Diversity in Primate External Eye Morphology: Previously Undescribed Traits and Their Potential Adaptive Value
Abstract
:1. Introduction
2. Unreported Traits in External Primate Eye Morphology and Their Potential Adaptive Value
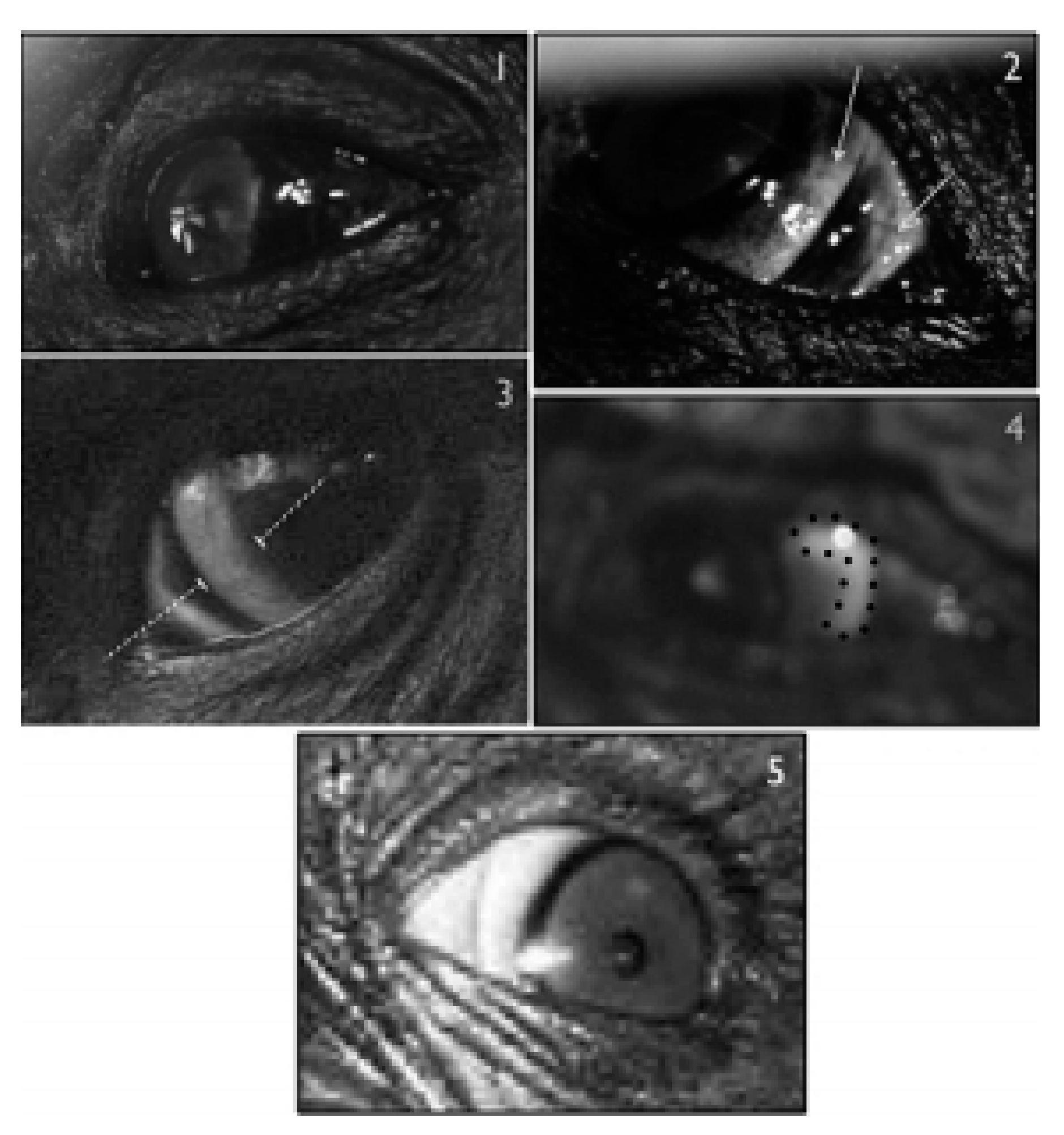
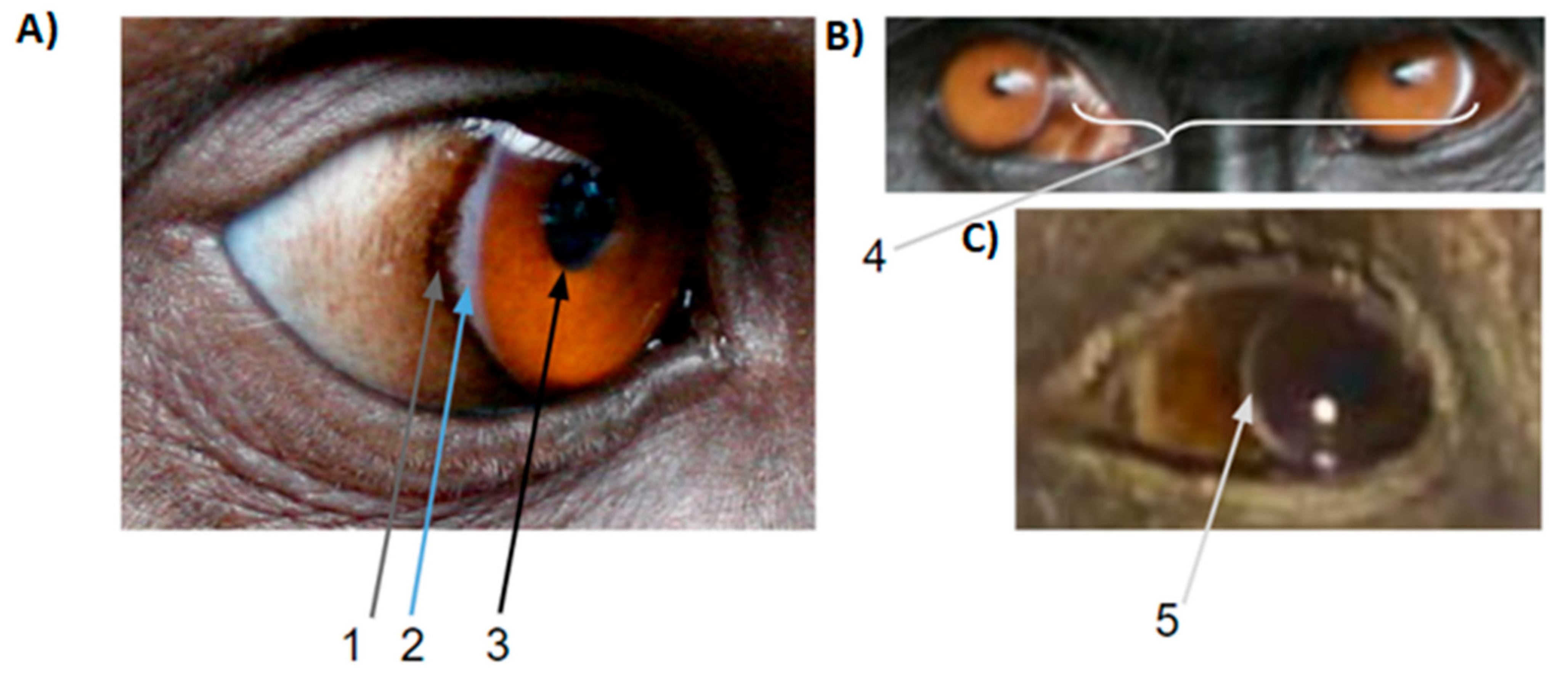
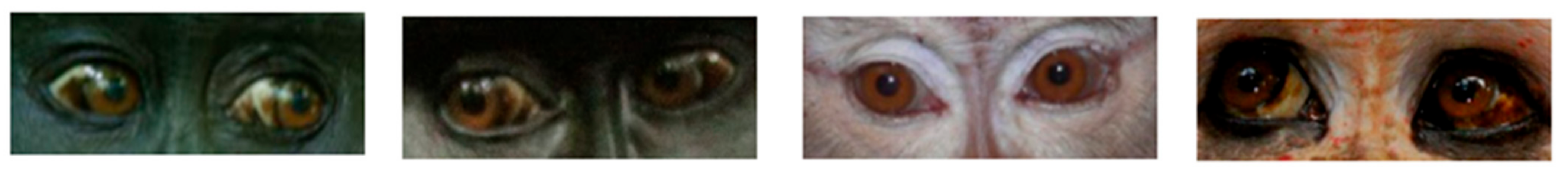
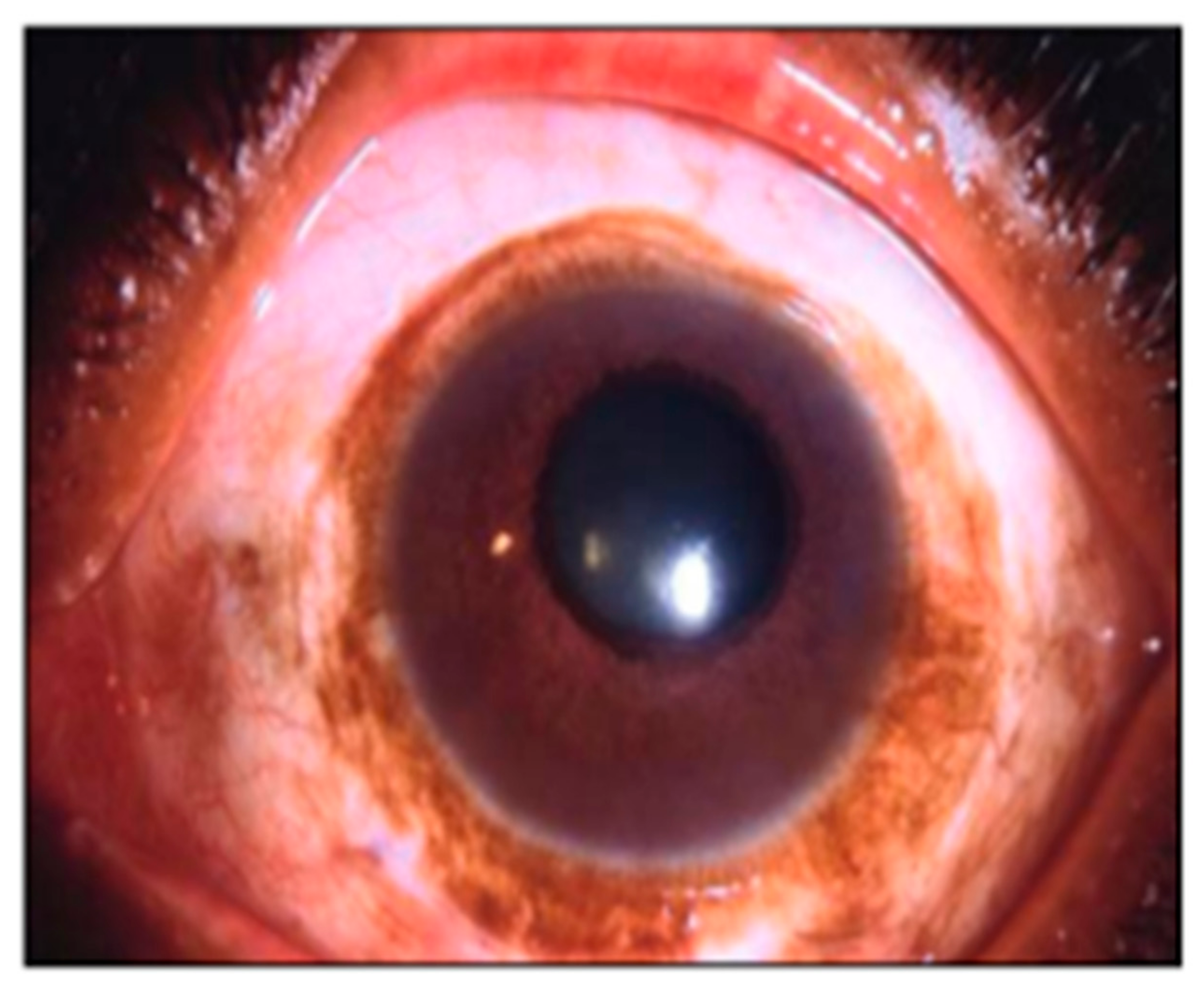
2.1. Dark Symmetric Rings Around the Iris
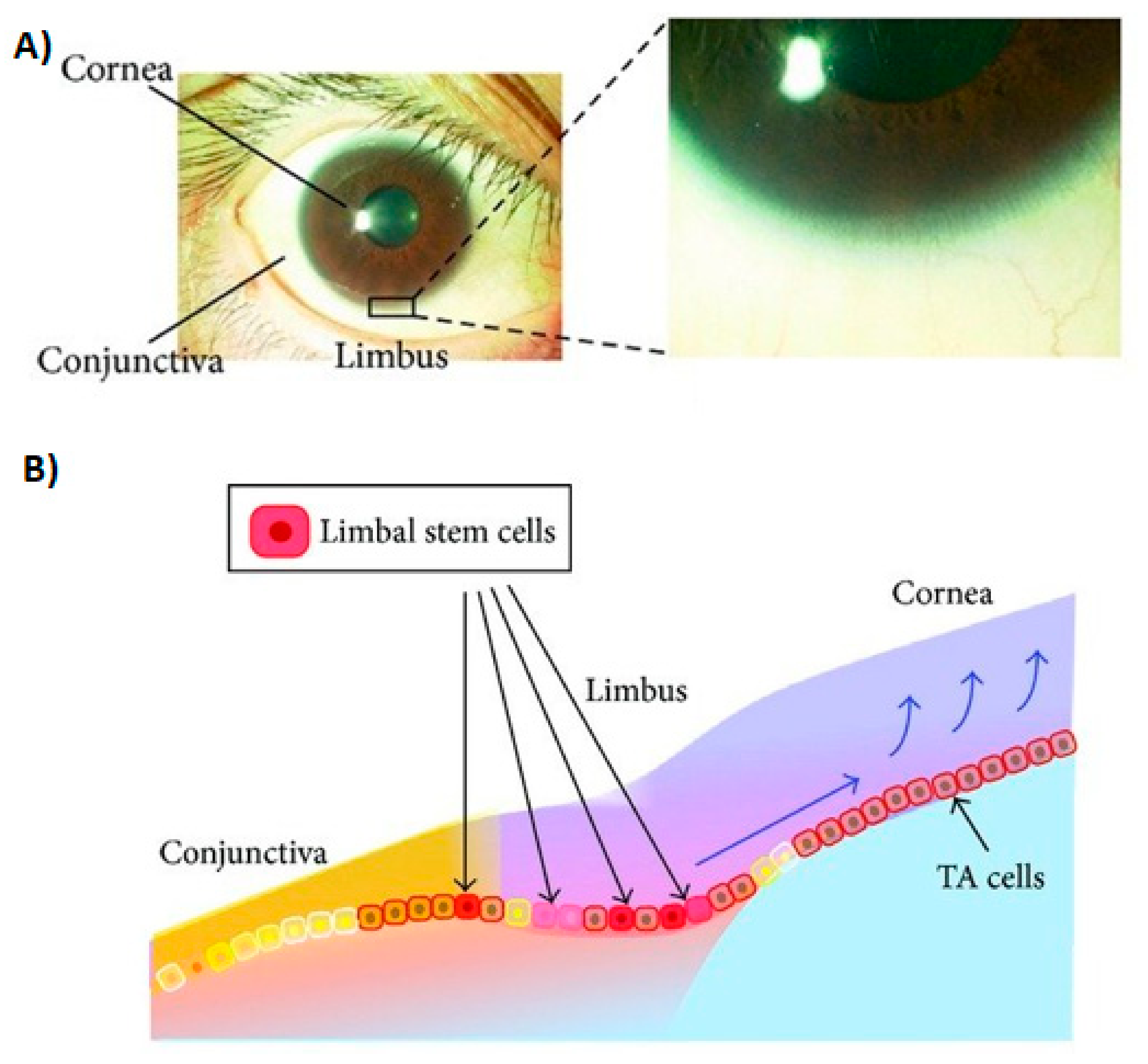
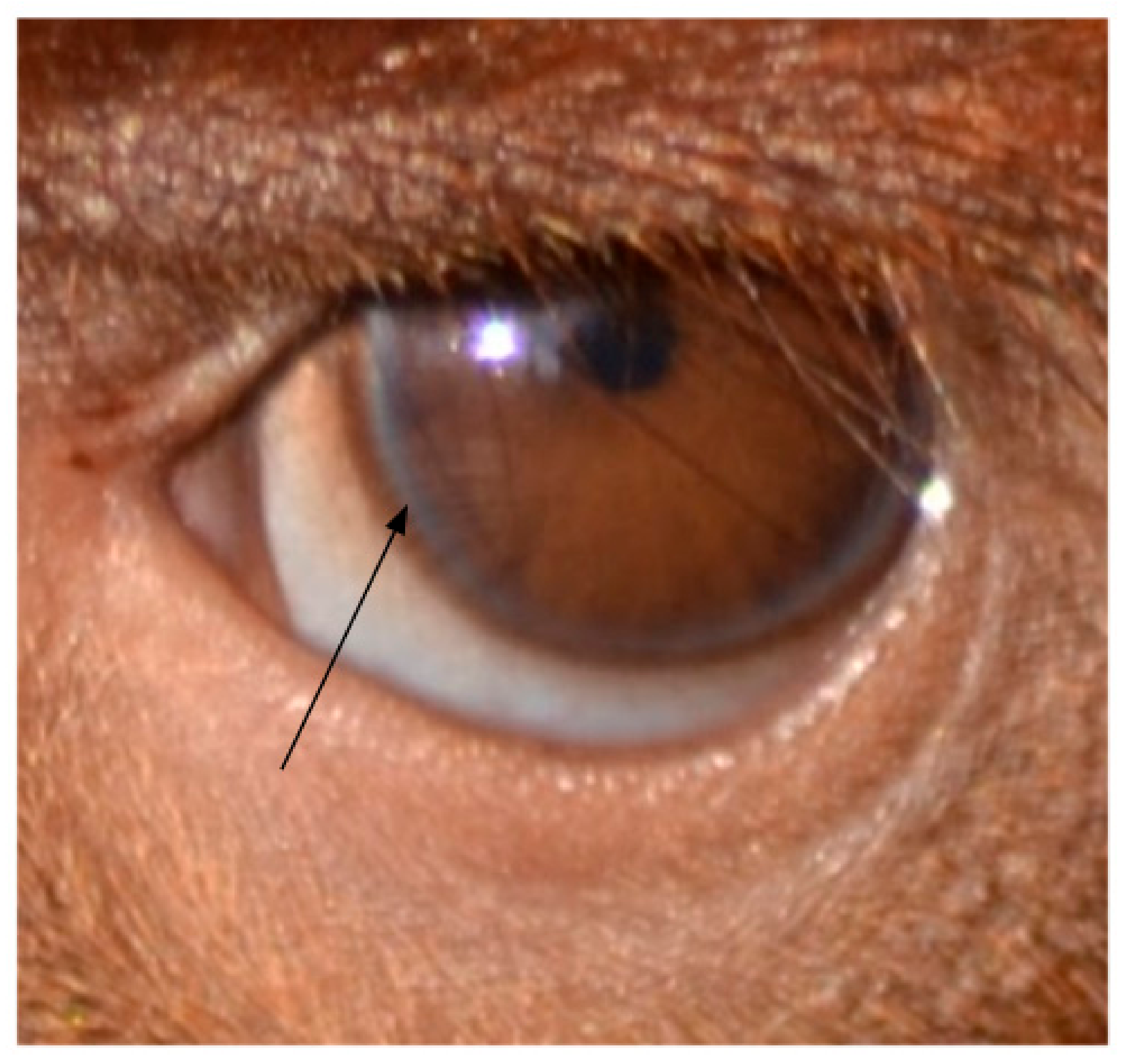
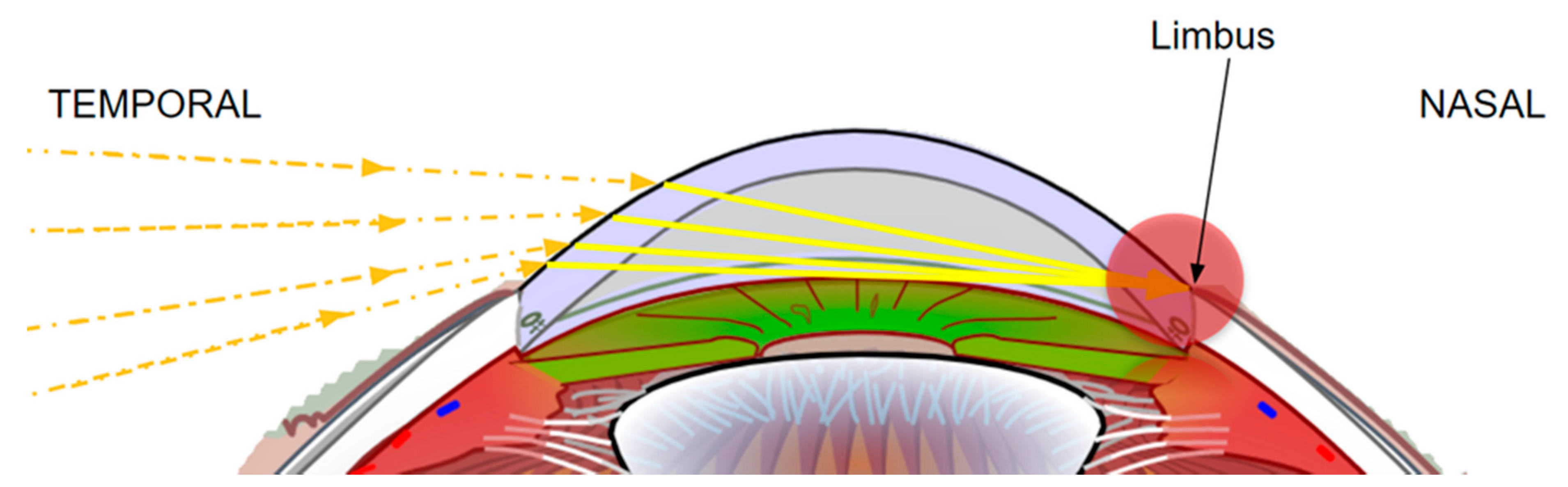
2.2. Temporal Wedge
2.3. White Symmetric Rings around the Iris
2.4. Iris and Pupil
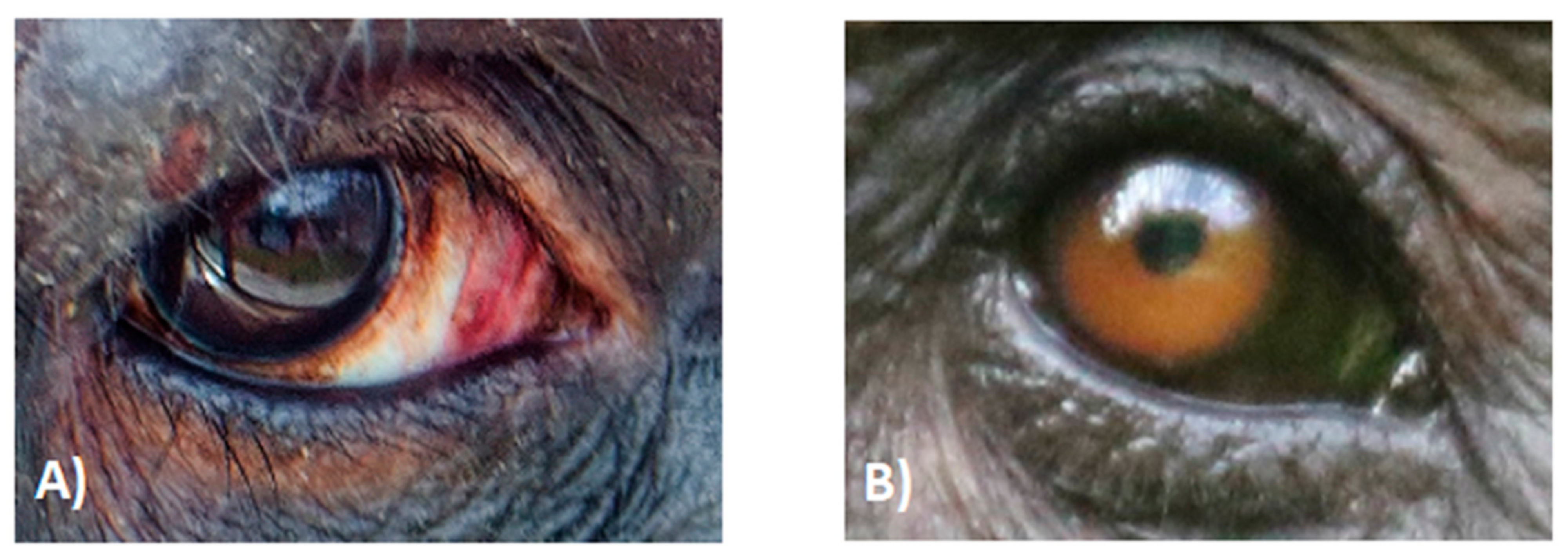
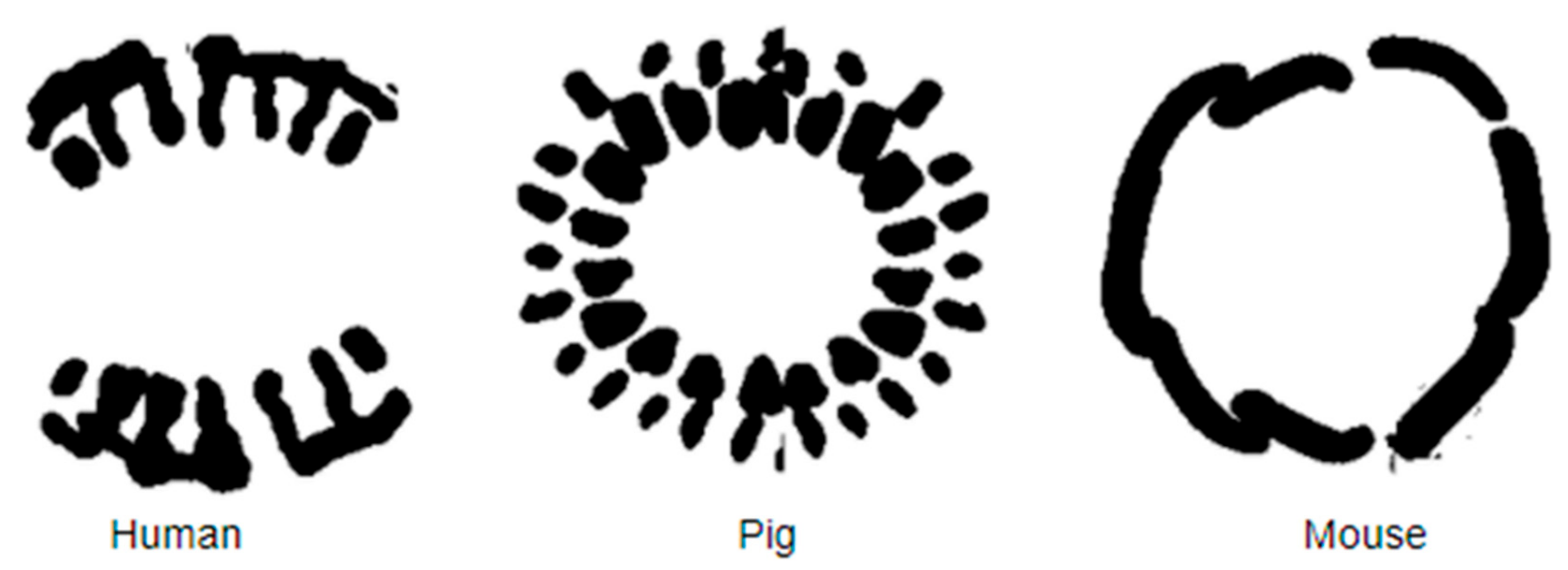
2.5. Local Variation in Scleral Pigmentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, H.; Kohshima, S. Unique morphology of the human eye. Nat. Cell Biol. 1997, 387, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kohshima, S. Unique morphology of the human eye and its adaptive meaning: Comparative studies on external morphology of the primate eye. J. Hum. Evol. 2001, 40, 419–435. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.; Rogers, L.J. Patterns of Gazing in Orangutans (Pongo pygmaeus). Int. J. Primatol. 2002, 23, 501–526. [Google Scholar] [CrossRef]
- Danel, D.P.; Wacewicz, S.; Kleisner, K.; Lewandowski, Z.; Kret, M.E.; Żywiczyński, P.; Perea-Garcia, J.O. Sex differences in ocular morphology in Caucasian people: A dubious role of sexual selection in the evolution of sexual dimorphism of the human eye. Behav. Ecol. Sociobiol. 2020, 74, 1–10. [Google Scholar] [CrossRef]
- Mayhew, J.A.; Gomez, J.-C. Gorillas with white sclera: A naturally occurring variation in a morphological trait linked to social cognitive functions. Am. J. Primatol. 2015, 77, 869–877. [Google Scholar] [CrossRef] [Green Version]
- García, J.O.P. Quantifying ocular morphologies in extant primates for reliable interspecific comparisons. J. Lang. Evol. 2016, 1, 151–158. [Google Scholar] [CrossRef]
- Perea-García, J.O.; Kret, M.E.; Monteiro, A.; Hobaiter, C. Scleral pigmentation leads to conspicuous, not cryptic, eye morphology in chimpanzees. Proc. Natl. Acad. Sci. USA 2019, 116, 19248–19250. [Google Scholar] [CrossRef] [Green Version]
- Perea-García, J.O.; Grenzner, T.; Hešková, G.; Mitkidis, P. Not everything is blue or brown: Quantification of ocular coloration in psychological research beyond dichotomous categorizations. Commun. Integr. Biol. 2017, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Caspar, K.R.; Biggemann, M.; Geissmann, T.; Begall, S. Ocular pigmentation in humans, great apes, and gibbons is not suggestive of communicative functions. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Mearing, A.S.; Koops, K. Quantifying gaze conspicuousness: Are humans distinct from chimpanzees and bonobos? J. Hum. Evol. 2021, 157, 103043. [Google Scholar] [CrossRef] [PubMed]
- Shyu, B.P.; Wyatt, H.J. Appearance of the human eye: Optical contributions to the ‘limbal ring’. Optom. Vis. Sci. 2009, 86, E1069–E1077. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Al-Aqaba, M.; Otri, A.M.; Fares, U.; Said, D.G.; Faraj, L.; Dua, H.S. In vivo confocal microscopic features of normal limbus. Br. J. Ophthalmol. 2012, 96, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.S.; Holland, A.J. The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev. 2018, 32, 620–638. [Google Scholar] [CrossRef] [Green Version]
- Coroneo, M. Ultraviolet Radiation and the Anterior Eye. Eye Contact Lens Sci. Clin. Pract 2011, 37, 214–224. [Google Scholar] [CrossRef]
- Das, P.; Pereira, J.A.; Chaklader, M.; Law, A.; Bagchi, K.; Bhaduri, G.; Chaudhuri, S.; Law, S. Phenotypic alteration of limbal niche–associated limbal epithelial stem cell deficiency by ultraviolet-B exposure–induced phototoxicity in mice. Biochem. Cell Biol. 2013, 91, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Peshek, D.; Semmaknejad, N.; Hoffman, D.; Foley, P. Preliminary Evidence that the Limbal Ring Influences Facial Attractiveness. Evol. Psychol. 2011, 9, 137–146. [Google Scholar] [CrossRef]
- Ilicic, J.; Baxter, S.M.; Kulczynski, A. White eyes are the window to the pure soul: Metaphorical association and overgeneralization effects for spokespeople with limbal rings. Int. J. Res. Mark. 2016, 33, 840–855. [Google Scholar] [CrossRef]
- Brown, M.; Sacco, D.F. Put a (Limbal) Ring on It: Women Perceive Men’s Limbal Rings as a Health Cue in Short-Term Mating Domains. Pers. Soc. Psychol. Bull. 2017, 44, 80–91. [Google Scholar] [CrossRef]
- Brown, M.; Sacco, D.F.; Medlin, M.M. Women’s short-term mating goals elicit avoidance of faces whose eyes lack limbal rings. Evol. Behav. Sci. 2019, 13, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Sacco, D.F.; Brown, M.; Medlin, M. Perfectionism and Relationship Status Influence Health Evaluations of Faces with Limbal Rings. Evol. Psychol. Sci. 2019, 5, 447–453. [Google Scholar] [CrossRef]
- Brown, M.; Keefer, L.A.; Sacco, D.F. Relational insecurity heightens sensitivity to limbal rings in partnered women. Pers. Relatsh. 2020, 27, 61–75. [Google Scholar] [CrossRef]
- Oie, Y.; Nishida, K. Regenerative Medicine for the Cornea. BioMed Res. Int. 2013, 2013, 428247. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Frikke-Schmidt, R.; Schnohr, P.; Jensen, G.B.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Xanthelasmata, arcus corneae, and ischaemic vascular disease and death in general population: Prospective cohort study. BMJ 2011, 343. [Google Scholar] [CrossRef] [Green Version]
- Brenes, J.A. White Rings: Arcus Lipoides. Am. J. Med. 2013, 126, 112–113. [Google Scholar] [CrossRef]
- Harrison, N.A.; Wilson, C.E.; Critchley, H. Processing of observed pupil size modulates perception of sadness and predicts empathy. Emotion 2007, 7, 724–729. [Google Scholar] [CrossRef]
- Kret, M.E. The role of pupil size in communication. Is there room for learning? Cogn. Emot. 2017, 32, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, F.; Moulder, R.G.; Boker, S.M.; Kret, M. Quantifying physiological synchrony through windowed cross-correlation analysis: Statistical and theoretical considerations. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kret, M.E.; de Dreu, C.K. Pupil-mimicry conditions trust in partners: Moderation by oxytocin and group membership. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162554. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, C.; Arslan, M.; Falck-Ytter, T.; Roeyers, H.; Gredebäck, G. Human eyes with dilated pupils induce pupillary contagion in infants. Sci. Rep. 2017, 7, 9601. [Google Scholar] [CrossRef] [Green Version]
- Kret, M.E.; Tomonaga, M.; Matsuzawa, T. Chimpanzees and humans mimic pupil-size of conspecifics. PLoS ONE 2014, 9, 8. [Google Scholar] [CrossRef]
- West, R.W. Perceived Direction of Gaze from Eyes with Dark vs. Light Irises. Optom. Vis. Sci. 2011, 88, 303–311. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Thorstenson, C.A.; Nguyen, T.P. Sclera and Iris Color Interact to Influence Gaze Perception. Front. Psychol. 2021, 12, 676. [Google Scholar] [CrossRef]
- Meyer, W.K.; Zhang, S.; Hayakawa, S.; Imai, H.; Przeworski, M. The convergent evolution of blue iris pigmentation in primates took distinct molecular paths. Am. J. Phys. Anthr. 2013, 151, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Pedersen, N.L.; Stattin, H. Associations between iris characteristics and personality in adulthood. Biol. Psychol. 2007, 75, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Watanabe, K. Preliminary study on eye colour in Japanese macaques (Macaca fuscata) in their natural habitat. Primates 2006, 48, 122–129. [Google Scholar] [CrossRef]
- Frost, P. European hair and eye color: A case of frequency-dependent sexual selection? Evol. Hum. Behav. 2006, 27, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Bradley, B.J.; Pedersen, A.; Mundy, N.I. Brief communication: Blue eyes in lemurs and humans: Same phenotype, different genetic mechanism. Am. J. Phys. Anthr. 2009, 139, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Terman, M.; Terman, J.S. Depressive symptomatology differentiates subgroups of patients with seasonal affective disorder. Depress. Anxiety 2002, 15, 34–41. [Google Scholar] [CrossRef]
- Workman, L. Blue Eyes Keep away the Winter Blues: Is Blue Eye Pigmentation an Evolved Feature to Provide Resilience to Seasonal Affective Disorder. OA J. Behav. Sci. Psychol. 2018, 1, 180002. [Google Scholar]
- Münch, M.; Nowozin, C.; Regente, J.; Bes, F.; De Zeeuw, J.; Hädel, S.; Wahnschaffe, A.; Kunz, D. Blue-Enriched Morning Light as a Countermeasure to Light at the Wrong Time: Effects on Cognition, Sleepiness, Sleep, and Circadian Phase. Neuropsychobiology 2016, 74, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef]
- Goldberg, B.; Klein, W. Variations in the spectral distribution of daylight at various geographical locations on the earth’s surface. Sol. Energy 1977, 19, 3–13. [Google Scholar] [CrossRef]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coroneo, M.T.; Müller-Stolzenburg, N.W.; Ho, A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg. 1991, 22, 705–711. [Google Scholar] [PubMed]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Thouvenin, O.; Bouheraoua, N.; Paques, M.; Borderie, V. Three-dimensional structure of the mammalian limbal stem cell niche. Exp. Eye Res. 2015, 140, 75–84. [Google Scholar] [CrossRef]
- Alqahtani, J.M. Conjunctival Melanosis: Review of the Literature. Bahrain Med Bull. 2013, 35, 206–210. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; Mashayekhi, A.; Marr, B.P.; Benavides, R.; Thangappan, A.; Phan, L.; Eagle, R.C., Jr. Primary acquired melanosis of the conjunctiva: Experience with 311 eyes. Trans. Am. Ophthalmol. Soc. 2007, 105, 61. [Google Scholar] [PubMed]
- Leung, T.S.; Outlaw, F.; Macdonald, L.W.; Meek, J. Jaundice Eye Color Index (JECI): Quantifying the yellowness of the sclera in jaundiced neonates with digital photography. Biomed. Opt. Express 2019, 10, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Provine, R.R.; Nave-Blodgett, J.; Cabrera, M.O. The emotional eye: Red sclera as a uniquely human cue of emotion. Ethology 2013, 119, 993–998. [Google Scholar] [CrossRef]
- Provine, R.R.; Cabrera, M.O.; Nave-Blodgett, J. Red, Yellow, and Super-White Sclera. Hum. Nat. 2013, 24, 126–136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea-García, J.O.; Danel, D.P.; Monteiro, A. Diversity in Primate External Eye Morphology: Previously Undescribed Traits and Their Potential Adaptive Value. Symmetry 2021, 13, 1270. https://doi.org/10.3390/sym13071270
Perea-García JO, Danel DP, Monteiro A. Diversity in Primate External Eye Morphology: Previously Undescribed Traits and Their Potential Adaptive Value. Symmetry. 2021; 13(7):1270. https://doi.org/10.3390/sym13071270
Chicago/Turabian StylePerea-García, Juan Olvido, Dariusz P. Danel, and Antónia Monteiro. 2021. "Diversity in Primate External Eye Morphology: Previously Undescribed Traits and Their Potential Adaptive Value" Symmetry 13, no. 7: 1270. https://doi.org/10.3390/sym13071270
APA StylePerea-García, J. O., Danel, D. P., & Monteiro, A. (2021). Diversity in Primate External Eye Morphology: Previously Undescribed Traits and Their Potential Adaptive Value. Symmetry, 13(7), 1270. https://doi.org/10.3390/sym13071270





