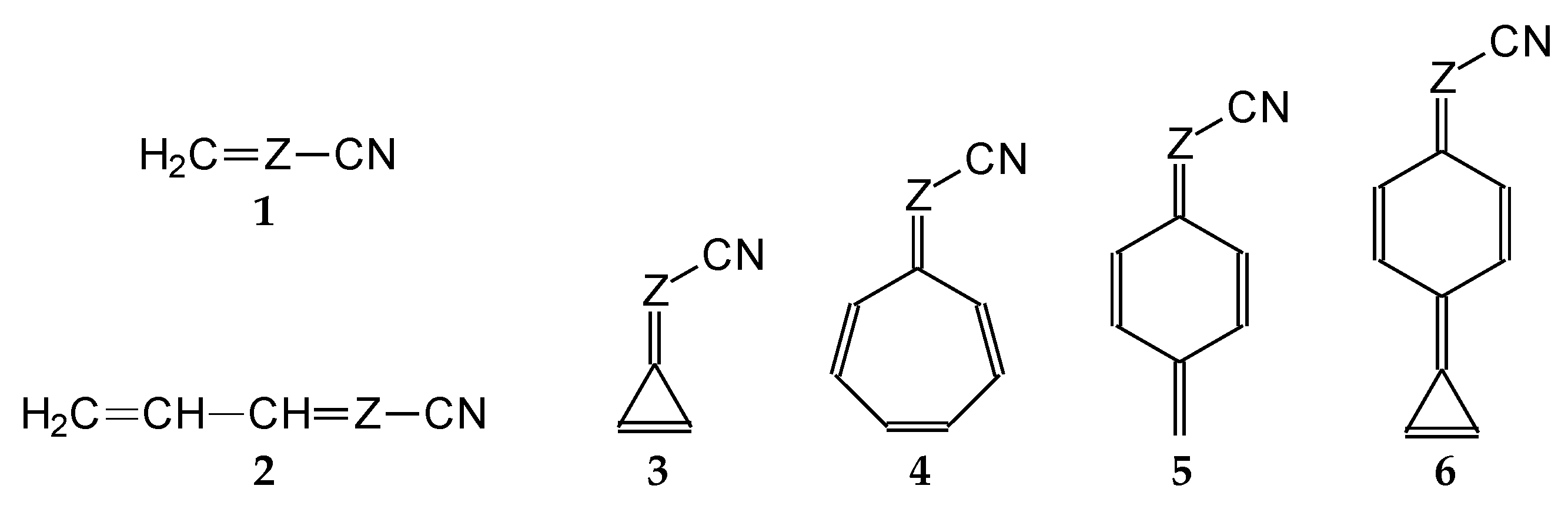
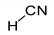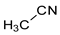3.1. Gas-Phase Basicity of Simple Nitriles
The two DFT levels of theory for B3LYP/311+G(d,p) and B3LYP/6-311++G(d,p), referred to as DFT1 and DFT2, respectively, applied for selected derivatives lead to very similar basicity parameters (PA and GB) for the cyano and imino N sites, and consequently to analogous DFT gas-phase basicity scales. For simple molecules optimized at the two DFT levels, the geometry parameters are identical. Only their energy parameters slightly differ. Note that the geometry and energy (including basicity) parameters for compounds I.9 and II.9 depend not much on Me conformation. In our case, the differences in gas-phase basicity data predictions were not larger than 0.5 kJ mol−1.
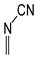
For simple nitriles, the PAs calculated here for the cyano and imino N (Z) atoms, together with data for DFT, G2, and G4 from the literature, are summarized in
Table 1. Generally, applications of the DFT methods lead to considerably higher PAs (and GBs) for the N-cyano site than those of the G
n theories (differences 10–20 kJ mol
−1), with the exception of HCN. On the other hand, the DFT and G2 methods predict similar PAs for the N-imino site in series
II (differences lower than 10 kJ mol
−1). Consequently, the differences between the PA(N-cyano) and PA(N-imino) dramatically change when going from DFT to G2 levels. This means that the favored site of monoprotonation can be predicted without any doubt when the DFT-calculated ΔPA for the cyano and imino N atoms is higher than 20 kJ mol
−1. In other cases, a conclusion on favored monoprotonation sites should be carefully derived on the basis of DFT calculations.
3.2. Isomeric Phenomena in Neutral Forms
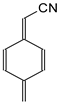
Among derivatives of simple π-π-conjugated series
I and
II (
Figure 1), two geometrical isomers about the C=C and C=N double bonds (
a and
b) and their two rotational isomers about the C–C bond (
c and
d) can be distinguished for compounds
I.2 and
II.2 (
Chart S1 in SM). Four isomeric structures with different stability levels are, thus, possible for the neutral forms of
I.2 and
II.2. We found that at the DFT level of theory, the Gibbs energy of structure
I.2a is lower than that of
I.2b (by only 1 kJ mol
−1) and is significantly lower than those of the less favorable structures
c and
d. On the other hand, the G2 level of theory predicted the reverse. In the case of neutral α-imino nitriles
II.2, the stability order of structures
a–
d at the DFT levels is analogous to that for
I.2. The presence of the imino N atom only affects the relative Gibbs energies (Δ
Gs), although by no more than 4 kJ mol
−1. Slight differences occur at the G2 level. The isomer
a has the lowest
G similarly, as found at the DFT levels. The isomer
b has higher
G by 2 kJ mol
−1. For both derivatives (
I.2 and
II.2), the isomers
c and
d may be neglected in the isomeric mixtures owing to their high Δ
Gs (≥10 kJ mol
−1); hence, their basicity data calculated at different levels of theory are not compared in
Table 1.
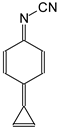
The structural investigations for simple π-π-conjugated nitriles encouraged us to perform an extended analysis of isomeric phenomena for the series, in which the push–pull effect is transmitted by the
CPC or by the
CPN scaffolds (
Figure 2). When large donor substituents (guanidino N=C(NR
2)
2 and phosphazeno N=P(NR
2)
3 groups) are introduced at the cyclopropene ring, rotational isomerism about the single C–X bonds can affect geometric and energetic parameters and cannot be neglected for these derivatives. Note that rotation of the Me groups was not studied here in detail for
I.11,
I.12,
II.11, and
II.12, because for compounds
I.9 and
II.9, we observed only slight structural and energetic effects.
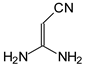
Although prototropic tautomerism (amino–imino conversions) may additionally occur for guanidino derivatives with two N=C(NH
2)
2 groups, this phenomenon can be neglected owing to the very strong electron-accepting effect of the C
2C=Z–C≡N fragment, as well as the commonly known rule based on the Brønsted theory and substituent effects that pulling groups strongly stabilize the imino tautomer of weaker basicity [
64,
65]. According to this rule, the labile protons are preferentially bonded to the amino N atoms in guanidine and the imino N atoms of the two guanidine groups are linked to the C
2C=Z–C≡N fragment, leading to the following favored tautomeric form, [(H
2N)
2C=N]
2C
2C=Z–C≡N, for
I.10 (Z: CH) and
II.10 (Z: N). Replacement of H by Me at the amino N atoms in
I.11 and
II.11 eliminates amino-imino tautomerism in the guanidine group. The same is true for the diphosphazeno derivatives
I.12 and
II.12.
In order to reduce the number of drawings for the representation of the possible isomers of
I.10–
I.12 and
II.10–
II.12, the guanidino and phosphazeno groups are abbreviated here as N=Y(NR
2)
n, where R is H or Me, Y is C or P, and n is 2 or 3, respectively. If we look at the single-bonds of C
ring–N=Y(NR
2)
n, about which rotations are possible, four major isomers are possible (not including methyl group rotations), which we examined separately for each derivative regarding the C=Z moiety; therefore, we decided to analyze these four particular isomers (
a, b, c, and d in Chart S2 in SM) for derivatives
I.10–
I.12 and
II.10–
II.12. In our analyses, we neglected other intermediate positions as well as the minor conformational changes induced by the methyl group rotations. The relative Gibbs energies calculated at the DFT1 or DFT2 level for
a–
d of the neutral CH and N derivatives are summarized in
Chart S2 (SM). They indicate some differences in stability of
a–
d in
I and
II. Generally, the isomer
a is favored (except
I.10), and in some cases
c is also favored (
I.10,
I.11, and
I.12). Possible intramolecular interactions in selected nitriles that can influence their stability are shown in
Figures S1 and S2 (SM).
3.3. Possible and Favored Sites of Monoprotonation
For compounds containing different functional groups, such as cyano, imino, and amino groups, the N atom(s) are potential sites of protonation. Quantum chemical methods have an advantage in that they allow the possibility of investigating all potential sites of protonation, then indicating the atom(s) that can preferentially bind one proton. On the basis of our calculations performed for series
I and
II of simple π-π-conjugated nitriles and imino nitriles given in
Figure 1, we can conclude that the cyano N atom is the favored site of protonation. Generally, imines are much stronger bases than nitriles [
1,
2,
3,
4,
32]; however, the combination of the strong electron-accepting effect of the cyano group, considerably decreasing the basicity of the imino N atom, and the push–pull effect on the nitrile, increasing its basicity, reverse the usual order. Consequently, the difference in Gibbs energies between the imino-protonated cation (C=NH
+) and the cyano-protonated cation (C≡NH
+) is not lower than 20 kJ mol
−1; therefore, the imino-protonated isomers can be neglected (<0.1%) for monoprotonated forms, at least for the simple systems considered in
Chart S3 (SM).
We paid particular attention to the two derivatives,
I.2 and
II.2, which display isomeric phenomena about the double C=C and C=N bonds and about the single C–C bond, not only for the neutral molecules (
Chart S1 in SM), but also for their monoprotonated forms (
Chart S3 in SM). We found that for the cyano-protonated forms of
I.2 and
II.2, the structure
a is favored at each level of theory applied here for basicity prediction (DFT and G
n). Its Gibbs energy is lower than that of structure
b by more than 5 kJ mol
−1. The other cyano-protonated isomers (
c and
d) have higher Gibbs energies than
a and can be considered as minor (
c) or even as rare (
d) forms in the isomeric mixture of monoprotonated
I.2 and
II.2. The imino-protonated isomers
a–
d of
II.2 can be neglected, as their Δ
Gs are exceptionally high (≥50 kJ mol
−1).
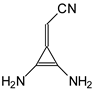
The cyano N atom also appears to be the preferred site of protonation for the derivatives given in
Figure 2, containing additional N atoms in the substituents, for which Z is CH or N. For example, protonation of the amino N atoms at the
syn- (
a) and
anti-position (
b) regarding C≡N for the diamino derivatives
I.8 and
II.8 leads to monocations (
Chart S4 in SM), for which the DFT1-calculated
Gs are dramatically higher than those of the cyano-protonated forms (by ca. 180 and 210 kJ mol
−1, respectively). For
II.8 (Z: N), this effect seems to be stronger due to an additional pull effect of the imino group. Since it is commonly recognized that amines are stronger bases than nitriles, the reverse orders of
Gs for the cyano- and amino-protonated forms of
I.8 and
II.8 confirm very strong push–pull effects in these molecules between the amino and cyano groups through the
CPC or
CPN scaffolds. The protonation at the imino nitrogen in
II.8 is unfavored by the strong electron-accepting effect of the cyano group combined with the push–pull effect on the nitrile, as already mentioned. The Gibbs energy of the imino-protonated form is higher than that of the cyano monocation by more than 40 kJ mol
−1. An analogous difference (37 kJ mol
−1 at the DFT2 level) is found for the cyclopropenimine derivative
II.9 containing two NMe
2 groups, indicating also that the cyano N atom is preferentially protonated.
Taking into account the negligible probability of amino protonation in
I.8 and
II.8, as well as in the investigated earlier series of amino, amidino, guanidino, and phosphazeno nitriles possessing the substituent directly linked with the cyano group, X–C≡N [
5,
6], the amino-protonated forms for other
CPC (
I.10–
I.12) and
CPN derivatives (
II.10–
II.12) were not considered in this study; however, two extreme conformations (
syn and
anti regarding C=Z) for the guanidino and phosphazeno groups, which are possible for the cyano and imino-protonated forms and analogously as neutral forms (
Chart S2 in SM), have to be considered. As such, DFT calculations were performed for four isomers (
a–
d) of each monoprotonated form (cyano and imino). Their relative Gibbs energies are summarized in
Chart S5 (SM).
The DFT results indicate N
cyano as the favored site of protonation in the gas phase for all disubstituted derivatives. Generally, the isomer
b has the lowest Gibbs energy for the cyano monocation, probably owing to possible favorable intramolecular interactions (see
Figure S3 and discussion in SM for diguanidino derivatives). For the diphosphazeno CH derivative
I.12, isomer
d is favored in the monoprotonation reaction leading to the cyano monocation of the lowest Gibbs energy. For other derivatives with large substituents, this isomer belongs to the minor form. The probability of protonation of the imino N (Z) seems to be very low (Δ
G > 15 kJ mol
−1). The same is true for the imino N (X), particularly for N derivatives, where the electron-accepting effect of the imino C=N(Z) group increases Δ
Gs between the cyano and imino (X) protonated forms. An exception is the diguanidino CH derivative
I.11, for which some isomers of the N
imino (guanidino) protonated forms (Δ
G < 20 kJ mol
−1) cannot be neglected in the isomeric mixture. A tentative explanation for this phenomenon is the polarizability effect of Me-groups in
I.11 that increases the PA of imino N(X) to a higher degree than that of cyano N (
Table S5 in SM). When compared to
I.10, the Δ
Gs decrease for isomers
a–
c of
I.11.
3.4. Gas-Phase Basicity of N Atoms in Isomers
For compounds
I.2 and
II.2, for which two types of isomerism (geometrical about a double C=C or C=N bond and rotational about a single C–C bond) can be considered for neutral (
Chart S1 in SM) and monoprotonated forms (
Chart S3 in SM), we calculated the basicity parameters, namely the PAs and GBs (
Table S5 in SM), for each potential site of protonation (cyano and imino N atoms) in four isomers (
a–
d). Comparing the DFT-calculated PAs for two pairs of isomers,
a and
b and separately
c and
d (
Figure 3), the effects of
E/Z isomerism on basicity of the cyano N atom are slightly stronger for the second pair of these isomers (ca. 10 kJ mol
−1) than for the first one (3–6 kJ mol
−1). On the other hand, comparing the PAs of the two other pairs of isomers,
a and
c and separately
b and
d, the effects of rotational isomerism seem to be slightly weaker (1–2 and 6–7 kJ mol
−1, respectively) than those of
E/Z isomerism. Generally, the structural (isomeric) effects on PA(N
cyano) are not very strong for
I.2 and
II.2 (ΔPA ≤ 10 kJ mol
−1). For
II.2, the analogous effects on PA(N
imino) are only slightly stronger; ΔPAs do not exceed 13 kJ mol
−1.
For
I.8 and
II.8 containing the
CPC or
CPN scaffold with two NH
2 groups, gas-phase basicity values were estimated for the cyano, imino, and amino N atoms.
Figure 4 presents the PAs calculated for these sites. When we compare the PAs estimated for N atoms in
I.8 and
II.8 to those estimated at the same level of theory (DFT1) for cross-n-π-conjugated derivatives without the
CPC or
CPN fragment, (H
2N)
2C=CH–C≡N and (H
2N)
2C=N–C≡N [
6], we can see that the
CPC or
CPN part increases the PA of the cyano N atom in
I.8 and
II.8 by ca. 60 kJ mol
−1. To a higher degree (by ca. 70 kJ mol
−1), this part also increases the PA of the imino N atom in
II.8, but it does not change the general order of basicity parameters: PA(N
cyano) > PA(N
imino) > PA(N
amino). All of these increases of gas-phase basicity for the imino and cyano N atoms give information about the particular transmission of the electron-donating (push) effects of two NH
2 groups through the
CPC or
CPN transmitter to C=N and next to C≡N, and also about particular electron delocalization effects in the system. Note that the
CPC or
CPN part only slightly changes the PAs of the amino N atoms (by 0–15 kJ mol
−1). The PAs estimated for the amino groups in
I.8 and
II.8 are considerably lower (by 50–100 kJ mol
−1) than the experimental PA of ammonia at 853.6 kJ mol
−1 [
3]. This observation, together with earlier reports on strong PA decreases for amino groups in push–pull nitriles [
5,
6], allows us to omit PA estimations for all NH
2 and for all NMe
2 groups in other derivatives.
Taking into account the rotational isomerism of substituent X about the single C–X bond for neutral (
Chart S2 in SM) and monoprotonated forms (
Chart S5 in SM) of disubstituted
CPC or
CPN derivatives bearing large groups (
I.10–
I.12 and
II.10–
II.12), we calculated the PAs and GBs for the potential sites of protonation (N
cyano and N
imino) in possible individual isomers, where substituent X adopts a
syn or
anti conformation regarding Z=N. The basicity data (PA and GB) estimated by the DFT1 or DFT2 methods are included in
Table S5 (SM). Since the neutral isomer
d of
I.10–
I.12 and
II.10–
II.12 is not stable and during optimization goes to another more stable isomer (
b or
c) or to another molecule, PAs and GBs are given in
Table S5 (SM) only for three rotamers (
a–
c). Some exceptions were also found for cyano-N-protonated
II.12c, which is not stable at the DFT2 level and after optimization transforms into
II.12b. This observation shed some light on the energy barrier of the CNH
+ group rotation about the C=Z bond. This barrier seems to be negligible in the protonated form.
The differences in PAs for extreme rotamers
a–
c of
I.10–
I.12 and
II.10–
II.12 are considerably larger than those for conformational isomers of compounds
I.2 and
II.2 (
Table S5 in SM). For the favored site of protonation (N
cyano) in CH derivatives, the strongest effect (ΔPA 34 kJ mol
−1) is observed for isomers of
I.10 possessing two N=C(NH
2)
2 groups, for which PA varies from 985 (isomer
a) to 1019 kJ mol
−1 (isomer
b), and the weakest effect (ΔPA 22 kJ mol
−1) for isomers of
I.12 containing two N=P(NMe
2)
3 groups, for which PA varies from 1066 (isomer
a) to 1088 kJ mol
−1 (isomer
b). For isomers of
I.11 with two N=C(NMe
2)
2 groups, the difference in PAs (27 kJ mol
−1) is lower than that for isomers of
I.10, although larger than that for isomers of
I.12. Analogous PA variations are found for isomers of N derivatives (37, 28, and 22 kJ mol
−1 for
II.10,
II.11, and
II.12, respectively). For the N-imino sites, changes in the conformation of X substituents lead to ΔPAs higher than 20 kJ mol
−1 and lower than 40 kJ mol
−1 for the CH derivative.
The differences in PAs for individual isomers quantitatively inform us about the strong sensitivity of N-sites in terms of structural changes in compounds with large flexible substituents. They also provide information how significant errors can be made in PA prediction when conformational analysis is not considered and when PA is calculated only for rotamers of neutral and protonated forms that do not correspond to true energy minima. For example, on the basis of DFT calculations performed only for conformation
a of
I.11a (
Figure 5), one could conclude that the CH derivative does not belong to the family of nitriles, because PA(N
cyano) is lower (by ca. 11 kJ mol
−1) than the PA of the imino N atom in the guanidino group at the
syn-position regarding C≡N.
3.5. Substituent Effects in the Simple Nitrile Series
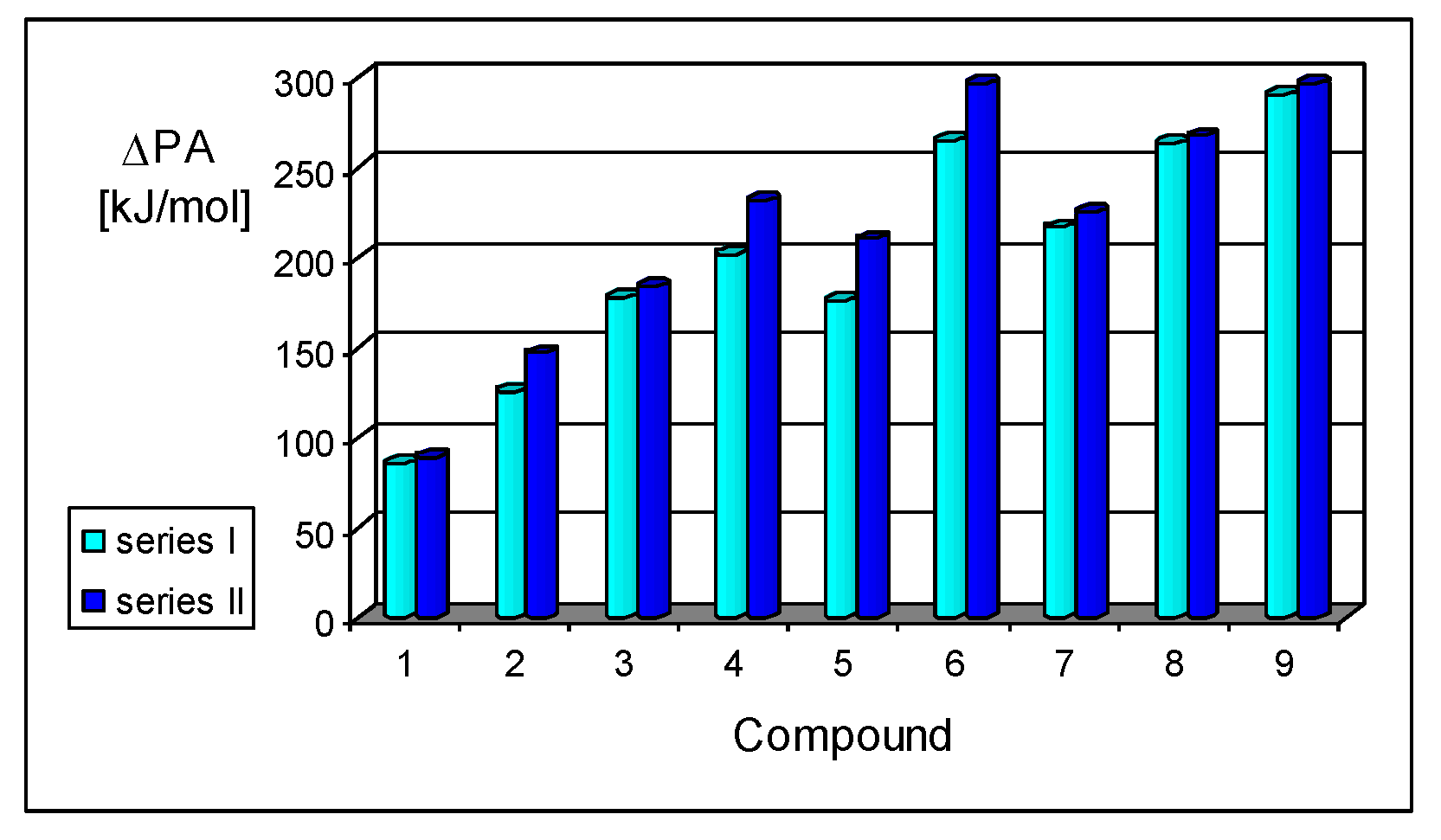
On the basis of our DFT calculations, we considered all compounds studied as functional nitriles in the gas phase, because protonation occurs on the nitrile group. Consequently, we investigated the substituent effects in the two series of nitriles (I and II). First, we compared the PAs of acyclic and cyclic nitriles containing H2C=CH–CH=Z (2) and (HC)2C=Z (3) with those possessing the simplest π-electron group, H2C=Z (1). Compounds 2 and 3 are the π-π-conjugated systems and have the same numbers of heavy atoms and π-electrons, although they exhibit completely different PAs. When proceeding from I.1 to I.2 and I.3, and also from II.1 to II.2 and II.3, the total electronic effects for the cyclic part (HC=CH closed in the ring) in I.3 and II.3 (ca. 90 kJ mol−1) are about twice those for the acyclic part (H2C=CH in acyclic group) in I.2 and II.2 (ca. 40 kJ mol−1). The analogous effects of the HC=CH part in the ring increase the PAs of I.6 and II.6 (by ca. 90 kJ mol−1) when going from I.5 and II.5, respectively. Interestingly, the π-π-conjugated compounds I.4 and II.4 possess higher PAs (by 20–30 kJ mol−1) than their constitutional isomers I.5 and II.5. This analysis of substituent effects on the PAs of simple nitriles evidently showed an exceptionally large increase in basicity for derivatives containing CPC, methylene-1,3,5-cycloheptatriene, methylenequinoid, methylenequinoidcyclopropene, and their heteroanalog-transmitting systems. The basicity orders of the investigated nitriles are a consequence of their exceptional internal electronic effects, which are discussed in detail below.
Assessment of the gas-phase basicity data for all simple derivatives of series
I and
II summarized in
Table S5a (SM) led to additional observations concerning gas-phase internal effects. The total electronic substituent effects, measured as differences between the PAs of simple nitriles of series
I and
II (compounds
1–
9, including only
2a) and that of HCN calculated at the same level of theory, vary from ca. 90 kJ mol
−1 for
1 to ca. 300 kJ mol
−1 for
9 (
Figure 6). The greater size of the π-π-conjugated group and stronger electronic pushing effects of n-π-conjugated X substituents are related to the higher PA values. Moreover, replacement of the CH group in series
I by the imino N (Z) atom in series
II does not always affect the basicity of nitriles in the same way. For example, differences between PAs of the
CPC derivatives
I.3 and
I.7–
I.9 and of the
CPN derivatives
II.3 and
II.7–
II.9 are close to those between PAs of
I.1 and
II.1 (<10 kJ mol
−1), whereas those between the other π-π-conjugated derivatives are considerable higher, being close to 20 and 30 kJ mol
−1 for acyclic (
I.2 and
II.2) and cyclic (
I.4–
I.6 and
II.4–
II.6) molecules, respectively.
This variations of the relative PAs indicate that the electron-donating effects of π-π-conjugated groups in series
I of simple nitriles
1–
9 containing different transmitter parts are not identical but somewhat parallel to those in series
II; however, we can distinguish some subfamilies for the investigation of substituent effects, with one subfamily containing compounds
4,
5, and
6 (1,3,5-cycloheptatriene, quinoid, and quinoid cyclopropene derivatives, respectively), for which the electron-donating effect is considerably stronger in series
II than in series
I; and the other subfamily with compounds
3, 7,
8, and
9, for which the differences in electron-donating effects in series
I and
II are smaller than those for the first family but analogous to those in CH and N derivatives of
1 (see
Figure S4 in SM). Interestingly, all simple nitriles
3, 7,
8, and
9 in the second subfamily possess a cyclopropene part with two X (X: H, Me, NH
2, and NMe
2). Taking this simple observation into account, we included derivatives (
10–
12) containing larger X substituents (X: N=C(NH
2)
2, N=C(NMe
2)
2, and N=P(Nme
2)
3), for which the effects of isomerism on PAs were investigated. In this way, we could directly compare the total electronic effects of all X substituents in series
I of
CPC derivatives with those in the
CPN series
II.
3.6. Substituent Effects in the Methylenecyclopropene and Cyclopropeneimine Series
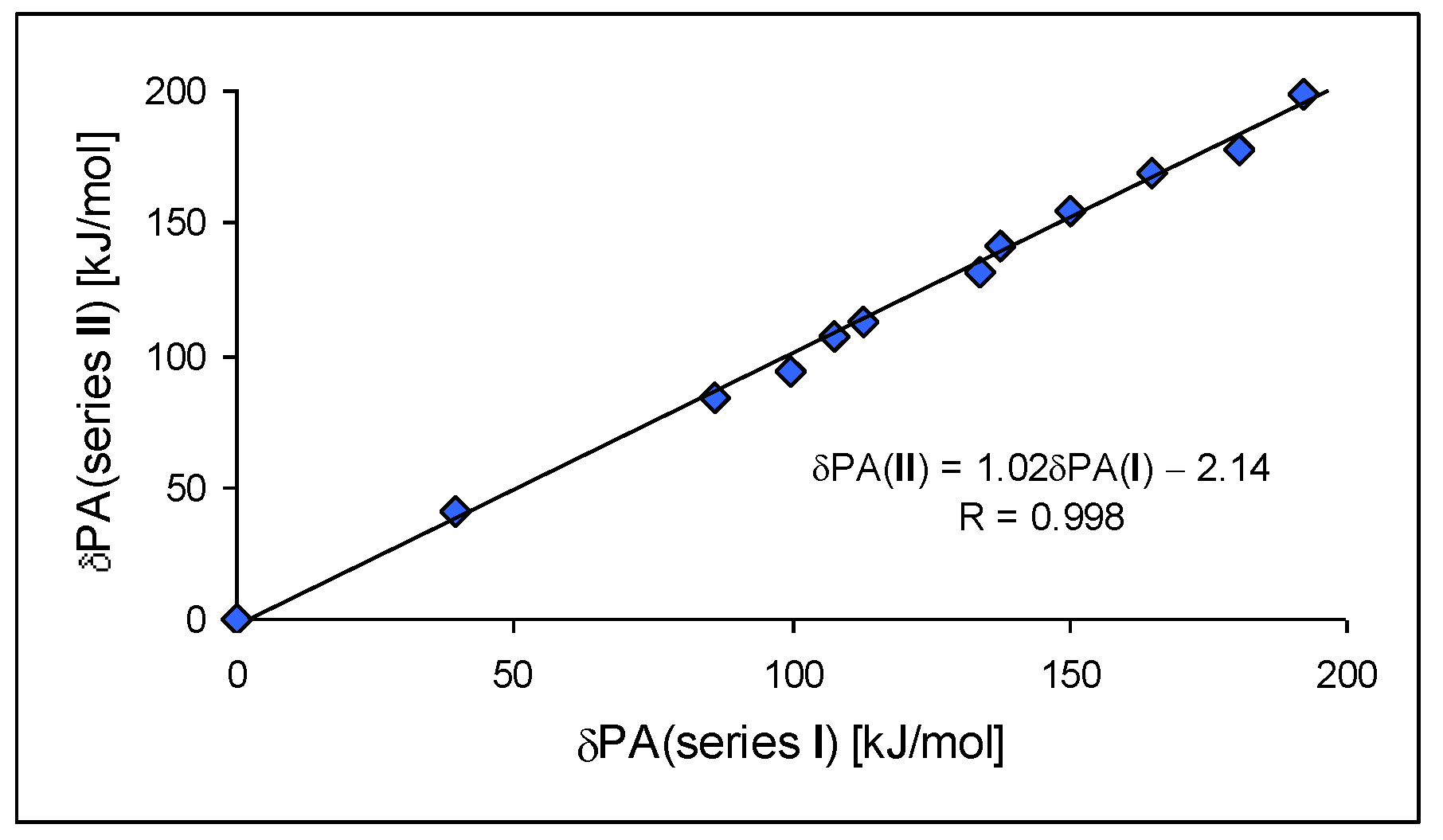
For investigation of the substituent effects in the
CPC (
I.7–
I.12) and
CPN series (
II.7–
II.12), we used the unsubstituted CH (
I.3) and N (
II.3) compounds as reference (parent) molecules. We calculated the differences between the PA of substituted derivatives (or its isomers) and the PA of the parent molecules. These differences, abbreviated as δPA, correspond to the total substituent effects of two X groups in
CPC and
CPN derivatives. They vary from 0 (for H) to ca. 200 kJ mol
−1 (for two phosphazeno groups in
b conformation). Since the basicity data found at the DFT1 and DFT2 levels for simple derivatives did not differ significantly (differences in PAs lower than 0.5 kJ mol
−1), we compared the estimated δPAs of CH derivatives with those of N derivatives, without indication of the DFT method used for calculation, as shown in
Figure 7. We found an interesting linear relationship between δPAs determined for series
I and
II. The slope of the regression line close to unity (correlation coefficient
R = 0.998) indicates that introduction of the N imino atom in series
II does not significantly perturb π-π conjugation or transmission of substituent (pushing) effects through the π-π-conjugated systems to the cyano N site.
The strong electron-donating effects of the two X substituents (push–pull cross-effects) can additionally explain the higher PA/GB values of disubstituted versus unsubstituted series
I and
II. As expected, the orders of basicity increase for series
I and
II are analogous to that for nitriles X–C≡N, substituted directly by the X group, containing H, Me, NH
2, NMe
2, N=C(NH
2)
2, N=C(NMe
2)
2, and N=P(NMe
2)
3 [
6]. Conformation of the large substituents, and consequently favorable and unfavorable intramolecular interactions of functional groups, are additional significant (but not main) factors that affect basicity parameters for investigated disubstituted
CPC and
CPN derivatives.
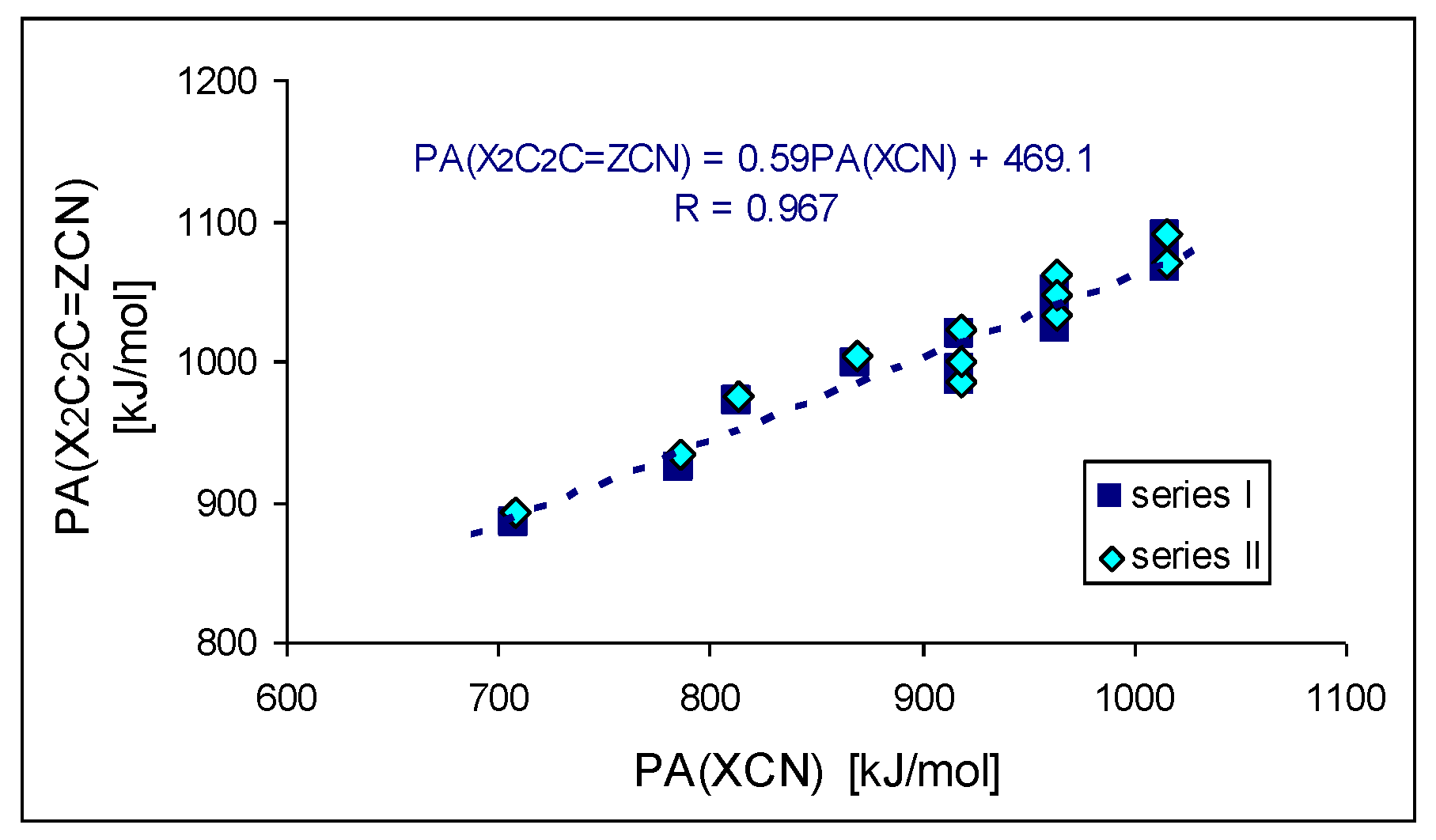
Figure 8 shows the linear trend (
R > 0.95) between the DFT-estimated PAs of series
I and
II and X–C≡N studied previously [
6]. The cross-push–pull effects of the two X substituents in series
I and
II (
CPC and
CPN derivatives, respectively) seem to be attenuated by the cyclo-C
2C=Z scaffold (slope ca. 0.6). For a more limited series of substituents (X: H, NH
2, NMe
2, data from
Table 1 and ref. [
6]), the comparison of calculated PAs for the two disubstituted cyclopropene systems cyclo-X
2C
2C=Z–C≡N and X
2C=Z–C≡N (substituted by
3 and
1, respectively) give a slope (
Figure 8) of 0.7, which can be viewed as a transmission factor of substituent effects (see
Figure S5 in SM). The cyclopropene scaffolds somewhat reduce the electron donor effect as compared to direct substitution in X–C≡N or to transmission by intercalating the C=Z double bond. Nevertheless, the substituted
CPC and
CPN molecules still present PAs larger than X–C≡N and X
2C=Z–C≡N analogs. This is due to the basicity enhancement of the nitrile functional group by the intrinsic effects (polarizability and resonance) of the scaffold cyclo-X
2C
2C=Z.
3.7. Changes in Electron Delocalization Caused by Protonation
Electron delocalization in organic conjugated systems can be related to their bond lengths. For delocalized systems, single and double bond lengths are usually different from those for the corresponding non-conjugated molecules. Depending on the conjugation strength, they are more or less close to those of aromatic systems (e.g., CC 1.394 and CN 1.334 Å for benzene and
s-triazine, respectively, calculated at the DFT1 level) [
51]. Observations of DFT-calculated geometric parameters for simple π-π-conjugated nitriles and imino nitriles, as well as for more complex disubstituted
CPC and
CPN derivatives, show significant variations of single and double bond lengths when proceeding from neutral to protonated forms; for example, the Z=C double bond lengthens and the Z–C
cyano single bond shortens (see
Table S6 in SM for selected nitriles). Analogous changes also take place for other single and double bonds in investigated systems. Generally, single bonds shorten and double bonds lengthen. This alternation is a consequence of differences in resonance hybrids for the neutral and protonated forms (
Scheme S1 in SM), but does not lead to full equalization of bond lengths—even for cyclic fragments—as it does benzene and
s-triazine.
To quantitatively describe changes in electron delocalization for simple neutral and cyano N protonated derivatives, the geometry-based HOMED index [
51,
54] has been applied. Based on the geometries, optimized at the DFT1 level, HOMEDs have been calculated for selected fragments using equation (3) and parameters from
Table S4 (SM). Generally, the estimated HOMEDs increase when the number of heavy atoms and π bonds increase, and also when proceeding from the neutral to cyano N protonated forms (
Figure 9). Moreover, they are higher for the cyclic derivatives
I.3 and
II.3 than for the acyclic ones
I.2 and
II.2 containing the same number of heavy atoms and π bonds. They are also higher for cyclic
I.4 and
II.4 than for cyclic
I.5 and
II.5, both being isomeric forms, and additionally for bicyclic
I.6 and
II.6 than for monocyclic
I.5 and
II.5. The highest HOMEDs (>0.9), close to those for aromatic compounds (HOMED ≈ 1), are found for the entire group R in R–C≡NH
+ of
I.3,
II.3,
I.4,
II.4,
I.6, and
II.6 as well as for their cyclic fragment(s). For these monocations, we can distinguish the resonance structures with well delocalized 3-membered (cyclopropenyl) and 7-membered cyclic (cycloheptatrienyl) cations as well as of the neutral benzene ring (
Scheme S1 in SM). This tendency confirms strong electron delocalization in the cyclic fragments that become aromatic for unsubstituted systems.
In the case of
CPC and
CPN derivatives substituted by two X groups (
I.7–
I.12 and
II.7–
II.12): Me, NH
2, NMe
2, N=C(NH
2)
2, N=C(NMe
2)
2, and N=P(NMe
2)
3, substituents X additionally participate in electron and charge delocalization (
Scheme S2 in SM). The Me groups are σ-π hyperconjugated and the other groups are n-π conjugated with the ring, and next with the cyano group. Hence, larger number of resonance structures can be written and stronger electron delocalization can be expected than for
I.3 and
II.3 (
Scheme S1 in SM), although differences between the resonance hybrids of the neutral and cyano protonated forms have analogous origin as those for simple π-π conjugated systems (
Scheme S1 in SM). Consequently, the HOMED indices increase for disubstituted derivatives when compared to unsubstituted ones (
I.3 and
II.3).
For example, the DFT1-estimated HOMEDs change for the cyclopropenylidene scaffold (C
2C=) when going from the neutral to protonated form of
I.7,
I.8,
I.9,
I.10a,
I.10b, and
I.10c (
Table 2). Analogous changes are found for the C
2C=CH fragment with slightly lower HOMEDs. The corresponding imino derivatives of series
II display similar HOMED trends for the cyclopropene ring and cyclopropenimine part (C
2C=N) when going from the neutral to protonated form. The introduction of N atom in series
II increases HOMEDs already for neutral molecules. This is a consequence of possible intramolecular interactions between functional groups that increase electron delocalization. Hence, smaller HOMED changes take place in protonation reaction for derivatives of series
II.
Note that for n-π-conjugated pushing substituents (X: NH
2, NMe
2, N=C(NH
2)
2), saturation of electron delocalization can be observed for neutral disubstituted 3-membered cycles (HOMEDs between 0.9 and 1.0). The effect of the strong pushing groups causes a step toward electron delocalization in the high PA/GB range for these derivatives (
Figure 10). Their exceptionally strong gas-phase basicity is rather a consequence of push–pull effects—analogous to those for series X–C≡N (
Figure 8)—which occur between the substituent X and site of protonation (cyano N) through the C
2C=Z fragment. Electron delocalization appears be the second factor that enhances the PA/GB range for push–pull nitriles.
The additional estimations of the HOMA and NICS indices for cyclic parts in selected simple nitriles shown in
Table 3 confirm increases of the electron delocalization and the magnetic susceptibility of the ring when proceeding from the neutral molecule to its cyano N-protonated form (C≡NH
+) in both series of nitriles
I and
II, and also in series
II when going from the neutral form to its imino monocation (ZH
+ for Z = N). Generally, negative NICS values are found for the neutral and monoprotonated structures. An exception is the neutral nitrile
I.4, for which a positive NICS value (close to zero) is found.
3.8. Macroscopic Basicity Values
For polyfunctional compounds displaying isomerism, gas-phase basicity parameters (PAs and GBs) can be estimated using quantum chemical methods for separate isomers, as well as for their isomeric mixtures. A distinction between the two approaches can be made using different terminology and different microscopic basicity levels for individual isomers and for their mixtures. The microscopic basicity parameters discussed in the previous subchapter refer to partial equilibria for individual isomers, BiH+ → Bi + H+, whereas the macroscopic basicity parameters correspond to the global equilibrium for the isomeric mixture, y1B1H+ + y2B2H+ + y3B3H+ +… → x1B1 + x2B2 + x3B3 +…+ H+. Each isomer mole fraction xi in the neutral isomeric mixture can be found on the basis of the relative Gibbs energies calculated for neutral individual isomers: xi ≈ (exp(–ΔG(Bi)/RT))/(Σ1n[exp(–ΔG(Bi)/RT)]). In a similar way, the isomer mole fractions yi in the isomeric mixture of monocations can be estimated on the basis of the relative Gibbs energies calculated for the corresponding protonated isomers: yi ≈ (exp(–ΔG(BiH+)/RT))/(Σ1n[exp(–ΔG(BiH+)/RT)]). As discussed above, the microscopic PAs and GBs indicate the basicity of selected sites of protonation for separate isomers, which can be distinguished as major, minor, and rare, as appropriate. They can be applied for analysis of the pure internal effects in individual isomers. The macroscopic PA or GB values give general information on the basicity of the isomeric mixture in the monoprotonation reaction, and can be compared with basicity data for other molecules already placed in the PA or GB scales in order to characterize its strength. For newly synthesized compounds, the predicted PA or GB can also be useful when selecting reference bases for experimental PA or GB determination.
To our knowledge, the gas-phase basicity values of the simple derivatives
I.2 and
II.2, which exhibit various types of isomerism (
E/Z and rotational) and can exist in mixtures of the four isomers, have not yet been experimentally determined. There is only a theoretical report on the microscopic basicity values of the two isomers
I.2a and
I.2b [
2]. Considering the four isomers (
a–
d), we found that microscopic PAs and GBs do not differ very much (
Figure 3), while the favored neutral and protonated forms correspond to the same isomer. In such cases, the macroscopic PAs and GBs are not very different from the microscopic ones for the favored isomer. Using the relative Gibbs energies for the rotamers
a and
c of the
E form and separately for the rotamers
b and
d of the
Z form, we obtained the following PAs and GBs at the DFT1 level: 834.0 and 805.6 for
E-
I.2, 828.3 and 799.5 for
Z-
I.2, 854.7 and 826.7 for
E-
II.2, 851.8 and 824.3 kJ mol
−1 for
Z-
II.2.
If we assume that protonation of the cyano N atom reduces the energy barrier for
E/
Z isomerization, we can estimate the macroscopic basicity for the mixture of the four isomers
a–
d (
Scheme S3 in SM). For this consideration, we take the following observation into account. First, the DFT-calculated double C=Z bonds significantly lengthen (e.g., from 1.348 to 1.369 Å for
I.2a and from 1.289 to 1.312 Å for
II.2a) and the single Z–CN bonds shorten (e.g., from 1.421 to 1.384 Å for
I.2a and from 1.332 to 1.269 Å for
II.2a) when going from the neutral to cyano-protonated forms. For estimation of the isomer mole fractions
xi and
yi, in the neutral and protonated isomeric mixtures of the four isomers
a–
d, we used the corresponding relative Gibbs energies between
b–
d and
a found at the DFT1 level (
Chart S1 and S3 in SM). The percentage contents calculated for individual isomers in the isomeric mixtures of the neutral and protonated forms and the macroscopic PAs and GBs estimated for the monoprotonation equilibria are included in
Scheme S3 (SM). Indeed, the macroscopic PAs and GBs shown in
Table 4 are not very different from the microscopic PAs and GBs of the favored isomer
a given in
Table S5 (SM).
The macroscopic PAs and GBs were estimated for all derivatives (Z: CH and N) with two large substituents. For the estimations, we considered only major and minor isomers with Δ
G < 25 kJ mol
−1 that contribute significantly in the PAs and GBs of the isomeric mixtures. As shown below, the macroscopic PAs and GBs estimated for
I.10–
I.12 and
II.10–
II.12 differ from the microscopic ones found for individual isomers. The reasons are as follows. First, the microscopic basicity data strongly depend on the
syn and
anti conformation of X groups. Second, the favored conformation for the neutral form (
Chart S2 in SM) is not the same as that for the monocation (
Chart S5 in SM). Next, intramolecular interactions in
I.10–
I.12 and
II.10–
II.12 that influence their relative Gibbs energies seem to not always be analogous when proceeding from guanidino to phosphazeno groups and from CH (series
I) to N (series
II) derivatives (vide infra). Consequently, the estimated macroscopic PAs and GBs may be placed between the lowest and highest PAs and GBs for individual isomers or close to one of them.
For the CH and N derivatives containing two N=C(NH
2)
2 groups (
I.10 and
II.10,
Scheme S4 in SM), in the isomeric mixture three isomers are important for the neutral form (
Chart S2 in SM) and four isomers for the monoprotonated form (
Chart S5 in SM). The imino-protonated forms are not significant and they can be neglected in the isomeric mixture of monocation. For estimation of the isomer mole fractions
xi and
yi in the isomeric mixtures, we used the corresponding relative Gibbs energies found for neutral and protonated isomers. The macroscopic PAs and GBs estimated at the DFT1 level (
Table 4) are between the lowest (for isomers
a) and the highest (for isomer
b) microscopic PAs and GBs (
Table S5 in SM).
Some imino-N-protonated forms, particularly guanidino protonated isomers (
Chart S5 in SM), have to be considered for the CH and N derivatives containing two N=C(NMe
2)
2 groups (
I.11 and
II.11). Methylation of amino groups seems to increase the basicity of the guanidino N imino atom in comparison to the cyano N atom. For estimation of the macroscopic PAs and GBs, we used the isomers mole fractions calculated from the Δ
Gs of three neutral isomers (
Chart S2 in SM), four isomers of cyano monocations, and the isomers of imino-N-protonated forms, for which Δ
G < 25 kJ mol
−1 (
Chart S5 in SM). The DFT2-estimated macroscopic PAs and GBs (
Table 4) are also between the lowest and highest microscopic PAs and GBs calculated for the isomers
a and
b (
Table S5 in SM).
Particular cases are diphosphazeno CH and N derivatives (
I.12 and II.12), for which isomeric preferences are different for both the neutral (isomer
c for
I.12 and isomer
a for
II.12, see in
Chart S2 in SM) and monoprotonated forms (isomer
d for
I.12 and isomer
b for
II.12, see in
Chart S5 in SM); hence, these differences strongly affect the macroscopic PAs and GBs estimated at the DFT2 level (
Scheme S6 in SM). For the N derivative, they are between the lowest and highest microscopic PA and GB calculated for the isomers
a and
b of
II.12, although for the CH derivative they are close to the highest microscopic PA and GB values found for
I.12b (
Table S5 in SM). Consequently,
II.12 seems to be a weaker base than
I.12 at the DFT2 level (
Table 4). This order of basicity data is reversed in comparison to other derivatives of series
I and
II. For simple nitriles investigated here that do not exhibit rotational isomerism, N derivatives are stronger bases than CH derivatives (
Figure 6). The analogous trend is found for individual isomers of
I.10–
I.12 and
II.10–
II.12. They obey linear relationship given in
Figure 7. The exceptionally high macroscopic PA and GB values for the diphosphazeno CH derivative
I.12 result from the strong stability of the cyano-protonated isomer
d. For the N derivative
II.12, this isomer belongs to the rare form, similar to the diguanidino N derivative
II.10d.
3.9. New π-π- and n-π-Conjugated Nitriles on the DFT-PA Scale
In our previous studies on the gas-phase basicity of push–pull nitriles [
5,
6], we investigated series of compounds with strong pushing groups, such as R
2N, R
2N–CH=CH, R
2N–CH=N, (R
2N)
2C=N, (R
2N)
3P=N, and (R
2N)
3P=N(R
2N)
2P=N, directly linked to the pulling C≡N group. In this way, we extended the DFT basicity scale for nitriles up to superbasic 1,8-bis(dimethylamino)naphthalene (DMAN, experimental PA 1028.2 kJ mol
−1 [
3]). The most basic nitrile in this series with the biphosphazene group (Me
2N)
3P=N(Me
2N)
2P=N has a DFT-calculated PA value even higher than that of DMAN by ca. 30 kJ mol
−1 [
6].
The new simple π-π-conjugated nitriles investigated here containing methylenecyclopropene, methylene-1,3,5-cycloheptatriene, methylenequinoid, and methylenequinoidcyclopropene, as well as their heteroanalog transmitter systems, seem to be very promising for the future extension of the PA scale for nitriles. Their unsubstituted derivatives (
I.3–
I.6 and
II.3–
II.6) already display higher PAs (
Table 1) than the push–pull dimethylcyanamide Me
2N–C≡N (DFT-calculated PA 868.7 kJ mol
−1 [
6]). Substitution of
CPC and
CPN parts by two NMe
2 groups in
I.9 and
II.9 leads to derivatives with PAs (
Table 1) higher than those of phosphazenenitrile (H
2N)
3P=N–C≡N (DFT calculated PA 978.8 kJ mol
−1 [
6]). The positions of all these simple new π-π-conjugated nitrile bases (
I.1–
I.9 and
II.1–
II.9) on the DFT-calculated PA scale are given in
Figure 11.
Although the simple nitriles with unsubstituted transmitter parts (
I.3–
I.6 and
II.3–
II.6) investigated here are weaker bases than the strongest base studied previously [
6], namely biphosphazenenitrile (Me
2N)
3P=N(Me
2N)
2P=N–C≡N (DFT-calculated PA 1055.5 kJ mol
−1), we show here that substitution of the cyclopropene ring in
I.3 and
II.3 by two pushing groups increases the PAs by ca. 200 kJ mol
−1 (
Figure 7). Diphosphazene derivatives (
I.12 and
II.12, macroscopic PA 1088 and 1079 kJ mol
−1, respectively;
Table 4) are the strongest bases in the current DFT-calculated PA scale for nitriles. This observation suggests that analogous substitution of the methylenequinoid, methylene-1,3,5-cycloheptatriene, and methylenequinoidcyclopropene transmitters and their N analogs by two strong pushing groups in
I.4–
I.6 and
II.4–
II.6 may lead to derivatives for which the DFT-calculated PAs will be close to the limit of the experimental PA scale for push–pull N imino bases (PA < 1200 kJ mol
−1) [
1]. Transmission of the strong pushing effects in such π-π-conjugated systems has already been proved in the literature for imines by quantum-chemical methods [
7,
8,
10].