Sex-Related Left-Lateralized Development of the Crus II Region of the Ansiform Lobule in Cynomolgus Monkeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
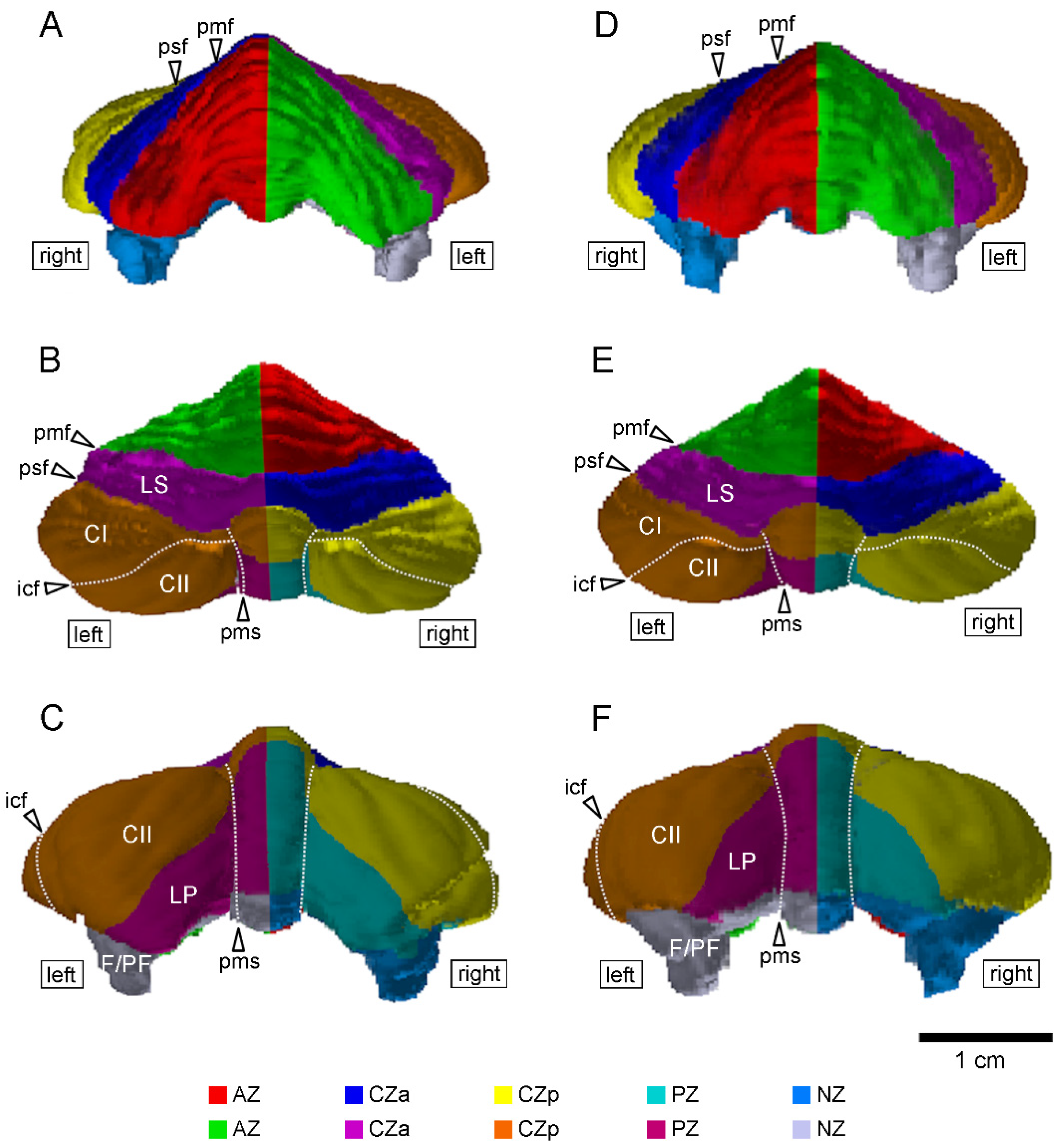
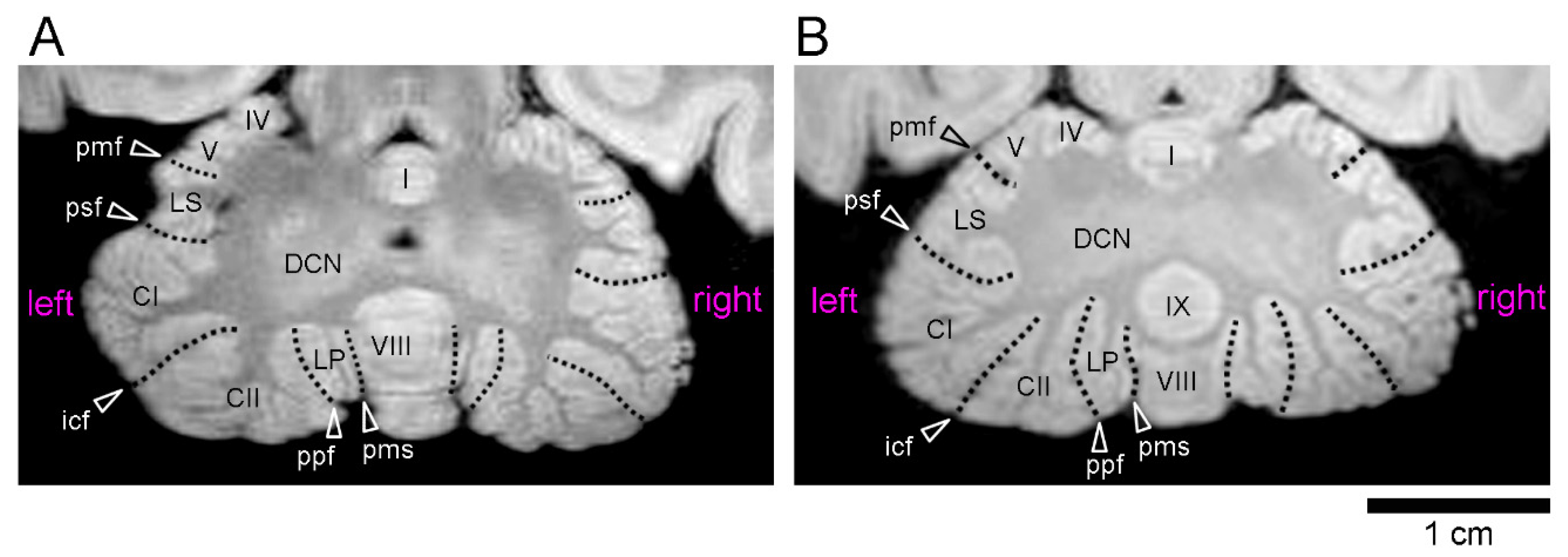
2.2. Volumetry
2.3. Statistical Analysis
3. Results
3.1. Cerebellar Volumes of Males and Females
3.2. Left/Right-Side Differences in Cerebellar Volumes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Lombardino, L.J.; Walczak, A.R.; Bonihla, L.; Leonard, C.M.; Binder, J.R. Manual and automated measures of superior temporal gyrus asymmetry: Concordant structural predictors of verbal ability in children. Neuroimage 2008, 41, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Iuricich, F.; Vaden , K.I., Jr.; Glaze, B.T. Dyslexia Data Consortium. The topology of pediatric structural asymmetries in language-related cortex. Symmetry 2020, 12, 1809. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.; Hildebolt, C.; Cheverud, J.; Vannier, M.; Helmkamp, R.C.; Konigsberg, L. Cortical asymmetries in frontal lobes of rhesus monkeys (Macaca mulatta). Brain Res. 1990, 512, 40–45. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Nir, T. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): The effect of handedness and comparison within findings in humans. Behav. Brain Res. 2010, 208, 436–443. [Google Scholar] [CrossRef]
- Sawada, K. Cerebral sulcal asymmetry in macaque monkeys. Symmetry 2020, 12, 1509. [Google Scholar] [CrossRef]
- Synder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Phillips, K.; Hopkins, W.D. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella). Neuropsychologia 2007, 45, 2333–2339. [Google Scholar] [CrossRef]
- Rosch, R.E.; Ronan, L.; Cherkas, L.; Gurd, J.M. Cerebellar asymmetry in a pair of monozygotic handedness-discordant twins. J. Anat. 2010, 217, 38–47. [Google Scholar] [CrossRef]
- Koyun, N.; Aydinlioğlu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Aoki, I. Age-dependent sexually-dimorphic asymmetric development of the ferret cerebellar cortex. Symmetry 2017, 9, 40. [Google Scholar] [CrossRef]
- Sawada, K.; Kamiya, S.; Aoki, I. Asymmetry of cerebellar lobular development in ferrets. Symmetry 2020, 12, 735. [Google Scholar] [CrossRef]
- Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S., Jr.; Howard, J.; McGrath, L.; Steele, S.; Frazier, J.A.; Tager-Flusberg, H.; Harris, G.J. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010, 40, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Q.; Zuo, C.; Zhao, L.; Hao, L. Longitudinal changes of cerebellar thickness in autism. spectrum disorder. Neurosci. Lett. 2020, 728, 134949. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Gunning-Dixon, F.; Ashtari, M.; Snyder, P.J.; Lieberman, J.A.; Bilder, R.M. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol. Psychiatry 2003, 53, 450–459. [Google Scholar] [CrossRef]
- Hu, D.; Shen, H.; Zhou, Z. Functional asymmetry in the cerebellum: A brief review. Cerebellum 2008, 7, 304–313. [Google Scholar] [CrossRef]
- Iglói, K.; Doeller, C.F.; Paradis, A.L.; Benchenane, K.; Berthoz, A.; Burgess, N.; Rondi-Reig, L. Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cereb. Cortex 2015, 25, 4146–4154. [Google Scholar] [CrossRef]
- Ciricugno, A.; Ferrari, C.; Rusconi, M.L.; Cattaneo, Z. The left posterior cerebellum is involved in orienting attention along the mental number line: An online-TMS study. Neuropsychologia 2020, 143, 107497. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Biederman, J.; Poldrack, R.A.; Seidman, L.J. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 439–447. [Google Scholar] [CrossRef]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Sawada, K.; Fukunishi, K.; Sakata-Haga, H.; Fukui, Y. Sexual dimorphism of sulcal length asymmetry in cerebrum of adult cynomolgus monkeys (Macaca fascicularis). Congenit. Anom. 2011, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sillitoe, R.V.; Hawkes, R. Whole-mount immunohistochemistry: A high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002, 50, 235–244. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Hulliger, M.; Dyck, R.; Hawkes, R. Antigenic compartmentation of the cat cerebellar cortex. Brain Res. 2003, 977, 1–15. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Malz, C.R.; Rockland, K.; Hawkes, R. Antigenic compartmentation of the primate and tree shrew cerebellum: A common topography of zebrin II in Macaca mulatta and Tupaia belangeri. J. Anat. 2004, 204, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Marzban, H.; Hawkes, R. On the architecture of the posterior zone of the cerebellum. Cerebellum 2011, 10, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Franchini, D.; Pepe, A.M.; Sasso, R.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Volumetric assessment of cerebral asymmetries in dogs. Laterality 2011, 16, 528–536. [Google Scholar] [CrossRef]
- Miller, E.K.; Walls, J.D. The frefrontal cortex and executive brain functions. In Fundamental Neuroscience, 2nd ed.; Squire, L., Berg, D., Bloom, F.E., McConnell, S., Roberts, J.L., Spitzer, N.C., Zigmond, M.J., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 1353–1376. [Google Scholar]
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 2012, 61, 1213–1225. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Sofuoglu, M.; DeVito, E.E.; Waters, A.J.; Carroll, K.M. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 2013, 64, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Lentini, E.; Kasahara, M.; Arver, S.; Savic, I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex 2013, 23, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tang, Y.; Sun, B.; Gong, G.; Chen, Z.J.; Lin, X.; Yu, T.; Li, Z.; Evans, A.C.; Liu, S. Sexual dimorphism and asymmetry in human cerebellum: An MRI-based morphometric study. Brain Res. 2010, 1353, 60–73. [Google Scholar] [CrossRef]
- Raz, N.; Dupuis, J.H.; Briggs, S.D.; McGavran, C.; Acker, J.D. Differential effects of age and sex on the cerebellar hemispheres and the vermis: A prospective MR study. AJNR Am. J. Neuroradiol. 1998, 19, 65–71. [Google Scholar] [PubMed]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Williamson, A.; Acker, J.D. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. AJNR Am. J. Neuroradiol. 2001, 22, 1161–1167. [Google Scholar]
- Giedd, J.N.; Raznahan, A.; Mills, K.L.; Lenroot, R.K. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012, 3, 19. [Google Scholar] [CrossRef]
- Ratcliffe, S.G.; Read, G.; Pan, H.; Fear, C.; Lindenbaum, R.; Crossley, J. Prenatal testosterone levels in XXY and XYY males. Horm. Res. 1994, 42, 106–109. [Google Scholar] [CrossRef]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef]
- Bishop, K.M.; Wahlsten, D. Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res. 1999, 815, 358–366. [Google Scholar] [CrossRef][Green Version]
| Males (n = 5) | Females (n = 5) | |
|---|---|---|
| Volumes (mm3) | ||
| Whole cerebellum | 3441 ± 147 | 3338 ± 204 |
| Anterior zone | 916 ± 81 | 844 ± 68 |
| Central zone anterior | 491 ± 46 | 489 ± 48 |
| Central zone posterior | 1050 ± 86 | 1033 ± 70 |
| Posterior zone | 493 ± 34 | 442 ± 57 |
| Nodular zone | 492 ± 87 | 530 ± 36 |
| Males (n = 5) | Females (n = 5) | ||
|---|---|---|---|
| Volumes (mm3) | |||
| Crus I | 520 ± 66 | 541 ± 44 | |
| Crus II | 455 ± 92 | 409 ± 64 | |
| Paramedian lobule | 286 ± 29 | 247 ± 31 | |
| Flocculus/Paraflocculus | 404 ± 83 | 436 ± 22 | |
| Left Side (mm3) | Right Side(mm3) | AQ Value | |
|---|---|---|---|
| Whole cerebellum | |||
| Males (n = 5) | 1791 ± 116 | 1650 ± 67 | −0.080 ± 0.070 |
| Females (n = 5) | 1703 ± 126 | 1635 ± 96 | −0.040 ± 0.056 |
| Anterior zone | |||
| Males (n = 5) | 479 ± 64 | 437 ± 24 | −0.085 ± 0.118 |
| Females (n = 5) | 439 ± 40 * | 404 ± 33 * | −0.083 ± 0.068 |
| Central zone anterior | |||
| Males (n = 5) | 252 ± 30 | 239 ± 21 | −0.048 ± 0.111 |
| Females (n = 5) | 245 ± 27 | 243 ± 23 | 0.007 ± 0.062 |
| Central zone posterior | |||
| Males (n = 5) | 547 ± 47 ** | 502 ± 41 ** | −0.085 ± 0.030 # |
| Females (n = 5) | 536 ± 51 | 497 ± 40 | −0.075 ± 0.114 |
| Posterior zone | |||
| Males (n = 5) | 251 ± 16 | 243 ± 31 | −0.038 ± 0.141 |
| Females (n = 5) | 215 ± 34 | 227 ± 29 | 0.058 ± 0.125 |
| Nodular zone | |||
| Males (n = 5) | 262 ± 51 | 229 ± 43 | −0.131 ± 0.135 |
| Females (n = 5) | 267 ± 15 | 264 ± 23 | −0.014 ± 0.055 |
| Left Side | Right Side | AQ Value | |
|---|---|---|---|
| Crus I | |||
| Males (n = 5) | 263 ± 24 | 257 ± 43 | −0.030 ± 0.094 |
| Females (n = 5) | 277 ± 33 | 267 ± 17 | −0.044 ± 0.095 |
| Crus II | |||
| Males (n = 5) | 240 ± 52 * | 215 ± 41 * | −0.108 ± 0.034 # |
| Females (n = 5) | 213 ± 33 | 196 ± 38 | −0.087 ± 0.165 |
| Paramedian lobule | |||
| Males (n = 5) | 141 ± 11 | 145 ± 20 | 0.025 ± 0.112 |
| Females (n = 5) | 119 ± 21 | 127 ± 11 | 0.072 ± 0.094 |
| Flocculus/Paraflocculus | |||
| Males (n = 5) | 211 ± 48 | 193 ± 40 | −0.081 ± 0.136 |
| Females (n = 5) | 222 ± 11 | 214 ± 19 | −0.034 ± 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, K.; Saito, S. Sex-Related Left-Lateralized Development of the Crus II Region of the Ansiform Lobule in Cynomolgus Monkeys. Symmetry 2022, 14, 1015. https://doi.org/10.3390/sym14051015
Sawada K, Saito S. Sex-Related Left-Lateralized Development of the Crus II Region of the Ansiform Lobule in Cynomolgus Monkeys. Symmetry. 2022; 14(5):1015. https://doi.org/10.3390/sym14051015
Chicago/Turabian StyleSawada, Kazuhiko, and Shigeyoshi Saito. 2022. "Sex-Related Left-Lateralized Development of the Crus II Region of the Ansiform Lobule in Cynomolgus Monkeys" Symmetry 14, no. 5: 1015. https://doi.org/10.3390/sym14051015
APA StyleSawada, K., & Saito, S. (2022). Sex-Related Left-Lateralized Development of the Crus II Region of the Ansiform Lobule in Cynomolgus Monkeys. Symmetry, 14(5), 1015. https://doi.org/10.3390/sym14051015






