An Examination of Factors Influencing Small Proton Chemical Shift Differences in Nitrogen-Substituted Monodeuterated Methyl Groups
Abstract
:1. Introduction
2. N-CHD-2-methylpiperidine
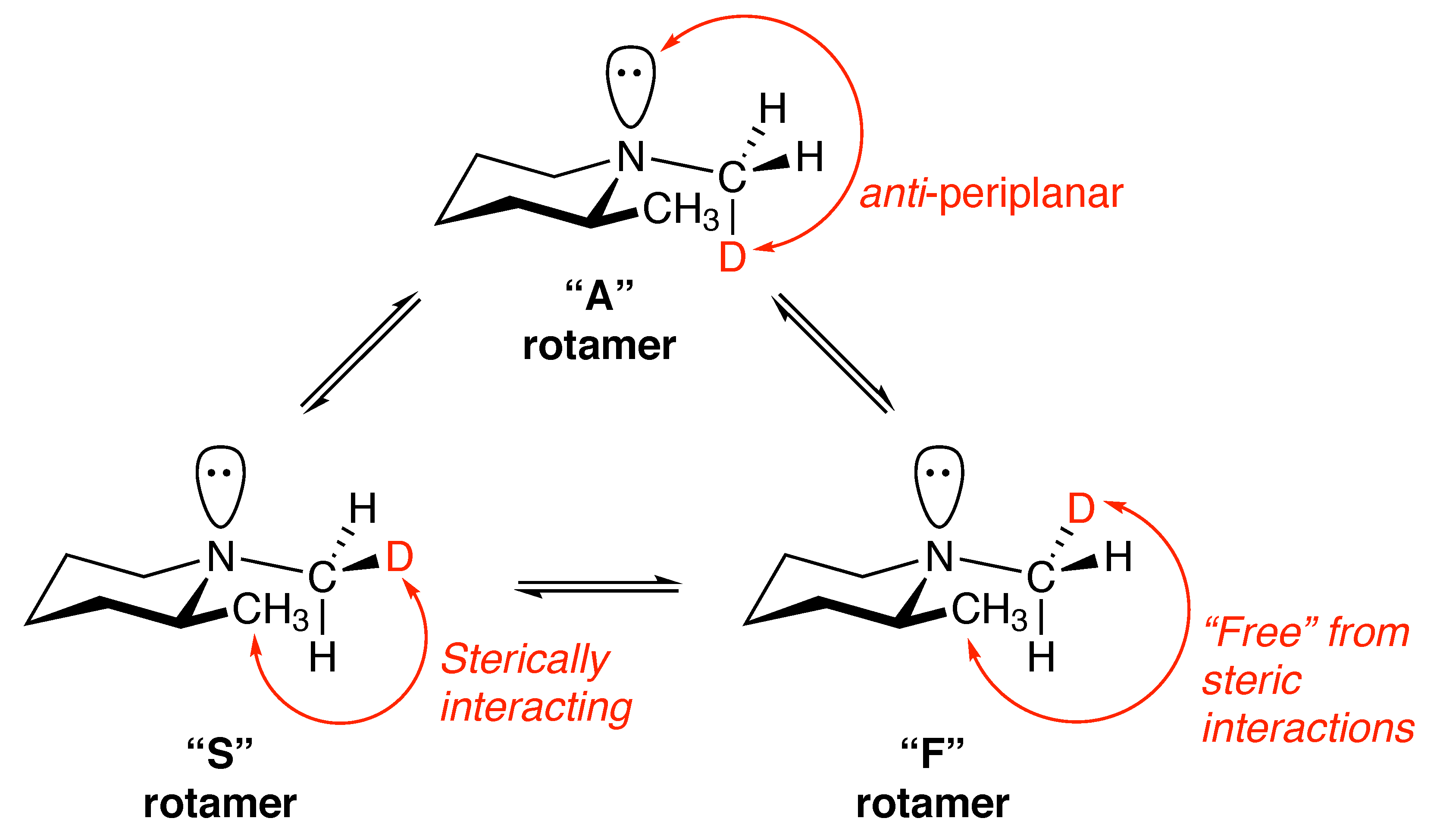
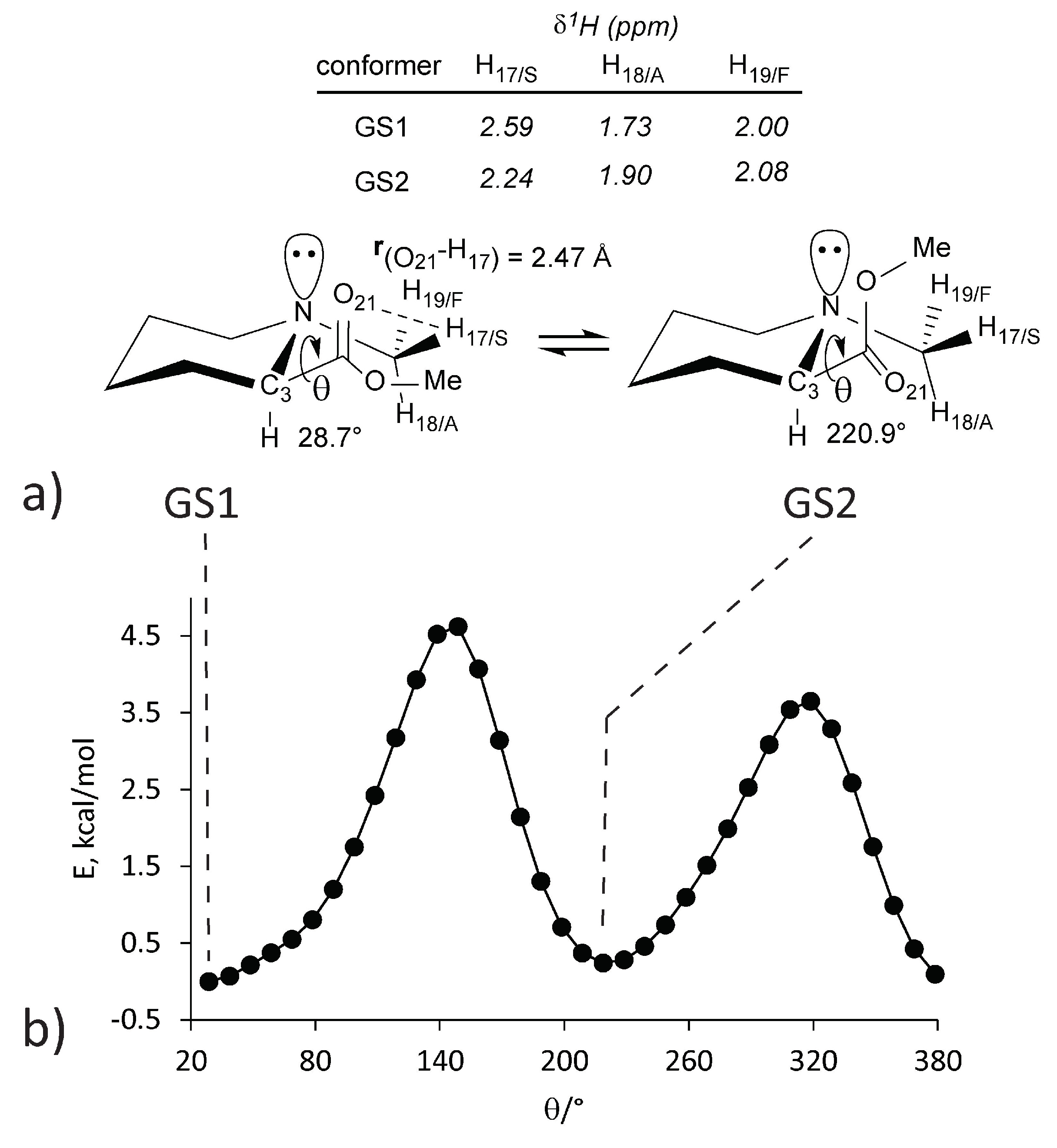
2.1. Symmetry Breaking
- A considerable rotameric preference/aversion for a particular CHD group rotamer.
- Distinct magnetic environments at each site occupied by a CHD group proton.
- S. The deuteron is sterically interacting with the 2-position CH group.
- F. The deuteron is free from interaction with the 2-position CH group.
- A. The deuterium is anti to the lone pair of electrons on the nitrogen atom.
- Compute and the relative populations of the rotamers i = S, F and A.
- Determine the chemical shift difference between the CHD protons of the rotamers i = S, F and A.
- Weight the chemical shift difference of each rotamer by the corresponding relative population .
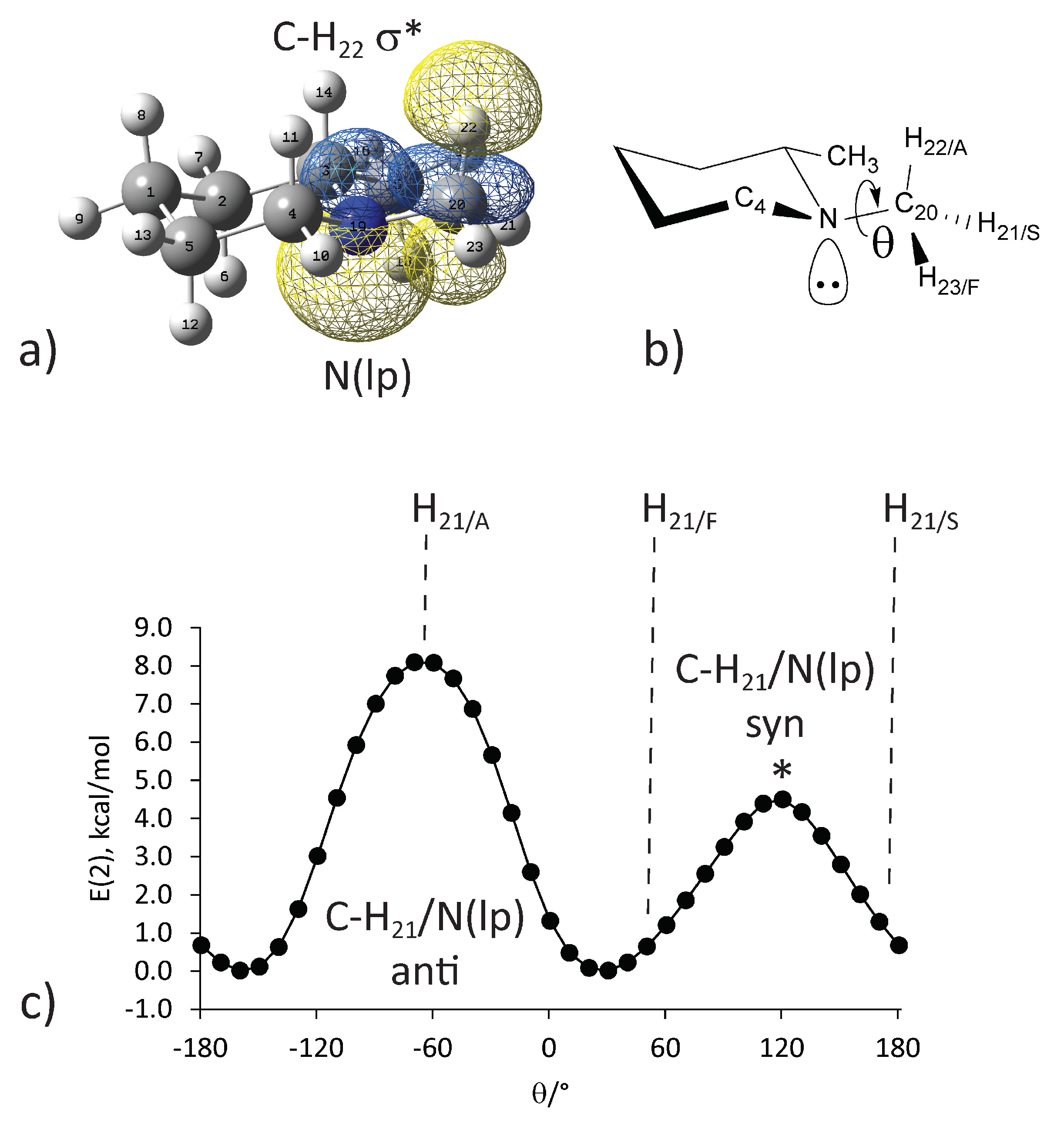
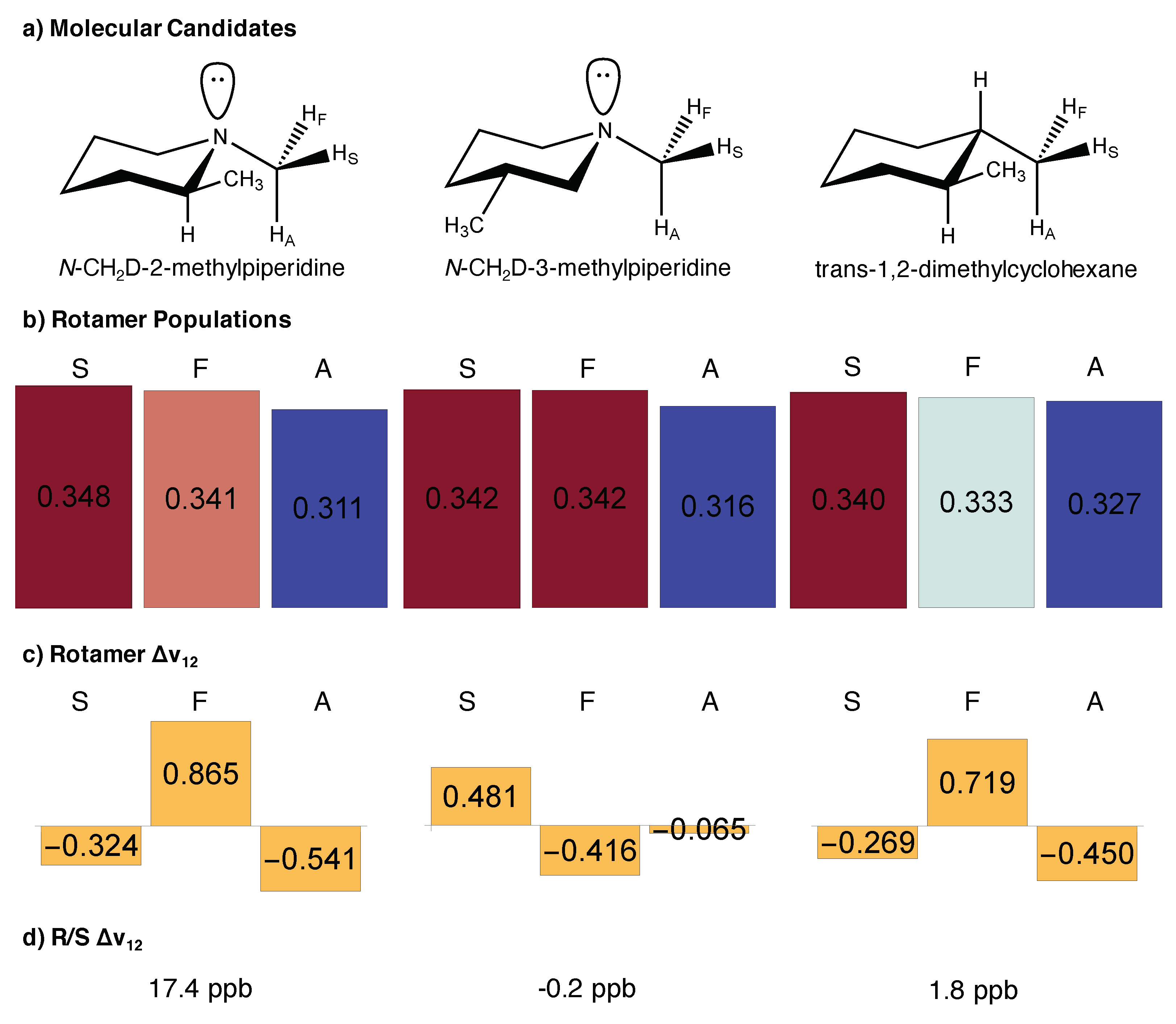
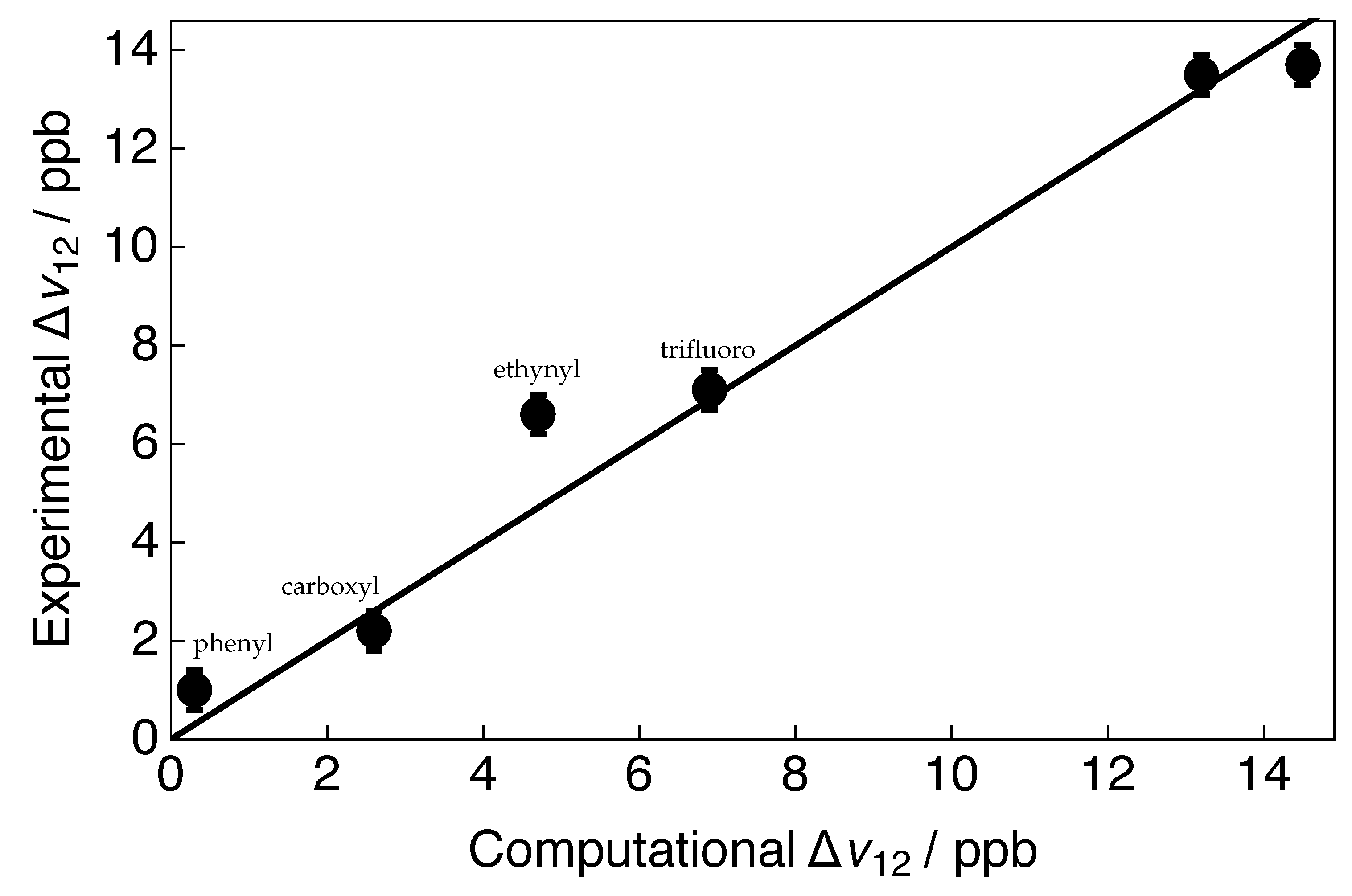
2.2. Illustrative Examples
2.3. The Role of Nitrogen
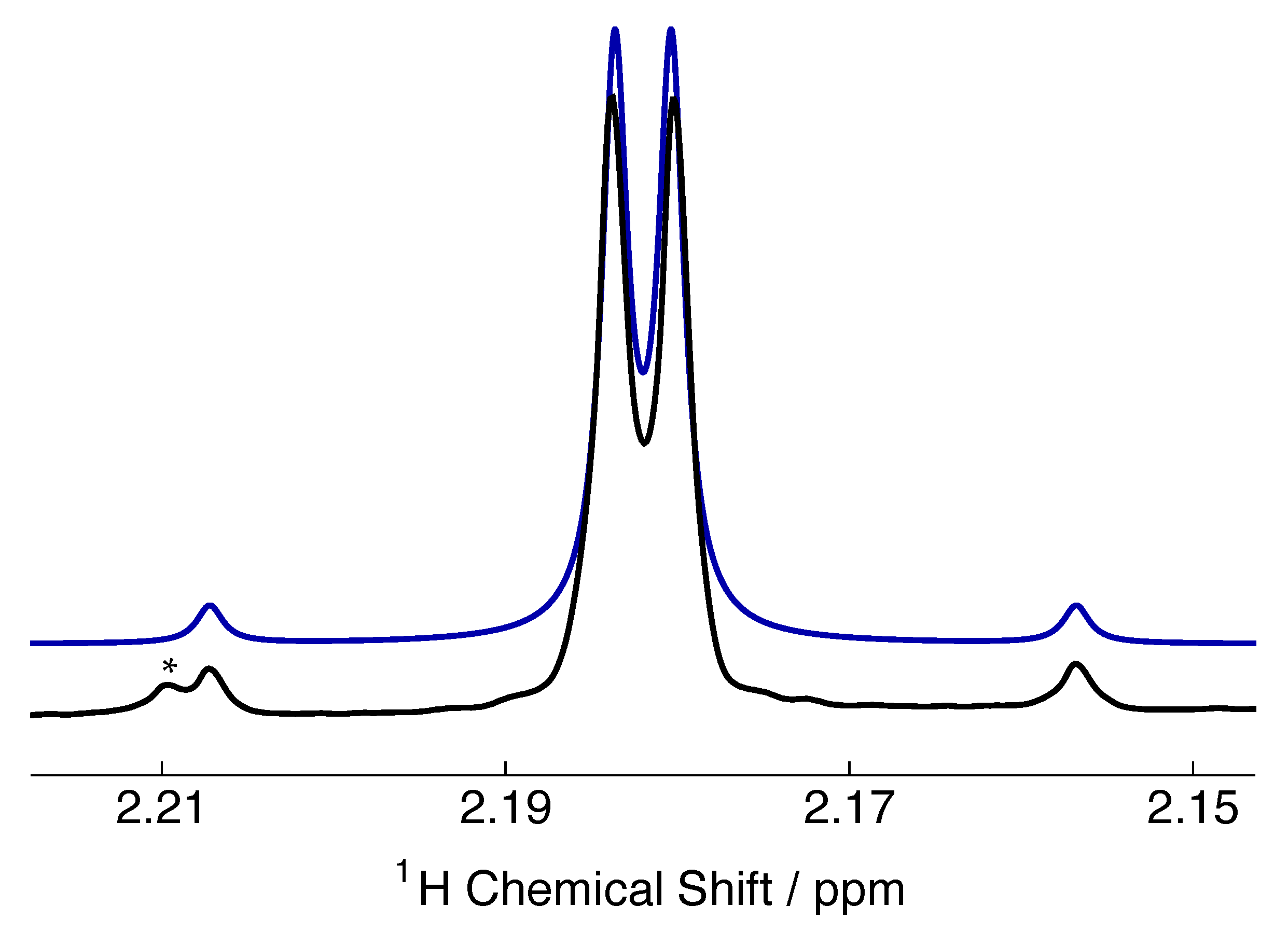
3. H NMR Spectrum
4. Environmental Influences
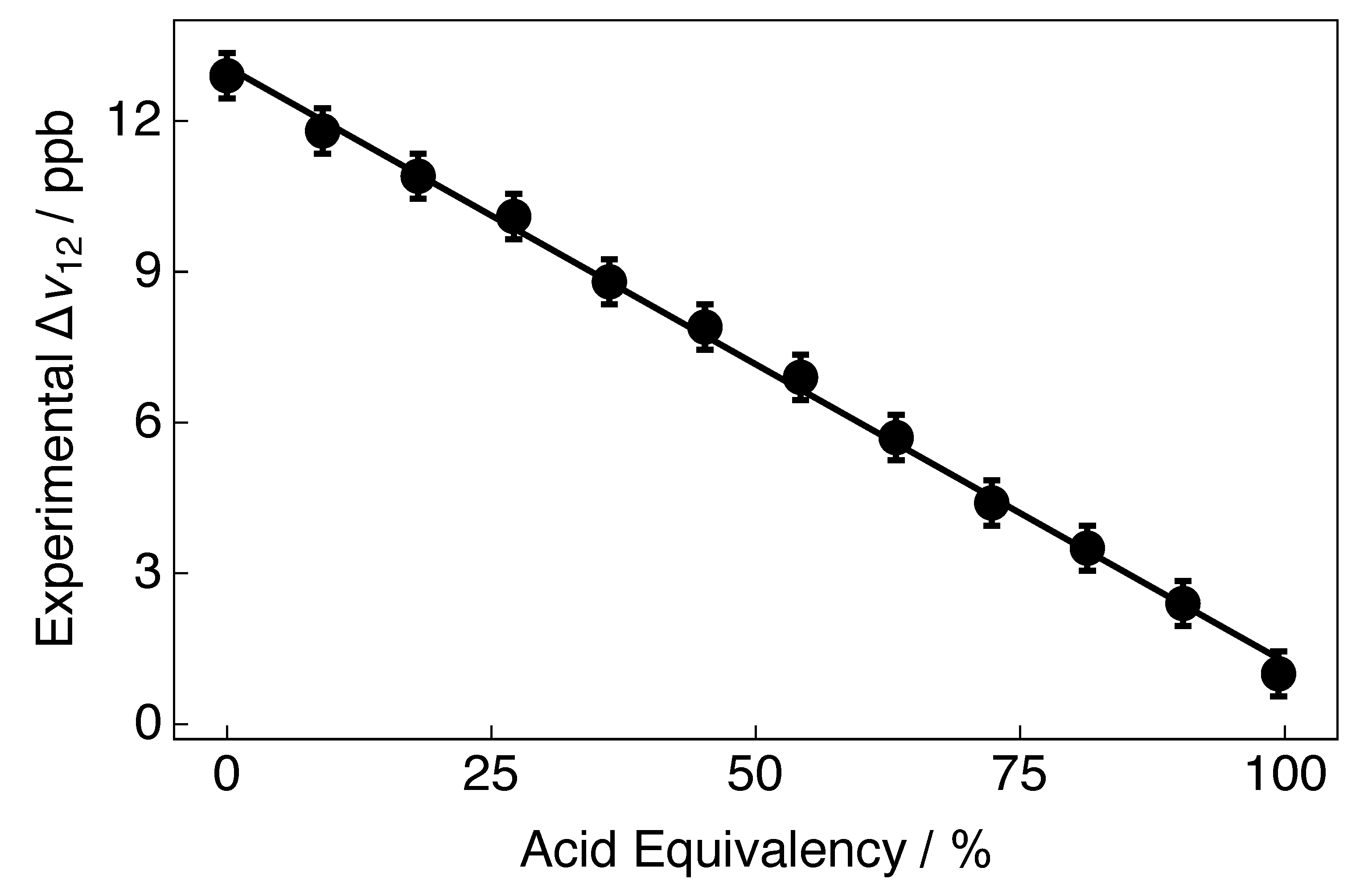
5. Tuning
6. CHD LLS
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carravetta, M.; Johannessen, O.G.; Levitt, M.H. Beyond the T1 Limit: Singlet Nuclear Spin States in Low Magnetic Fields. Phys. Rev. Lett. 2004, 92, 153003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, M.H. Singlet Nuclear Magnetic Resonance. Annu. Rev. Phys. Chem. 2012, 63, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.H. Long live the singlet state! J. Magn. Reson. 2019, 306, 69–74. [Google Scholar] [CrossRef]
- Long-Lived Nuclear Spin Order; New Developments in NMR; The Royal Society of Chemistry: London, UK, 2020; pp. 1–441. [CrossRef]
- Pileio, G.; Carravetta, M.; Hughes, E.; Levitt, M.H. The Long-Lived Nuclear Singlet State of 15N-Nitrous Oxide in Solution. J. Am. Chem. Soc. 2008, 130, 12582–12583. [Google Scholar] [CrossRef] [Green Version]
- Dumez, J.N.; Hill-Cousins, J.T.; Brown, R.C.D.; Pileio, G. Long-lived localization in magnetic resonance imaging. J. Magn. Reson. 2014, 246, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Pileio, G.; Dumez, J.N.; Pop, I.A.; Hill-Cousins, J.T.; Brown, R.C.D. Real-space imaging of macroscopic diffusion and slow flow by singlet tagging MRI. J. Magn. Reson. 2015, 252, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.J.; Kadeřávek, P.; Brown, L.J.; Sabba, M.; Glöggler, S.; O’Leary, D.J.; Brown, R.C.D.; Ferrage, F.; Levitt, M.H. Field-cycling long-lived-state NMR of 15N2 spin pairs. Mol. Phys. 2018, 117, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Erriah, B.; Elliott, S.J. Experimental evidence for the role of paramagnetic oxygen concentration on the decay of long-lived nuclear spin order. RSC Adv. 2019, 9, 23418–23424. [Google Scholar] [CrossRef] [Green Version]
- Kiryutin, A.S.; Zimmermann, H.; Yurkovskaya, A.V.; Vieth, H.M.; Ivanov, K.L. Long-lived spin states as a source of contrast in magnetic resonance spectroscopy and imaging. J. Magn. Reson. 2015, 261, 64–72. [Google Scholar] [CrossRef]
- Salvi, N.; Buratto, R.; Bornet, A.; Ulzega, S.; Rentero Rebollo, I.; Angelini, A.; Heinis, C.; Bodenhausen, G. Boosting the Sensitivity of Ligand-Protein Screening by NMR of Long-Lived States. J. Am. Chem. Soc. 2012, 134, 11076–11079. [Google Scholar] [CrossRef] [Green Version]
- Bornet, A.; Ji, X.; Mammoli, D.; Vuichoud, B.; Milani, J.; Bodenhausen, G.; Jannin, S. Long-Lived States of Magnetically Equivalent Spins Populated by Dissolution-DNP and Revealed by Enzymatic Reactions. Chem. A Eur. J. 2014, 20, 17113–17118. [Google Scholar] [CrossRef] [Green Version]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Vasos, P.R.; Comment, A.; Sarkar, R.; Ahuja, P.; Jannin, S.; Ansermet, J.P.; Konter, J.A.; Hautle, P.; van den Brandt, B.; Bodenhausen, G. Long-lived states to sustain hyperpolarized magnetization. Proc. Natl. Acad. Sci. USA 2009, 106, 18469–18473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pileio, G.; Bowen, S.; Laustsen, C.; Tayler, M.C.D.; Hill-Cousins, J.T.; Brown, L.J.; Brown, R.C.D.; Ardenkjær-Larsen, J.H.; Levitt, M.H. Recycling and Imaging of Nuclear Singlet Hyperpolarization. J. Am. Chem. Soc. 2013, 135, 5084–5088. [Google Scholar] [CrossRef]
- Elliott, S.J.; Meier, B.; Vuichoud, B.; Stevanato, G.; Brown, L.J.; Alonso-Valdesueiro, J.; Emsley, L.; Jannin, S.; Levitt, M.H. Hyperpolarized long-lived nuclear spin states in monodeuterated methyl groups. Phys. Chem. Chem. Phys. 2018, 20, 9755–9759. [Google Scholar] [CrossRef] [Green Version]
- Pileio, G.; Carravetta, M.; Levitt, M.H. Storage of nuclear magnetization as long-lived singlet order in low magnetic field. Proc. Natl. Acad. Sci. USA 2010, 107, 17135–17139. [Google Scholar] [CrossRef] [Green Version]
- Tayler, M.C.D.; Levitt, M.H. Singlet nuclear magnetic resonance of nearly-equivalent spins. Phys. Chem. Chem. Phys. 2011, 13, 5556–5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengs, C.; Sabba, M.; Jerschow, A.; Levitt, M.H. Generalised magnetisation-to-singlet-order transfer in nuclear magnetic resonance. Phys. Chem. Chem. Phys. 2020, 22, 9703–9712. [Google Scholar] [CrossRef] [Green Version]
- Hill-Cousins, J.T.; Pop, I.A.; Pileio, G.; Stevanato, G.; Håkansson, P.; Roy, S.S.; Levitt, M.H.; Brown, L.J.; Brown, R.C.D. Synthesis of an Isotopically Labeled Naphthalene Derivative That Supports a Long-Lived Nuclear Singlet State. Org. Lett. 2015, 17, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, G.; Hill-Cousins, J.T.; Håkansson, P.; Roy, S.S.; Brown, L.J.; Brown, R.C.D.; Pileio, G.; Levitt, M.H. A Nuclear Singlet Lifetime of More than One Hour in Room-Temperature Solution. Angew. Chem. Int. Ed. 2015, 54, 3740–3743. [Google Scholar] [CrossRef] [Green Version]
- Tayler, M.C.D.; Levitt, M.H. Accessing Long-Lived Nuclear Spin Order by Isotope-Induced Symmetry Breaking. J. Am. Chem. Soc. 2013, 135, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Pravdivtsev, A.N.; Sönnichsen, F.D.; Hövener, J.B. In vitro singlet state and zero-quantum encoded magnetic resonance spectroscopy: Illustration with N-acetyl-aspartate. PLoS ONE 2020, 15, e0239982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Soon, P.C.; Jerschow, A.; Canary, J.W. Long-Lived 1H Nuclear Spin Singlet in Dimethyl Maleate Revealed by Addition of Thiols. Angew. Chem. Int. Ed. 2014, 53, 3396–3399. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Kopelevich, M. Detection and assignments of diastereotopic chemical shifts in partially deuteriated methyl groups of a chiral molecule. J. Am. Chem. Soc. 1989, 111, 3429–3431. [Google Scholar] [CrossRef]
- Restelli, A.; Siegel, J.S. Cryptoclastic diastereotopism: NMR evidence for the chirotopicity of the methyl group in (α-deuterio-o-chlorotoluene)chromium tricarbonyl. J. Am. Chem. Soc. 1992, 114, 1091–1092. [Google Scholar] [CrossRef]
- Allen, B.D.; O’Leary, D.J. Fomenting Proton Anisochronicity in the CH2D Group. J. Am. Chem. Soc. 2003, 125, 9018–9019. [Google Scholar] [CrossRef]
- Allen, B.D.; Cintrat, J.C.; Faucher, N.; Berthault, P.; Rousseau, B.; O’Leary, D.J. An Isosparteine Derivative for Stereochemical Assignment of Stereogenic (Chiral) Methyl Groups Using Tritium NMR: Theory and Experiment. J. Am. Chem. Soc. 2005, 127, 412–420. [Google Scholar] [CrossRef]
- Elliott, S.J.; Brown, L.J.; Dumez, J.N.; Levitt, M.H. Long-lived nuclear spin states in monodeuterated methyl groups. Phys. Chem. Chem. Phys. 2016, 18, 17965–17972. [Google Scholar] [CrossRef] [Green Version]
- Elliott, S.J.; Brown, L.J.; Dumez, J.N.; Levitt, M.H. Long-lived nuclear spin states in rapidly rotating CH2D groups. J. Magn. Reson. 2016, 272, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Ogba, O.M.; Elliott, S.J.; Kolin, D.A.; Brown, L.J.; Cevallos, S.; Sawyer, S.; Levitt, M.H.; O’Leary, D.J. Origins of Small Proton Chemical Shift Differences in Monodeuterated Methyl Groups. J. Org. Chem. 2017, 82, 8943–8949. [Google Scholar] [CrossRef]
- Binsch, G.; Franzen, G.R. Intrinsically anisochronous nuclei in propeller molecules. J. Am. Chem. Soc. 1969, 91, 3999–4000. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Kopelevich, M. Anomeric and conformational deuterium isotope effects in saturated sulphur and nitrogen heterocycles. J. Chem. Soc. Chem. Commun. 1987, 8, 595–597. [Google Scholar] [CrossRef]
- Forsyth, D.A.; Hanley, J.A. Conformationally dependent intrinsic and equilibrium isotope effects in N-methylpiperidine. J. Am. Chem. Soc. 1987, 109, 7930–7932. [Google Scholar] [CrossRef]
- Forsyth, D.A.; Prapansiri, V. Conformational equilibrium isotope effects in 3-azabicyclo[3.2.2]nonanes. Tetrahedron Lett. 1988, 29, 3551–3554. [Google Scholar] [CrossRef]
- Anet, F.A.L.; O’Leary, D.J.; Beale, J.M.; Floss, H.G. Stereogenic (chiral) methyl groups: Determination of configuration by direct tritium NMR spectroscopy. J. Am. Chem. Soc. 1989, 111, 8935–8936. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.; Glendening, E. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Alabugin, I.V.; dos Passos Gomes, G.; Abdo, M.A. Hyperconjugation. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2019, 9, e1389. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, Version 3.1; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Available online: https://nbo6.chem.wisc.edu/tutorial.html (accessed on 24 May 2021).
- Atkins, P.; de Paula, J. Atkins Physical Chemistry; University Press: Oxford, UK, 2014. [Google Scholar]
- O’Leary, D. Nuclear Magnetic Resonance Relaxation by the Antisymmetric Component of the Chemical Shift Tensor: A Longer Transverse Than Longitudinal Relaxation Time, and Intrinsic Deuterium Isotope Effects in Methane, The Conformational Preference of Deuterium and Tritium in Cyclohexane, and a Tritium NMR Method to Assign the Configuration of a Stereogenic (Chiral) Methyl Group. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 1991. [Google Scholar]
- Bengs, C.; Levitt, M.H. SpinDynamica: Symbolic and numerical magnetic resonance in a Mathematica environment. Magn. Reson. Chem. 2018, 56, 374–414. [Google Scholar] [CrossRef] [Green Version]
- Erxleben, N.D.; Kedziora, G.S.; Urban, J.J. Anomeric effects in fluoro and trifluoromethyl piperidines: A computational study of conformational preferences and hydration. Theor. Chem. Acc. 2014, 133, 1491. [Google Scholar] [CrossRef]
- Corey, E.; Rohde, J.J. The application of the formyl CH–O hydrogen bond postulate to the understanding of enantioselective reactions involving chiral boron lewis acids and aldehydes. Tetrahedron Lett. 1997, 38, 37–40. [Google Scholar] [CrossRef]
- Cannizzaro, C.E.; Houk, K.N. Magnitudes and Chemical Consequences of R3N+-C-H···O=C Hydrogen Bonding. J. Am. Chem. Soc. 2002, 124, 7163–7169. [Google Scholar] [CrossRef]
- Scheiner, S. Weak H-bonds. Comparisons of CH···O to NH···O in proteins and PH···N to direct P···N interactions. Phys. Chem. Chem. Phys. 2011, 13, 13860–13872. [Google Scholar] [CrossRef]
- Sandoval-Lira, J.; Fuentes, L.; Quintero, L.; Höpfl, H.; Hernández-Pérez, J.M.; Terán, J.L.; Sartillo-Piscil, F. The Stabilizing Role of the Intramolecular C–H···O Hydrogen Bond in Cyclic Amides Derived From α-Methylbenzylamine. J. Org. Chem. 2015, 80, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, E.; Scheiner, S. CH···F Hydrogen Bonds. Dimers of Fluoromethanes. J. Phys. Chem. A 2004, 108, 2527–2535. [Google Scholar] [CrossRef]
- Jain, R.; Bally, T.; Rablen, P.R. Calculating Accurate Proton Chemical Shifts of Organic Molecules with Density Functional Methods and Modest Basis Sets. J. Org. Chem. 2009, 74, 4017–4023. [Google Scholar] [CrossRef] [Green Version]
- Bally, T.; Rablen, P.R. Quantum-Chemical Simulation of 1H NMR Spectra. 2. Comparison of DFT-Based Procedures for Computing Proton–Proton Coupling Constants in Organic Molecules. J. Org. Chem. 2011, 76, 4818–4830. [Google Scholar] [CrossRef]
- Meier, B.; Dumez, J.N.; Stevanato, G.; Hill-Cousins, J.T.; Roy, S.S.; Håkansson, P.; Mamone, S.; Brown, R.C.D.; Pileio, G.; Levitt, M.H. Long-Lived Nuclear Spin States in Methyl Groups and Quantum-Rotor-Induced Polarization. J. Am. Chem. Soc. 2013, 135, 18746–18749. [Google Scholar] [CrossRef]
- Dumez, J.N.; Håkansson, P.; Mamone, S.; Meier, B.; Stevanato, G.; Hill-Cousins, J.T.; Roy, S.S.; Brown, R.C.D.; Pileio, G.; Levitt, M.H. Theory of long-lived nuclear spin states in methyl groups and quantum-rotor induced polarisation. J. Chem. Phys. 2015, 142, 044506. [Google Scholar] [CrossRef]
- Roy, S.S.; Dumez, J.N.; Stevanato, G.; Meier, B.; Hill-Cousins, J.T.; Brown, R.C.; Pileio, G.; Levitt, M.H. Enhancement of quantum rotor NMR signals by frequency-selective pulses. J. Magn. Reson. 2015, 250, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Dumez, J.N.; Vuichoud, B.; Mammoli, D.; Bornet, A.; Pinon, A.C.; Stevanato, G.; Meier, B.; Bodenhausen, G.; Jannin, S.; Levitt, M.H. Dynamic Nuclear Polarization of Long-Lived Nuclear Spin States in Methyl Groups. J. Phys. Chem. Lett. 2017, 8, 3549–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, S.J.; Ogba, O.M.; Brown, L.J.; O’Leary, D.J. An Examination of Factors Influencing Small Proton Chemical Shift Differences in Nitrogen-Substituted Monodeuterated Methyl Groups. Symmetry 2021, 13, 1610. https://doi.org/10.3390/sym13091610
Elliott SJ, Ogba OM, Brown LJ, O’Leary DJ. An Examination of Factors Influencing Small Proton Chemical Shift Differences in Nitrogen-Substituted Monodeuterated Methyl Groups. Symmetry. 2021; 13(9):1610. https://doi.org/10.3390/sym13091610
Chicago/Turabian StyleElliott, Stuart J., O. Maduka Ogba, Lynda J. Brown, and Daniel J. O’Leary. 2021. "An Examination of Factors Influencing Small Proton Chemical Shift Differences in Nitrogen-Substituted Monodeuterated Methyl Groups" Symmetry 13, no. 9: 1610. https://doi.org/10.3390/sym13091610
APA StyleElliott, S. J., Ogba, O. M., Brown, L. J., & O’Leary, D. J. (2021). An Examination of Factors Influencing Small Proton Chemical Shift Differences in Nitrogen-Substituted Monodeuterated Methyl Groups. Symmetry, 13(9), 1610. https://doi.org/10.3390/sym13091610





