Abstract
This paper presents the results of investigations on the pyrolysis of tyre waste in a laboratory fixed-bed batch reactor. The results regarding the influence of either the reaction temperature (425, 450, 475, and 500 °C) and the flow of the inert gas (0, 100, 300, and 500 mL/min) on product yield (referred to as pyrolysis of waste tyres) are also considered and discussed. On the basis of the abovementioned findings, the most appropriate experimental conditions were selected to contribute to a higher yield of pyrolysis oil. The sample of pyrolysis oil obtained from the experiments carried out in the selected optimal conditions (reaction time 120 min, temperature 450 °C and the inert gas flow of 100 mL/min) was subjected to calorimetric and infrared spectroscopy analysis.
1. Introduction
Nowadays, communication in its broadest sense is the basis of the development of civilization and cannot even be imagined without developed traffic, in which road traffic plays a leading role in many elements. It is estimated that there are approximately 1.446 billion cars on the planet [1]. The consequences of the massive use of road vehicles are, in addition to the emission of various air pollutants, approximately 30.9 million tonnes of end-of-life tyres to manage only in 2019 [2]. European Union (EU) legislation regarding end-of-life tyres can be found in [3].
One of the techniques for tyre recycling is pyrolysis or thermal cracking. Pyrolysis is a thermochemical process that causes the decomposition of organic matter upon heating in an inert atmosphere. Unlike mechanical tyre recycling, where long polymer chains remain preserved, pyrolysis products are fragments of lower molecular mass. As a product of pyrolysis, a solid phase is formed as a pyrolysis char, basically char or soot, and a volatile fraction that is further decomposed into condensable hydrocarbons (pyrolysis oil) and gas. The relative share of individual phases is determined both by the chemical composition and the choice of the reactor for pyrolysis, and by the process operating conditions. As a matter of fact, by adjusting the temperature, the reaction time, the carrier gas flow rate, the heating rate, the product cooling rate, the pressure, the particle size of the starting raw material, the addiction of catalyst, etc., it is possible to control the rate and extent of decomposition, i.e., change the relative proportion of individual phases and the presence and yield of different products [4,5].
Pyrolysis of car tyre waste has been studied relatively extensively in the past, similarly to what has been conducted with other polymer-based materials, like plastics [6,7,8,9,10,11,12]. In a series of papers, an overview of significant research is given [13,14,15,16,17,18,19,20,21,22,23,24]. In some review papers [4,25,26,27], the latest achievements were presented in the field of pyrolysis plants for processing of tyre waste, while all the problems encountered in the management of this kind of waste, as well as the yield of pyrolysis depending on the type of reactor, catalyst and operating conditions are considered and critically discussed in [6,28,29,30,31,32,33].
Most research is based on the use of thermogravimetry (TG), and on examining the kinetics of the degradation process (under inert atmosphere) or examining the influence of various process parameters on the product yield. These experiments are carried out on small samples and most often in autoclaves, dedicated devices for conducting TG analysis. Under such conditions, there are no limitations in the heat transfer to the raw material particles, so these results cannot be fully applied to industrial processes [17]. Certain authors mention the possibility of controlling and changing the heating rate of the process, and the application of multiphase pyrolysis, in order to use the heat of the exothermic contribution of the process for providing energy to the next step of the sequence, endothermic in nature, thus reducing the required energy consumption [17,34,35].
As already mentioned, the yield and distribution of pyrolysis products depend on the selection of the process parameters as well as on the performance of the reactor systems in which the process takes place. Various data can be found in the literature that differ from author to author, but most fall within the following yield ranges (expressed in mass percentage of pyrolysis products): 10–30% for gas, 40–60% for oil and 30–40% for solid residue (basically pyrolysis char, char or carbon black) [36,37].
Tyre pyrolysis oil, also known as TPO, can be used in petroleum refineries and as a source of chemicals like BTX or limonene, which are used in the production of resins, surfactants, plastics and pharmaceutical compounds. TPO can contain significant amounts of sulphur (in form of SO2), which lead to the corrosion of pyrolysis equipment. Therefore, a desulfurization method should be performed. However, TPO can be directly used in boilers or after desulfurization, moisture removal, distillation or mixing with diesel fuel, and it can be used in engines with internal combustion [38,39,40]. Toteva and Stanulov [41] provided an overview of desulfurization methods for TPO.
Non-condensable gases are called pyro-gas or pyrolytic gas. These gases can be applied as a combustible fuel that can be used for the energy requirements for the pyrolysis process. The gaseous products from tyre pyrolysis are mixture of olefins, carbon oxides, hydrogen and sulfur and nitrogen compounds [39,42]. The temperature and heating rate are directly connected to the gas evolution during the pyrolysis process. When the gas production increases, the liquid products decrease, and vice versa [38].
The solid product of tyre pyrolysis is known as carbon black or char. Its contents vary depending on the tyre composition and pyrolysis condition. After the activation of the process, carbon black can be used as activated carbon [38,39]. Char can be used in printing ink or filler material in rubber goods [38]. Carbon black is also used as a solid fuel, with calorific value ranging between 25 and 34 MJ/kg, depending on the feedstock used [42].
Several authors investigated this residue with the aim of using it as activated carbon, after the activation process. Activated carbon is the product of two processes, namely the carbonization of raw material and the activation process. The first process increases the carbon content and builds the porosity, while the latter expands the structures of pores. Physical and chemical activation processes can be used for the production of activated carbon [43]. The following studies provide more details on carbon activation [44,45,46,47,48,49].
Rodriguez et al. [50] performed a chemical analysis of char obtained by pyrolysis at different temperatures. Chemical analysis determined that the solid residue contains approximately 84% m/m carbon, 2.3–2.6% m/m sulfur, which is important from the point of view of using char as a fuel, and that its heating value is lying in the interval 27–29 MJ/kg. Some authors state that the heating value of char is approximately 30 MJ/kg [36,51]. Choi et al. [52] conducted one- and two-stage pyrolysis of tyre waste. The authors studied the concentration of sulfur in the obtained pyrolysis oil and concluded that that pyrolysis oil obtained at temperatures around 500 °C had a lower sulfur content than the pyrolysis oil obtained at temperatures around 600 °C.
The liquid phase is considered the most important product of pyrolysis of tyre waste. Gas chromatography-mass spectrometry (GC/MS) is the most commonly used technique for analysing not only liquids, but all pyrolysis products. In their work, Laresgoiti et al. [53] provided one of the most complete descriptions of the liquid products of pyrolysis by applying GS/MS analysis, elemental analysis (proximate analysis), where the heating value of the corresponding products is also given. The liquid products of pyrolysis represent a complex mixture of C6-C24 organic compounds, including a lot of aromatics (53.4–74.8%), a certain amount of nitrogen compounds (2.47–3.5%) and oxide compounds (2.29–4.85%). Their calorific value is approximately 42 MJ/kg, which is a higher value than in commercial heating oils, but the presence of sulfur (1–1.4% m/m) is close to the upper limit, or higher than the permitted values. Rodriguez et al. [50] also reported a heating value of approximately 42 MJ/kg, with a nitrogen and sulfur content of 0.4 and 1.2 m/m, respectively. Furthermore, approximately 30% m/m of the total amount of the liquid phase is a light fraction with a boiling point between 70 and 210 °C, which is essentially the same as commercial petroleum, while 60% m/m has a boiling point in the range of 150–370 °C, which is within the range of typical commercial oils.
The yield and composition of pyrolysis gases vary and take on different values from one study to another, which can be attributed to different operating conditions associated with the process, reactor, etc. The approximate gas yield in tyre pyrolysis is 10–30% m/m [3,54] and essentially the pyrolysis gas yield increases with increasing the temperature. Its heating value is between 30 and 40 MJ/kg. Basically, different authors agree with the fact that pyrolysis gases are H2, H2S, CO, CO2, CH4, C2H4, C3H6, and other light carbohydrates [14].
Regarding the quantitative and qualitative composition of the products of the pyrolysis process, a very wide range of published results can be observed. The representation of each individual step depends on the operating conditions of the process, i.e., temperature, pressure, heating rate, size of raw materials, heat transfer method, catalysts, etc., as well as the type of reactor used for pyrolysis.
Depending on the temperature range, the pyrolysis process can be divided into three categories: slow pyrolysis when the temperature does not exceed 300 °C, moderate or medium-temperature pyrolysis when the temperature of the pyrolysis process is between 300 and 500 °C and fast pyrolysis or high-temperature pyrolysis, when temperature is above 500 °C [55].
Most authors examine tyre pyrolysis in the temperature range of 400–600 °C [15,25,56,57,58,59,60], mainly focusing their attention on the liquid phase yield. By increasing the pyrolysis temperature, the proportion of pyrolysis gas increases and the proportion of char and liquid phase decreases. The increase in gas yield and decrease in liquid products yield are directly related to the increase in temperature, due to decomposition of vapours into stable gases and the occurrence of secondary re-polymerization. The pyrolysis process begins at around 237 °C, where weaker molecular bonds break and new, shorter molecules are created. These new molecules have a lower molecular weight than the parent molecule. Long exposure to high temperatures causes the breakdown of organic molecules that eventually leave the char. Akkouche et al. [61] studied the pyrolysis of waste truck powder in a fixed-bed reactor with a water-cooled liquid recovery system and a gas sampling valve. They varied the heating rates between 5 and 25 °C/min and concluded that heating rate had only a significant influence on the gas yield. Heating rates between 10 and 15 °C/min minimize the evolution of CO, CO2 and H2 and promote the formation of C2H6, C3H6, C4H6 and H2S.
Williams et al. [62] performed pyrolysis of tyre waste in a temperature range of 300–720 °C and a heating rate ranging from 5 to 80 °C/min, and found that the maximum conversion of tyres occurs at 600 °C, if the pyrolysis oil yield is considered as a reference. Similar research results are presented by Clark et al. [63].
Laresgoiti et al. [64] demonstrated that pyrolysis temperatures above 500 °C have no significant effect on gas yield and carbon residue. However, the temperature change affects the composition of the pyrolysis gas products. Rodriguez et al. [50] performed pyrolysis with a raw material with a cross section of 2–3 cm, as a simulation of the whole tyre, at temperatures in the range of 300–700 °C. Their report states that the distilled liquid products are a mixture of hydrocarbons, containing 0.4% m/m of nitrogen and 1.2% m/m of sulfur. Of this, approximately 30% m/m is a volatile fraction with a boiling point of 70–210 °C and approximately 60% m/m boils in the temperature range of 150–370 °C. After the occurrence of the pyrolysis of the tyres, the hydrogen sulphide content is below 0.3% m/m, while in the laboratory analyses that preceded the pyrolysis, approximately 2% m/m of sulfur was present in the raw material. After pyrolysis, sulfur remained in the char in the form of zinc sulphide and calcium sulphide [16]. These data are extremely important if pyrolysis oil is to be used as fuel, and at the same time indicate the disadvantages of using char as an energy source due to the increased sulfur content. The yield of liquid products increases at temperatures between 400 and 500 °C. Subsequently, at temperatures above 500 °C, no significant changes occur and the yield of liquid products is constant. The increase in gas yield in relation to the temperature change is 2.4% m/m at 400 °C, while with an increase in temperature up to 700 °C the gas yield increases to 4.4% m/m [65]. Similar behaviour is found by Xu et al. [66] with the FTIR spectrum at different temperatures.
Islam et al. [67] examine the influence of temperature, raw material size and heating rate on pyrolysis yield and product composition; the maximum yield of the liquid phase (49%) is obtained at 475 °C, raw material in the form of a cube of 4 cm side, with a heating rate of 5 °C/min under nitrogen carrier gas in a reactor with tubular flame heaters.
Zabanioti and Stavropolos [68] performed pyrolysis under helium atmosphere in the temperature range 390–890 °C and at a heating rate of 70–90 °C/min, and under these conditions they conclude that the char yield decreases with temperature to a final value of 20% m/m from the initial total mass of the raw material at around 830 °C. The gas yield also increases with increasing temperature and reaches even at around 830 °C a maximum of 73% m/m of the initial total mass of the raw material. Lee et al. [20] in similar temperature ranges (700–880 °C) obtain a char yield of approximately 32%, the gas yield with increasing temperature increases from 30% m/m to a maximum of 40% m/m to the detriment of liquid products which ultimately contain around 25%. Increasing the temperature does not significantly affect the char yield.
Chang [69] showed in his research that the yield of tyre pyrolysis is distributed between 30 and 53% m/m gas, 28 and 42% m/m liquid distillates and 14 and 28% m/m of char. Barobboti et al. [70] performed pyrolysis in the temperature range of 400–460 °C, with nitrogen as the carrier gas, which had a flow rate of 0.2–0.5 m3/h, while the size of the raw material was in the range 2–20 mm. As optimal conditions, seen from the point of view of the yield of liquid distillates, they indicated a temperature of 430 °C, with a N2 flow rate of 0.35 m3/h and a raw material size of 10 mm. Under the aforementioned experimental conditions, the yield ratio is 32.5% m/m char and 51% m/m liquid distillates and 16.5% m/m pyrolysis gas. The introduction of a carrier gas into the pyrolysis process of tyre waste increases the yield of the liquid phase at the expense of the solid residue and pyrolysis gas [71]. The yield depends on the carrier gas, so the use of water vapor as a carrier leads to a lower content of sulfur in the liquid phase (0.129% m/m), while its content increases in the solid phase (2.5% m/m). However, the use of N2 or H2 as a carrier gas produces the opposite effect, which is again important from the point of view of using pyrolysis products as a fuel. Murena et al. [72] performed pyrolysis with hydrogen in order to better saturate the broken bonds of the polymer chain. They came to the conclusion that, using this method, pyrolysis can be performed at slightly lower temperatures and that the reaction takes place in the temperature range of 390–430 °C. At such parameters, the yield of liquid distillates is maximized, while the char is reduced to a minimum. Roy et al. [51] performed pyrolysis with a constant temperature of 500 °C, but varied the pressure in the range of 0.8–28 kPa. It was shown that the pressure change did not significantly affect the pyrolysis yield as a whole and that the change in pressure did not affect the change in the yield of any individual product. Nevertheless, the pressure change significantly affects the composition of the obtained products, especially the composition of char and liquid products. An overview of the influence of pyrolysis process parameters on yield investigated by various authors is presented in the review paper by Juma et al. [14].
Various types of reactors are used in tyre pyrolysis, such as fixed-bed reactors, vacuum reactors, fluidized bed reactors, etc. Williams [25] provided an overview of pyrolysis yields for different types of reactors, showing the conditions under which the experiment was performed and the type of pyrolysis reactor. From the above review, one can observe a fairly wide range of yields of different products, depending on the conditions applied and type of reactor. Generally, in fixed-bed reactors the yield of liquid, solid and gaseous products varied between 20.9, 40.7 and 23.9% m/m (at 950 °C, below ~2 °C/min), while between 63, 30 and 7% m/m (at 350–450 °C, below 30 °C/min), respectively.
In general, based on the abovementioned findings, it can be stated that research in the field of pyrolysis of tyre waste indicates different results; some authors state that changes in process parameters do not have a significant impact on products; others have focused their research mainly on temperature, as a process parameter. The characteristic of the research cited is that it was carried out under different conditions, often not sufficiently explained, and in different types of reactors. In addition, the types of samples are mostly different, both in terms of shape and mass, the tests in most cases are reduced to TG analysis, coupled with DTG data (first order derivative of TG) [73]. It can be stated that there is a significant data variation on the working conditions of the process, on the configuration of the reactor systems when it comes to tyre pyrolysis.
Taking into account all the previously mentioned facts, this study aims to shed light by analysing a significant number of appropriate cases studied with the aim of finding the most suitable temperature and inert gas flow rate to obtain the maximum pyrolysis oil yield in a fixed-bed reactor. Therefore, this study focused on investigating the influence of temperature on the yield of pyrolysis products in a narrower temperature range and with a lower carrier gas flow rate than other similar studies.
2. Materials and Methods
2.1. Materials and Materials Characterization
Waste tyres from cars, trucks and work machines were shredded and a granulometric analysis of a sample of shredded tyres was carried out and the average diameter of the particles was determined to be 1.28 mm. Sieves with an opening size of 2.0 were used, followed by those with 1.6, 1.1, 0.8, 0.5 and finally with 0.25 mm. Proximate or technical analysis of the sample was carried out, according to the regulations for proximate analysis of solid fuel. The results of the proximate analysis of the sample are presented in Table 1. The listed results essentially correspond to the average results published by other authors [74].

Table 1.
Results of proximate analysis of tyre waste.
2.2. Reactor Setup
The pyrolysis experiments were conducted in a lab-scale fixed-bed batch reactor, which allows for examining the influence of the most important process parameters (temperature, reaction time, carrier gas flow, heat consumption), with a process control system. All temperatures were measured with K-type thermocouples, and recorded using the OMRON CX-Thermo Support software Ver. 4.0. The block diagram of the reactor used is presented in Figure 1.
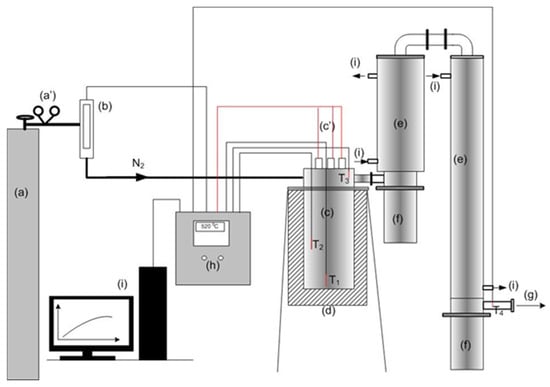
Figure 1.
Block diagram of the experimental fixed-bed pyrolysis reactor: (a) cylinder with nitrogen gas; (b) gas mass flow meter; (c) the pyrolysis reactor vessel; (d) thermal insulation; (e) steam condensation system; (f) separation system-vessels for receiving condensate; (g) discharge of non-condensable gases in the gas washing system; (h) control box; (i) PC; (a′) reducing valve; (c′) electric heater connection wiring.
The reactor has a shape of a vertical cylinder (101.6 × 2 mm). The reactor vessel contains the bottom or body and a lid or upper body, with flanged bottle joint with gaskets. The reactor body is 200 mm high and is separated from the upper part. It also works as a dosing system. A second cylinder is connected to the reactor body from the upper side. It is a cover with a total length of 40 mm. It also carries three K-type thermocouples and three stainless steel tubes (A304), in which the electric cartridge heaters are housed (total power 3 × 350 W). When the lid is connected to the reactor body, electric heaters and thermocouples are placed in the reactor body. The heaters extend to the bottom of the reactor and the thermocouples are arranged in the reactor body, at different heights. The thermocouple T1 is placed 7 mm from the bottom of the reactor, T2 at 90 mm and T3 at 220 mm. The reactor is made of stainless steel and a 3 cm thick layer of thermal insulation stone wool is applied to the outer wall of the reactor.
The regulation of the operation of the heater, i.e., the temperature control, was carried out using the CelciuX temperature controller (EJ1N-TC4A-QQ), from the OMRON Corporation (Kyoto, Japan), after adjustment of the PID constants. Inert gas flows were measured with a mass flow meter, Bronkhorst High-Tech B.V., model MASS-VIEW MV-304 (Ruurlo, The Nederland), which has the additional possibility of fine flow adjustment and covers measurement ranges between 40 and 20,000 mL/min. Nitrogen with a purity of 99.99% was used as the carrier gas. The description of the experimental techniques and reactors can be found in previous studies [75,76,77].
The yield of the liquid phase, pyrolysis oil, was determined by measuring the mass of the products obtained after their collection in the separation system. A smaller portion of the product remaining in the inner walls of the condensation system was also collected and measured and presented together with the mass of the liquid phase from the separation system. The condensation system is oversized to ensure complete condensation and consists of two double pipe heat exchangers, Figure 1e. The solid residue, i.e., pyrolysis char or carbon black, was determined by measuring the mass of residue, found in the reactor after the end of the process and cooling. The yield of the gaseous phase was determined by the material balance, calculated as the difference between the mass of the raw material and the sum represented by the mass of the liquid phase and that of the solid residue. All samples, as well as products, were weighed on a KERN PLJ 3500-2NM laboratory scale. The sulfur content was determined by X-ray-fluorescence spectrometry (XRF Oxford), according to the ISO 20847 method [78]. The qualitative chemical analysis of the fuels obtained was performed by FTIR spectroscopy (FTIR 1600 Perkin Elmer), CEI IEC 590 method. The heating value of the obtained biofuels was determined with a Parr calorimeter (mode 6400 Automatic Isoperibol Calorimeter) using the dynamic measurement method. All measurements were carried out at least 3 times, and the results listed in the tables represent the arithmetic means of the measurements.
3. Results
When choosing temperatures for conducting experiments, most researchers start from the results of TG or DTG analyses, and, based on the corresponding curves, the temperature ranges of thermal decomposition are observed while during the reaction a suitable range of time is usually chosen, so that maximum decomposition can be expected at the selected temperature. In the literature, it is possible to find a series of published TG and DTG curves of tyre pyrolysis or, as often stated, thermal decomposition in an inert atmosphere, which may differ depending on different conditions of conducting experiments [14,37,69,71,79,80,81]. Considering the abovementioned facts, the reactor temperature of 425 °C was chosen for the initial recording, because only at that temperature can higher decomposition rates be expected, as subsequent experiments will demonstrate. For the second temperature, 500 °C was chosen, since according to the results of numerous TG and DTG curves, the maximum decomposition rates of the examined polymers are lower than the given temperature. Furthermore, numerous studies examine processes at ranges above 500 °C [52,82,83,84]. Regarding the reaction time, 120 min was chosen for the initial experiment, considering that the indicated time is sufficient for complete thermal decomposition to take place at the selected temperature.
3.1. Effect of Temperature on Tyre Pyrolysis
Table 2 shows the effect of temperature on the yield of the pyrolysis process. A significant increase in raw material conversion, i.e., reduction of the mass of the solid residue in the reactor, is observed in the range from 425 to 500 °C. The maximum yield of the liquid phase (pyrolysis oil) is obtained at 450 °C. At temperatures of 475 and 500 °C, the pyrolysis oil yield decreases at the expense of a greater yield of gaseous products. This can be explained by the fact that secondary reactions start to dominate at higher temperatures, i.e., reactions in which molecular chains break down further, producing less oil and more gaseous products [21,82,85].

Table 2.
Product yield as a function of temperature (120 min, inert gas flow rate of 500 mL/min, heat rate 14 °C/min).
In the pilot plant tested for pyrolysis, i.e., the tested reactor with fixed-bed and characteristic heating of the mixture, the optimal temperature of the reactor for the pyrolysis of tyre waste, in terms of the maximum yield of liquid products, is 450 °C at time of 120 min. The specified values were used as fixed values for the next test series.
3.2. Effect of Inert Gas Flow on Tyre Pyrolysis
A range from static air up to 500 mL/min was chosen to examine the influence of the flow of carrier gas, nitrogen, on the yield of the pyrolysis process in the investigated plant. Higher flow rates were not tested due to the possibility that the contents of the reactor, raw material and solid product are transported in the gas flow, i.e., due to fluidization of the bed. Since inert gas is not only used to create an inert atmosphere, it is also used to remove steam and gaseous products from the reactor. It is clear that when the flow of the carrier gas varies, the residence time of these products in the reactor actually changes.
Table 3 shows the yields of pyrolysis products as a function of the carrier gas flow.

Table 3.
Product yield as a function of the carrier gas flow rate.
The experiment was performed at a previously defined optimal reaction time of 120 min and a temperature of 450 °C.
This plot shows an increase in the yield of pyrolysis oil from the initial value of 37.13% m/m with no carrier gas flow up to a maximum of 43.63% m/m at a flow rate of 100 mL/min, after which there is a relatively negligible decrease in the yield of pyrolysis oil, at the expense of increasing the solid residue up to a maximum of 43.53% m/m at the highest value of nitrogen flow. From Table 3 it can be seen that with nitrogen flow values of 100, 300, and 500 the yield of the individual products almost did not differ. It can however be emphasized that there is no increase in the yield of liquid products with higher flows of inert atmosphere, i.e., carrier gas, due to the shorter retention time of the products of primary reactions in the high temperature zone, which causes further secondary reactions, i.e., shortening of molecular chains and formation of gaseous (non-condensable) products. It is obvious that with higher carrier gas flows, purely fluid dynamic conditions begin to prevail in the tested reactor, i.e., it can be assumed that there is more intensive contact of steam and gaseous products with the heater surface in the upper part of the reactor, whose temperature is normally higher than that of the lower part of the reactor, and more intense secondary reactions take place, which give a greater yield of gaseous products.
From the information provided it can be seen that the process still depends on the carrier gas flow. In the experiment with static air (no gas flow), the raw material conversion is significantly lower (52.55% m/m of unreacted raw material) compared to all other experiments where a carrier gas flow was present. It can be concluded that the introduction of carrier gas into the tyre waste pyrolysis process increases the yield of the liquid phase at the expense of the solid residue or pyrolysis char and pyrolysis gas.
Based on the results presented in Table 3, it can be concluded that for the tested laboratory scale pyrolysis reactor, the optimal carrier gas flow, in terms of maximizing the amount of liquid products, is 100 mL/min.
It should be noted that in all experiments an extremely high yield of solid residue is obtained and it is necessary to examine the qualitative and energetic value of the obtained char in future research, as well as the possibility of further gasification of the char.
3.3. Calorimetry, Chemical and Proximate Analysis
Pyrolysis oil samples obtained from this experiment, carried out under selected optimal conditions (120 min reaction time, 450 °C and 100 mL/min inert gas), were subjected to calorimetric analysis, i.e., determination of the heating value. The heating value of the obtained fuels was determined calorimetrically, and was 42 MJ/kg, which is slightly lower than the heating value of higher quality coals (43 MJ/kg), as well as the heating value of oil (44 MJ/kg).
The determination of the composition of pyrolytic oil, i.e., the distribution of carbon atoms in the paraffin chains, aromatic and naphthenic rings, was carried out using the CEI IEC 590 method. In general, this method is based on the relationship between measured absorbance at 1610 cm−1, 720 cm−1, and n-d-M analysis (ASTM D3238 [86]), which allows the determination of the fraction of carbon atoms in aromatic ring structures. Figure 2 shows the IR spectra of pyrolysis oil obtained under the selected optimal pyrolysis conditions (120 min reaction time, 450 °C and 100 mL/min inert gas). On the IR spectra, the characteristic absorption peaks at the wave numbers 1610 cm−1 and 720 cm−1 were identified and measured. This was used to determine the fraction of carbon content in aromatic rings-CA (corresponding to the peak at 1610 cm−1), the fraction of carbon content in paraffin chains-CP (corresponding to the peak at 720 cm−1) and the rest of the carbon contained in naphthenic rings-CN as a sum up to 100:
CN = 100 − (CP + CA)
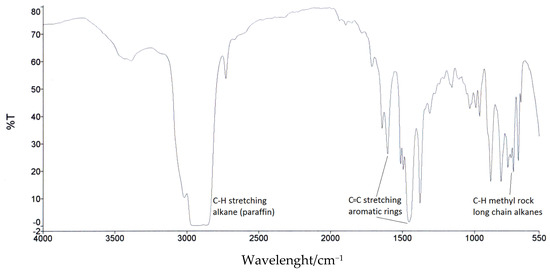
Figure 2.
FTIR spectrum (4000–500 cm−1) of pyrolysis oil.
In general, the presence of aromatic compounds can be identified by the presence of medium intensity bands at 1640 and 1450 cm−1 due to the tension in −C=C bonds. This is also confirmed by the presence of medium intensity bands at 690 and 730 cm−1 and a band at 3000 cm−1, due to «out of plane» bending of C(sp2)-H and tension of the C(sp2)-H, respectively. The presence of paraffinic compounds can be confirmed by bands located between 2900 and 2850 cm−1, which correspond to C(sp3)-H tension or single bonds typical of alkanes. The characteristic band at 720–725 cm−1 can only be seen in long chain alkanes corresponding to C-H methyl rock.
Furthermore, the results of sulfur content in the obtained pyrolysis oil (0.407% m/m) (Table 4), indicate its potential for use as an energy source.

Table 4.
Analysis of the optimal pyrolysis oil sample (tyre pyrolysis at defined optimal conditions 120 min, 100 mL/min, 450 °C).
3.4. Proximate Analysis of Pyrolysis Char
These tests included the proximate/technical analysis of char, i.e., the determination of the following parameters: moisture content, ash, char residue, combustible materials, volatile materials, and fixed carbon. The tests were conducted according to the procedures for the proximate analysis of solid fuel, and a detailed description of the methods for determining individual components can be found in [87]. The results of laboratory tests on char obtained by pyrolysis of tyre waste are shown in Table 5.

Table 5.
Results of the proximate analysis of the char sample.
According to the results of proximate analysis of tyre samples (Table 5), it can be seen that the content of volatile matter is 5.44%, while the content of fixed carbon is 81.72%, which is approximately the same as data in the literature [58,66]. In general, moisture, ash and combustible materials make up 100% of the total matter. Fixed carbon (81.72%) is only part of combustible matter, while the remaining part (5.44%) is volatile matter. The sum of fixed carbon (81.72%) and ash (12.11%) constitutes the char residue (93.83%). The starting raw material, i.e., rubber granulate, contains traces of metal tyre parts which ultimately end up in ash as part of the final product, i.e., char, which essentially gives a higher ash content. In this sense, before further application of char, separation of those metal parts, for example by using electromagnetic separation, should be considered. However, ash content of pyrolytic char (12.11 wt%) is in agreement with already published results regarding tyre-derived char ranging from 12.32 to 14.58 wt% [59]. Galvagano et al. [88] reported an ash content in tire pyrolytic char of between 11.78 and 15.33 wt%.
Pyrolysis char samples obtained from the pyrolysis of tyres under the optimal conditions indicated to obtain the maximum yield of the liquid phase (reaction time 120 min, 450 °C and inert gas flow 100 mL/min) were subjected to calorimetric analysis. Using a dynamic measurement method, the heating value of 31 MJ/kg was recorded. Based on all the above results of the proximate analysis, the proportion of char residue, and the high heating value, this product has exceptional potential to be used as an energy source. It is worth mentioning that this solid product of tyre pyrolysis can also be used as an adsorbent or catalyst carrier. That is, after the activation process, physical or chemical, changes occur in the irregular pore structure of the carbon black matter, whereby the entire structure becomes more voluminous and more crystalline in nature, that is, more symmetrical.
4. Discussion
In the study of Rodriguez et al. [50], tyre pyrolysis was examined from the point of view of liquid phase yield, in a reactor with a fixed bed. It is shown that the yield of the liquid phase constantly increased in the temperature interval 300–500 °C, and thereafter no significant changes occur when increasing the temperature. Suhanya et al. [74] stated in their study of the yield of the liquid phase that the proportion of light oils in the liquid phase, such as benzene and kerosene, increases with an increasing temperature, while the yield of pyrolysis char shows no significant variations. In the study of Rofiqulisam et al. [63] in the temperature range 375–575 °C, a yield of liquid products of 42 ± 2.3% m/m (at a temperature of 375 °C) was obtained. Initially, the yield increases with an increasing temperature up to a maximum of 49 ± 1.3% m/m (at the temperature of 475 °C), while with a further increase in temperature the yield decreases to 42 ± 1.4% m/m (at a temperature of 575 °C) [67]. Aydin and Ilkilic [89] performed pyrolysis of tyre waste in a 1.15 L fixed-bed reactor with nitrogen as a carrier in the temperature range 400–700 °C. In their study, it was stated that the pyrolysis oil yield varies from 31% m/m at 400 °C, increases to 40% m/m at 500 °C and that further changes in yield with increasing temperature were negligible. They also investigated the effect of carrier gas flow on yield and found that the effect was negligible. In the research of Kar et al. [57], the results of the study on the influence of temperature on tyre pyrolysis in a fixed-bed reactor were presented. A 10 g sample of raw material, nitrogen as a carrier gas, was used at a heating rate of 10 °C/min and in the temperature range 375–500 °C, whereby the maximum pyrolysis oil yield of 60% m/m at 425 °C has been achieved. This is significantly different from our study or the studies mentioned above, but the result is not comparable because the sample is much smaller, so heat transfer problems in such small systems are almost negligible. For example, in experimental conditions similar to Kar et al. [57], Banar et al. [56] achieved a maximum pyrolysis oil yield of 38.8% m/m, char 34% m/m and gas 27.2% m/m. The experiment was conducted at 400 °C with a heating rate of 5 °C/min. Pyrolysis oil yields of 38% m/m at a temperature of 500 °C, under a heating rates of 15 °C/min, were also obtained by Laresgoiti et al. [53], while according to another study [90], a yield of 40% m/m of oil, 40% w/m of char and 20% w/m of gas was obtained.
From the above discussion, it can be seen that most studies on tyre waste pyrolysis consider a wider temperature range than that considered in this study. These studies conclude that a higher temperature, generally above 500 or 475 °C, is not necessary to achieve maximum pyrolysis oil yield. Contrary to those studies, a narrower temperature interval was observed in this study, and it is shown that even a lower temperature, up to a maximum of 450 °C, is sufficient to obtain the maximum oil yield.
It can be concluded that the yield of liquid products, as well as the conversion of the raw material, depend on experimental conditions (process parameters), the composition of the raw material, the type of plant in which the process is performed, the sample size, from the reaction time. Therefore, for each system, or plant in which the pyrolysis process takes place, it is necessary to clearly indicate the specified parameters, and examine the corresponding yield.
According to the results found in this study, the introduction of carrier gas into the tyre waste pyrolysis process increases the yield of the liquid phase at the expense of the solid residue or pyrolysis char and pyrolysis gas. However, the study of Hopa et al. [91] analysed the effect of the inert gas flow rate at a temperature of 450 °C, with a heating rate of 10 °C/min. The nitrogen flow rate varied between 500, 750, and 1000 mL/min. The maximum oil yield was obtained with a nitrogen flow rate of 1000 L/min (53.33 wt%). However, this study analysed oil yield at higher inert gas flow rates than our study. Nevertheless, we concluded that at lower carrier gas flow rates, the liquid oil yield did not change significantly. It is sufficient to introduce a minimum flow rate of the carrier gas (100 mL/min in this case) just to maintain an inert atmosphere and facilitate the removal of steam from the reactor.
According to current EU norms, prescribed by Directive 2016/802 of the European Parliament and of the Council [92] on the reduction of the sulfur content of certain liquid fuels, the obtained oil could be used as a marine fuel, since the sulfur content does not exceed 0.5% by mass. The pyrolysis oil obtained from tyres has a high heating value of 42 MJ/kg and its direct use as a fuel is possible, from the point of view of heating value, viscosity and sulfur content. Other authors also state that the heating value of pyrolysis oil obtained in similar experiments is 42 MJ/kg [50,53,61].
The FTIR results are slightly different from the statements of other authors who analysed the pyrolysis of tyre waste, which was to be expected considering the different conditions of the experiments and the type of reactor, as already described. For example, when testing tyres in a fixed-bed reactor, it is stated that the FTIR results indicate that the distribution of hydrocarbons by peaks is as follows: aromatic hydrocarbons 35.60% m/m; paraffinic 55.10% m/m and naphthenic 9.3% m/m [93]. Gonzalez et al. [94] obtained similar results by FTIR analysis of pyrolysis oil obtained from tyres, such that the distribution of hydrocarbons according to the peaks is: aromatic hydrocarbons 36.70% m/m; paraffinic 51.40% m/m and naphthenic 11.90% m/m.
5. Conclusions
Based on the results presented and discussed in this study, the following conclusions can be drawn:
- Research on the influence of process parameters on the maximum pyrolysis oil yield during the pyrolysis of tyres in a fixed-bed reactor shows that the optimal conditions are as follows: a reaction time of 120 min, reactor temperature of 450 °C, inert gas flow of 100 mL/min, with an installed heating power of 1000 W and a heating rate of 14 °C/min. This demonstrated the possibility of choosing a lower optimal temperature than in previous similar studies.
- This study demonstrates that selecting a minimum carrier gas flow rate of 100 mL/min is sufficient just to maintain the inert atmosphere and support the steam flow from the reactor.
- Under optimal conditions, pyrolysis of tyre waste gives a product composed of: 43.63% m/m of pyrolysis oil, 13.61% m/m gas and 42.76% m/m solid residue. The total conversion was 57.24%.
- The oil obtained from waste tyres is the most suitable for energy use, due to its high heating value (42 MJ/kg). This is close to the heating value of high-quality coals (43 MJ/kg) and the heating value of oil (44 MJ/kg). The results of FTIR analysis of the pyrolysis oil show the following content in mass percentages: aromatic compounds 32.59%, paraffins 51.06% and naphthenes 16.35%.
- The low sulfur content (0.407%) in the obtained pyrolysis oil also indicates its potential use as an energy source. The prescribed value of sulfur content in heating oil is 1% m/m, for four types of liquid petroleum fuels (light special LS, light L, medium LUS and heavy oil LUT).
- Pyrolysis char or carbon black obtained from the pyrolysis of tyre waste also has a high calorific value (31 MJ/kg), and can be used as a solid fuel as well as an adsorbent, catalyst or catalyst carrier after the activation process. Considering the increase in ash content of 12.11 wt% due to the presence of trace metal particles, an additional step for their separation must be considered before its further use as a raw material.
Author Contributions
Conceptualization, S.P., M.D. and S.V.C.; methodology, S.P. and G.T.; software, S.P. and G.T.; investigation, G.T., M.D. and S.P.; writing—original draft preparation, G.T., M.D. and S.P.; writing—review and editing, S.P. and M.D; supervision, S.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- How Many Cars Are There in the World? PD Insurance Blog. 2022. Available online: https://www.pd.com.au/blogs/how-many-cars-in-the-world/ (accessed on 5 October 2023).
- Valentini, F.; Pegoretti, A. End-of-life options of tyres. A review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Radley-Gardner, O.; Beale, H.; Zimmermann, R. (Eds.) Fundamental Texts On European Private Law; Hart Publishing Ltd.: Oxford, UK, 2016; ISBN 978-1-78225-867-4. [Google Scholar]
- Gao, N.; Wang, F.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire pyrolysis char: Processes, properties, upgrading and applications. Prog. Energy Combust. Sci. 2022, 93, 101022. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Papuga, S.; Djurdjevic, M.; Ciccioli, A.; Vecchio Ciprioti, S. Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry 2023, 15, 38. [Google Scholar] [CrossRef]
- Cafiero, L.; Castoldi, E.; Tuffi, R.; Vecchio Ciprioti, S. Identification and characterization of plastics from small appliances and kinetic analysis of their thermally activated pyrolysis. Polym. Degrad. Stab. 2014, 109, 307–318. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Pyrolytic degradation of common polymers present in packaging materials. J. Therm. Anal. Calorim. 2019, 138, 2683–2689. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Polymer packaging waste recycling: Study of the pyrolysis of two blends via TGA. J. Therm. Anal. Calorim. 2020, 142, 1891–1895. [Google Scholar] [CrossRef]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Vecchio Ciprioti, S. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef]
- Mavukwana, A.; Sempuga, C. Recent developemnts in waste tyre pyrolysis and gasification processes. Chem. Eng. Comm. 2022, 209, 485–511. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, T.H.; Nguyen, H.P. Scrap tire pyrolysis as a potential strategy for waste management pathway: A review. In Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; Taylor and Francis, Ltd.: London, UK, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Juma, M.; Kore, Z. Pyrolysis and combustion of scrap tire. Pet. Coal. 2006, 48, 15–26. [Google Scholar]
- Cunliffe, A.M.; Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Kaminsky, W. Recycling of polymers by pyrolysis. J. Phys. IV 1993, 3, C7-1543–C7-1552. [Google Scholar] [CrossRef]
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Lee, C.W.; Hui, C.W. Integrated kinetics and heat flow modelling to optimise waste tyre pyrolysis at different heating rates. Fuel Process Technol. 2011, 92, 856–863. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. Liquefaction of waste tires by pyrolysis for oil and chemicals—A review. J. Anal. Appl. Pyrolysis 2013, 101, 1–16. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Fan, Z.; Hu, Q.; Zhou, N.; Xia, M.; Song, M.; Qi, Z.; Zhou, Z. In-situ catalytic pyrolysis of waste tires over clays for high quality pyrolysis products. Int. J. Hydrog. Energy 2021, 46, 6937–6944. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.S.; Kim, J.R.; Kim, S.D. Pyrolysis of waste tires with partial oxidation in a fluidized-bed reactor. Energy 1995, 20, 969–976. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of waste tires in a circulating fluidized-bed reactor. Energy 2001, 26, 385–399. [Google Scholar] [CrossRef]
- Chen, G.; Sun, B.; Li, J.; Lin, F.; Xiang, L.; Yan, B. Products distribution and pollutants releasing characteristics during pyrolysis of waste tires under different thermal process. J. Hazard. Mater. 2022, 424, 127351. [Google Scholar] [CrossRef]
- Nkosi, N.; Muzenda, E. A Review and Discussion of Waste Tyre Pyrolysis and Derived Products. In Proceedings of the World Congress on Engineering, London, UK, 2–4 July 2014. [Google Scholar]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Al Qubeissi, M.; Ui Hassan, M.; Mutjaba, M.A. Current Status and Potential of Tire Pyrolysis Oil Production as an Alternative Fuel in Developing Countries. Sustainability 2021, 13, 3214. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.; Rutto, H.; Seodigeng, T. Waste tire pyrolysis and desulfurization of tire pyrolytic oil (TPO)—A review. J. Air Waste Manag. Assoc. 2023, 73, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Afash, H.; Ozarisoy, B.; Altan, H.; Budayan, C. Recycling of Tire Waste Using Pyrolysis: An Environmental Perspective. Sustainability 2023, 15, 14178. [Google Scholar] [CrossRef]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Tomic, T.; Kremer, I.; Vecchio Ciprioti, S.; Schneider, D.R. Efficiency of municipal packaging waste recovery chain and suitability of separated residual waste fractions for use in alternative fuels production. J. Environ. Manag. 2022, 322, 116056. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Hrnjak-Murgić, Z.; Erceg, M.; Vecchio Ciprioti, S.; Schneider, D.R. Effect of Zeolite Catalyst on the Pyrolysis Kinetics of Multi-Layered Plastic Food Packaging. Symmetry 2022, 14, 1362. [Google Scholar] [CrossRef]
- Czarna-Juszkiewicz, D.; Kunecki, P.; Cader, J.; Wdowin, M. Review in Waste Tire Management—Potential Applications in Mitigating Environmental Pollution. Materials 2023, 16, 5771. [Google Scholar] [CrossRef]
- Rahman, M.d.M.; Yu, Y.; Wu, H. Valorisation of waste tyre via pyrolysis: Advances and Perspectives. Energy Fuels 2022, 36, 12429–12477. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency: Scarp Tire Cleanup Guidebook: A Resource for Solid Waste Managers Across the United States. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100DCR8.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000031%5CP100DCR8.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 3 September 2023).
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Chan, T.Y.; Lee, C.W.; Hui, C.W. Operation strategy for multi-stage pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 165–182. [Google Scholar] [CrossRef]
- Abdallah, R.; Juaidi, A.; Assad, M.; Salameh, T.; Manzano-Agugliaro, F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energy 2020, 13, 1817. [Google Scholar] [CrossRef]
- Jasminská, N.; Brestovič, T.; Čarnogurská, M. The effect of temperature pyrolysis process of used tires on the quality of output products. Acta Mech. Autom. 2013, 7, 20–25. [Google Scholar] [CrossRef][Green Version]
- Tripathi, D.; Sharma, R.K. (Eds.) Energy Systems and Nanotechnology. In Advances in Sustainability Science and Technology; Springer Nature Singapore Pte Ltd.: Singapore, 2021; ISBN 978-981-16-1256-5. [Google Scholar]
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products—A review. Sust. Mat. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Martinez, J.D.; Puy, N.; Murillo, R.; Garcia, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Toteva, V.; Stanulov, K. Waste tires pyrolysis oil as a source of energy: Methods for refining. Prog. Rubber Plast. Recycl. Technol. 2019, 36, 143–158. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Rawat, M.; Agrawal, A. Tyre pyrolysis oil as an alternative fuel: A review. Mater. Today Proc. 2020, 28, 2481–2484. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Kotkowski, T.; Cherbański, R.; Molga, E. Adsorption on activated carbons from end-of-life tyre pyrolysis for environmental applications. Part I. preparation of adsorbent and adsorption from gas phase. J. Anal. Appl. Pyrolysis 2021, 157, 105205. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Jones, I.; Preciado-Hernandez, J.; Zhu, M.; Zhang, J.; Zhang, Z.; Zhang, D. Utilisation of spent tyre pyrolysis char as activated carbon feedstock: The role, transformation and fate of Zn. Waste Manag. 2021, 126, 549–558. [Google Scholar] [CrossRef]
- Mikulova, Z.; Sedenkova, I.; Matejova, L.; Večeř, M.; Dombek, V. Study of carbon black obtained by pyrolysis of waste scrap tyres. J. Therm. Anal. Calorim. 2013, 111, 1475–1481. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F.; Shahid, M. Conversion of Waste Tyres into Carbon Black and Their Utilization as Adsorbent. J. Chin. Chem. Soc. 2006, 53, 1085–1089. [Google Scholar] [CrossRef]
- Matandabuzo, M.; Dovorogwa, D. Activated Carbons from Waste Tyre Pyrolysis: Application. In Recent Perspectives in Pyrolysis Research; Bartoli, M., Giorcelli, M., Eds.; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/chapters/80798 (accessed on 21 September 2023).
- Muttil, N.; Jagadeesan, S.; Chanda, A.; Duke, M.; Singh, S.K. Production, Types, and Applications of Activated Carbon Derived from Waste Tyres: An Overview. Appl. Sci. 2022, 13, 257. [Google Scholar] [CrossRef]
- De Marco Rodriguez, I.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomón, M.J.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Roy, C.; Labrecque, B.; De Caumia, B. Recycling of scrap tires to oil and carbon black by vacuum pyrolysis. Resour. Conserv. Recycl. 1990, 4, 203–213. [Google Scholar] [CrossRef]
- Choi, G.G.; Oh, S.J.; Kim, J.S. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content. Appl. Energy 2016, 170, 140–147. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; De Marco, I.; Torres, A.; Caballero, B.; Cabrero, M.A.; Chomón, M.J. Chromatographic analysis of the gases obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2000, 55, 43–54. [Google Scholar] [CrossRef]
- CalRecovery, Inc. in association with American Consulting and Commodities, Inc., ICF, Inc. and Recycling by Nature. Environmental Factors of Waste Tire Pyrolysis, Gasification, and Liquefaction; California Integrated Waste Management Board: Sacramento, CA, USA, 1995; Report No.: 1364. [Google Scholar]
- Chatterjee, R.; Sajjadi, B.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Banar, M.; Akyıldız, V.; Özkan, A.; Çokaygil, Z.; Onay, Ö. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Kar, Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011, 8, 1772–1782. [Google Scholar] [CrossRef]
- Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of rubber tyre wastes: A kinetic study. Fuel 2013, 103, 542–552. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Islam, M.R.; Joardder, M.U.H.; Hasan, S.M.; Takai, K.; Haniu, H. Feasibility study for thermal treatment of solid tire wastes in Bangladesh by using pyrolysis technology. Waste Manag. 2011, 31, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating rate effects on pyrolytic vapors from scrap truck tires. J. Anal. Appl. Pyrolysis 2017, 123, 419–429. [Google Scholar] [CrossRef]
- Williams, P.T.; Beslerm, S.; Taylor, D.T. The pyrolysis of scrap automotive tyres. Fuel 1990, 69, 1474–1482. [Google Scholar] [CrossRef]
- Clark, C. (Ed.) Scrap Tire Technology and Markets; Noyes Data Corp: Park Ridge, NJ, USA, 1993; p. 316, (Pollution technology review). [Google Scholar]
- Laresgoiti, M.F.; Caballero, B.M.; De Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Rofiqulislam, M.; Haniu, H.; Rafiqulalambeg, M. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Chang, Y.M. On pyrolysis of waste tire: Degradation rate and product yields. Resour. Conserv. Recycl. 1996, 17, 125–139. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J. Anal. Appl. Pyrolysis 2004, 72, 165–170. [Google Scholar] [CrossRef]
- Yongrong, Y.; Jizhong, C.; Guibin, Z. Technical advance on the pyrolysis of used tires in China. In Proceedings of the China-Japan International Academic Symposium, Environmental Problem in Chinese Iron-Steelmaking industries and Effective Technology Transfer, Sendai, Japan, 6 March 2000. [Google Scholar]
- Murena, F. Kinetics of sulphur compounds in waste tyres pyrolysis. J. Anal. Appl. Pyrolysis 2000, 56, 195–205. [Google Scholar] [CrossRef]
- Acevedo, B.; Fernández, A.M.; Barriocanal, C. Identification of polymers in waste tyre reinforcing fibre by thermal analysis and pyrolysis. J. Anal. Appl. Pyrolysis 2015, 111, 224–232. [Google Scholar] [CrossRef]
- Suhanya, M.; Thirumarimurugan, M.; Kannadasan, T. Recovery of oil from waste tyres using pyrolysis method: A review. IJRET 2013, 1, 81–90. [Google Scholar]
- Kremer, I.; Tomić, T.; Katančić, Z.; Erceg, M.; Papuga, S.; Vuković, J.P.; Schneider, D.R. Catalytic pyrolysis of mechanically non-recyclable waste plastics mixture: Kinetics and pyrolysis in laboratory-scale reactor. J. Environ. Manag. 2021, 296, 113145. [Google Scholar] [CrossRef] [PubMed]
- Kremer, I.; Tomić, T.; Katančić, Z.; Hrnjak-Murgić, Z.; Erceg, M.; Schneider, D.R. Catalytic decomposition and kinetic study of mixed plastic waste. Clean. Technol. Environ. Policy 2021, 23, 811–827. [Google Scholar] [CrossRef]
- Papuga, S.; Gvero, P.; Vukic, L. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm. Sci. 2016, 20, 731–741. [Google Scholar] [CrossRef]
- International Organization for Standardization. Petroleum Products—Determination of Sulfur Content of Automotive Fuels—Energy-Dispersive X-ray Fluorescence Spectrometry (ISO 20847:2004). Available online: https://www.iso.org/standard/34285.html (accessed on 1 October 2023).
- Castaldi, M.J.; Kwon, E. An Investigation of the Thermal Degradation Mechanisms of a Waste Tire Through Chemical Analysis Including Hydrocarbons, Benzene Derivatives, and Polycyclic Aromatic Hydrocarbons (PAHs) at High Temperature. In Proceedings of the 16th Annual North American Waste-to-Energy Conference, Philadelphia, PA, USA, 19–21 May 2008; ASME: New York, NY, USA, 2008; pp. 97–105. [Google Scholar]
- Ramírez Arias, A.M.; Moreno-Piraján, J.C.; Giraldo, L. Kinetic Study of Waste Tire Pyrolysis Using Thermogravimetric Analysis. ACS Omega 2022, 7, 16298–16305. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Valorisation of End of Life Tyres (ELTs) in a Newly Developed Pyrolysis Fixed-Bed Batch Process. Process Saf. Environ. Prot. 2020, 138, 167–175. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Pyrolysis of end of life tyres reclaimed from lorry trucks: Part I—Oil recovery and characterisation. WIT Trans. Eng. Sci. 2021, 133, 107–112. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Kinetics and product distribution of end of life tyres (ELTs) pyrolysis: A novel approach in polyisoprene and SBR thermal cracking. J. Hazard. Mater. 2009, 172, 1690–1694. [Google Scholar] [CrossRef]
- Wang, H.; Hu, H.; Yang, Y.; Liu, H.; Tang, H.; Xu, S.; Li, A.; Yao, H. Effect of high heating rates on products distribution and sulfur transformation during the pyrolysis of waste tires. Waste Manag. 2020, 118, 9–17. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials (ASTM). Standard Test Method for Calculation of Carbon Distribution and Structural group Analysis of Petroleum Oils by the n-d-M Method ASTM D3238-95(2000). Available online: https://www.astm.org/d3238-95r00.html (accessed on 1 October 2023).
- Rekalic, V.; Vitorovic, O. Analytical Tests in Technological Production: Principles and Procedures [Original Serbian: Analitička Ispitivanja u Tehnološkoj Proizvodnji: Principi i Postupci]; Faculty of Technology and Metallurgy: Belgrade, Serbia, 1988. [Google Scholar]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scarp tyres: Preliminary experimental results. Waste Manag. 2022, 22, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Aydın, H.; İlkılıç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- Rada, E.C.; Ragazzi, M.; Panaitescu, V.N. Energy recovery from tyres waste through thermal option. UPB Sci. Bull. Ser. D Mech. Eng. 2012, 74, 201–210. [Google Scholar]
- Hopa, D.Y.; Yilmaz, A.; Bahtli, T.A. Recovery of waste tyres by pyrolysis in a fixed bed reactor for liquid fuel production: Effects of pyrolysis conditions on oil yield. Res. Eng. Struct. Mater. 2017, 3, 186–191. [Google Scholar] [CrossRef]
- Council Directive 2016/802 on the reduction in the Sulphur content of certain liquid fuels (2016). Official Journal of the European Union L132/58. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016L0802&rid=1 (accessed on 1 October 2023).
- Islam, M.R.; Beg, M.R.A.; Haniu, H. Fuel Based Liquids From Scrap Tire By Pyrolysis Technology. In Proceedings of the 3rd BSME-ASME International Conference on Thermal Engineering, Dhaka, Bangladesh, 20–22 December 2006. [Google Scholar]
- González, J.F.; Encinar, J.M.; Canito, J.L.; Rodríguez, J.J. Pyrolysis of automobile tyre waste. Influence of operating variables and kinetics study. J. Anal. Appl. Pyrolysis 2001, 58–59, 667–683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).