Abstract
Background: Although some studies showed distinct electrophysiological correlates of emotions in men and women about 300 ms after the stimulus onset, little is known about the automatic visual phases of emotional processing. Investigating both early and late event-related potential (ERP) components (e.g., the P1, P300) could allow us to clarify the effect of gender on the temporal dynamics underlying emotional processing. Methods: Twenty men and twenty women similar in age, education and empathy traits passively viewed emotional and neutral IAPS pictures during EEG recording, providing their subjective evaluations about valence and arousal. ERP and source analyses were implemented to examine gender effects on emotional processing. Results: The P1 analysis revealed gender-related asymmetries, consisting of the greater amplitude of right vs. left parietal sites for women, and bilateral activation for men, almost for each emotional category. These findings were also supported by source analyses. Conversely, during the fear stimulus processing, women showed an involvement of the left cuneus, and men of the right homologous. No group differences appeared in the P300 component analysis. Conclusions: Our findings support the hypothesis that men and women adopt different strategies when processing visual emotional information, and suggest that gender is a crucial variable in emotional research.
1. Introduction
Investigating the brain correlates of emotional processing has received considerable recent attention. Over the last few years, different methods have been implemented for eliciting affective states in participants, such as the presentation of words, pictures or video clips with various emotional content [1,2,3,4]. At the same time, several psychophysiological, electrophysiological and neuroimaging indices have been used to assess the neural responses induced by particular emotional stimulation (e.g., pleasant vs. unpleasant) [5,6,7]. Event-related potentials (ERPs) represent a powerful tool for studying the temporal dynamics of emotions, especially for their millisecond temporal resolution (for a review, see [8,9]). Among others, a significant number of ERP studies analyzed the temporal course of emotional responses, presenting to participants images from the International Pictures Affective System [3], which is characterized by standardized stimuli having low-to-high valence and arousal. These researches contributed to clarifying the temporal dynamics of positive and negative information processing (e.g., P200, P300, late positive potential or LPP) [10,11,12,13]. For example, Carretié and colleagues showed that negative pictures elicited greater P200 amplitudes at frontal and central sites than positive stimuli [11]. At later temporal intervals (around 300–400 ms after stimulus onset), both pleasant and unpleasant pictures induced greater LPP than neutral ones [14]. Moreover, regardless of valence, emotional stimuli with a higher arousal increased the LPP amplitude compared to stimuli with a lower arousal [14]. Other studies investigated the modulation of the P300 component and revealed that, compared to neural stimuli, the processing of emotional information increases the P300 amplitude [15]. Interestingly, this greater amplitude was found for both pleasant and unpleasant material, suggesting that the P300 modulation is independent of stimulus valence [16,17]. A limitation of the above-mentioned experiments is that they did not consider the potential effect of gender on emotional reactivity, tacitly assuming that men and women process affective stimuli in a similar way. However, there is evidence of marked gender differences in several fields, including hormonal levels, brain anatomy, hemispheric lateralization, and also emotional processing (for details, see [18,19]). Notably, the investigation of gender effects on emotional responses is particularly relevant because they may represent a critical risk factor for psychopathology development [20]. Behavioral investigations revealed that women reported greater arousal for unpleasant pictures depicting war, blood and injury scenes compared to men [7], suggesting that stressful, dangerous and fear information elicits different responses in the two groups. Conversely, men were found to be more emotionally aroused by erotic visual stimuli, showing greater subjective ratings and skin conductance responses [21,22]. Gender differences also emerged at the electrophysiological level. Greater N200 amplitude was found for women compared to men during the processing of unpleasant visual stimuli [23]. The modulation of the N200 component was also analyzed for comparing the emphatic brain responses induced by positive and negative IAPS images depicting humans or not humans. Compared to males, females exhibited enhanced N200 in anterior sites after the processing of positive pictures with humans. Women also showed greater late positivity (LPP, 500–700 ms after stimulus presentation) than men for negative pictures with humans [24]. Similar results were also found by Groen and colleagues that observed increased anterior N200 and parietal LPP amplitudes for females vs. males for emotional stimuli depicting humans [25]. However, this effect was independent of the emotional valence of the pictures (i.e., positive or negative). In another study, unpleasant pictures elicited greater P300 amplitude in the right hemisphere for men and in the left for women [26]. Overall, these findings highlighted the presence of distinct electrophysiological correlates of emotional processing in males and females, notably suggesting that gender represents a critical variable in emotional experimental settings. Nevertheless, given the heterogeneity of some results, it is still not clear whether the differences found between genders are mainly due to particular emotional content (positive vs. negative) or whether, regardless of the stimulus valence, men and women use different strategies to process affective information. In this regard, investigating the early ERP components (e.g., the P1) could clarify whether the gender effect occurs in the initial stage of stimulus processing. The P1 component depends on the activity of neurons in the extrastriate visual cortex, and it is engaged in sensory processes and in the allocation of attentional resources to visual stimuli [27]. The P1 amplitude appears sensitive to various stimulus characteristics, as it is modulated by the processing of emotional faces [28,29,30] and by the valence of the stimulus [31] and its alteration was observed in various psychiatric conditions [32]. In the present study, we aimed to investigate whether gender would affect brain responses to pleasant and unpleasant stimuli focusing on the early phases of emotional processing. To explore the potential gender effect, we compared two groups of participants (i.e., men vs. women) that passively viewed emotional and neutral pictures during EEG recordings. Afterward, they provided their subjective judgments about the valence and arousal of each stimulus, using the self-assessment manikin (SAM). To control other confounding variables, age, education and empathy were matched in-group. Compared to previous works, we included a wide range of emotional stimuli from the IAPS database: erotic and extreme sports pictures as positive stimuli and mutilation and fear pictures as negative stimuli. In addition, neutral pictures were also presented as control conditions. This allowed us to: (i) assess the brain responses elicited by emotional pictures comparing male and female groups; (ii) clarify whether the possible gender differences depended on the kind of emotion processed (positive vs. negative); and (iii) explore if the contents of the two positive (erotic and sport) or two negative (mutilation and fear) categories resulted in similar or different ERP responses. We investigated both early (P1) and later (P300) ERP components to grab a more complete picture of the temporal dynamics underlying emotional processing in men and women. Finally, source analysis was data-driven, computed in the time windows of P1 and P300 to localize the active neural generators underlying emotional stimuli. In line with previous literature, we expect to observe gender differences at the behavioral level, consisting in greater arousal of women for unpleasant stimuli and greater valence of men for erotic stimuli [7,21,22]. With respect to ERP data, we hypothesize that men and women could differ in the early processing (P1 component) of emotional vs. neutral information, in terms of amplitude and/or neural generators (e.g., left vs. right hemisphere). Discrepancies in the P1 responses could be the result of differences in the allocation of attentional resources to emotional visual stimuli.
2. Materials and Methods
2.1. Participants
A preliminary online questionnaire was completed by a sample of 215 young and healthy adults. This online screening included questions about age, educational levels, neurological or psychiatric disorders, and use of drugs, as well as empathy and a fear inventory. Specifically, as the content of erotic pictures consisted of heterosexual couples, we enrolled heterosexual participants only. We then excluded participants with specific phobias (e.g., phobia of firearms, knives, and blood) and/or with neurological or psychiatric disorders. The final sample was thus composed of 40 participants (20 women, mean age: 22.45 ± 2.91 years; 20 men, mean age: 22.40 ± 2.41 years; t(38) = 1.24, ns) with normal or corrected-to-normal vision. All participants were more than 18 years old and gave their written informed consent to take part in the experiment, according to the Declaration of Helsinki. The experimental procedure was approved by the Psychology Ethics Committee of the University of Padova (Protocol n. 4440).
2.2. Behavioral Measures
During the online screening, participants were administered the Interpersonal Reactivity Index (IRI) questionnaire [33] for assessing their empathy levels. This test comprises 4 subscales (i.e., fantasy, empathic concern, perspective taking and personal distress), each measuring a different empathy trait. Participants also completed an ad hoc short Italian version of the Fear Survey [34] for assessing the presence of particular phobias. Before the experiment was carried out, each participant completed two additional questionnaires: the STAI and the PANAS questionnaires. The State-Trait Anxiety Inventory (STAI Y1 and Y2) [35] is a test aimed at measuring trait and state anxiety. The Positive and Negative Affect Schedule (PANAS) [36] is a self-report tool aimed at assessing positive and negative affect (PA and NA).
2.3. Experimental Setting
The experiment was carried out in a dedicated EEG laboratory and included a passive viewing condition during which 130 pictures with five different contents were administered. Each stimulus was presented in full-screen mode for 2 s, followed by a black screen (interstimulus duration ranging between 1–3 s). Stimuli were all part of the International Affective Picture System (IAPS) and comprised two emotional positive categories (i.e., 26 erotic and 26 sports pictures), two emotional negative categories (i.e., 26 mutilation and 26 fear images) and neutral pictures (n = 26) depicting objects used in everyday life activities. Pictures were randomly interspersed, but the same order of presentation was maintained for all subjects. After this passive viewing task, participants were asked to rate both valence and arousal elicited by each stimulus using the SAM, including a scale ranging from 1 (low) to 9 (high). The EEG sessions occurred both during the morning and the afternoon, counterbalanced by gender.
2.4. EEG Preprocessing
EEG data were collected using a standard 64-channel cap (Acticap, BrainProduct System) according to the international 10–20 system. The EEG was recorded in DC mode and the activity of each electrode was referred to FCz online. The sampling rate was set at 1000 Hertz (Hz), and the impedance was kept below 5 KiloOhm (kΩ) throughout the recording. The activity of FCz was reconstructed, and data were off-line re-referenced to the average reference. Bad channels were identified and interpolated using the triangulation and linear interpolation method provided by BrainVision Analyzer software (BrainProduct GmbH, Gilching, Germany). All the other preprocessing steps were computed through the Brainstorm toolbox [37], including data filtering (lower cutoff = 0.5 Hz; upper cutoff = 125 Hz; stop-band attenuation = 60 dB) and Independent Component Analysis (ICA; method = Infomax EEGLAB/RunICA) to identify and then remove ocular artifactual components (e.g., blinks, vertical or horizontal movements). The baseline correction (−100/0 ms) was then computed on each epoch (−500 and 2000 ms before and after the stimulus presentation, respectively). Epochs with residual noise were deleted first by using a peak-to-peak procedure (threshold value = ± 100 microVolts, μV) that rejected the entire epoch, and lastly by visually inspecting the residual artifact-free epochs. For each subject, the epochs were averaged per condition (mean = 75.60% ± 12.00%; minimum = 14, maximum = 26 epochs) and a second filter was applied (0.5–30 Hz).
2.5. Behavioral Statistical Analysis
As groups showed different empathy levels only in the personal distress (PD) IRI subscale, we carried out statistical analyses on subjective data using PD scores as a covariate. Two separate ANCOVAs were carried out to analyze subjective valence and arousal judgments (2 genders [males vs. females] × 5 stimuli [sport vs. erotic vs. neutral vs. mutilation vs. fear]). Data analyses were performed using Statistica 6.1 (StatSoft GmbH, Hamburg, Germany) software. Post-hoc comparisons were computed using the Tukey HSD method (p < 0.05), and the Huynh Feldt correction was applied when necessary (i.e., for df > 2).
2.6. ERP Analysis
P1 and P300 components were analyzed. The time window of the P1 component corresponded to the 110–130 ms temporal interval after stimulus onset. The P300 component included the 200–340 ms interval after stimulus onset. Two clusters of electrodes were created, including 5 parieto-occipital sites in the left hemisphere (P7-P5-PO7-PO3-O1) and 5 parieto-occipital electrodes in the right hemisphere (P8-P6-PO8-PO4-O2). The clusters of electrodes selected to investigate the P1 and P300 components were selected according to the maximal peaks seen in the brain waves analysis (Figure 1). Preliminary analyses, including PD scores as the covariate, revealed no significant effect of this variable, and were in line with the results found for subjective data analyses. Therefore, ERP data were analyzed by means of ANOVA (2 gender [males vs. females] × 5 stimuli [sport vs. erotic vs. neutral vs. mutilation vs. fear] × 2 laterality [left vs. right]).
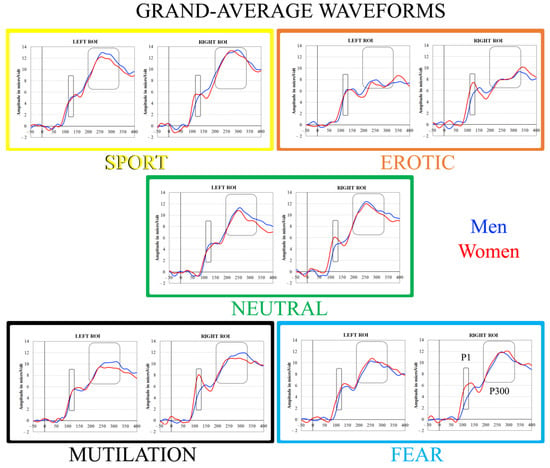
Figure 1.
Mean grand-average waveforms of all regions of interest showing the time course of IAPS processing following stimulus onset. The black full lines represent the P1 temporal intervals (110–130 ms), whereas the back dotted lines depict the P300 temporal windows (200–340 ms).
2.7. EEG Source Localization
Localization of the neural sources underlying the effects of the emotional stimulation in male and female groups was computed using the standardized low-resolution brain electromagnetic tomography (sLORETA) method [38]. Since sLORETA computes the smoothest possible 3D-distributed current-source density solution constrained to grey matter, this approach was particularly suited for our analysis since, due to the smoothness constraint, it does not need an a priori definition of known sources. Separate t-tests (5000 permutations) were carried out for the following: (a) between-group analysis, regardless of stimulus content, on each component (i.e., P1, in the 110–130 ms interval, and P300, in the 200–340 ms interval after stimulus onset); (b) by comparing, for each group and stimulus, the electrical activity within the P1 interval (110–130 ms after stimulus onset) with that of an interval with no active linguistic processing (the 20-ms baseline prior to stimulus onset); and (c) within the P300 interval (200–340 ms) with an equivalent neutral interval (i.e., the 140-ms baseline). All results are expressed in MNI coordinates.
3. Results
3.1. Empathy Traits, Valence and Arousal
Men and women showed similar results to the IRI questionnaire total scores (Mean [M] = 95.90 for both men and women, standard error [SE] ± 2.80 and 2.37, respectively; t38 = 1.00, ns) as well as on the fantasy (25.40 ± 1.15 vs. 24.00 ± 1.07; t38 = 0.89, ns), empathic concern (26.45 ± 1.08 vs. 25.75 ± 0.89; t38 = 0.50, ns) and perspective taking (27.95 ± 0.89 vs. 27.15 ± 0.82; t38 = 0.66, ns) IRI subscales. However, the personal distress scores were greater in women (19.00 ± 0.87) than in men (16.10 ± 0.97; t38 = −2.23, p = 0.03). For this reason, PD scores were considered in the analysis of subjective valence and arousal ratings.
The ANCOVA carried out on the valence ratings revealed a gender main effect (F1, 37 = 6.85, p = 0.005), with overall men’s ratings being higher than those of women when considering the five stimulus categories together (M = 4.65, SE ± 0.27, F = 4.09, SE ± 0.27, respectively). A stimulus main effect was also significant (F4, 148 = 6.42, HF ε = 0.76, p < 0.001), showing significant differences between the contents of images presented, in line with the inclusion of those images in the category of positive-/negative-valenced stimuli. Furthermore, the 2-way gender by stimulus interaction was statistically significant (F4, 148 = 3.61, HF ε = 0.76, p = 0.007). As shown in Figure 2A, men showed statistically different valence ratings among all stimuli (all ps < 0.001), whereas in the women’s group, differences can be found in erotic/sport vs. neutral (p < 0.001), neutral vs. mutilation/fear (p < 0.001) and fear vs. mutilation (p < 0.05). With respect to between-group comparisons, men’s ratings for erotic images (p < 0.001) and for fear images (p < 0.01) were higher than women’s.
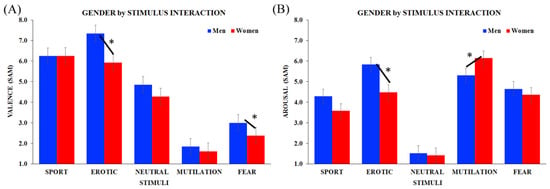
Figure 2.
Analyses of participants’ subjective evaluations through self-assessment manikin (SAM). Significant gender x stimulus interaction for (A) valence and (B) arousal scores in men (blue) and women (red). Bars depict standard errors [SE], asterisks significant post hoc comparisons.
Similar results were obtained when analyzing results collected from the subjective arousal scores, without main effects. The 2-way gender by stimulus interaction was significant (F4, 148 = 5.88, HF ε = 0.89, p < 0.001), and the typical V pattern appeared in both groups (Figure 2B). However, in men arousal was higher in erotic/sport vs. neutral (all ps < 0.001), erotic vs. sport (p < 0.001) and mutilation/fear vs. neutral (all ps p < 0.001), whereas women showed greater arousal levels in erotic/sport vs. neutral (p < 0.001), erotic vs. sport (p < 0.05), mutilation/fear vs. neutral (all ps < 0.001) and mutilation vs. fear (p < 0.001). Focusing on between-group comparisons, erotic pictures elicited greater arousal in men compared with women (p < 0.001), whereas women’s ratings were higher than men’s scores for mutilation stimuli (p < 0.001).
3.2. ERP Analysis: P1 Component
ANOVA was conducted on the P1 component (110–130 ms after stimulus onset) and revealed a stimulus main effect (F4,152 = 4.20, HF ε = 0.95, p = 0.002). As can be seen in Figure 3A, significant differences emerged between both erotic and mutilation vs. neutral (p = 0.04 and p = 0.03, respectively), with emotional stimuli eliciting greater P1 amplitude than neutral images. In addition, erotic pictures showed greater P1 amplitudes compared with the sport stimuli (p = 0.05).
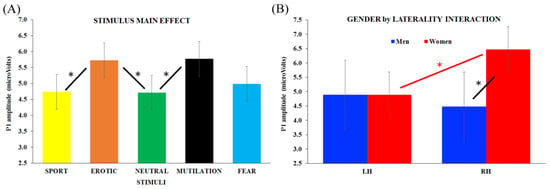
Figure 3.
Analysis of the P1 component (110–130 ms after stimulus onset revealing (A) the significant stimulus main effect and (B) the significant gender x laterality interaction. Bars depict standard errors [SE], asterisks significant post hoc comparisons.
To summarize, the P1 amplitude reached the maximum amplitude when associated with mutilation and erotic stimulus processing, the neutral and sport categories had the lowest activation, and there was no significant difference between all stimuli and fear stimuli.
Notably, results also showed a 2-way significant interaction between group and laterality (F1, 38 = 7.44, p = 0.001), revealing that the P1 amplitude in the right hemisphere was significantly higher for women than men (p = 0.002; Figure 3B). In addition, for women only, greater P1 amplitude was found in the right vs. left cluster (p = 0.019). No laterality effect emerged for the men group, as no differences were found between the left and right hemispheres.
3.3. Source Analysis on P1 Components
The source analysis carried out on the P1 components, including all stimuli between groups (thus following the ERP results), showed a significantly greater activation in women vs. men (t = 1.27, p < 0.001) in MNI coordinates X = 5, Y = −80, Z = 10, corresponding to the right cuneus (Brodmann area, BA, 17; see Figure 4).
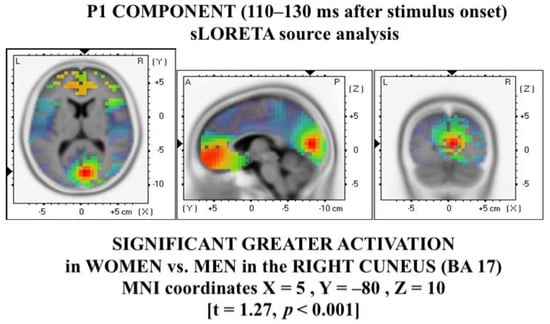
Figure 4.
P1 source analysis (sLORETA) revealed greater women’s activation in the right cuneus, compared with men.
3.4. Separate ANOVAs and Source Analysis on Emotional Stimuli (P1 Component)
Given the main effect of the stimulus observed before, together with the 2-way group by laterality interaction, separate ANOVAs were conducted for each stimulus, to carry out a more fine-grained analysis aimed at revealing which IAPS contents were the most responsible for gender differences.
The only case in which there were no significant P1 results was for the sports category. For the erotic stimulus category, the 2-way interaction gender x laterality was found (F1, 38 = 6.92, p = 0.01): as shown in Figure 5A (in orange), women showed greater P1 amplitude in the right vs. left cluster (p = 0.048) but also greater amplitude than men in the right hemisphere cluster (p = 0.006). No laterality effect was found for the men’s group.
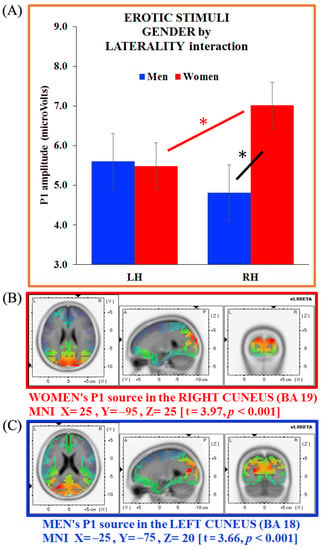
Figure 5.
(A) Gender x laterality interaction for the P1 component on erotic stimuli and source analysis on P1 generators separate for (B) women (in red) and (C) men (in blue). Bars depict standard errors [SE], asterisks significant post hoc comparisons.
As the between-group sLORETA analysis carried out on the P1 showed no significant gender differences, we carried out separate analyses on women’s and men’s P1 generators (erotic pictures). These latter analyses revealed a neural generator in the right cuneus (BA 19, MNI X = 25, Y = −95, Z = 25; t = 3.97, p < 0.001) for women, and in the left cuneus (BA 18, MNI X = −25, Y = −75, Z = 20; t = 3.66, p < 0.001) for men. Therefore, the P1 generator appeared located in the cuneus in both groups, with an opposite hemisphere engagement for women (right side) and men (left side; Figure 5B,C, respectively).
For the neutral category, the 2-way interaction gender x laterality was also observed (F1, 38 = 7.812, p = 0.008): whereas no laterality effect was found in men, in women there was greater P1 amplitude in the right vs. left cluster (p = 0.002), but no significant between-group differences were found (Figure 6A, in green).
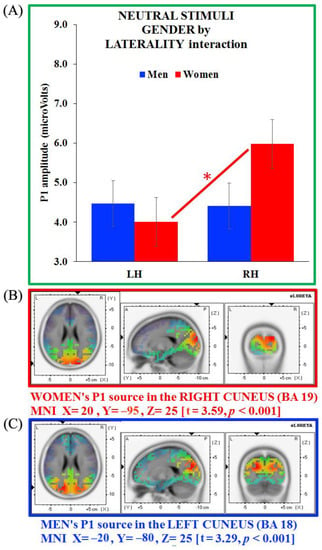
Figure 6.
(A) Gender x laterality interaction for the P1 component on neutral stimuli and source analysis on P1 generators separate for (B) women (in red) and (C) men (in blue). Bars depict standard errors [SE], asterisks significant post hoc comparisons.
sLORETA analyses revealed neural generators in the right cuneus for women (BA 19, MNI X = 20, Y = −95, Z = 25; t = 3.59, p < 0.001), and in the left cuneus for men (BA 18, MNI X = −20, Y = −80, Z = 25; t = 3.29, p < 0.001). Thus, as for the erotic images, the P1 generator appeared located in the cuneus in both groups, with an opposite hemisphere engagement for women (right side) and men (left side; Figure 6B,C, respectively).
Moving onto the negative categories, for the mutilation stimuli there was a significant 2-way gender x laterality interaction, shown in Figure 7A (in black; F1, 38 = 7.29, p = 0.01): a laterality effect was observed in women, who exhibited greater P1 amplitude in the right vs. left cluster (p = 0.004) and also a greater amplitude than men in the right cluster (p = 0.002).
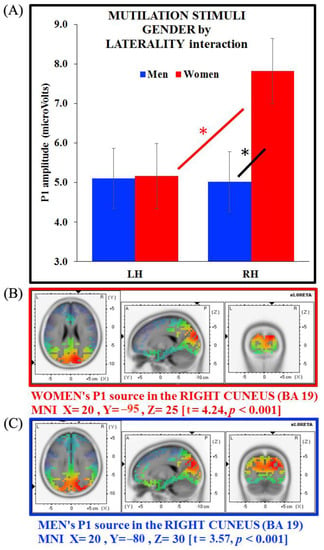
Figure 7.
(A) Gender x laterality interaction for the P1 component on mutilation stimuli and source analysis on P1 generators separate for (B) women (in red) and (C) men (in blue). Bars depict standard errors [SE], asterisks significant post hoc comparisons.
sLORETA analysis carried out on each group showed a neural generator in the right cuneus for both women and men (BA 19, MNI X = 20, Y = −95, Z = 25; t = 4.24, p < 0.001; and BA 19, MNI X = 20, Y = −80, Z = 30; t = 3.57, p < 0.001, respectively). Therefore, unlike erotic and neutral images, mutilation images activated the right cuneus in both groups, thus revealing that this kind of emotional content is not affected by gender bias (Figure 7B,C, for women and men respectively).
Lastly, for the fear category, the significant 2-way gender x laterality interaction was observed (F1, 38 = 4.28, p = 0.045): a gender effect appeared in the right cluster, with women showing greater P1 amplitudes than men (Figure 8A in sky-blue).
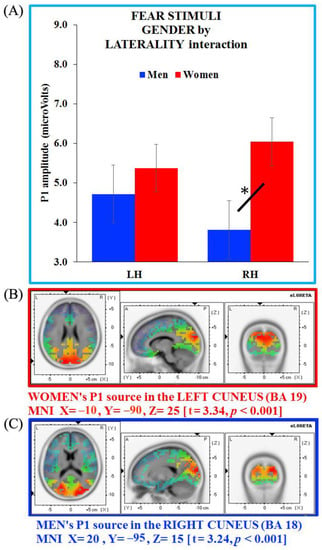
Figure 8.
(A) Gender x laterality interaction for the P1 component on fear stimuli and source analysis on P1 generators separate for (B) women (in red) and (C) men (in blue). Bars depict standard errors [SE], asterisks significant post hoc comparisons.
The source analyses computed showed neural generators located in the left cuneus (BA 19, MNI X = −10, Y = −90, Z = 25; t = 3.34, p < 0.001) for women, and in the right cuneus for men (BA 18, MNI X = 20, Y = −95, Z = 15; t = 3.24, p < 0.001). Thus, the P1 generator appeared to be located in the cuneus in both groups, with an opposite hemisphere engagement for women (left side) and men (right side; Figure 8B,C, respectively).
3.5. ERP Analysis: P300 Component
An ANOVA was computed for analyzing the amplitude of the P300 component, revealing a stimulus main effect (F4, 152 = 43.76, HF ε = 0.98, p < 0.001): significant differences were found for the sport and erotic images vs. all the other stimuli (all ps < 0.001). Therefore, regardless of gender, the greater P300 amplitude was measured in response to sport stimuli, whereas the lowest amplitude was measured in response to erotic stimuli. No significant differences were found when comparing the amplitudes of neutral, mutilation and fear stimuli. Furthermore, a laterality main effect was found (F1, 38 = 12.59, p = 0.001), as the right cluster showed greater P300 amplitude (mean = 10.45 ± 1.57 μV) than the left one (mean = 9.42 ±1.48 μV). Notably, no main effects or interactions with gender were found.
4. Discussion
The goal of the present study was to investigate the role of gender in the processing of different emotions (i.e., pleasant and unpleasant). To ensure that the differences found could be attributed just to the gender variable, all other variables in the participants’ sample were controlled, including age, education level, possible psychological and psychiatric disorders, drug abuse, as well as empathy level, which was previously found to affect the emotional responses [39]. A wide range of emotional contents was selected from the International Affective Pictures System, including erotic and sport stimuli as the positive-valenced category, mutilation and fear stimuli as the negative-valenced category and neutral stimuli as the control condition. Our results revealed several differences in the men’s and women’s groups both at the behavioral and electrophysiological levels. The self-reported data showed the expected pattern of responses for the valence dimension: men reported higher ratings for the erotic and fear pictures relative to women [40,41], whereas the other stimuli elicited similar scores. Arousal evaluations also showed that emotions are differently perceived by men and women. Erotic pictures were judged as more arousing for men than women; however, unpleasant mutilation stimuli induced the opposite arousal evaluation. On the one hand, erotic stimuli are the ones that mainly differentiate the two groups, given the high valence and arousal elicited in men vs. women. On the other hand, women appear more sensitive than men to negative emotional material (i.e., fear/mutilation images), in agreement with previous studies [7,21,42]. The effect found for erotic pictures could be explained by a cultural bias, according to which women are less interested in this kind of stimuli, whereas men are allowed to express their liking in a freer way. This bias could have forced female participants to rate erotic stimuli as less pleasurable than they actually would have if there were no societal pressure. Focusing on unpleasant stimuli, negative feelings (e.g., anger and aggression) are commonly considered socially acceptable for men, but this is not the case for women [19]. Consequentially, men tend to be evolutionarily less affected by dangerous and fearful situations, but women are more inclined to show their concern about dangerous and disgusting situations.
A main goal of our study was also to investigate the temporal dynamics of emotional processing, analyzing both early and late ERP components. Our intent was to clarify whether the gender differences only concern components reflecting high cognitive processes (i.e., P300) or also influence the initial phases of processing (i.e., early visual processing or P1). Our findings provided evidence of distinct brain correlates in men and women when exposed to particular emotional stimulation. The analysis carried out on the P1 component included two clusters of posterior electrodes, one in the left hemisphere and one in the right. Overall, we observed greater amplitude modulation during mutilation and erotic pictures presentation compared to neutral stimuli, and greater amplitude for erotic vs. sport images. No differences emerged in the fear category. This finding confirms that, in general, erotic and mutilation represent biologically relevant stimuli that elicit greater brain responses compared to other kinds of emotional and non-emotional pictures. Furthermore, regardless of the stimulus content, women showed greater P1 amplitude in the right posterior cluster compared to the left, but also greater amplitude in the right hemisphere compared to men. The P1 amplitude of men was instead similar in the two hemispheres. This right-lateralized pattern for the women’s group was also confirmed by the source analysis that revealed greater posterior right cuneus activation compared with men. Given this result was not related to emotional information, it could be due to different image processing, consisting of bilateral visual-area recruitment for the masculine brain and prominent right recruitment for the feminine brain. Gender differences in brain laterality have been previously observed for language and visuo-spatial processes: men are more left-lateralized in language tasks, while women are more right-lateralized in visuo-spatial tasks [43]. Moreover, it has been proposed that men and women differ in basic visual processing (e.g., contrast sensitivity, visual acuity and color and motor perception) [44]. With respect to our data, we found that in a very early phase of processing (P1 interval), men reported comparable amplitude in left and right visual occipital areas, while women showed larger amplitude in the right sites. This suggests that visual information might be processed in a different way by masculine and feminine brains.
To better explore the gender effect in specific emotional contexts, we computed separate ERP and source analyses within each stimulus category. No modulation was found for sport stimuli. The absence of an effect for the sport images could be due to the ambiguous content of these stimuli, depicting highly arousing outdoor sports, marked by extreme (and therefore hypothetically dangerous) activities, such as climbing or hang gliding. Notably, sport and fear stimuli elicited similar arousal levels and P1 modulation, together with neutral ones (as reported in the main ANOVA). An interesting result was that, regardless of picture content, men always showed similar P1 amplitude in the two hemispheres, whereas women maintained a greater amplitude in right vs. left posterior areas. In other words, men exhibited a bilateral pattern of P1 activation, whereas women revealed a right-lateralized P1 distribution in posterior visuo-associative sites. The only case in which women showed bilateral ERP responses, in agreement with the men’s pattern of early activation, was during the processing of fear images. This particular set of images is the only one in which we chose two subtypes of fear contents, i.e., dangerous animals (such as sharks, snakes and poison spiders) and attack scenes (e.g., a man pointing a knife at a woman’s throat or soldiers/terrorists with rifles and machine guns). As we selected our images ensuring that arousal levels were a priori similar among all emotional stimuli, these were the only options we had. The use of different fear contents, considering the visual gestalt/complexity of these images probably contributed to altering the picture processing, in particular in women: the typical right-lateralized activation [43] is, indeed, replaced by a wider network, involving both hemispheres.
Focusing on gender effects, the parieto-occipital regions of the right hemisphere showed greater activation in the women’s but not the men’s brains when erotic, mutilation and fear contents were passively viewed. Interestingly, no gender differences were found in these electrodes in the control condition, i.e., when pictures of objects were administered, nor in the left hemisphere homologs. This finding suggests that highly arousing stimuli, regardless of their valence, significantly engage the right parieto-occipital regions in women only. This pattern was also replicated in the separate source analyses. In particular, women mainly activated the right cuneus (BA 19) during passive watching of erotic and mutilation pictures, as well as neutral images, a pattern that suggests this is probably the typical network engaged by feminine brains’ visual scene processing (Note that emotional and neutral stimuli were randomly interspersed during the task in a single block, consisting of the presentation of 52 positive pictures (i.e., 26 sport and 26 erotic), 52 negative pictures (i.e., 26 fear and 26 mutilation) and 26 neutral pictures. Therefore, the number of neutral stimuli was half the positive and negative ones: it is possible that the greater number of emotional pictures influenced the processing of neutral pictures, resulting in the similar pattern observed for both emotional and neutral conditions). In addition, fear IAPS elicited greater activation in women’s right parieto-occipital regions, compared with men, but no right-hemisphere dominance (i.e., similar levels of left and right parieto-occipital sites were found in women), and the source generator revealed significant left cuneus (BA 19) activation. A possible interpretation may depend on the complexity of this type of mixed stimuli, which may require a more in-depth analysis of stimulus gestalt/contents, to interpret the meaning of the scene represented. On the other hand, men showed a bilateral P1 amplitude to all stimuli but significant activation of the left cuneus (BA 18) to erotic/neutral stimuli and the right cuneus (BA 18) to negative-valenced stimuli (i.e., both mutilation and fear pictures). This pattern suggests that (a) men tend to activate opposite parieto-occipital regions depending on the stimulus valence (left for positive-valenced stimuli, right for negative-valenced stimuli); (b) that only extremely negative, biologically relevant negative stimuli, such as mutilations, engage a shared mechanism in the parieto-occipital regions of the right hemisphere regardless of gender, thus suggesting a sort of preferred cortical pathway devoted to the automatic processing of stimuli critical for surviving; and (c) that masculine and feminine brains tap different networks to process emotionally relevant stimuli.
In an fMRI study using IAPS pleasant, neutral, and unpleasant pictures, women showed greater activation in the right extra-striate visual cortex when viewing unpleasant compared to pleasant pictures, whereas men showed greater activation for pleasant vs. unpleasant stimuli [45]. Similarly, we always observed the activation of Broadmann areas 18–19 during picture processing; however, both the left and right hemispheres were differently recruited by men and women. Importantly, in our research, we did not contrast positive and negative stimuli with neutral ones because our aim was to describe the neural underpinnings elicited by each emotion in the two samples. Indeed, in our experimental paradigm, the number of positive and negative stimuli was twice the number of neutral images: the greater amount of emotional pictures probably affected the processing of neutral stimuli, inducing a physiological activation similar to that of positive-valenced stimuli. Future studies should consider choosing a block design, in which stimuli with the same content are grouped together. However, it is interesting to note that only during emotional processing (i.e., erotic, fear and mutilation stimuli) was the P1 amplitude in the right posterior cluster higher for women than men, whereas similar amplitudes were found for the neutral control pictures. It has been proposed that the P1 component might be considered an index of attention allocation to valenced stimuli, and its amplitude was measured while participants evaluated pleasant and unpleasant images [31]. The results provided evidence for the extremely rapid (<120 ms) discrimination of positive and negative stimuli: P1 amplitudes to frequent stimuli and to rare negative stimuli were larger than P1 amplitudes to rare positive stimuli. Our data showed that two conditions in particular (i.e., erotic and mutilation) elicited the maximum P1 amplitude compared to other stimuli, with right hemispheric lateralization for women only. This increased amplitude is in line with previous visual-evocated potential studies reporting that early components (e.g., P50, N70 and P100) have greater amplitudes in women compared with men (for a review, see [44]). Hemispheric asymmetry for visual scene processing has been reported in an fMRI study showing that the right hemisphere is specialized in form-specific visual processing and the left hemisphere in form-abstract visual processing [46].
No gender effect emerged for the P300 analysis. The only modulation we found was for the sport pictures, which evoked significantly greater amplitude than erotic ones. Starting from the results of Gasbarri and colleagues, we expected to observe different responses for male and female participants, at least for unpleasant pictures [26]. In this study, EEG was collected from 19 channels, and linked earlobes were used as an online reference. This latter choice, in particular, is crucial as past literature suggests that linked mastoids or earlobes may significantly contribute to an increase in the P300 amplitude (e.g., [47]). Furthermore, the authors analyzed only two fronto-parietal electrodes within each hemisphere (F3-F4 and P3-P4) for pleasant, neutral and unpleasant IAPS pictures [26]. The differences in the references (linked earlobes vs. FCz) and the cluster of electrodes for statistical analyses (fronto-parietal vs. parieto-occipital sites) probably explain the different results from Gasbarri and colleagues’ study and the present one.
In conclusion, although our data refers to the initial stimulus processing, we propose that men and women use different strategies in the processing of emotional visual information. At the source level, we observed the same right activation in both groups during the mutilation presentation, and this suggests that this kind of image is not affected by gender. On the contrary, it is plausible that the mechanism triggered by these kinds of stimuli is shared and phylogenetically determined. Another interesting result was the processing of fear pictures. At a behavioral level, women and men showed similar arousal levels, and valence was slightly larger in men. Notwithstanding these few differences, we found different neural generators. In particular, this was the only condition in which we observed an opposite asymmetry: females showed activation in the left cuneus, males in the right. This finding reveals that men showed the same electrophysiological reaction for negative stimuli (both mutilation and fear), but women’s processing of mutilation images induced right hemisphere lateralization and greater right P1 amplitude, whereas that of fear images elicited left hemisphere lateralization and bilateral P1 amplitude. The present study contributed to the investigation of gender bias during the passive view of emotional information and showed that at the early stage of visual processing (110–130 ms after stimulus presentation), men and women recruit different neural circuits. We observed specific hemispheric asymmetries both for ERP amplitude and source activation that were associated with given emotional contents. Our findings support the idea that men and women adopt different strategies when processing emotions and suggest that gender is a crucial variable in emotional research.
Author Contributions
Conceptualization, methodology, project administration, funding acquisition, supervision, C.S.; data curation, Z.R.; investigation, formal analysis, Z.R and C.S.; writing—original draft preparation, Z.R.; writing—review and editing, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a grant, PRIN 2017, from the Italian Ministry of University and Research (MIUR), project n. 20178NNRCR_003 to C.S., and supported by a grant from the Italian Ministry of University and Research (MIUR), Dipartimenti di Eccellenza DM 11/05/2017 n.262 to the Department of General Psychology.
Data Availability Statement
The anonymized data that support the findings of this study are available on request from the corresponding author. These data are not publicly available, due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romeo, Z.; Fusina, F.; Semenzato, L.; Bonato, M.; Angrilli, A.; Spironelli, C. Comparison of Slides and Video Clips as Different Methods for Inducing Emotions: An Electroencephalographic Alpha Modulation Study. Front. Hum. Neurosci. 2022, 16, 901422. [Google Scholar] [CrossRef]
- Alves, N.T.; Aznar-Casanova, J.A.; Fukusima, S.S. Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality 2009, 14, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.; Cuthbert, B.N. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Cent. Study Emot. Atten. 1997, 1, 39–58. [Google Scholar]
- Maddock, R.J.; Garrett, A.S.; Buonocore, M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003, 18, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Moser, E.; Peper, M. fMRI of Emotion. In fMRI Techniques and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; Volume 119, pp. 451–494. [Google Scholar]
- Rahman, M.M.; Sarkar, A.K.; Hossain, M.A.; Hossain, M.S.; Islam, M.R.; Hossain, M.B.; Quinn, J.M.W.; Moni, M.A. Recognition of human emotions using EEG signals: A review. Comput. Biol. Med. 2021, 136, 104696. [Google Scholar] [CrossRef]
- Bianchin, M.; Angrilli, A. Gender differences in emotional responses: A psychophysiological study. Physiol. Behav. 2012, 105, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.K.; Nordin, S.; Sequeira, H.; Polich, J. Affective picture processing: An integrative review of ERP findings. Biol. Psychol. 2008, 77, 247–265. [Google Scholar] [CrossRef]
- Ding, R.; Li, P.; Wang, W.; Luo, W. Emotion Processing by ERP Combined with Development and Plasticity. Neural Plast. 2017, 2017, 5282670. [Google Scholar] [CrossRef]
- Carretié, L.; Mercado, F.; Tapia, M.; Hinojosa, J.A. Hinojosa Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. Int. J. Psychophysiol. 2001, 41, 75–85. [Google Scholar] [CrossRef]
- Carretié, L.; Martín-Loeches, M.; Hinojosa, J.A.; Mercado, F. Emotion and attention interaction studied through event-related potentials. J. Cogn. Neurosci. 2001, 13, 1109–1128. [Google Scholar] [CrossRef]
- Conroy, M.A.; Polich, J. Affective valence and P300 when stimulus arousal level is controlled. Cogn. Emot. 2007, 21, 891–901. [Google Scholar] [CrossRef]
- Schupp, H.T.; Cuthbert, B.N.; Bradley, M.; Cacioppo, J.T.; Tiffany, I.; Lang, P.J. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology 2000, 37, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.; Birbaumer, N.; Lang, P.J. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Macnamara, A.; Olvet, D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol. 2010, 35, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Mini, A.; Palomba, D.; Angrilli, A.; Bravi, S. Emotional Information Processing and Visual Evoked Brain Potentials. Percept. Mot. Skills 1996, 83, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Palomba, D.; Angrilli, A.; Mini, A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int. J. Psychophysiol. 1997, 27, 55–67. [Google Scholar] [CrossRef]
- Cahill, L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Kret, M.E.; De Gelder, B. A review on sex differences in processing emotional signals. Neuropsychologia 2012, 50, 1211–1221. [Google Scholar] [CrossRef]
- Davidson, R.J. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol. Psychiatry 2002, 51, 68–80. [Google Scholar] [CrossRef]
- Bradley, M.; Codispoti, M.; Sabatinelli, D.; Lang, P.J. Emotion and motivation II: Sex differences in picture processing. Emotion 2001, 1, 300–319. [Google Scholar] [CrossRef]
- Chivers, M.L.; Seto, M.C.; Lalumière, M.L.; Laan, E.; Grimbos, T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch. Sex. Behav. 2010, 39, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Lithari, C.; Frantzidis, C.A.; Papadelis, C.; Vivas, A.B.; Klados, M.A.; Kourtidou-Papadeli, C.; Pappas, C.; Ioannides, A.A.; Bamidis, P.D. Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M.; Adorni, R.; Zani, A.; Trestianu, L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia 2009, 47, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Groen, Y.; Wijers, A.A.; Tucha, O.; Althaus, M. Are there sex differences in ERPs related to processing empathy-evoking pictures? Neuropsychologia 2013, 51, 142–155. [Google Scholar] [CrossRef]
- Gasbarri, A.; Arnone, B.; Pompili, A.; Pacitti, F.; Pacitti, C.; Cahill, L. Sex-related hemispheric lateralization of electrical potentials evoked by arousing negative stimuli. Brain Res. 2007, 1138, 178–186. [Google Scholar] [CrossRef]
- Luck, S.J.; Woodman, G.F.; Vogel, E.K. Event-related potential studies of attention. Trends Cogn. Sci. 2000, 4, 432–440. [Google Scholar] [CrossRef]
- Müller-Bardorff, M.; Bruchmann, M.; Mothes-Lasch, M.; Zwitserlood, P.; Schlossmacher, I.; Hofmann, D.; Miltner, W.; Straube, T. Early brain responses to affective faces: A simultaneous EEG-fMRI study. Neuroimage 2018, 178, 660–667. [Google Scholar] [CrossRef]
- Schindler, S.; Bublatzky, F. Attention and emotion: An integrative review of emotional face processing as a function of attention. Cortex 2020, 130, 362–386. [Google Scholar] [CrossRef]
- Denefrio, S.; Simmons, A.; Jha, A.; Dennis-Tiwary, T.A. Emotional cue validity effects: The role of neurocognitive responses to emotion. PLoS ONE 2017, 12, e0179714. [Google Scholar] [CrossRef]
- Smith, N.K.; Cacioppo, J.T.; Larsen, J.T.; Chartrand, T.L. May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia 2003, 41, 171–183. [Google Scholar] [CrossRef]
- Spironelli, C.; Romeo, Z.; Maffei, A.; Angrilli, A. Comparison of automatic visual attention in schizophrenia, bipolar disorder, and major depression: Evidence from P1 event-related component. Psychiatry Clin. Neurosci. 2019, 73, 331–339. [Google Scholar] [CrossRef]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–126. [Google Scholar] [CrossRef]
- Wolpe, J.; Lang, P.J. A fear survey schedule for use in behaviour therapy. Behav. Res. Ther. 1964, 2, 27–30. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual For the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Bock, E.; Niso, G.; Mosher, J.C.; Cousineau, M.; Pantazis, D.; Leahy, R.M.; Baillet, S. MEG/EEG group analysis with brainstorm. Front. Neurosci. 2019, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 91–95. [Google Scholar]
- Maffei, A.; Spironelli, C.; Angrilli, A. Affective and cortical EEG gamma responses to emotional movies in women with high vs low traits of empathy. Neuropsychologia 2019, 133, 107175. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.; Pinheiro, A.P.; Costa, A.; Frade, C.S.; Comesaña, M.; Pureza, R. Adaptation of the International Affective Picture System (IAPS) for European Portuguese. Behav. Res. Methods 2014, 47, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Lang, P.J. The International Affective Digitized Sounds (IADS): Stimuli, Instruction Manual and Affective Ratings; The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Poláčková Šolcová, I.; Lačev, A. Differences in male and female subjective experience and physiological reactions to emotional stimuli. Int. J. Psychophysiol. 2017, 117, 75–82. [Google Scholar] [CrossRef]
- Clements, A.M.; Rimrodt, S.L.; Abel, J.R.; Blankner, J.G.; Mostofsky, S.H.; Pekar, J.J.; Denckla, M.B.; Cutting, L.E. Sex differences in cerebral laterality of language and visuospatial processing. Brain Lang. 2006, 98, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Vanston, J.E.; Strother, L. Sex differences in the human visual system. J. Neurosci. Res. 2017, 95, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.; Fitzsimmons, J.R.; Cuthbert, B.N.; Scott, J.D.; Moulder, B.; Nangia, V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 1998, 35, 199–210. [Google Scholar] [CrossRef]
- Stevens, W.D.; Kahn, I.; Wig, G.S.; Schacter, D.L. Hemispheric asymmetry of visual scene processing in the human brain: Evidence from repetition priming and intrinsic activity. Cereb. Cortex 2012, 22, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xin, X.; Zhu, H.; Li, F.; Xiong, H.; Zhang, T.; Lai, Y. A comparative study on the dynamic EEG center of mass with different references. Front. Neurosci. 2017, 11, 509. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).