Analysis of Short-Term Heart Rate Asymmetry in High-Performance Athletes and Non-Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Autonomic Function Test and Recording of RR Interbeat Intervals
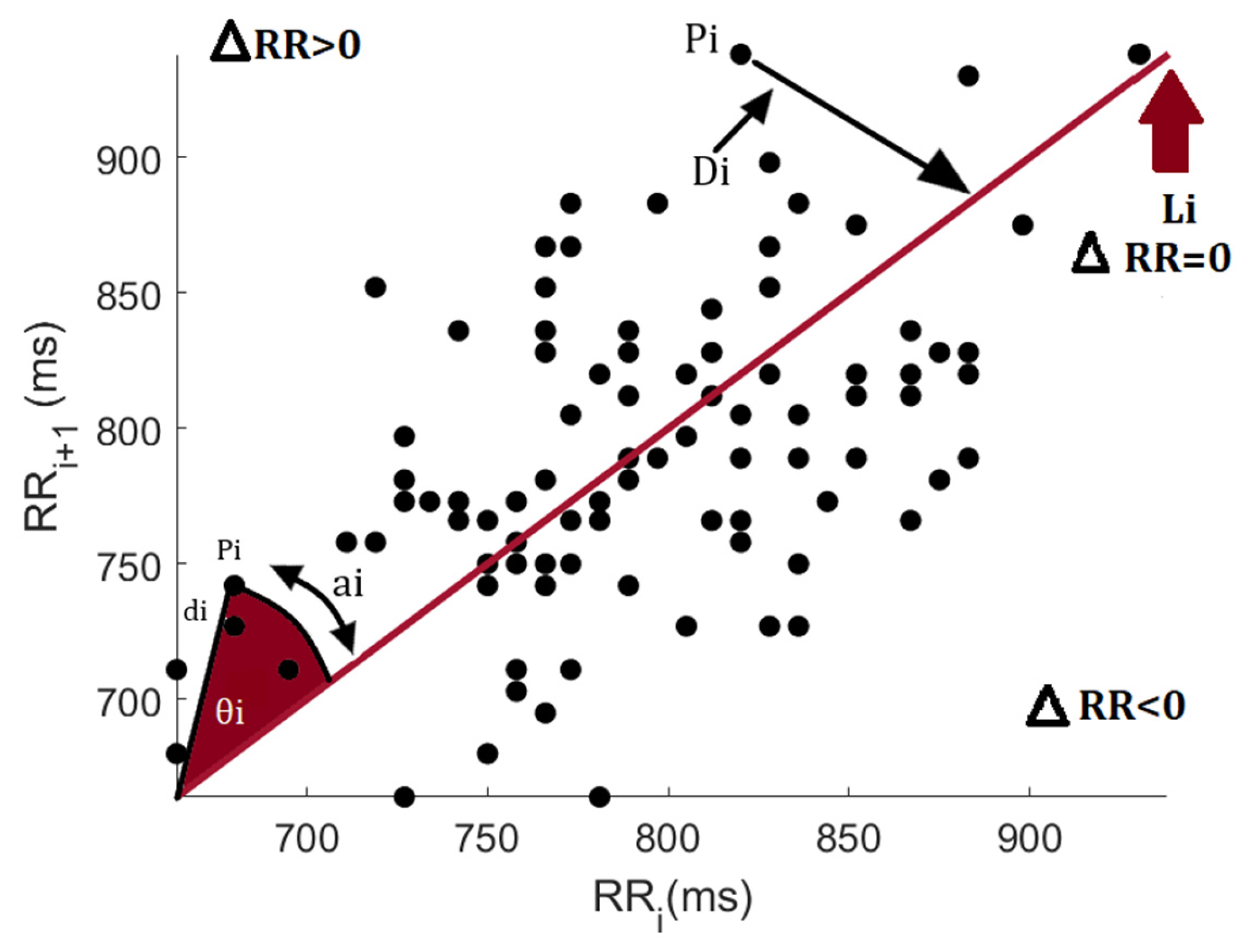
2.3. Heart Rate Asymmetry Assessment
2.3.1. Porta’s Index (PI%)
2.3.2. Guzik’s Index (GI%)
2.3.3. Ehler’s Index (EI)
2.3.4. Slope Index (SI)
2.3.5. Asymmetric Spread Index (ASI)
2.3.6. Statistical Analysis
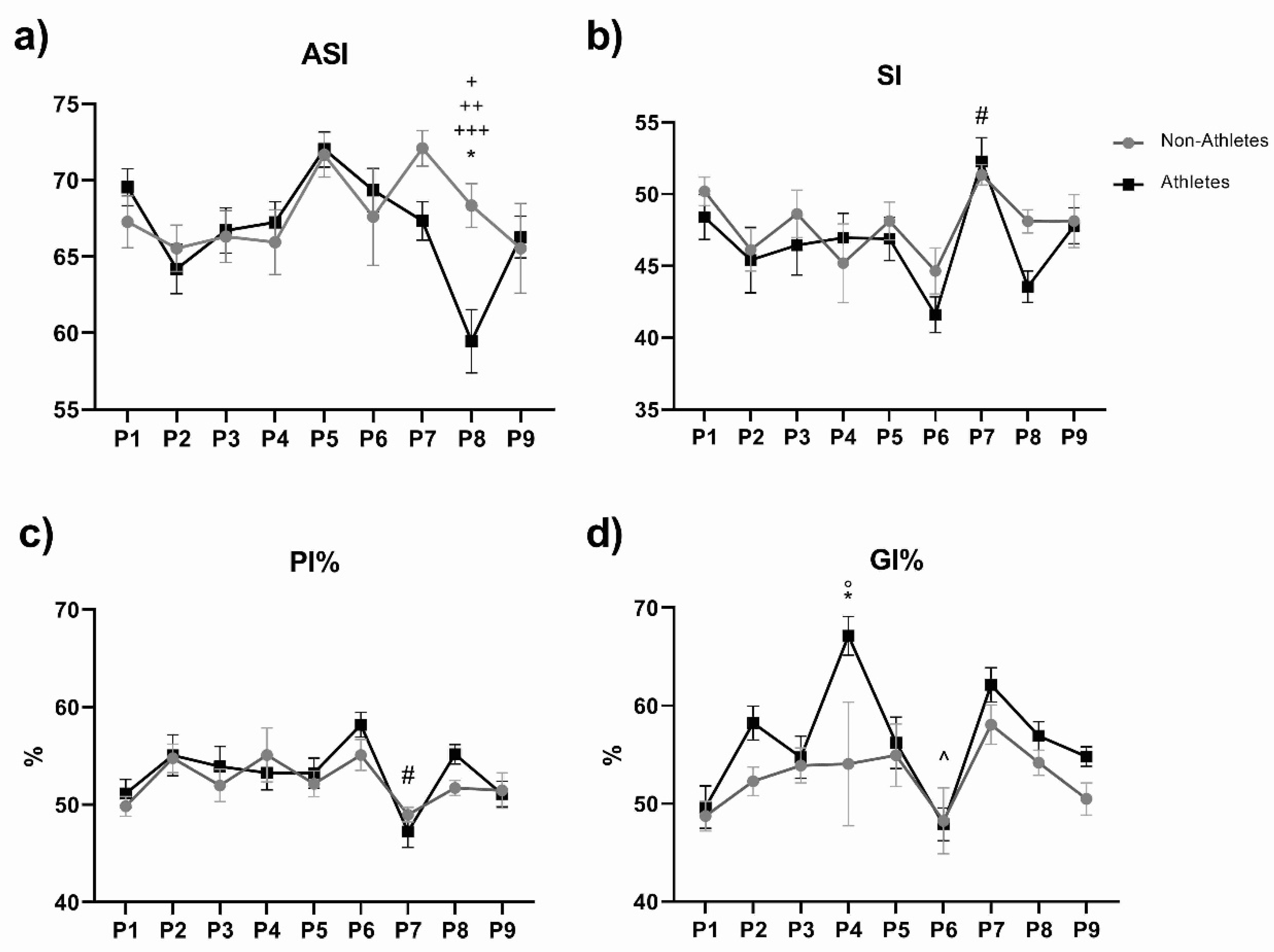
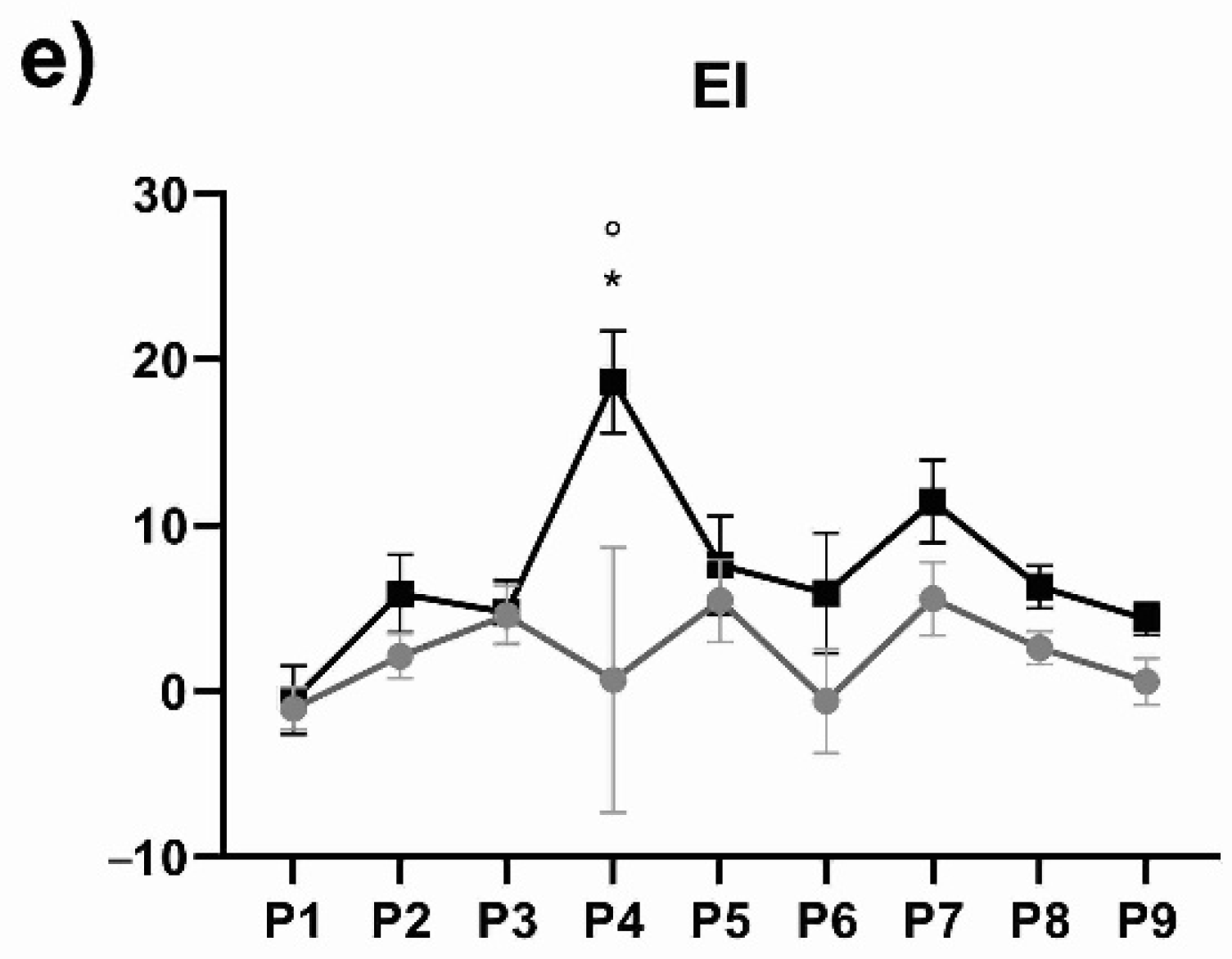
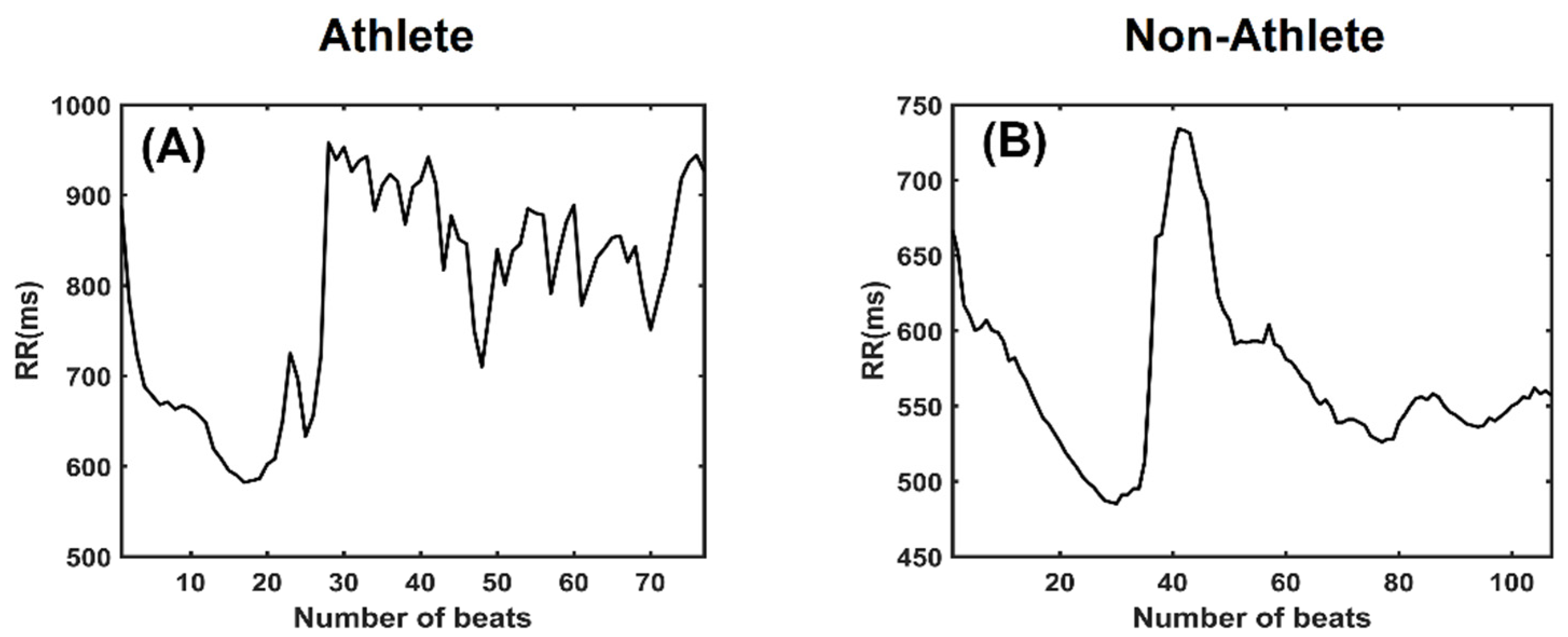
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, M. Task Force Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of The European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Zabel, M. CrossTalk proposal: Heart rate variability is a valid measure of cardiac autonomic responsiveness. J. Physiol. 2019, 597, 2595–2598. [Google Scholar] [CrossRef]
- Makivić, B.; Nikić, M.D.; Willis, M.S. Heart Rate Variability (HRV) as a Tool for Diagnostic and Monitoring Performance in Sport and Physical Activities. J. Exerc. Physiol. Online 2013, 16, 103–131. [Google Scholar]
- Bricout, V.A.; DeChenaud, S.; Favre-Juvin, A. Analyses of heart rate variability in young soccer players: The effects of sport activity. Auton. Neurosci. Basic Clin. 2010, 154, 112–116. [Google Scholar] [CrossRef]
- Udayanga, M.A.S. Heart Rate Variability (HRV) for sports and exercise training. Sri Lankan J. Sport. Exerc. Med. 2018, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.G. The role of heart rate variability in sports physiology (Review). Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Schroeder, R.; Vallverdú, M.; Schulz, S.; Cygankiewicz, I.; Vázquez, R.; Bayés de Luna, A.; Caminal, P. Short-term vs. long-term heart rate variability in ischemic cardiomyopathy risk stratification. Front. Physiol. 2013, 4, 364. [Google Scholar] [CrossRef] [Green Version]
- Spasić, S.Z.; Kesić, S. Editorial: Nonlinearity in living systems: Theoretical and practical perspectives on metrics of physiological signal complexity. Front. Physiol. 2019, 10, 298. [Google Scholar] [CrossRef]
- Klintworth, A.; Ajtay, Z.; Paljunite, A.; Szabados, S.; Hejjel, L. Heart rate asymmetry follows the inspiration/expiration ratio in healthy volunteers. Physiol. Meas. 2012, 33, 1717–1731. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Shi, B.; Liu, C.; Karmakar, C.; Li, P. Does the Temporal Asymmetry of Short-Term Heart Rate Variability Change during Regular Walking? A Pilot Study of Healthy Young Subjects. Comput. Math. Methods Med. 2018, 2018, 3543048. [Google Scholar] [CrossRef]
- Visnovcova, Z.; Mestanik, M.; Javorka, M.; Mokra, D.; Gala, M.; Jurko, A.; Calkovska, A.; Tonhajzerova, I. Complexity and time asymmetry of heart rate variability are altered in acute mental stress. Physiol. Meas. 2014, 35, 1319–1334. [Google Scholar] [CrossRef]
- Kaczmarek, L.D.; Behnke, M.; Enko, J.; Kosakowski, M.; Hughes, B.M.; Piskorski, J.; Guzik, P. Effects of emotions on heart rate asymmetry. Psychophysiology 2019, 56, e13318. [Google Scholar] [CrossRef]
- Tonhajzerová, I.; Ondrejka, I.; Farský, I.; Višňovcová, Z.; Mešťaník, M.; Javorka, M.; Jurko, A.; Čalkovská, A. Attention deficit/hyperactivity disorder (ADHD) is associated with altered heart rate asymmetry. Physiol. Res. 2014, 63, S509–S519. [Google Scholar] [CrossRef]
- Shi, B.; Wang, L.; Yan, C.; Chen, D.; Liu, M.; Li, P. Nonlinear heart rate variability biomarkers for gastric cancer severity: A pilot study. Sci. Rep. 2019, 9, 13833. [Google Scholar] [CrossRef] [Green Version]
- Pawłowski, R.; Buszko, K.; Newton, J.L.; Kujawski, S.; Zalewski, P. Heart Rate Asymmetry Analysis During Head-Up Tilt Test in Healthy Men. Front. Physiol. 2021, 12, 657902. [Google Scholar] [CrossRef]
- Kiss, O.; Sydó, N.; Vargha, P.; Vágó, H.; Czimbalmos, C.; Édes, E.; Zima, E.; Apponyi, G.; Merkely, G.; Sydó, T.; et al. Detailed heart rate variability analysis in athletes. Clin. Auton. Res. 2016, 26, 245–252. [Google Scholar] [CrossRef]
- Hottenrott, L.; Gronwald, T.; Hottenrott, K.; Wiewelhove, T.; Ferrauti, A. Utilizing Heart Rate Variability for Coaching Athletes During and After Viral Infection: A Case Report in an Elite Endurance Athlete. Front. Sport. Act. Living 2021, 3, 612782. [Google Scholar] [CrossRef]
- Tafur Tascón, L.J.; Cabrera Hernandez, M.A.; Neisa Herrera, L.F.; García-Corzo, S.A.; Povea Combariza, C.H.; Tejada Rojas, C.X. Autonomic modulation and association with the performance in a jump test in university Colombian players of rugby seven. J. Hum. Sport Exerc. 2019, 14, S1102–S1111. [Google Scholar]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. Eur. J. Appl. Physiol. 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Wessel, N.; Voss, A.; Malberg, H.; Ziehmann, C.; Voss, H.U.; Schirdewan, A.; Meyerfeldt, U.; Kurths, J. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmachertherapie Elektrophysiologie 2000, 11, 159–173. [Google Scholar] [CrossRef]
- Silva, L.E.V.; Fazan, R.; Marin-Neto, J.A. PyBioS: A freeware computer software for analysis of cardiovascular signals. Comput. Methods Programs Biomed. 2020, 197, 105718. [Google Scholar] [CrossRef]
- Porta, A.; Casali, K.R.; Casali, A.G.; Gnecchi-Ruscone, T.; Tobaldini, E.; Montano, N.; Lange, S.; Geue, D.; Cysarz, D.; Van Leeuwen, P. Temporal asymmetries of short-term heart period variability are linked to autonomic regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Piskorski, J.; Guzik, P. Geometry of the Poincaré plot of RR intervals and its asymmetry in healthy adults. Physiol. Meas. 2007, 28, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Havstad, J.; Prichard, D.; Theiler, J. Low doses of ethanol reduce evidence for nonlinear structure in brain activity. J. Neurosci. 1998, 18, 7474–7486. [Google Scholar] [CrossRef]
- Karmakar, C.K.; Khandoker, A.H.; Palaniswami, M. Phase asymmetry of heart rate variability signal. Physiol. Meas. 2015, 36, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Rohila, A.; Sharma, A. Asymmetric spread of heart rate variability. Biomed. Signal Process. Control 2020, 60, 101985. [Google Scholar] [CrossRef]
- Martinelli, F.S.; Chacon-Mikahil, M.P.T.; Martins, L.E.B.; Lima-Filho, E.C.; Golfetti, R.; Paschoal, M.A.; Gallo-Junior, L. Heart rate variability in athletes and non-athletes at rest and during head-up tilt. Braz. J. Med. Biol. Res. 2005, 38, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Ackland, G.L. Cardiac vagus and exercise. Physiology 2019, 34, 71–80. [Google Scholar] [CrossRef]
- Grant, C.C.; Viljoen, M.; Janse van Rensburg, D.C.; Wood, P.S. Heart Rate Variability Assessment of the Effect of Physical Training on Autonomic Cardiac Control. Ann. Noninvasive Electrocardiol. 2012, 17, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Rubchenya, I.N.; Sukach, E.S.; Merkis, A.P. Analysis of Heart Rate Variability in Young Athletes of a School of Olympic Reserve. Health Ecol. Issues 2019, 4, 70–75. [Google Scholar] [CrossRef]
- Mendes, J.; Pereira, J.; Pereira, T. Variability of Heart Rate in Athletes and Non Athletes. Eur. J. Public Health 2019, 29, ckz034.098. [Google Scholar] [CrossRef]
- Molina, G.E.; Fontana, K.E.; Porto, L.G.G.; Junqueira, L.F. Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin. Auton. Res. 2016, 26, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Karavirta, L.; Thomson, R.L.; Robertson, E.Y.; Davison, K.; Buckley, J.D. Contextualizing parasympathetic hyperactivity in functionally overreached athletes with perceptions of training tolerance. Int. J. Sports Physiol. Perform. 2016, 11, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, D.; Gjerdalen, G.F.; Solberg, E.E.; Khokhlova, M.; Badtieva, V.; Herzig, D.; Trachsel, L.D.; Noack, P.; Karavirta, L.; Eser, P.; et al. Sex differences in heart rate variability: A longitudinal study in international elite cross-country skiers. Eur. J. Appl. Physiol. 2015, 115, 2107–2114. [Google Scholar] [CrossRef]
- Brar, T.K.; Singh, K.D.; Kumar, A. Effect of different phases of menstrual cycle on heart rate variability (HRV). J. Clin. Diagn. Res. 2015, 9, CC01–CC04. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.A.; Ferreira, J.B.; De Oliveira Schein, A.S.; Dartora, D.R.; Casali, A.G.; Scassola, C.M.C.; Tobaldini, E.; Montano, N.; Guzzetti, S.; Porta, A.; et al. Optimization of vagal stimulation protocol based on spontaneous breathing rate. Front. Physiol. 2018, 9, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.; Rössler, A.; Lackner, H.K.; Trozic, I.; Laing, C.; Lorr, D.; Green, D.A.; Hinghofer-Szalkay, H.; Goswami, N. Effect of postural changes on cardiovascular parameters across gender. Medicine 2016, 95, e4149. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, P.; Ji, L.; Yao, L.; Karmakar, C.; Liu, C. Area asymmetry of heart rate variability signal. Biomed. Eng. Online 2017, 16, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Maria, B.; Dalla Vecchia, L.A.; Maestri, R.; Pinna, G.D.; Parati, M.; Perego, F.; Bari, V.; Cairo, B.; Gelpi, F.; la Rovere, M.T.; et al. Lack of association between heart period variability asymmetry and respiratory sinus arrhythmia in healthy and chronic heart failure individuals. PLoS ONE 2021, 16, e0247145. [Google Scholar] [CrossRef]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart Rate Variability in Athletes. Sport. Med. 2003, 33, 889–919. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mina-Paz, Y.; Santana-García, V.N.; Tafur-Tascon, L.J.; Cabrera-Hernández, M.A.; Pliego-Carrillo, A.C.; Reyes-Lagos, J.J. Analysis of Short-Term Heart Rate Asymmetry in High-Performance Athletes and Non-Athletes. Symmetry 2022, 14, 1229. https://doi.org/10.3390/sym14061229
Mina-Paz Y, Santana-García VN, Tafur-Tascon LJ, Cabrera-Hernández MA, Pliego-Carrillo AC, Reyes-Lagos JJ. Analysis of Short-Term Heart Rate Asymmetry in High-Performance Athletes and Non-Athletes. Symmetry. 2022; 14(6):1229. https://doi.org/10.3390/sym14061229
Chicago/Turabian StyleMina-Paz, Yecid, Victoria Noemí Santana-García, Luis Javier Tafur-Tascon, Moisés Arturo Cabrera-Hernández, Adriana Cristina Pliego-Carrillo, and José Javier Reyes-Lagos. 2022. "Analysis of Short-Term Heart Rate Asymmetry in High-Performance Athletes and Non-Athletes" Symmetry 14, no. 6: 1229. https://doi.org/10.3390/sym14061229
APA StyleMina-Paz, Y., Santana-García, V. N., Tafur-Tascon, L. J., Cabrera-Hernández, M. A., Pliego-Carrillo, A. C., & Reyes-Lagos, J. J. (2022). Analysis of Short-Term Heart Rate Asymmetry in High-Performance Athletes and Non-Athletes. Symmetry, 14(6), 1229. https://doi.org/10.3390/sym14061229





