New High-Pressure Structures of Transition Metal Carbonates with O3C–CO3 Orthooxalate Groups
Abstract
1. Introduction
2. Computational Methods
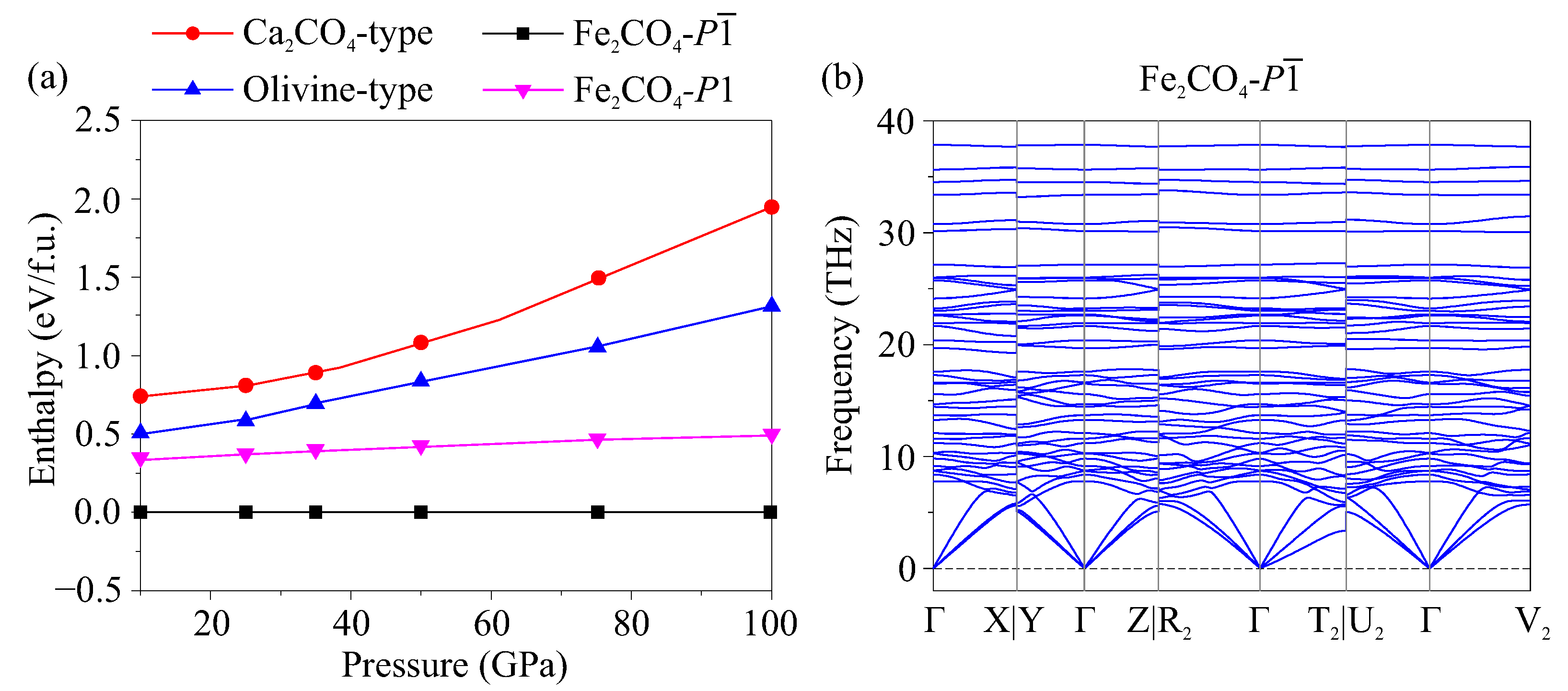
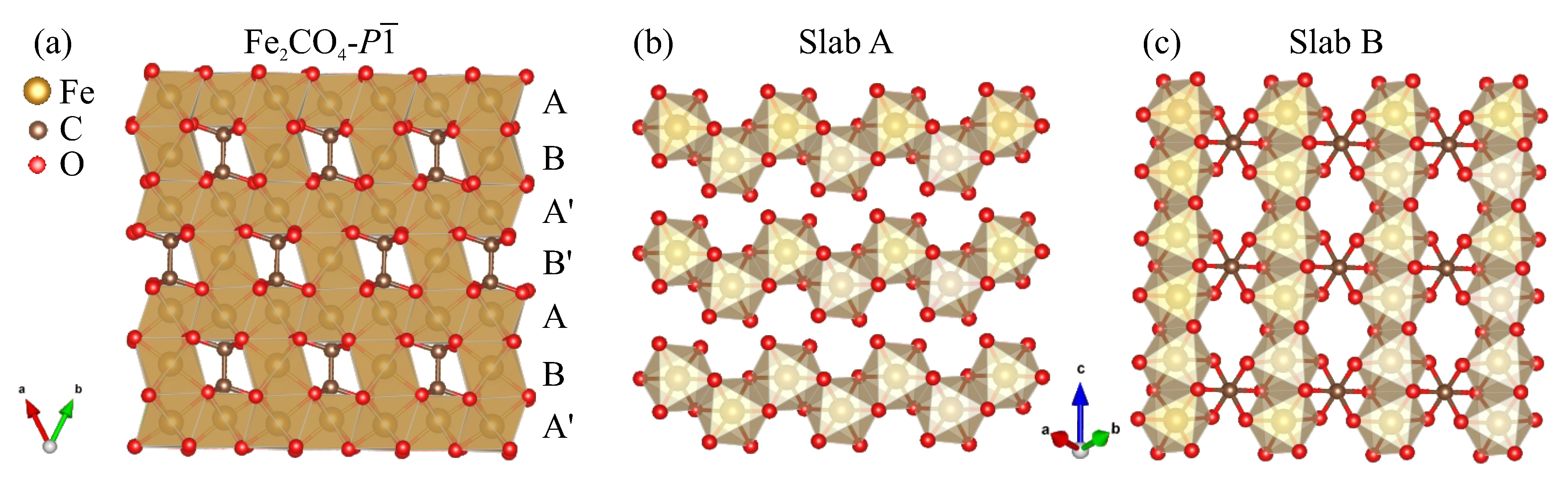
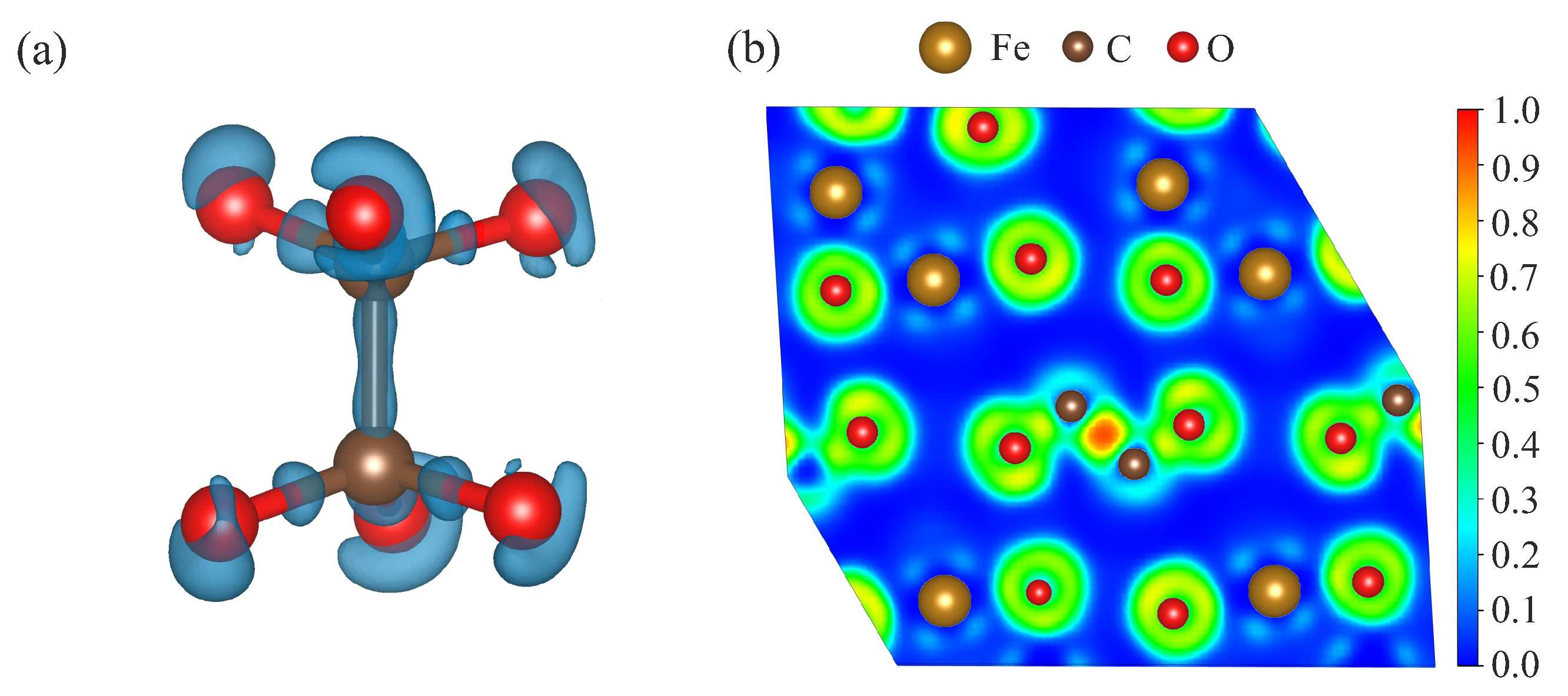
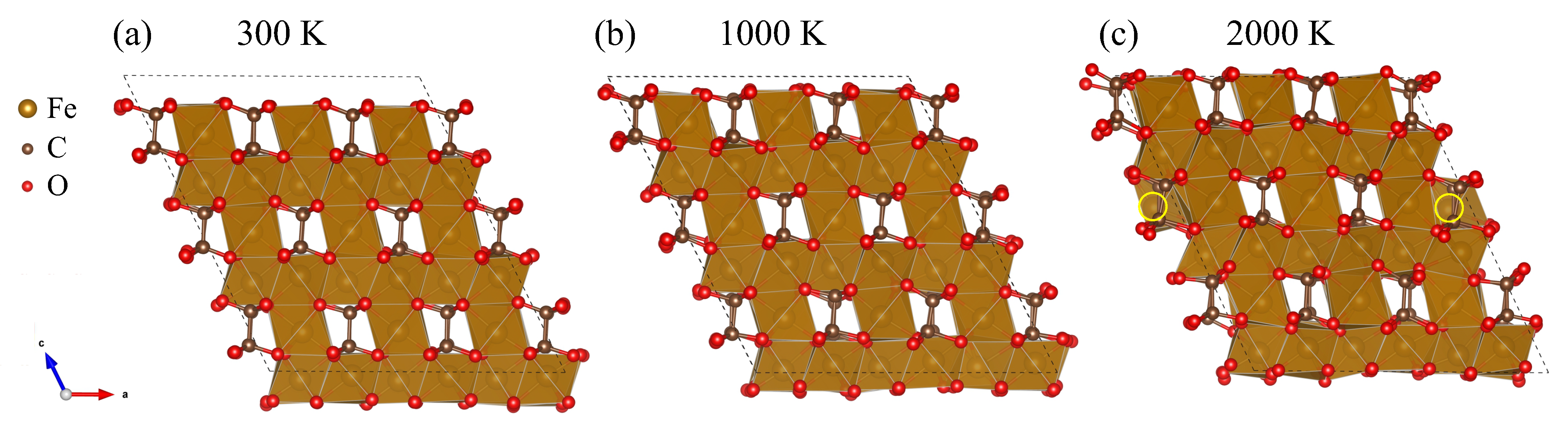
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, X.; Xie, C.; Dong, X.; Oganov, A.R.; Zeng, Q. Novel high-pressure calcium carbonates. Phys. Rev. B 2018, 98, 014108. [Google Scholar] [CrossRef]
- Sagatova, D.; Shatskiy, A.; Sagatov, N.; Gavryushkin, P.N.; Litasov, K.D. Calcium orthocarbonate, Ca2CO4-Pnma: A potential host for subducting carbon in the transition zone and lower mantle. Lithos 2020, 370, 105637. [Google Scholar] [CrossRef]
- Gavryushkin, P.N.; Sagatova, D.N.; Sagatov, N.; Litasov, K.D. Formation of Mg-Orthocarbonate through the Reaction MgCO3+ MgO= Mg2CO4 at Earth’s Lower Mantle P–T Conditions. Cryst. Growth Des. 2021, 21, 2986–2992. [Google Scholar] [CrossRef]
- Gavryushkin, P.N.; Sagatova, D.N.; Sagatov, N.; Litasov, K.D. Orthocarbonates of Ca, Sr, and Ba—The Appearance of sp3-Hybridized Carbon at a Low Pressure of 5 GPa and Dynamic Stability at Ambient Pressure. ACS Earth Space Chem. 2021, 5, 1948–1957. [Google Scholar] [CrossRef]
- Banaev, M.V.; Sagatov, N.E.; Sagatova, D.N.; Gavryushkin, P.N. High-Pressure Crystal Structures of Pb2CO4 and PbC2O5 with Tetrahedral [CO4] and Pyrocarbonate [C2O5] atomic groups. ChemistrySelect 2022, 7, e202201940. [Google Scholar] [CrossRef]
- Gavryushkin, P.; Martirosyan, N.; Rashchenko, S.; Sagatova, D.; Sagatov, N.; Semerikova, A.; Fedotenko, T.; Litasov, K. First Experimental Synthesis of Mg Orthocarbonate by the MgCO3 + MgO= Mg2CO4 Reaction at Pressures of the Earth’s Lower Mantle. JETP Lett. 2022, 116, 477–484. [Google Scholar] [CrossRef]
- Binck, J.; Laniel, D.; Bayarjargal, L.; Khandarkhaeva, S.; Fedotenko, T.; Aslandukov, A.; Glazyrin, K.; Milman, V.; Chariton, S.; Prakapenka, V.B.; et al. Synthesis of calcium orthocarbonate, Ca2CO4-Pnma at P-T conditions of Earth’s transition zone and lower mantle. Am. Mineral. 2022, 107, 336–342. [Google Scholar] [CrossRef]
- König, J.; Spahr, D.; Bayarjargal, L.; Gavryushkin, P.N.; Sagatova, D.; Sagatov, N.; Milman, V.; Liermann, H.P.; Winkler, B. Novel Calcium sp3 Carbonate CaC2O5-I4d May Be a Carbon Host in Earth’s Lower Mantle. ACS Earth Space Chem. 2022, 6, 73–80. [Google Scholar] [CrossRef]
- Laniel, D.; Binck, J.; Winkler, B.; Vogel, S.; Fedotenko, T.; Chariton, S.; Prakapenka, V.; Milman, V.; Schnick, W.; Dubrovinsky, L.; et al. Synthesis, crystal structure and structure–property relations of strontium orthocarbonate, Sr2CO4. Acta Crystallogr. Sect. B 2021, 77, 131–137. [Google Scholar] [CrossRef]
- Spahr, D.; König, J.; Bayarjargal, L.; Gavryushkin, P.N.; Milman, V.; Liermann, H.P.; Winkler, B. Sr3[CO4]O Antiperovskite with Tetrahedrally Coordinated sp3-Hybridized Carbon and OSr6 Octahedra. Inorg. Chem. 2021, 60, 14504–14508. [Google Scholar] [CrossRef]
- Spahr, D.; König, J.; Bayarjargal, L.; Milman, V.; Perlov, A.; Liermann, H.P.; Winkler, B. Sr[C2O5] is an Inorganic Pyrocarbonate Salt with [C2O5]2– Complex Anions. J. Am. Chem. Soc. 2022, 144, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Spahr, D.; König, J.; Bayarjargal, L.; Luchitskaia, R.; Milman, V.; Perlov, A.; Liermann, H.P.; Winkler, B. Synthesis and Structure of Pb[C2O5]: An Inorganic Pyrocarbonate Salt. Inorg. Chem. 2022, 61, 9855–9859. [Google Scholar] [CrossRef] [PubMed]
- Sagatova, D.N.; Gavryushkin, P.N.; Sagatov, N.E.; Banaev, M.V. High-pressure transformations of CaC2O5—A full structural trend from double [CO3] triangles through the isolated group of [CO4] tetrahedra to framework and layered structures. Phys. Chem. Chem. Phys. 2022, 24, 23578–23586. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Glass, C.W.; Ono, S. High-pressure phases of CaCO3: Crystal structure prediction and experiment. Earth Planet. Sci. Lett. 2006, 241, 95–103. [Google Scholar] [CrossRef]
- Pickard, C.J.; Needs, R.J. Structures and stability of calcium and magnesium carbonates at mantle pressures. Phys. Rev. B 2015, 91, 104101. [Google Scholar] [CrossRef]
- Smith, D.; Lawler, K.V.; Martinez-Canales, M.; Daykin, A.W.; Fussell, Z.; Smith, G.A.; Childs, C.; Smith, J.S.; Pickard, C.J.; Salamat, A. Postaragonite phases of CaCO3 at lower mantle pressures. Phys. Rev. Mater. 2018, 2, 013605. [Google Scholar] [CrossRef]
- Binck, J.; Bayarjargal, L.; Lobanov, S.S.; Morgenroth, W.; Luchitskaia, R.; Pickard, C.J.; Milman, V.; Refson, K.; Jochym, D.B.; Byrne, P.; et al. Phase stabilities of MgCO3 and MgCO3-II studied by Raman spectroscopy, x-ray diffraction, and density functional theory calculations. Phys. Rev. Mater. 2020, 4, 055001. [Google Scholar] [CrossRef]
- Cerantola, V.; Bykova, E.; Kupenko, I.; Merlini, M.; Ismailova, L.; McCammon, C.; Bykov, M.; Chumakov, A.I.; Petitgirard, S.; Kantor, I.; et al. Stability of iron-bearing carbonates in the deep Earth’s interior. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Nagai, T.; Ishido, T.; Seto, Y.; Nishio-Hamane, D.; Sata, N.; Fujino, K. Pressure-induced spin transition in FeCO3-siderite studied by X-ray diffraction measurements. J. Phys. Conf. Ser. 2010, 215, 012002. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, L.; Gui, W.; Liu, J. Phase Stability and Vibrational Properties of Iron-Bearing Carbonates at High Pressure. Minerals 2020, 10, 1142. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How Evolutionary Crystal Structure Prediction Works–and Why. Accounts Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Bushlanov, P.V.; Blatov, V.A.; Oganov, A.R. Topology-based crystal structure generator. Comput. Phys. Commun. 2019, 236, 1–7. [Google Scholar] [CrossRef]
- Pickard, C.J.; Needs, R.J. High-Pressure Phases of Silane. Phys. Rev. Lett. 2006, 97, 045504. [Google Scholar] [CrossRef] [PubMed]
- Pickard, C.J.; Needs, R.J. Ab initio random structure searching. J. Phys. Condens. Matter 2011, 23, 053201. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Li, Z.; Stackhouse, S. Iron-rich carbonates stabilized by magnetic entropy at lower mantle conditions. Earth Planet. Sci. Lett. 2020, 531, 115959. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Aped, P.; Fuchs, B.; Goldberg, I.; Senderowitz, H.; Tartakovsky, E.; Weinman, S. Structure and conformation of heterocycles. 21. Probing the anomeric effect in orthoesters. Structure, conformation, and dynamic behavior of a unique orthooxalate: 2, 5, 7, 10, 11, 14-hexaoxa [4.4. 4] propellane. J. Am. Chem. Soc. 1992, 114, 5585–5590. [Google Scholar] [CrossRef]
- Junk, P.C. Supramolecular interactions in the X-ray crystal structure of potassium tris (oxalato) ferrate (III) trihydrate. J. Coord. Chem. 2005, 58, 355–361. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Lukinov, T.; Fu, J.; Zhao, J.; Davis, S.; Simak, S.I. Stabilization of body-centred cubic iron under inner-core conditions. Nat. Geosci. 2017, 10, 312–316. [Google Scholar] [CrossRef]
- Ishizawa, N. Calcite V: A hundred-year-old mystery has been solved. Powder Diffr. 2014, 29, S19–S23. [Google Scholar] [CrossRef]
- Gavryushkin, P.N.; Martirosyan, N.S.; Inerbaev, T.M.; Popov, Z.I.; Rashchenko, S.V.; Likhacheva, A.Y.; Lobanov, S.S.; Goncharov, A.F.; Prakapenka, V.B.; Litasov, K.D. Aragonite-II and CaCO3-VII: New High-Pressure, High-Temperature Polymorphs of CaCO3. Cryst. Growth Des. 2017, 17, 6291–6296. [Google Scholar] [CrossRef]
- Solomatova, N.V.; Caracas, R.; Manning, C.E. Carbon sequestration during core formation implied by complex carbon polymerization. Nat. Commun. 2019, 10, 789. [Google Scholar] [CrossRef]
- Kuang, H.; Tse, J.S. High-Temperature, High-Pressure Reactions of H2 with CaCO3 Melts. Phys. Status Solidi B 2022, 259, 2100644. [Google Scholar] [CrossRef]

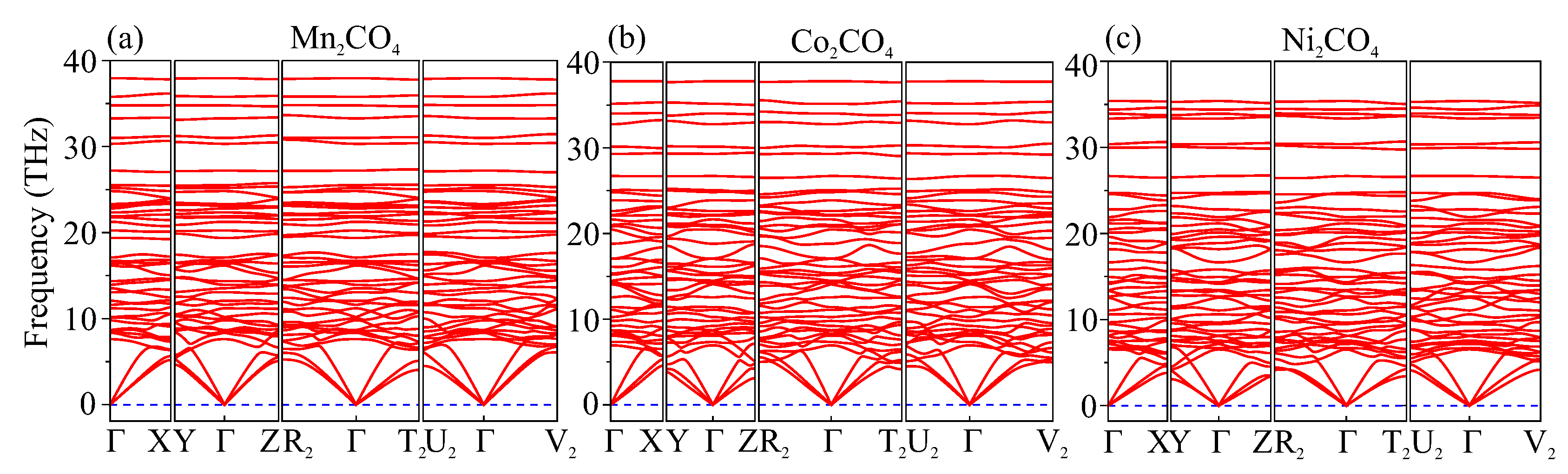
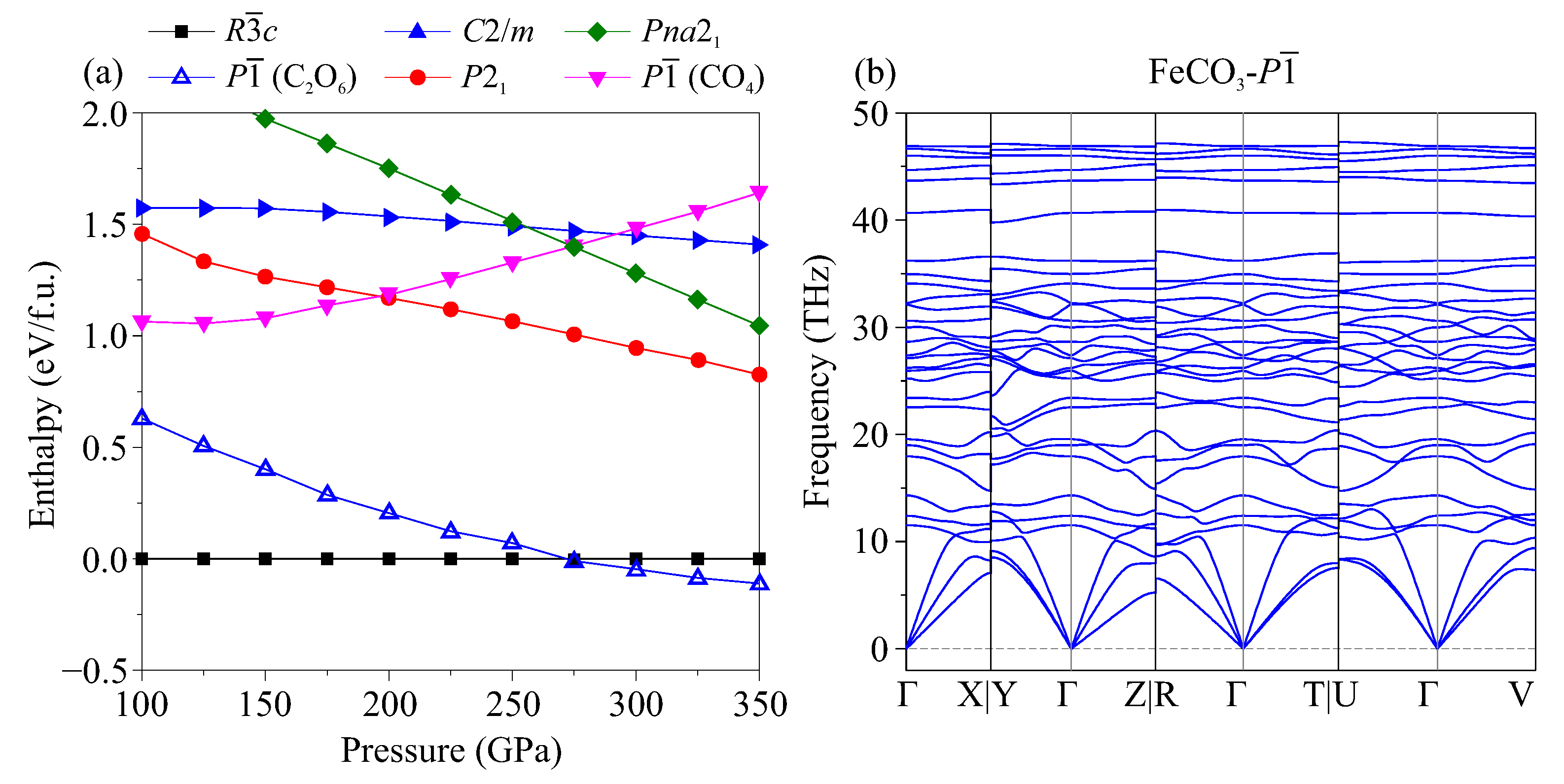
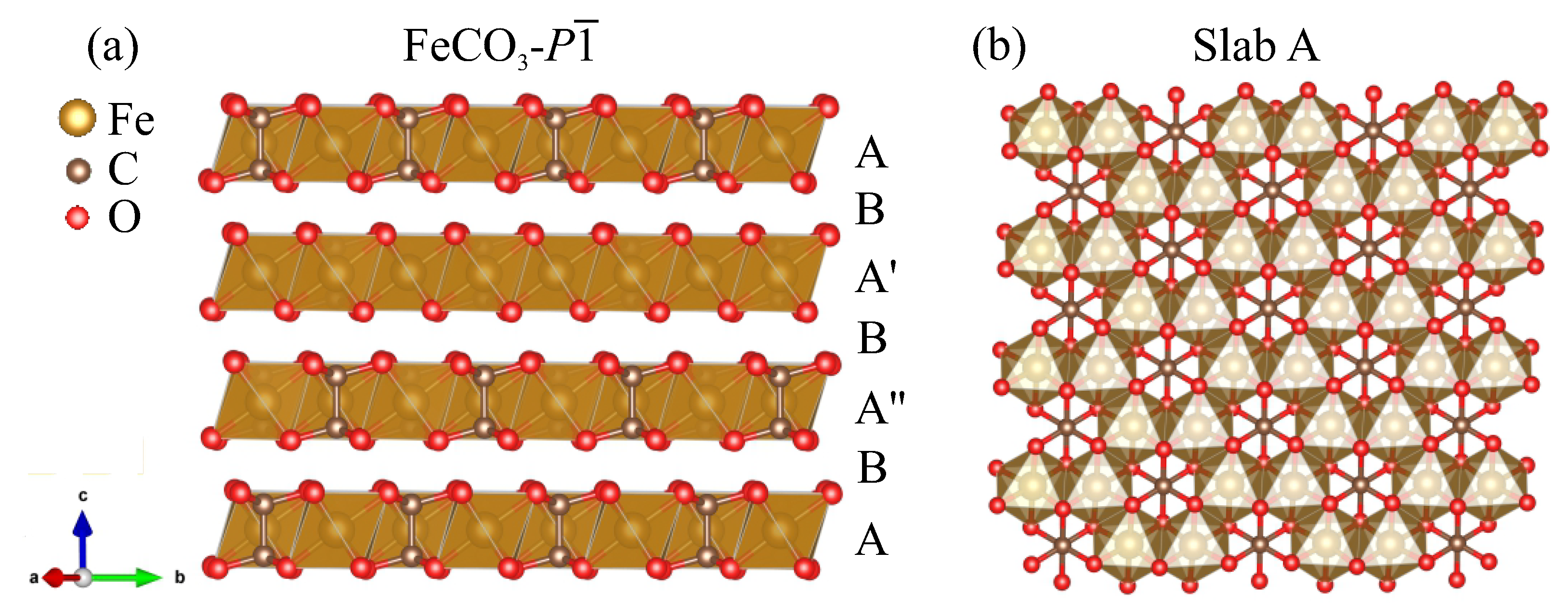
| Phase (#s.g.) | P (GPa) | Lattice Parameters (Å, deg) | Atom | Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| FeCO- | 50 | Fe | 0.7775 | 0.3775 | 0.0286 | |||
| = 103.63 | = 115.32 | = 90.81 | Fe | 0.4953 | 0.2401 | 0.4757 | ||
| C | −0.0905 | −0.0435 | 0.3287 | |||||
| O | 0.2903 | 0.2940 | 0.7483 | |||||
| O | 0.2825 | 0.8464 | 0.7596 | |||||
| O | 0.2239 | 0.4483 | 0.2571 | |||||
| O | 0.8404 | 0.0695 | 0.7651 | |||||
| FeCO- | 250 | Fe | 0.6729 | 0.8428 | 0.4959 | |||
| = 91.75 | = 116.73 | = 118.79 | C | 0.8665 | 0.4328 | 0.2999 | ||
| O | 0.5147 | 0.6023 | 0.7874 | |||||
| O | 0.8863 | 0.2857 | 0.7919 | |||||
| O | 0.1883 | −0.0950 | 0.8004 | |||||
| FeCO | FeCO | ||
|---|---|---|---|
| Atom | Bader Charge () | Atom | Bader Charge () |
| Fe | +1.195 | Fe1 | +1.282 |
| Fe2 | +1.215 | ||
| C | +2.228 | C | +1.609 |
| O | −1.141 | O1 | −1.045 |
| O2 | −1.078 | ||
| O3 | −0.924 | ||
| O4 | −1.059 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagatov, N.E.; Sagatova, D.N.; Gavryushkin, P.N.; Litasov, K.D. New High-Pressure Structures of Transition Metal Carbonates with O3C–CO3 Orthooxalate Groups. Symmetry 2023, 15, 421. https://doi.org/10.3390/sym15020421
Sagatov NE, Sagatova DN, Gavryushkin PN, Litasov KD. New High-Pressure Structures of Transition Metal Carbonates with O3C–CO3 Orthooxalate Groups. Symmetry. 2023; 15(2):421. https://doi.org/10.3390/sym15020421
Chicago/Turabian StyleSagatov, Nursultan E., Dinara N. Sagatova, Pavel N. Gavryushkin, and Konstantin D. Litasov. 2023. "New High-Pressure Structures of Transition Metal Carbonates with O3C–CO3 Orthooxalate Groups" Symmetry 15, no. 2: 421. https://doi.org/10.3390/sym15020421
APA StyleSagatov, N. E., Sagatova, D. N., Gavryushkin, P. N., & Litasov, K. D. (2023). New High-Pressure Structures of Transition Metal Carbonates with O3C–CO3 Orthooxalate Groups. Symmetry, 15(2), 421. https://doi.org/10.3390/sym15020421







