The Capture and Transformation of Carbon Dioxide in Concrete: A Review
Abstract
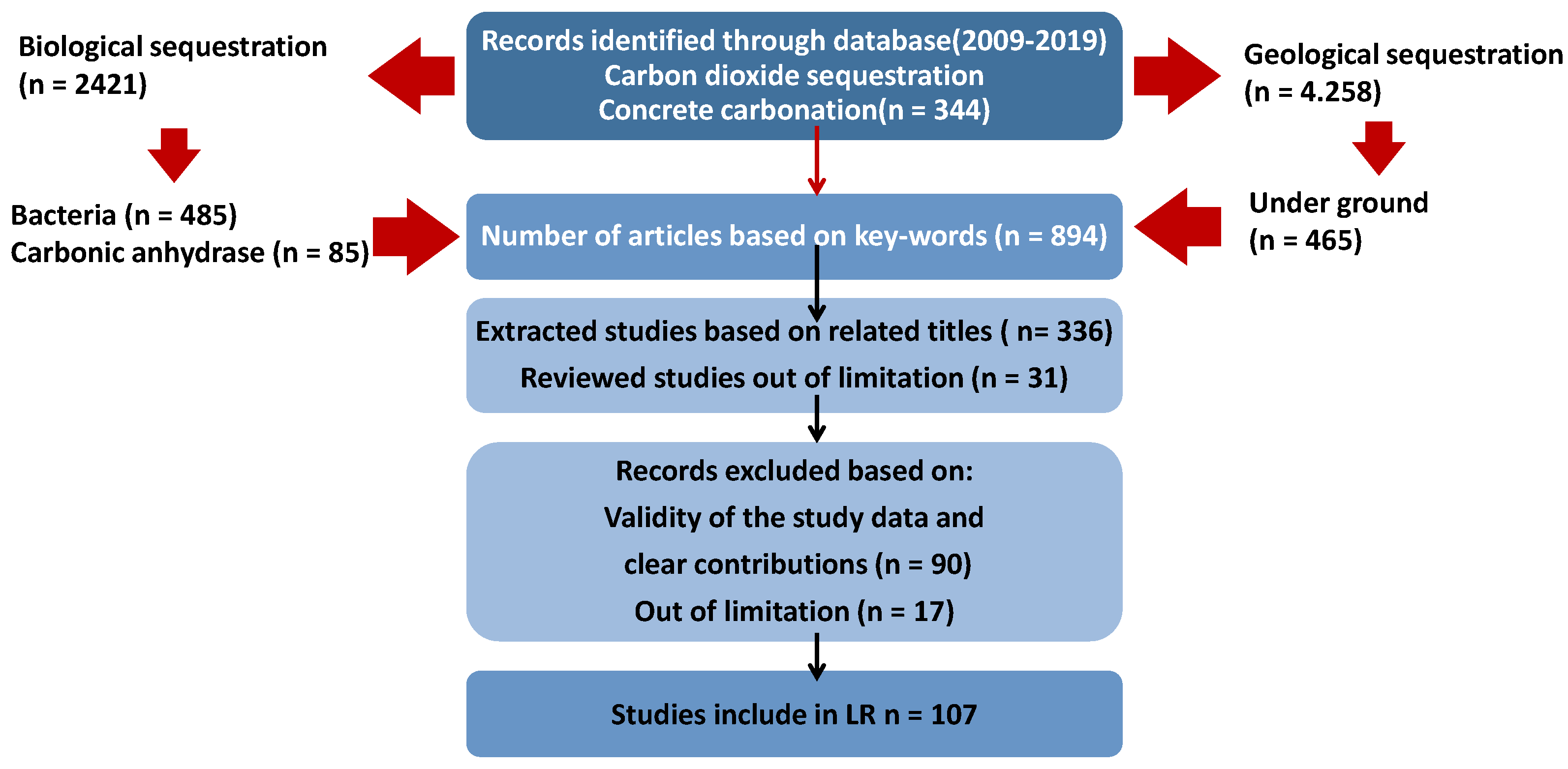
1. Introduction
2. Significance of Cement-Based Materials for Carbon Dioxide Absorption
2.1. The Process of Producing Carbon Dioxide and Absorbing Carbon Dioxide
2.1.1. CO2 Production Mechanism
2.1.2. CO2 Absorption Mechanism
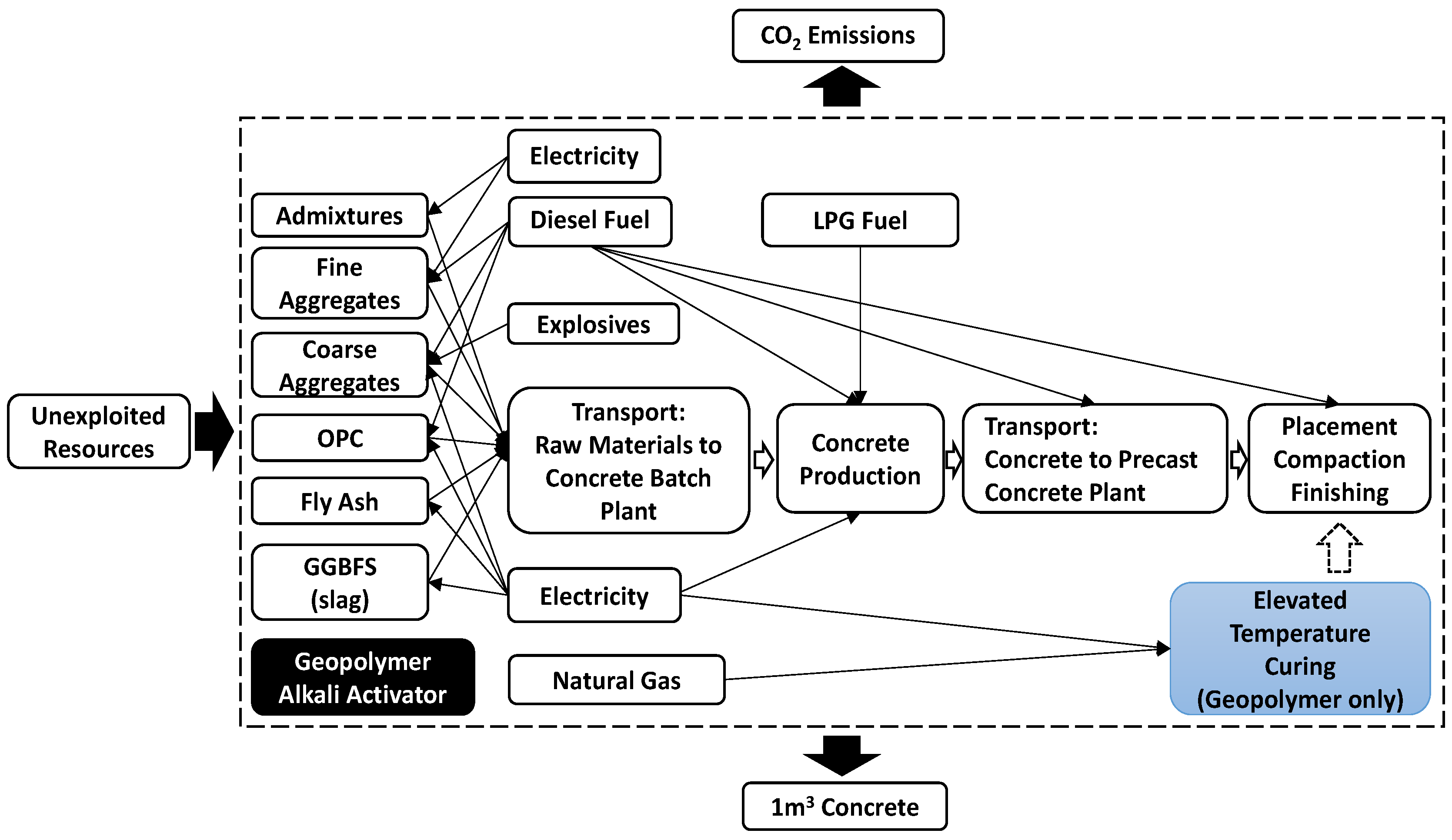
2.2. Cement Industry’s Role in Balancing the Release of CO2
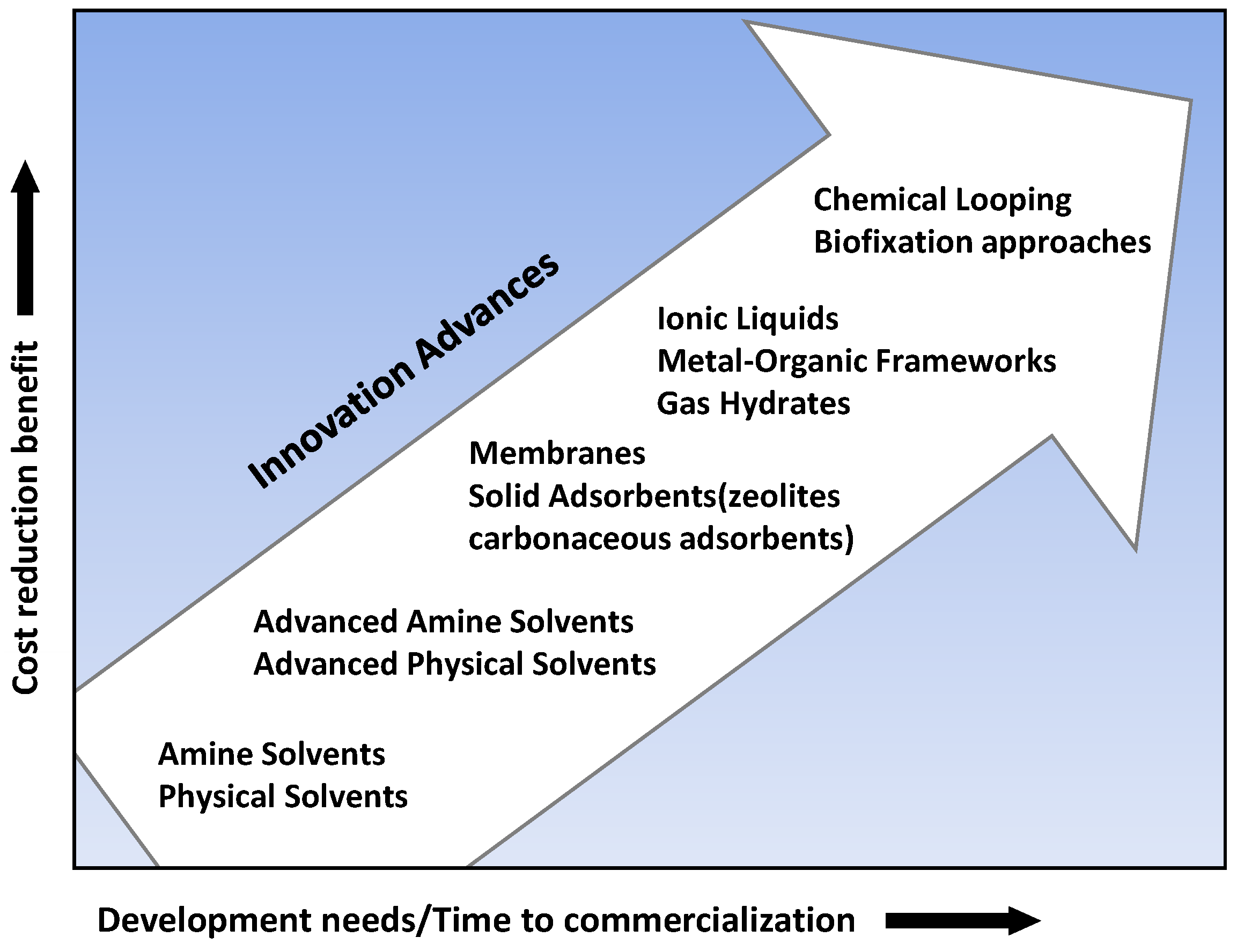
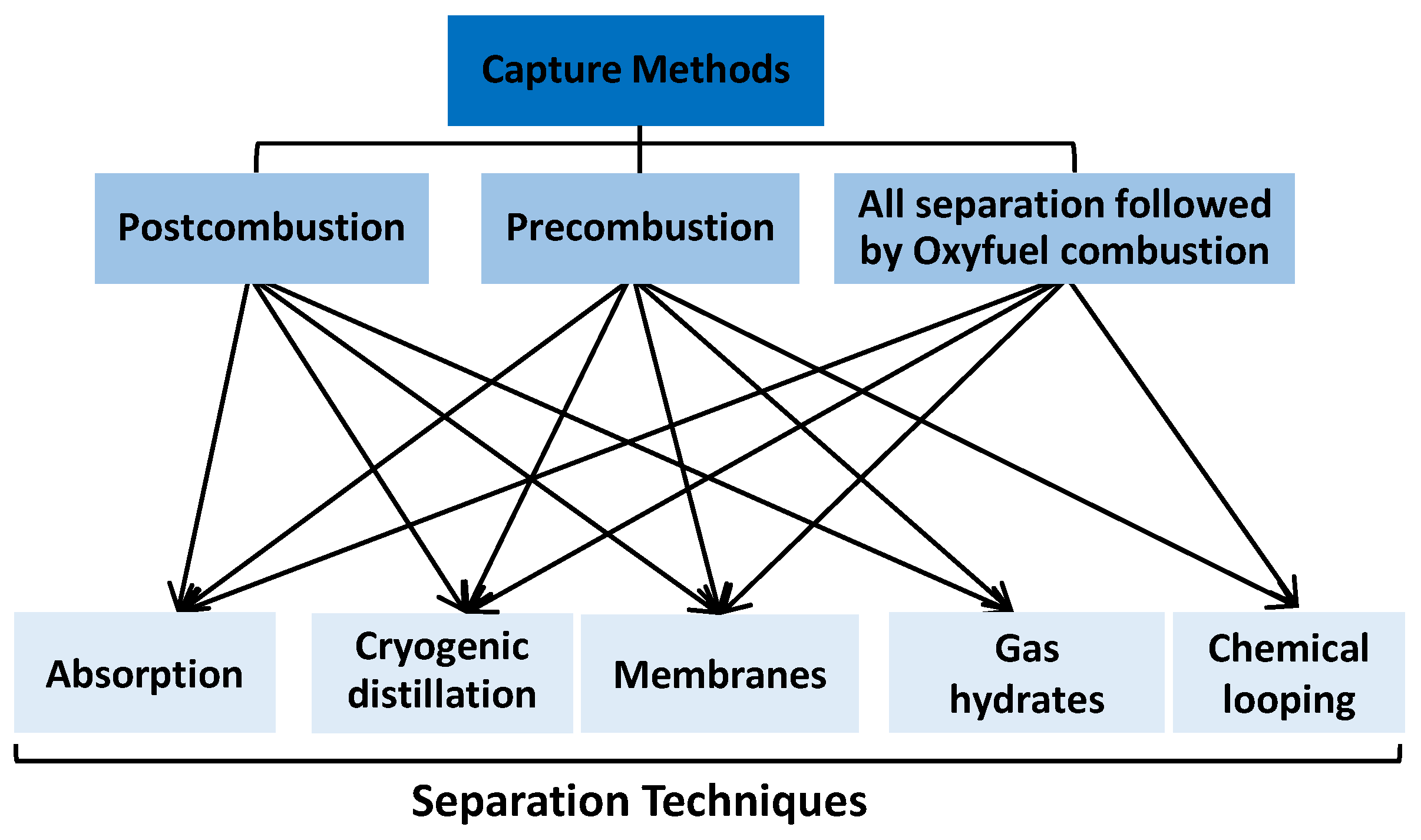
2.3. The Ways to Reduce Emissions in the Cement Industry
3. Carbonation of Concrete: An Investigation of Its Basic Characteristics
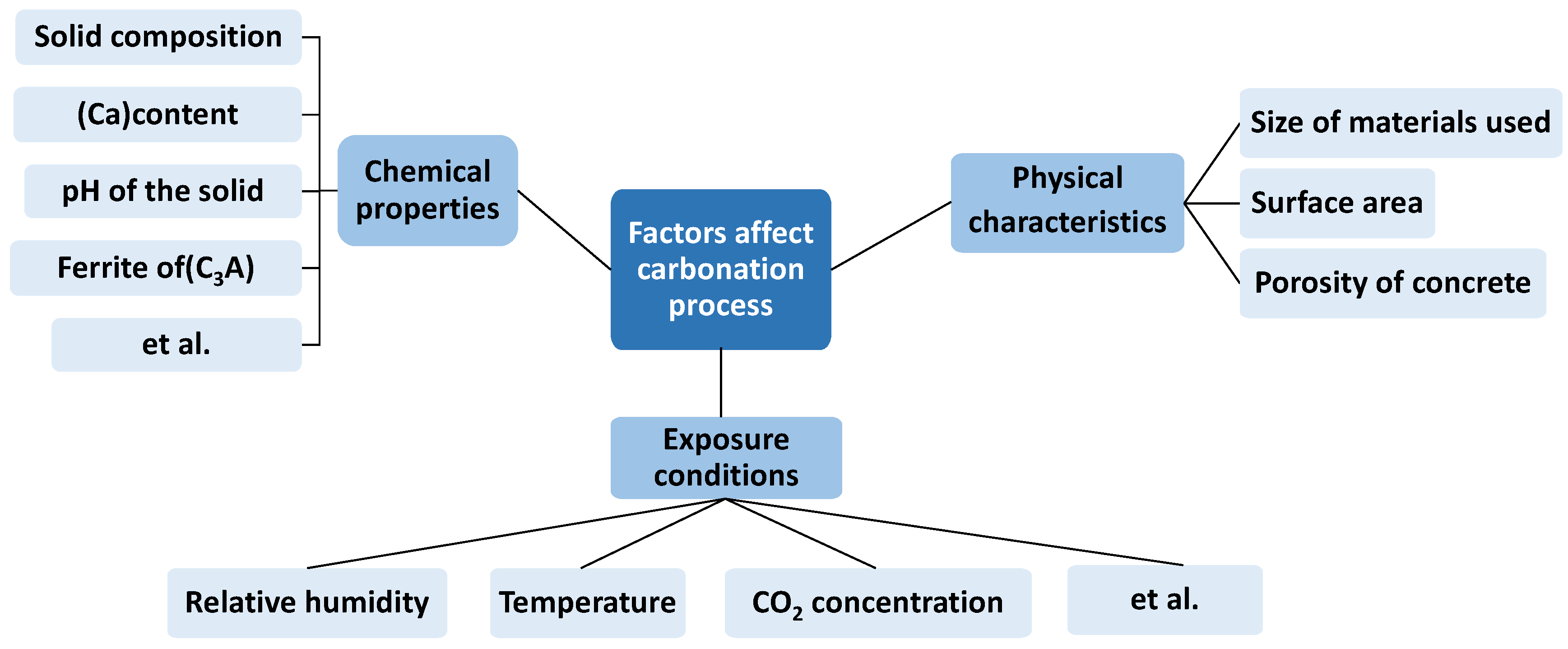
3.1. The Factors Affecting Carbonization
3.2. Modeling Carbonization Mathematically
3.3. Method for Detecting Carbonation
3.4. Applications and Findings of Carbonation Research
4. The Methods Increasing Concrete’s Ability to Adsorb Carbon
4.1. The Physical Method
4.2. The Chemical Method
- Basic carbonation mechanism
- 2.
- Mechanism applied to ammonia absorbent
4.3. The Microorganism’s Method
5. Conclusions and Prospect
- (1)
- Carbon dioxide has been sequestered in carbonating cement materials for a cumulative amount of 4.5 GtC since 1930. Over the same period, this project offset 43% of cement’s CO2 emissions. It does not include the fossil fuel emissions generated during cement production. The reverse process, carbonation in concrete, balances the release of carbon dioxide. Therefore, the significance of carbon sequestration by applying cement-based materials is that carbon neutrality of the cement industry can be achieved by the properties of cement itself.
- (2)
- Calcite sequestration into concrete is affected by various factors, including carbonation and acceleration. Temperature, relative humidity, and CO2 concentration are some of these curing conditions. Furthermore, solid physical characteristics as well as the material chemical properties affect carbonation. The aerated concrete carbonation rate is influenced significantly by concrete density and water–cement ratio (w/c), particularly by density and w/c ratio.
- (3)
- Carbon sequestration of materials mainly goes through two stages: carbon capture and carbon storage. As the first stage of carbon sequestration, carbon capture is the premise of carbon sequestration and determines the maximum amount of carbon sequestration. By optimizing the porous structure of cement-based materials to enhance the transmission to effectively improve the carbon capture performance, it can effectively enhance the carbon sequestration capacity. Carbon sequestration with carbonization reaction as the main way has been studied a lot, but there is little attention to carbon capture performance. Therefore, enhancing the carbon sequestration capacity of cement-based materials by increasing the total amount of carbon sequestration needs more attention, which can become an important research direction in the field of carbon sequestration by cement-based materials.
- (4)
- The future work in capturing carbon dioxide in concrete can be conducted based on the percolation theory and by changing the pore structure of concrete to improve the carbon sequestration capacity. The research direction includes changing the pore structure of concrete itself and changing the pore structure of concrete by adding porous materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alshalif, A.F.; Irwan, J.M.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S. A systematic review on bio-sequestration of carbon dioxide in bio-concrete systems: A future direction. Eur. J. Environ. Civ. Eng. 2022, 26, 1209–1228. [Google Scholar] [CrossRef]
- Anand, S.; Vrat, P.; Dahiya, R.P. Application of a system dynamics approach for assessment and mitigation of CO2 emissions from the cement industry. J. Environ. Manag. 2006, 79, 383–398. [Google Scholar] [CrossRef]
- Hendriks, C.A.; Worrell, E.; De Jager, D.; Blok, K.; Riemer, P. Emission reduction of greenhouse gases from the cement industry. In Proceedings of the Fourth International Conference on Greenhouse Gas Control Technologies, Interlaken, Switzerland, 30 August–2 September 1998; IEA GHG R&D Programme: Interlaken, Austria, 1998; pp. 939–944. [Google Scholar]
- Gonzalez, J.L.A. Technology Change and Environmental Management for Cement Manufacturing: The Cement Industry in the United States (2004–2050). Doctoral Dissertation, Carnegie Mellon University, Pittsburgh, PA, USA, 2005. [Google Scholar]
- World Business Council for Sustainable Development. The Cement Sustainability Initiative. Cement Industry Energy and CO2 Performance. 2010. Available online: http://www.wbcsd.org/DocRoot/LDqFZHcewCUmDh4v02pN/csi-gnr-reportwith-label.pdf (accessed on 17 September 2010).
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Deja, J.; Uliasz-Bochenczyk, A.; Mokrzycki, E. CO2 emissions from Polish cement industry. Int. J. Greenh. Gas Control 2010, 4, 583–588. [Google Scholar] [CrossRef]
- Hoenig, V.; Hoppe, H.; Emberger, B. Carbon Capture Technology—Options and Potentials for the Cement Industry; Tannenstrasse: European Cement Research Academy: Düsseldorf, Germany, 2007; p. 96. Available online: http://www.ecraonline.org/downloads/RepCCS.pdf (accessed on 16 August 2010).
- Qian, C.; Yu, X.; Zheng, T.; Chen, Y. Review on bacteria fixing CO2 and bio-mineralization to enhance the performance of construction materials. J. CO2 Util. 2021, 55, 101849. [Google Scholar] [CrossRef]
- Haselbach, L. Potential for carbon dioxide absorption in concrete. J. Environ. Eng. 2009, 135, 465–472. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of cement manufacturing: Comparing traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the eco-efficiency of cement use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Fang, J.; Yu, G.; Liu, L.; Hu, S.; Stuart Chapin, F. Climate change, human impacts, and carbon sequestration in China. Proc. Natl. Acad. Sci. USA 2018, 115, 4015–4020. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Çopuroğlu, O.; Park, K.B. Effect of global climatic change on carbonation progress of concrete. Atmos. Environ. 2007, 41, 7274–7285. [Google Scholar] [CrossRef]
- Pachauri, R.K. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on; IPCC: Geneva, Switzerland, 2014.
- Kiełbasa, K.; Bayar, Ş.; Varol, E.A.; Sreńscek-Nazzal, J.; Bosacka, M.; Michalkiewicz, B. Thermochemical conversion of lignocellulosic biomass-olive pomace-into activated biocarbon for CO2 adsorption. Ind. Crops Prod. 2022, 187, 115416. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.K.; Lee, H.W.; Chang, Y.S.; Han, K.; Kim, J.Y.; Rhee, C.H.; Chun, H.D.; Lee, M.W.; Park, J.M. Characterization of ammonia-based CO2 capture process using ion speciation. Int. J. Greenh. Gas Control 2011, 5, 1606–1613. [Google Scholar] [CrossRef]
- Leung DY, C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Naucler, T.; Campbell, W.; Ruijs, J. Carbon Capture and Storage: Assessing the Economics; Office of Scientific and Technical Information: London, UK, 2008.
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—the US Department of Energy’s Carbon Sequestration ProgramJ. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, H.; Guan, S.; Zhang, J.; Yao, D. Technology and method for applying biochar in building materials to evidently improve the carbon capture ability. J. Clean. Prod. 2020, 273, 123154. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
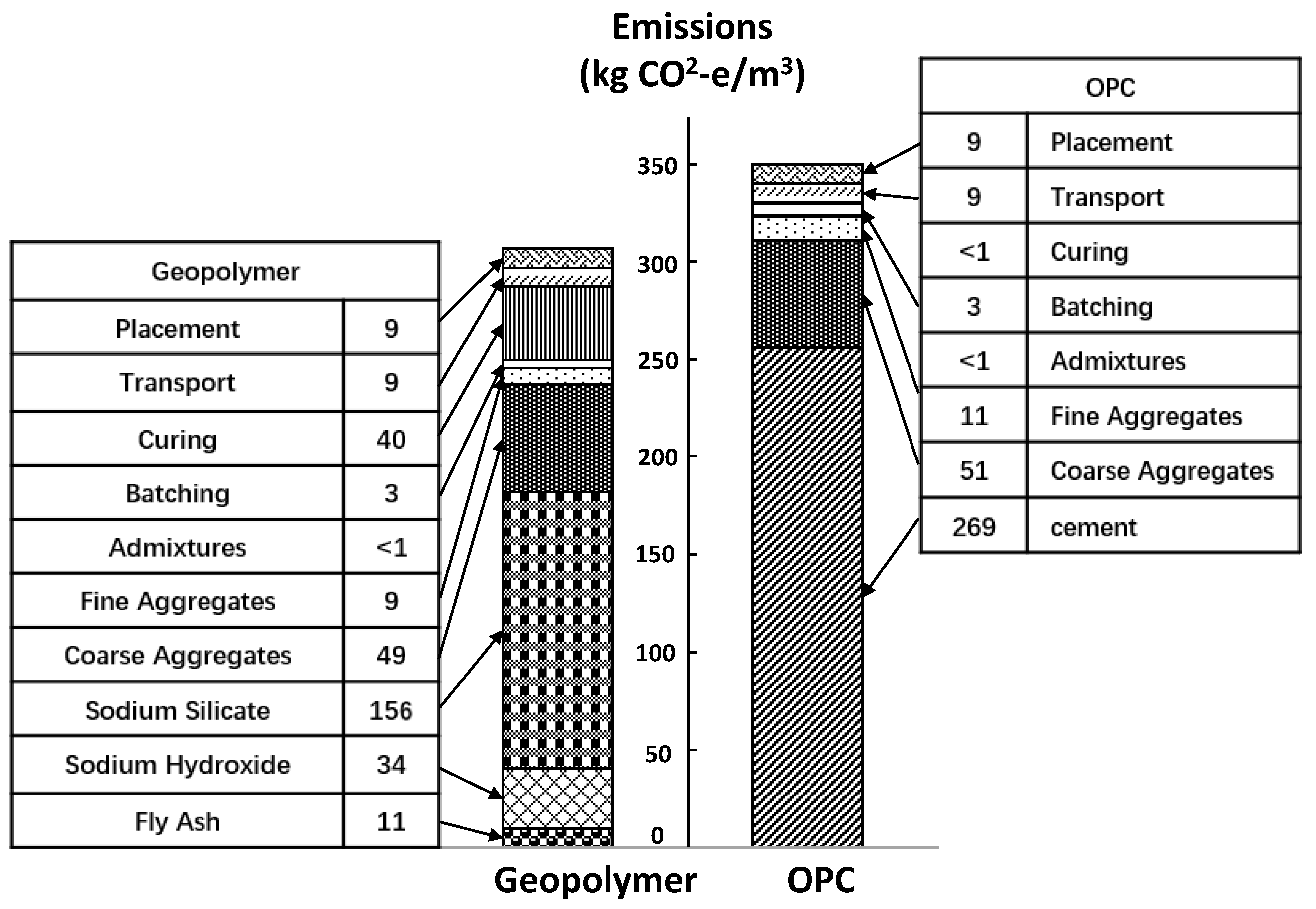
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete Structure, Properties, and Materials, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Bary, B.; Sellier, A. Coupled moisture–carbon dioxide–calcium transfer model for carbonation of concrete. Cem. Concr. Res. 2004, 34, 1859–1872. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z. Effects of temperature, relative humidity and carbon dioxide concentration on concrete carbonation. Mag. Concr. Res. 2020, 72, 936–947. [Google Scholar] [CrossRef]
- Newell, R.; Anderson, S. Prospects for Carbon Capture and Storage Technologies; Discussion Paper; IEA: Paris France, 2003; p. 70.
- ECDN. Capturing and Storing Carbon Dioxide: Technical Lessons Learned. 2004. Available online: http://www.co2net.eu/public/reports/technicallessonslearnedfifinalreport.pdf (accessed on 17 August 2010).
- Ogbeide, S. Developing an optimization model for CO2 reduction in cement production process. J. Eng. Sci. Technol. Rev. 2010, 3, 85–88. [Google Scholar] [CrossRef]
- Caruso, H. Reduction of Carbon Dioxide Emissions from Cement Plants. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2007. [Google Scholar]
- Metz, B. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005; p. 431. [Google Scholar]
- Bosoaga, A.; Masek, O.; Oakey, J. CO2 capture technologies for cement industry. Energy Procedia 2009, 1, 133–140. [Google Scholar] [CrossRef]
- Taylor, M.; Tam, C.; Gielen, D. Energy efficiency and CO2 emissions from the global cement industry. Korea 2006, 50, 61–67. [Google Scholar]
- Kellouche, Y.; Boukhatem, B.; Ghrici, M.; Tagnit-Hamou, A. Exploring the major factors affecting fly-ash concrete carbonation using artificial neural network. Neural Comput. Appl. 2019, 31, 969–988. [Google Scholar] [CrossRef]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle: State of the Art; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 2005. [Google Scholar]
- Liang, M.T.; Qu, W.-J.; Liao, Y.S. A study of carbonation in concrete structures at existing cracks. J. Chin. Inst. Eng. 2000, 23, 143–153. [Google Scholar] [CrossRef]
- Lee, M.G.; Wang, W.C.; Huang, Y.S.; Su, Y.M.; Jiang, Q.Z. Effect of carbon dioxide curing on strength development of cement mortar. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2017; Volume 748, pp. 323–327. [Google Scholar]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Kumazaki, K. A mathematical model of carbon dioxide transport in concrete carbonation process. Discret. Contin. Dyn. Syst. Ser. S 2014, 7, 113–125. [Google Scholar] [CrossRef]
- Muntean, A.; Böhm, M. A moving-boundary problem for concrete carbonation: Global existence and uniqueness of weak solutions. J. Math. Anal. Appl. 2009, 350, 234–251. [Google Scholar] [CrossRef]
- Aiki, T.; Kumazaki, K. Mathematical model for hysteresis phenomenon in moisture transport in concrete carbonation process. Physica B 2012, 407, 1424–1426. [Google Scholar] [CrossRef]
- Aiki, T.; Kumazaki, K. Well-posedness of a mathematical model for moisture transport appearing in concrete carbonation process. Adv. Math. Sci. Appl. 2011, 21, 361–381. [Google Scholar]
- Maekawa, K.; Ishida, T.; Kishi, T. Multi-scale modeling of concrete performance. J. Adv. Concr. Technol. 2003, 1, 91–126. [Google Scholar] [CrossRef]
- Maekawa, K.; Chaube, R.; Kishi, T. Modeling of Concrete Carbonation; Taylor and Francis: Abingdon upon Thames, UK, 1999. [Google Scholar]
- Kumazaki, K.; Aiki, T. Uniqueness of a solution for some parabolic type of equation with hysteresis in three dimensions. Netw. Heterog. Media 2014, 9, 683–707. [Google Scholar]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Aiki, T.; Kumazaki, K. Mathematical Modelling of Concrete Carbonation Process with Hysteresis Effect; RIMS, Kyoto University: Kyoto, Japan, 2012; Volume 1792, pp. 99–107. [Google Scholar]
- Chang, C.-F.; Chen, J.-W. The experimental investigation of carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Fardis, M.N.; Vayenas, C.G. Hydration and carbonation of pozzolanic cements. ACI Mater. J. 1992, 89, 119–130. [Google Scholar]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991, 46, 1333–1338. [Google Scholar] [CrossRef]
- Huntzinger, D.N. Carbon Dioxide Sequestration in Cement Kiln Dust through Mineral Carbonation. Ph.D. Dissertation, Michigan Technological University, Houghton, MI, USA, 2006. [Google Scholar]
- Huijgen, W.J.J.; Witkamp, G.-J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.F.W.; Mohan, K.; Moir, G.K. Analytical study of pure and extended portland cement pastes. I: Pure portland cement pastes. J. Am. Ceram. Soc. 1985, 68, 680–685. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Feldman, R.F.; Sereda, P.J. Application of differential thermal analysis in cement research. Highw. Res. Rec. 1964, 62, 40–61. [Google Scholar]
- Cole, W.F.; Kroone, B. Carbon dioxide in hydrated portland cement. J. Am. Concr. Inst. 1960, 31, 1275–1295. [Google Scholar]
- Stern, K.H. High Temperature Properties and Thermal Decomposition of Inorganic Salts With Oxyanions; CRC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Pan, X.; Shi, C.; Hu, X.; Ou, Z. Effects of CO2 surface treatment on strength and permeability of one-day-aged cement mortar. Constr. Build. Mater. 2017, 154, 1087–1095. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Z.; Niu, Y.; Li, J.; Zhang, Y. Study on the preparation and properties of high-porosity foamed concretes based on ordinary Portland cement. Mater. Des. 2016, 92, 949–959. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Li, K.; Yu, B.; Grigore, M.; Yang, Q.; Wang, X.L.; Cheng, Z.L.; Zeng, M.; Zhao, S.F. Integrated absorption-mineralisation for low-energy CO2 capture and sequestration. Appl. Energy 2018, 225, 356–366. [Google Scholar] [CrossRef]
- Mun, S.J.; Han, S.J.; Wee, J.H. Carbon dioxide fixation by precipitating NaHCO3 via carbonation of NaOH-dissolved ethanol aqueous solution. Energy Fuel 2018, 32, 8614–8622. [Google Scholar] [CrossRef]
- Grollier, K.; Vu, N.D.; Onida, K.; Akhdar, A.; Norsic, S.; Agosto, F.D.; Boisson, C.; Duguet, N. A theriomorphic polyethylene-supported imidazolium salt for the fixation of CO2 into cyclic carbonates. Adv. Synth. Catal. 2020, 362, 1696–1705. [Google Scholar] [CrossRef]
- Hong, J.L.; Chen, W.; Wang, Y.T.; Xu, C.Q.; Xu, X. Life cycle assessment of caustic. Aust. J. Basic Appl. Sci. 2013, 7, 421–431. [Google Scholar]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef]
- Karl, M.; Wright, R.F.; Berglen, T.F.; Denby, B. Worst case scenario study to assess the environmental impact of amine emissions from a CO2 capture plant. Int. J. Greenh. Gas Control. 2011, 5, 439–447. [Google Scholar] [CrossRef]
- Zheng, T.W.; Qian, C.X. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar]
- Maeshima, K.; Yoshimoto, M. Preparation and characterization of carbonic anhydrase-conjugated liposomes for catalytic synthesis of calcium carbonate particles. Enzym. Microb. Technol. 2017, 105, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Kiełbasa, K.; Michalkiewicz, B. The new tailored nanoporous carbons from the common polypody (Polypodium vulgare): The role of textural properties for enhanced CO2 adsorption. Chem. Eng. J. 2022, 429, 131751. [Google Scholar] [CrossRef]
- Murge, P.; Dinda, S.; Roy, S. Zeolite-based sorbent for CO2 capture: Preparation and performance evaluation. Langmuir 2019, 35, 14751–14760. [Google Scholar] [CrossRef]
- Liu, W.; Song, L.; Xu, C.C.; Rohani, S.; Chen, M.; Liang, B.; Li, C. Combined synthesis of Li4SiO4 sorbent with high CO2 uptake in the indirect carbonation of blast furnace slag process. Chem. Eng. J. 2019, 370, 71–80. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Science. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems design and economic analysis of direct air capture of CO2 through temperature vacuum swing adsorption using MIL-101 (Cr)-PEI-800 and mmen-Mg2 (dobpdc) MOF adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Zhang, W.; Li, Z.; Xing, F.; Tang, L. Application potential analysis of biochar as a carbon capture material in cementitious composites: A review. Constr. Build. Mater. 2022, 350, 128715. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Ma, Q.; Xie, L.; Zhang, X.; Guo, H. Enhancement of CO2 capture on biomass-based carbon from Black Locust by KOH activation and ammonia modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Ozsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Kami’nska, A.; Niedoba, O.; Michalkiewicz, B. CO2 adsorption on activated carbons prepared from molasses: A comparison of two and three parametric models. Materials 2021, 14, 7458. [Google Scholar] [CrossRef]
- Al Mesfer, M.K. Synthesis and characterization of high-performance activated carbon from walnut shell biomass for CO2 capture. Environ. Sci. Pollut. Res. 2020, 27, 15020–15028. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Chang, C.F.Y.; Wang, S.Y.; Chang, C.F.Y.; Chien, S.F.; Sun, H.F. Utilization of agricultural waste corn cob for the preparation of carbon adsorbent. J. Environ. Sci. Health Part B 2001, 36, 677–686. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R.M. Synthesis and characterization of activated carbon from biomass date seeds for carbon dioxide adsorption. J. Environ. Chem. Eng. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Shih, C.-H.; Lo, S.-L.; Sun, L.; Zhong, Y.; Qiu, C. Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High surface area porous carbon from cotton stalk agro-residue for CO2 adsorption and study of techno-economic viability of commercial production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Belver, C.; Rodriguez, J.J.; Bedia, J. Equilibrium, kinetics and breakthrough curves of acetaminophen adsorption onto activated carbons from microwave-assisted FeCl3-activation of lignin. Sep. Purif. Technol. 2021, 278, 119654. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Cabriga, C.K.C.; Clarete, K.V.B.; Zhang, J.A.T.; Pacia, R.M.P.; Ko, Y.S.; Castro, J.C. Evaluation of biochar derived from the slow pyrolysis of rice straw as a potential adsorbent for carbon dioxide. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Sun, M.; Li, W.; Hu, X. Adsorption of CO2 by nitrogen doped corn straw-based biochar. Arab. J. Geosci. 2021, 14, 1875. [Google Scholar] [CrossRef]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. CO2 adsorption of bagasse waste feedstock using thermogravimetric analyses. J. Therm. Anal. Calorim. 2022, 147, 5973–5984. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon dioxide capture from flue gas in biochar produced from spent coffee grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Z.; Du, Y.; Xu, J.; Kong, L.; Pan, Y.; Xiao, H.; Xie, Q. Thermodynamics of CO2 adsorption on cellulose-derived biochar prepared in ionic liquid. Can. J. Chem. Eng. 2021, 99, 1940–1961. [Google Scholar] [CrossRef]
- Salem, I.B.; El Gamal, M.; Sharma, M.; Hameedi, S.; Howari, F.M. Utilization of the UAE date palm leaf biochar in carbon dioxide capture and sequestration processes. J. Environ. Manag. 2021, 299, 113644. [Google Scholar] [CrossRef]
- Promraksa, A.; Rakmak, N. Biochar production from palm oil mill residues and application of the biochar to adsorb carbon dioxide. Heliyon 2020, 6, e04019. [Google Scholar] [CrossRef]
- Dilokekunakul, W.; Teerachawanwong, P.; Klomkliang, N.; Supasitmongkol, S.; Chaemchuen, S. Effects of nitrogen and oxygen functional groups and pore width of activated carbon on carbon dioxide capture: Temperature dependence. Chem. Eng. J. 2020, 389, 124413. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Dissanayake, P.D.; Shang, J.; Wang, C.H.; Yang, X.; Kim, S.; Tsang, D.C.W.; Lee, K.B.; Ok, Y.S. Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption. J. Hazard. Mater. 2020, 391, 121147. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Shang, J.; Hanif, A.; Dissanayake, P.D.; Tsang, D.C.; Kwon, J.H.; Lee, K.B.; Ok, Y.S. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Praneeth, S.; Guo, R.; Wang, T.; Dubey, B.K.; Sarmah, A.K. Accelerated carbonation of biochar reinforced cement-fly ash composites: Enhancing and sequestering CO2 in building materials. Constr. Build. Mater. 2020, 244, 118363. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Poon, C.S.; Wang, C.H.; Ok, Y.S.; Mechtcherine, V.; Tsang, D.C. Roles of biochar and CO2 curing in sustainable magnesia cement-based composites. ACS Sustain. Chem. Eng. 2021, 9, 8603–8610. [Google Scholar] [CrossRef]
- Gupta, S.; Kashani, A.; Mahmood, A.H.; Han, T. Carbon sequestration in cementitious composites using biochar and fly ash–Effect on mechanical and durability properties. Constr. Build. Mater. 2021, 291, 123363. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Cynthia, S.Y.T. Use of biochar-coated polypropylene fibers for carbon sequestration and physical improvement of mortar. Cem. Concr. Compos. 2017, 83, 171–187. [Google Scholar] [CrossRef]
- Tan, K.; Pang, X.; Qin, Y.; Wang, J. Properties of cement mortar containing pulverized biochar pyrolyzed at different temperatures. Constr. Build. Mater. 2020, 263, 120616. [Google Scholar] [CrossRef]
- Maljaee, H.; Paiva, H.; Madadi, R.; Tarelho, L.A.; Morais, M.; Ferreira, V.M. Effect of cement partial substitution by waste-based biochar in mortars properties. Constr. Build. Mater. 2021, 301, 124074. [Google Scholar] [CrossRef]
- Sirico, A.; Bernardi, P.; Sciancalepore, C.; Vecchi, F.; Malcevschi, A.; Belletti, B.; Milanese, D. Biochar from wood waste as additive for structural concrete. Constr. Build. Mater. 2021, 303, 124500. [Google Scholar] [CrossRef]
- Gupta, S.; Tulliani, J.M.; Kua, H.W. Carbonaceous admixtures in cementitious building materials: Effect of particle size blending on rheology, packing, early age properties and processing energy demand. Sci. Total Environ. 2022, 807, 150884. [Google Scholar] [CrossRef]
- Gupta, S.; Wei, K.H.; Dai, P.S. Effect of biochar on mechanical and permeability properties of concrete exposed to elevated temperature. Constr. Build. Mater. 2020, 234, 16. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Novel biochar-concrete composites: Manufacturing, characterization and evaluation of the mechanical properties. Sci. Total Environ. 2018, 616, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Pang, X.; Tan, K.; Bao, T. Evaluation of pervious concrete performance with pulverized biochar as cement replacement. Cem. Concr. Compos. 2021, 119, 104022. [Google Scholar] [CrossRef]
- Haque, M.I.; Khan, R.I.; Ashraf, W.; Pendse, H. Production of sustainable, low-permeable and self-sensing cementitious composites using biochar. Sustain. Mater. Technol. 2021, 28, e00279. [Google Scholar] [CrossRef]
- Jami, T.; Rawtani, D.; Agrawal, Y.K. Hemp concrete: Carbon-negative construction. Emerg. Mater. Res. 2016, 5, 240–247. [Google Scholar] [CrossRef]
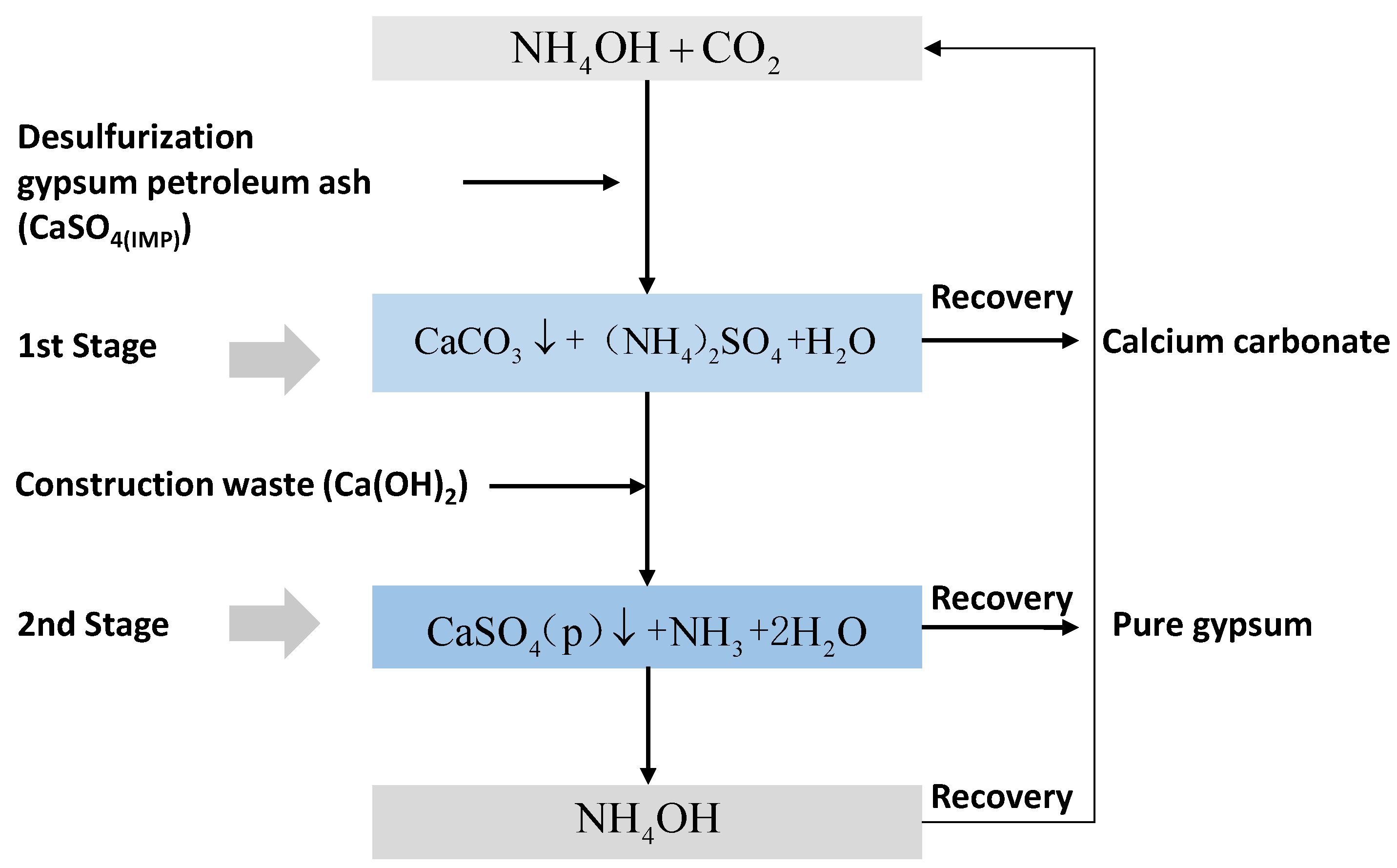
- Lee, M.G.; Kang, D.; Jo, H.; Park, J. Carbon dioxide utilization with carbonation using industrial waste-desulfurization gypsum and waste concrete. J. Mater. Cycles Waste Manag. 2016, 18, 407–412. [Google Scholar] [CrossRef]
- Arora, N.K. Bioremediation: A green approach for restoration of polluted ecosystems. Environ. Sustain. 2018, 1, 305–307. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of fly ash concrete. Constr. Build. Mater. 2012, 28, 351–356. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int. J. Greenh. Gas Control 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Zhang, Y.; Fu, X.; Tsang, Y.; Wu, J.; Le, Y. Variable decomposition of two plant litters and their effects on the carbon sequestration ability of wetland soil in the Yangtze River estuary. Geoderma 2018, 319, 230–238. [Google Scholar] [CrossRef]
- Achal, V.; Mukerjee, A.; Reddy, M.S. Biogenic treatment improves the durability and remediates the cracks of concrete structures. Constr. Build. Mater. 2013, 48, 1–5. [Google Scholar] [CrossRef]
- de Muynck, W.; Cox, K.; de Belie, N.; Verstraete, W. Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr. Build. Mater. 2008, 22, 875–885. [Google Scholar] [CrossRef]
| Ca(OH)2 TGA Decomposition | CaCO3 TGA Decomposition | Aragonite–Calcite Conversion | Vaterite–Calcite Conversion | Author |
|---|---|---|---|---|
| 425–550 | 550–950 | Chang and Chen [52] | ||
| 460 | Papadakis et al. [53] | |||
| 400–500 | 600–800 | Papadakis et al. [54] | ||
| 300–500 | 800-1000 unknown/chlorides | Huntzinger [55] | ||
| >500 | Huijgen et al. [56]—slag | |||
| 450–650 | Taylor et al. [57] | |||
| 464(DTA) | 850–950 calcite | Ramachandran et al. [58] | ||
| 600–750 poorly crystallinedls20 well crystallized | Cole and Kroone [59] | |||
| 827–927 calcite | ~460 | ~350–400 | Stern [60] |
| Material | Carbon Adsorption | Method | Author |
|---|---|---|---|
| Activated carbons | The superior CO2 adsorption at 1 bar equal to 5.67 and 9.05 mmol/g, for 298 and 273 K, respectively. | The isosteric heat of adsorption calculated on the basis of the Clausius–Clapeyron equation and Sips model | Serafin et al., 2019 [72] |
| Zeolites | At 30 degrees, zeolite-Y (designated as Z-Y-3, silica to alumina ratio of 2.25) sample exhibited maximum adsorption capacity, and the obtained values were around 114 and 190 mg CO2/g sorbent under atmospheric and 5 bar pressure | CO2 capture capacity of the sorbents was examined at various temperatures and pressures employing a fixed-bed flow reactor and simulated flue gas. | Murge et al., 2019 [73] |
| Silica gel | The sorbent synthesized with Li2CO3 and the BFS-derived silica gel at the Li/Si molar ratio of 4 (Li2CO3-BFS-4) possesses the best CO2 uptake performance with a sorption capacity of 0.329 g CO2/g, sorbent within 20 min. | Liu et al., 2019 [74] | |
| Metal–organic frameworks | The metal–organic frameworks (MOFs) are proving to be effective adsorbent material for CO2 capture due to their microporous structure. | Younas et al., 2020 [75] | |
| Polyethylenimine | The utility of polyethylenimine (PEI)-implanted MIL-101 for dilute CO2 capture; adsorption capacity of 1.0 mmol/gat 400 ppm. | Temperature Vacuum Swing Adsorption | Darunte et al. [76] |
| Biochar Materials | Studies | Author | |
|---|---|---|---|
| Agricultural waste | rice straw | In the presence of pure CO2 gas, biochar had 2.6 wt% adsorption capacity | C.K.C. Cabriga et al. [88] |
| corn stalks | It has been found that the biochar made from corn straw at 25 °C and 1 bar absorbs 4.51 mmol of CO2 per gram | Y.L. Zhou et al. [89] | |
| sugarcane bagasse | According to the studies conducted on sugarcane bagasse biochar, it has the capacity to adsorb CO2 at a temperature of 25 °C of 0.75 mmol/g | Christiano et al. [90] | |
| coffee grounds | With 30 °C adsorption temperature and a 30% CO2 concentration, coffee bagasse biochar could adsorb 2.8 mmol/g of CO2 at 600 °C pyrolysis | Alivia Mukherjee et al. [91] | |
| Wood waste | palm husk cellulose | As a result of maintaining 3.3 mmol/g of carbon dioxide adsorption at 25 °C and 1 bar, it is clear that good carbon capture is possible | Guo et al. [92] |
| date palm leaves | When date palm leaves were pyrolyzed to make biochar, they adsorb the maximum CO2 of 0.25 mol/kg | Salemet al. [93] | |
| palm kernel shells | At room temperature and standard atmospheric pressure, Promraksa et al. obtained a CO2 capture rate of 0.46 mmol/g using biochar produced from palm kernel shells | Promraksa et al. [94] | |
| bamboo | In their study, Waralee et al. used bamboo as the raw material for producing biochar, which absorbed 2.52 mmol/g of CO2 at 25 °C and a pressure of 1 bar. | Waralee et al. [95] | |
| Domestic garbage waste | pine wood biochar | Biochar produced at 550 °C absorbed CO2 at a rate of 1.66 mmol/g. | Igalavithan et al. [96] |
| paper mill sludge biochar | At 25 °C and 1 bar, 0.2 mmol/g of biochar produced from paper mill sludge was measured at 600 °C. | Igalavithan et al. [97] | |
| biochar made from 70% wood chips and 30% chicken manure | A combination biochar made from 70% wood chips and 30% chicken manure displayed 1.75 mmol/g CO2 adsorption capacity | P.D. Dissanayake et al. [98] | |
| pure wood chip biochar | Pure wood chip biochar had an adsorption capacity of 1.48 mmol/g at 30 °C and 1 bar of pressure | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Liu, R. The Capture and Transformation of Carbon Dioxide in Concrete: A Review. Symmetry 2022, 14, 2615. https://doi.org/10.3390/sym14122615
Wang Y, Li X, Liu R. The Capture and Transformation of Carbon Dioxide in Concrete: A Review. Symmetry. 2022; 14(12):2615. https://doi.org/10.3390/sym14122615
Chicago/Turabian StyleWang, Yixiao, Xiaolin Li, and Rui Liu. 2022. "The Capture and Transformation of Carbon Dioxide in Concrete: A Review" Symmetry 14, no. 12: 2615. https://doi.org/10.3390/sym14122615
APA StyleWang, Y., Li, X., & Liu, R. (2022). The Capture and Transformation of Carbon Dioxide in Concrete: A Review. Symmetry, 14(12), 2615. https://doi.org/10.3390/sym14122615







