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, A.; Suzuki, S.; Nakane, D.; Kashiwagi, Y.; Akitsu, T. Crystal structure and Hirshfeld surface analysis of (1H-imidazole-κN3)[4-methyl-2-({[2-oxido-5-(2-phenyldiazen-1-yl)phenyl]methylidene}amino)pentanoate-κ3O,N,O′]copper(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2024, 80, 468–471. [Google Scholar] [CrossRef]
- Boudraa, N.; Assabaa, R.; Ghichi, N.; Hannachi, D.; Djedouani, A.; Kraim, K.; Kashi, I.; Crouchet, A.; Stoeckli-Evans, H. Synthesis, crystal structure, theoretical studies and biological evaluation of nickel(II) complex derived from N2O2 tetradentate Schiff base. J. Mol. Struct. 2023, 1294, 136398. [Google Scholar] [CrossRef]
- Maity, M.; Maity, R.; Sarkar, T.; Bhakat, A.; Brandao, P.; Maity, T.; Das, P.; Sarkar, K.; Samanta, B.C. In Vitro Insight on Antifungal-Specific Potentiality of Ni(II) Complex against Colletotrichum siamense and Fusarium equisetum Phytopathogens. ACS Appl. Bio Mater. 2023, 6, 4836–4845. [Google Scholar] [CrossRef]
- Valentova, J.; Lintnerova, L.; Slavikova, B.; Baran, P. Chiral ligand induced geometrical type of isomerism in Schiff-base Copper (II) complexes with urease inhibitory activities. Inorg. Chim. Acta 2023, 558, 121707. [Google Scholar] [CrossRef]
- Hou, M.; Li, H.C.; An, N.; Li, W.G.; Tong, J. Synthesis, structure and anticancer studies of Cu(II), Ni(II) and Co(II) complexesbased on 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl) benzimidazole Schiff base. Arabian J. Chem. 2023, 16, 105144. [Google Scholar] [CrossRef]
- Paliwal, K.; Haldar, P.; Antharjanam, P.K.S.; Kumar, M. Synthesis, Characterization, DNA/HSA Interaction, and Cytotoxic Activity of a Copper(II) Thiolate Schiff Base Complex and Its Corresponding Water-Soluble Stable Sulfinato–O Complex Containing Imidazole as a Co-ligand. ACS Omega 2023, 8, 21948–21968. [Google Scholar] [CrossRef]
- Ranaweera, S.A.; Donnadieu, B.; Henry, W.P.; White, M.G. Effects of electron-donating ability of binding sites on coordination number: The interactions of acyclic Schiff base with copper ions. Acta Crystallogr. Sect. C Struct. Chem. 2023, 79, 142–148. [Google Scholar] [CrossRef]
- Yilmaz, S.K.; Agar, A.A.; Cinar, E.B.; Dege, N.; Vidya, V.G.; Kummar, V.G.V. Deciphering non-covalent interactions in unprecedented binuclear copper complex: Spectroscopic, Hirshfeld surface and DFT investigation. J. Mol. Struct. 2023, 1299, 137111. [Google Scholar] [CrossRef]
- Aprajita; Choudhary, M. Synthesis and characterization of phenoxy-bridged copper(II) complexes: Structural features, magnetic, thermal and redox properties, and theoretical studies. Inorg. Chim. Acta 2023, 557, 121708. [Google Scholar] [CrossRef]
- Ressler, A.J.; Brandt, O.N.; Weaver, A.; Poor, J.E.; Ream, A.; Summers, N.A.; McMillen, C.D.; Seeram, N.P.; Dougherty, W.G.; Henry, G.E. Chromene-based Schiff base ligand: DNA interaction studies and characterization of tetranuclear zinc, nickel and iron complexes. Inorg. Chim. Acta 2023, 557, 121363. [Google Scholar] [CrossRef]
- Lin, Y.-Q.; Tian, X.-M.; Zhu, B.-X.; Chen, D.-M.; Huang, C. Five Porous Complexes Constructed from a Racemic Ligand: Synthesis, Chiral Self-Assembly, Iodine Adsorption, and Desorption Properties. Inog. Chem. 2023, 62, 12099–12110. [Google Scholar] [CrossRef]
- Sepehrfar, S.; Salehi, M.; Parvarinezhad, S.; Grzeskiewicz, A.M.; Kubicki, M. New Cu(II), Mn(II) and Mn(III) Schiff base complexes cause noncovalent interactions: X-ray crystallography survey, Hirshfeld surface analysis and molecular simulation investigation against SARS-CoV-2. J. Mol. Struct. 2023, 1278, 134857. [Google Scholar] [CrossRef]
- Severin, T.; Karabtsova, V.; Börner, M.; Weiske, H.; Kuc, A.; Kersting, B. Synthesis, Structures and Photophysical Properties of Tetra- and Hexanuclear Zinc Complexes Supported by Tridentate Schiff Base Ligands. Chemistry 2023, 5, 1028–1045. [Google Scholar] [CrossRef]
- Karakousi, R.; Tsami, P.A.; Spanoudaki, M.-A.I.; Dalgarno, S.J.; Papadimitriou, V.C.; Milios, C.J. Blue-Emitting 2D- and 3D-Zinc Coordination Polymers Based on Schiff-Base Amino Acid Ligands. Chemistry 2023, 5, 1770–1780. [Google Scholar] [CrossRef]
- Hoque, A.; Islam, M.S.; Kahn, S.; Datta, B.; Zangrando, E.; Kole, G.K.; Alam, M.A. Crystallographic elucidation of an aluminium bound amido Schiff base chemosensor: A selective turn-on fluorescent chemosensor for Al3+ ions. Dalton Trans. 2023, 52, 10145–10154. [Google Scholar] [CrossRef]
- Jiang, Y.-R.; Su, D.; Wang, M.-Z.; Jia, A.-Q.; Zhang, Q.-F. Syntheses and characterizations of 2-nitrobenzaldehyde amino acid Schiff base reductive amination compounds. Indian J. Chem. 2023, 62, 425–430. [Google Scholar] [CrossRef]
- Break, M.K.B.; Fung, T.Y.; Koh, M.Z.; Ho, W.Y.; Tahir, M.I.M.; Elfar, O.A.; Syed, R.U.; Khojali, W.M.A.; Alluhaibi, T.M.; Huwaimel, B.; et al. Synthesis, Crystal Structure, Antibacterial and In Vitro Anticancer Activity of Novel Macroacyclic Schiff Bases and Their Cu (II) Complexes Derived from S-Methyl and S-Benzyl Dithiocarbazate. Molecules 2023, 28, 5009. [Google Scholar] [CrossRef]
- Aazam, E.S.; Majrashi, M.A. Novel Schiff Base Derived from Amino Pyrene: Synthesis, Characterization, Crystal Structure Determination, and Anticancer Applications of the Ligand and Its Metal Complexes. Molecules 2023, 28, 7352. [Google Scholar] [CrossRef] [PubMed]
- Bennour, H.A.M.; Elmagbari, F.M.; Hammouda, A.N.; El-Ferjani, R.N.; Amer, Y.O.B.; Soliman, S.M.; Jackson, G.E. Synthesis, characterisation and density functional theory (DFT) studies of a triazine ring with a mixed ligand Schiff base complexes. Results Chem. 2023, 5, 100775. [Google Scholar] [CrossRef]
- Ngoudjou, L.E.T.; Yepseu, A.P.; Paboudam, A.G.; Mbani, A.L.; Boulet, P.; Clemand, F.; Ndifon, P.T. Synthesis, Structure, Hirshfeld surface analysis and Anti-oxidant studies on 2-(4-dimethylamino) benzylidene) hydrazine-1-carbothioamide. Mor. J. Chem. 2023, 11, 932–947. [Google Scholar] [CrossRef]
- Majumdar, D.; Philip, J.E.; Tüzün, B.; Sutradhar, D.; Roy, S. Two adamantan-1-amine-based scaffolds: Synthesis, crystallographic synthons, TD/DFT calculations, in-depth molecular docking/ADME/T simulations, and shedding light on antibacterial/fungal activities. Results Chem. 2023, 5, 101228. [Google Scholar] [CrossRef]
- Esfahani, M.H.; Ghasemi, L.; Behzad, M.; Skorepova, E.; Dusek, M. Design, Spectroscopic, and Crystal Structural Characterization of New Pyrazolone-Based Schiff Bases: Molecular Docking Investigations against SARS-COVID-19 Main Proteases (PDB Ids: 6LU7 and 7TLL). Polycycl. Aromat. Compd. 2023, 43, 8933–8945. [Google Scholar] [CrossRef]
- Aprajita, C.M. New Schiff base copper(II) and nickel(II) complexes for biomedical applications with reference to SARS-CoV-2 and HIV virus. Indian J. Chem. 2022, 61, 1241–1256. [Google Scholar] [CrossRef]
- Singh, G.; Malik, P.; Pawan, M.; Devi, A.; Gupta, S.; Tamana, S.A.; Singh, K.N. Azomethine functionalized platform for the selective detection of Zr(IV) ion, biological evaluation and potent TLR-4 inhibitor. J. Mol. Struct. 2023, 1297, 136916. [Google Scholar] [CrossRef]
- Neela, M.; Premalatha, B.; Punitha, P.; Girisun, T.C.S. Synthesis, structural, third-order nonlinear optical properties and Hirshfeld surface analysis of 1-((E)-(4-chlorophenylimino)methyl) naphthalen-2-ol. Optik 2023, 290, 171302. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Xu, L.; Duan, Y.; Yuan, J.; Li, Z.; Han, T. A turn-on type vapofluorochromic methoxybenzylidene methanone Schiff base applied to wearable VOC sensor and smartphone-based detection. Sens. Actuators B Chem. 2024, 399, 134834. [Google Scholar] [CrossRef]
- Ati, G.A.; Chkirate, K.; El-Guourrami, O.; Chakchak, H.; Tüzün, B.; Mague, J.T.; Benzeid, H.; Achour, R.; Essassi, E.M. Schiff base compounds constructed from pyrazole–acetamide: Synthesis, spectroscopic characterization, crystal structure, DFT, molecular docking and antioxidant activity. J. Mol. Struct. 2023, 1295, 136637. [Google Scholar] [CrossRef]
- Singh, G.; Malik, S.; Mohit, P.; Diskit, T.; Kaur, H.; Khurana, S.; Mithun, K.A. Synthesis and structural characterization of new Schiff base probe: A conformation modulator for Co-C bond cleavage in coenzyme B12-dependent enzymes. J. Mol. Struct. 2023, 1293, 136279. [Google Scholar] [CrossRef]
- Misigo, W.O.; Njenga, L.W.; Odhiambo, R.A.; Meyer, M.; Jullius, L.; Sibuyi, N.; Lalancette, R.A.; Onani, M.O. New thiosemicarbazones and their palladium(II) complexes: Synthesis, spectroscopic characterization, X-ray structure and anticancer evaluation. Inorg. Chim. Acta 2023, 558, 121746. [Google Scholar] [CrossRef]
- Shaheen, S.; Liaqat, F.; Qamar, S.; Murtaza, I.; Rasheed, A.; Yousuf, S.; Ishtiaq, A.; Akhter, Z. Single crystal structure of nitro terminated Azo Schiff base: DNA binding, antioxidant, enzyme inhibitory and photo-isomerization investigation. J. Mol. Struct. 2023, 1284, 135376. [Google Scholar] [CrossRef]
- Shruthi, B.; Revannasiddappa, H.D.; Shivamallu, C.; Iqbal, M.; Amachawadi, R.G.; Majani, S.S.; Kollur, S.P. Highly selective fluorescent and colorimetric methylphenyl-based sensor towards Zn2+ ion detection: Synthesis, X-ray crystallography and selectivity studies. Inorg. Chim. Acta 2023, 556, 121614. [Google Scholar] [CrossRef]
- Behera, K.C.; Ravikanth, M. A white light emitting single halochromic hydrazine bridged bis(3-pyrrolyl BODIPY) fluorophore. Phys. Chem. Chem. Phys. 2023, 25, 32584–325893. [Google Scholar] [CrossRef]
- Khaidir, S.S.; Ahmad, S.N.; Kassim, K.; Sirat, S.S.; Ramasamy, K.; Yamin, B.M.; Tan, K.W.; Bahron, H. Synthesis, structural elucidation, Hirshfeld surface analysis and cytotoxicity studies of homometallic dinuclear Cu(II), Ni(II) and Zn(II) Schiff base complexes derived from m-phenylenediamine. J. Mol. Struct. 2023, 1294, 136386. [Google Scholar] [CrossRef]
- Sarkar, O.; Roy, M.; Pramanik, N.R.; Dey, P.; Seth, S.K.; Drew, M.G.B.; Chakrabarti, S. Metal–organic supramolecular architecture of oxo-bridged molybdenum(VI) complexes: Synthesis, structural elucidation and Hirshfeld surface analysis. J. Mol. Struct. 2023, 1301, 137125. [Google Scholar] [CrossRef]
- Kong, S.; Liu, R.; Hao, Z.; Han, Z.; Lu, G.-L.; Lin, J. Ruthenium(II) carbonyl complexes bearing schiff-base ligands: Syntheses, characterization, and their catalytic activities for benzylic C-H oxidation. J. Mol. Struct. 2023, 1296, 136870. [Google Scholar] [CrossRef]
- Muddassir, M.; Alarifi, A.; Abduh, N.A.Y.; Saeed, W.S.; Karami, A.M.; Afzal, M. Multifunctional Zn(II) Coordination Polymer as Highly Selective Fluorescent Sensor and Adsorbent for Dyes. Int. J. Mol. Sci. 2023, 24, 8512. [Google Scholar] [CrossRef]
- Ganczar, E.; Białon’ska, A. Bromine versus chlorine substituent in breathing crystals of a copper(I) coordination compound with a triazolamine Schiff base. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2023, 79, 392–398. [Google Scholar] [CrossRef]
- Wang, W.-M.; Qiao, N.; Xin, X.-Y.; Wu, Z.-L.; Cui, J.-Z. Octanuclear Ln(III)-Based Clusters Assembled by a Polydentate Schiff Base Ligand and a β-Diketone Co-Ligand: Efficient Conversion of CO2 to Cyclic Carbonates and Large Magnetocaloric Effect. Cryst. Growth Des. 2023, 23, 87–95. [Google Scholar] [CrossRef]
- Iorungwa, M.S.; Atagher, J.A.; Shimaibo, P.T.; Nanev, J.D. Synthesis, characterization and biological profiles of Schiff base aldimines derived from N-N1-Diphenyl-O-Pyrol-6 Methyleneacetate and its Cu2+ and Fe2+ complexes. Adv. J. Chem. Res. 2023, 1, 29–38. [Google Scholar]
- Kummar, S.; Choudhary, M. An in silico design of antivirus nickel (II) complexes as therapeutic drug candidates. Indian J. Chem. 2023, 62, 586–599. [Google Scholar] [CrossRef]
- Akitsu, T.; Iwama, J. Salen-type metal complexes based on structural database of X-ray crystallography. In Computational and Data-Driven Chemistry Using Artificial Intelligence; Elsevier: Amsterdam, The Netherlands, 2021; pp. 69–110. [Google Scholar]
- Akitsu, T. (Ed.) Introductory chapter. In Computational and Data-Driven Chemistry Using Artificial Intelligence; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–38. [Google Scholar]
- Takiguchi, Y.; Nakane, D.; Akitsu, T. The prediction of single-molecule magnet properties via deep learning. IUCrJ 2024, 11, 182–189. [Google Scholar] [CrossRef]
- Akitsu, T.; Takiguchi, Y.; Suda, S.; Nakane, D. Deep-learning prediction of safety moiety of salen-type complex crystals towards explosive perchlorate salts. FirePhysChem 2024, 4, 238–244. [Google Scholar] [CrossRef]
- Sgueglia, G.; Vrettas, M.D.; Hino, M.; De Simone, A.; Lombardi, A. MetalHawk: Enhanced Classification of Metal Coordination Geometries by Artificial Neural Networks. J. Chem. Inf. Model. 2024, 64, 2356–2367. [Google Scholar] [CrossRef]
- Akitsu, T.; Higashi, Y.; Tsuchiya, R.; Imae, T.; Komatsu, K.; Nakane, D.; Moon, D. Chemistry for Space Group Symmetry beyond Crystals. Symmetry 2024, 16, 319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).