EEG and fMRI Correlates of Insight: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli and Task
2.3. EEG Data Acquisition and Analysis
2.4. FMRI Data Acquisition and Analysis
3. Results
3.1. Behavioral Results
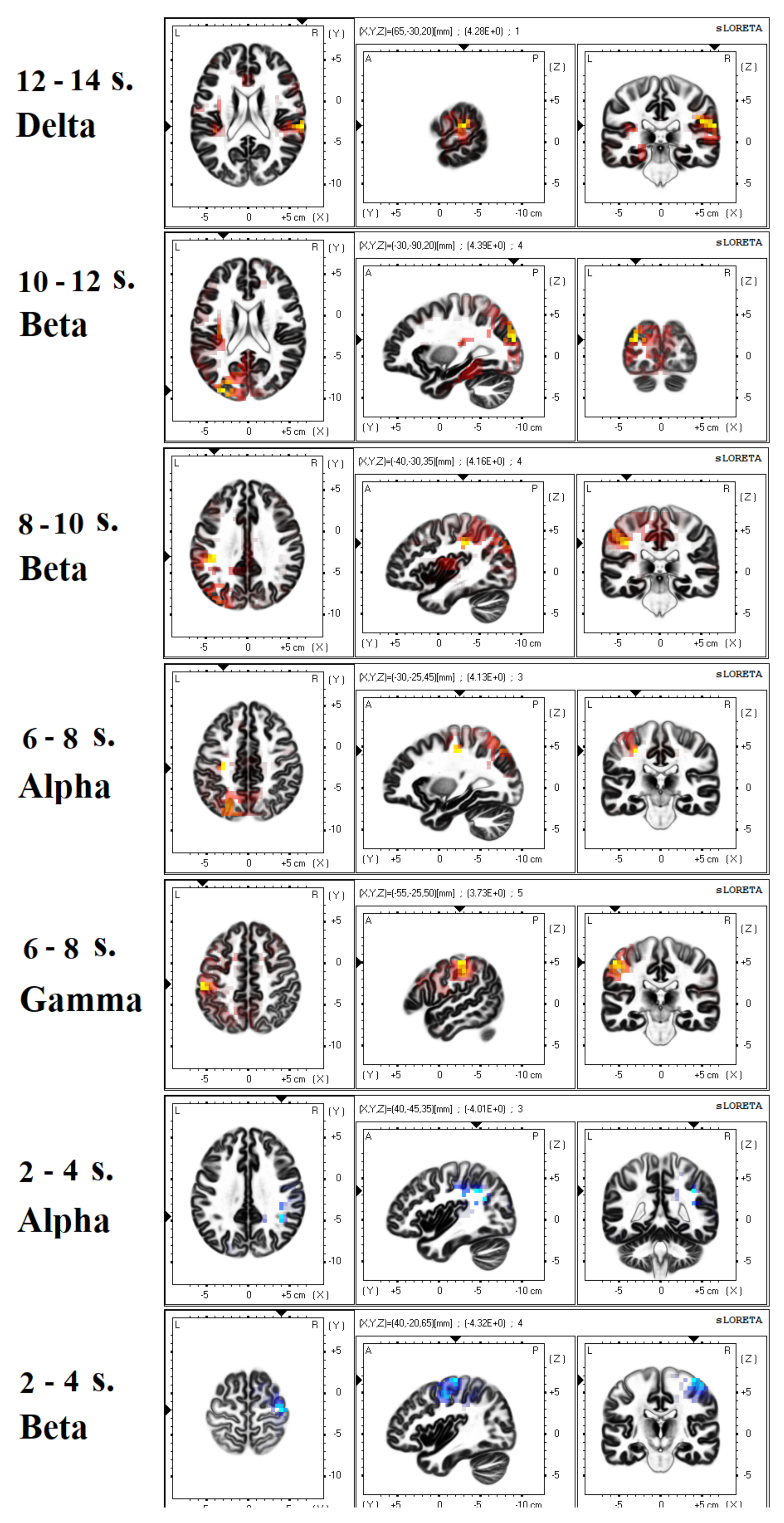
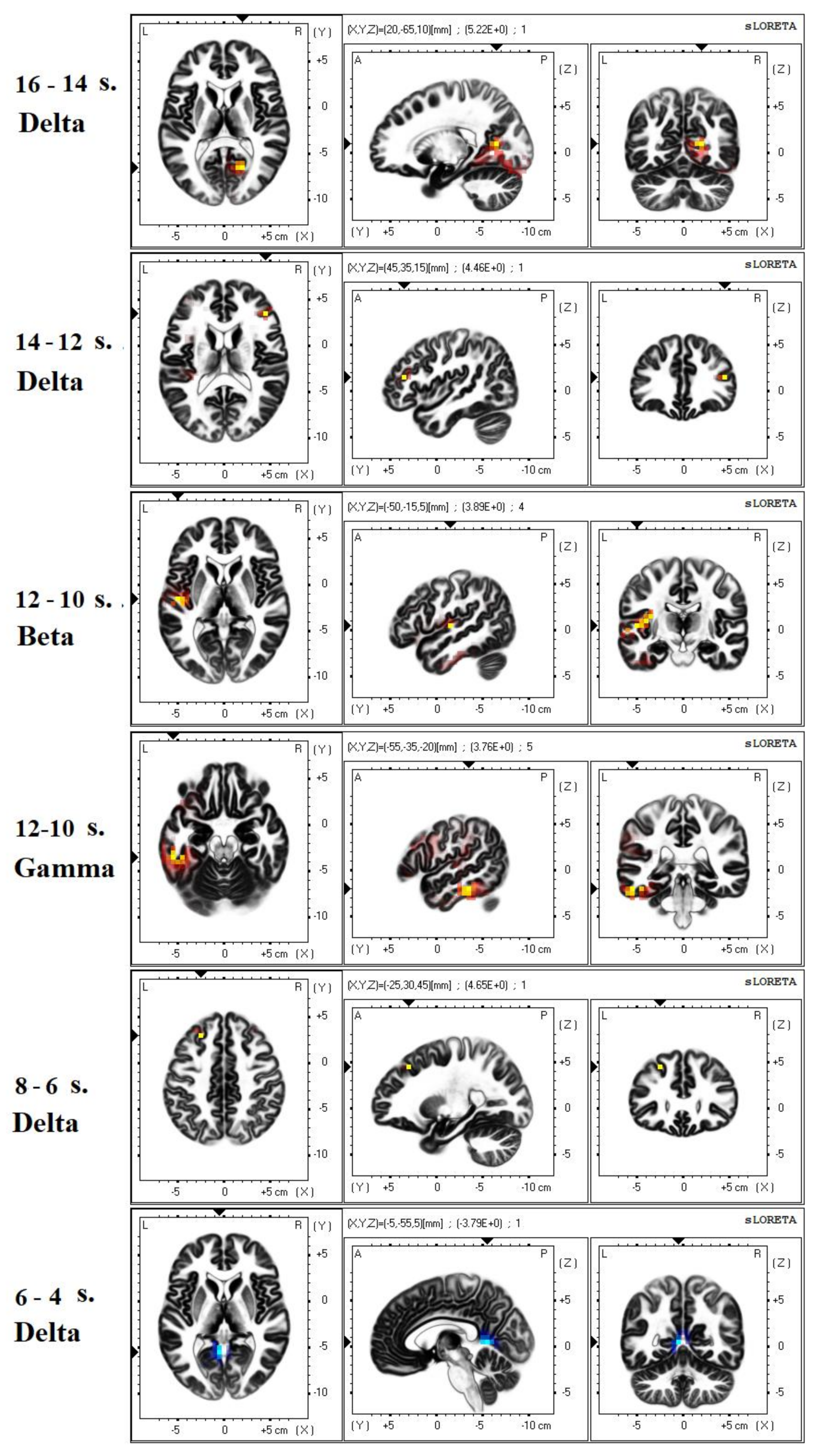
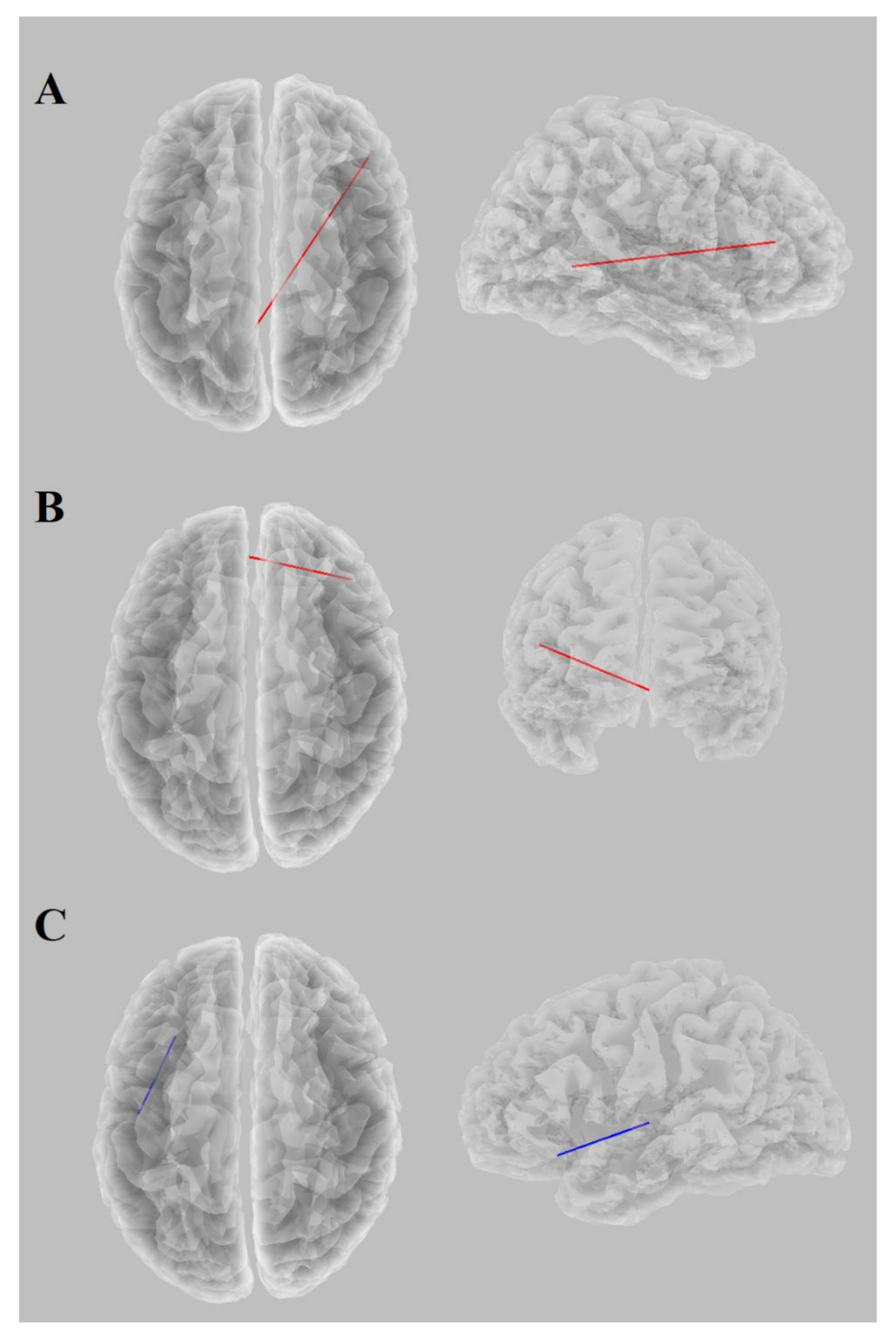
3.2. EEG Analysis
3.3. FMRI Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidson, J.E.; Sternberg, R.J. (Eds.) The Psychology of Problem Solving; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Cushen, P.J.; Wiley, J. Cues to solution, restructuring patterns, and reports of insight in creative problem solving. Conscious. Cogn. 2012, 21, 1166–1175. [Google Scholar] [CrossRef]
- Weisberg, R.W. Toward an integrated theory of insight in problem solving. Think. Reason. 2014, 21, 5–39. [Google Scholar] [CrossRef]
- Irvine, W.B. Aha! The Moments of Insight That Shape Our World; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Poincaré, H. The Foundations of Science; Science Press: Lancaster, PA, USA, 1913. [Google Scholar]
- Wallas, G. The Art of Thought; Harcourt Brace: NewYork, NY, USA, 1926. [Google Scholar]
- Kounios, J.; Jung-Beeman, M. The Aha! Moment the cognitive neuroscience of insight. Curr. Dir. Psychol. Sci. 2009, 18, 210–216. [Google Scholar] [CrossRef]
- Dietrich, A.; Kanso, R. A Review of EEG, ERP, and Neuroimaging Studies of Creativity and Insight. Psychol. Bull. 2010, 136, 822–848. [Google Scholar] [CrossRef]
- Bowden, E.M.; Jung-Beeman, M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychon. Bull. Rev. 2003, 10, 730–737. [Google Scholar] [CrossRef]
- Jung-Beeman, M.; Bowden, E.; Haberman, J.; Frymiare, J.; Aramber-Liu, S.; Greenblatt, R.; Kounios, J. Neural activity when people solve problems with insight. PloS Biol. 2004, 2, 500–510. [Google Scholar] [CrossRef]
- Kounios, J.; Fleck, J.; Green, D.; Payne, L.; Stevenson, J.; Bowden, E.; Jung-Beeman, M. The origins of insight in resting-state brain activity. Neuropsychologia 2008, 46, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Luo, Y.; Wu, Z.; Zhang, Q. A further study of ERP effects of “insight” in a riddle guessing task. Acta Psychol. Sin. 2006, 38, 507–514. [Google Scholar]
- Sandkuhler, S.; Bhattacharya, J. Deconstructing insight: EEG correlates of insightful problem solving. PLoS ONE 2008, 3, e1459. [Google Scholar] [CrossRef] [PubMed]
- Danko, S.; Starchenko, M.; Bechtereva, N. EEG local and spatial synchronization during a test on the insight strategy of solving creative verbal tasks. Hum. Physiol. 2003, 29, 129–132. [Google Scholar]
- Kounios, J.; Frymiare, J.; Bowden, E.; Fleck, J.; Subramaniam, K.; Parrish, T.; Jung-Beeman, M. The prepared mind: Neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol. Sci. 2006, 17, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Kanngieser, N.; Jaskowski, P.; Haider, H.; Rose, M.; Verleger, R. Precursors of insight in event-related brain potentials. J. Cogn. Neurosci. 2006, 18, 2152–2166. [Google Scholar] [CrossRef] [PubMed]
- Lavric, A.; Forstmeier, S.; Rippon, G. Differences in working memory involvement in analytical and creative tasks: An ERP study. NeuroReport 2000, 11, 1613–1618. [Google Scholar] [CrossRef][Green Version]
- Mai, X.; Luo, J.; Wu, J.; Luo, Y. “Aha!” effects in a guessing riddle task: An event related potential study. Hum. Brain Mapp. 2004, 22, 261–270. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Yang, D.; Luo, Y.; Li, Y.; Wu, Z.; Zhang, Q. The neural basis of insight problem solving: An event-related potential study. Brain Cogn. 2008, 68, 100–106. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Yang, D.; Luo, Y.; Li, Y.; Wu, Z.; Zhang, Q. Spatiotemporal cortical activation underlies mental preparation for successful riddle solving: An event-related potential study. Exp. Brain Res. 2008, 186, 629–634. [Google Scholar] [CrossRef]
- Goldman, R.I.; Stern, J.M.; Engel, J., Jr.; Cohen, M.S. Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport 2002, 13, 2487–2492. [Google Scholar] [CrossRef]
- Sadato, N.; Nakamura, S.; Oohashi, T.; Nishina, E.; Fuwamoto, Y.; Waki, A.; Yonekura, Y. Neural networks for generation and suppression of alpha rhythm: A PET study. NeuroReport 1998, 9, 893–897. [Google Scholar] [CrossRef]
- Mednick, S.A. The associative basis of the creative process. Psychol. Rev. 1962, 69, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Bowden, E.M. The effect of reportable and unreportable hints on anagram solution and the aha! experience. Conscious. Cogn. 1997, 6, 545–573. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, J.N.; Cunningham, J.B. Rebus puzzles as insight problems. Behav. Res. Methods 2008, 40, 263–268. [Google Scholar] [CrossRef]
- Valueva, E.A.; Belova, S.S. Creativity diagnostics: Methods, problems, perspectives. In Creativity: From Biological Prerequisites to Cultural Phenomena; Ushakov, D.V., Ed.; Institute of Psychology RAS: Moscow, Russia, 2011; pp. 625–647. (In Russian) [Google Scholar]
- Pascual-Marqui, R.D. Instantaneous and Lagged Measurements of Linear and Nonlinear Dependence between Groups of Multivariate Time Series: Frequency Decomposition. arXiv 2007, arXiv:0711.1455. Available online: http://arxiv.org/abs/0711.1455 (accessed on 9 November 2007).
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Jung, R.E.; Segall, J.M.; Bockholt, H.J.; Chavez, R.S.; Flores, R.; Haier, R.J. Neuroanatomy of creativity. Hum. Brain Mapp. 2010, 31, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Ritter, S.M.; Müller, B.C.N.; van Baaren, R.B.; Brass, M.; Dijksterhuis, A. The Importance of the Default Mode Network in Creativity—A Structural MRI Study. J. Creat. Behav. 2014, 48, 152–163. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.; Dufort, P.; Villani, M.; LaConte, S. BROCCOLI: Software for fast FMRI analysis on many-core CPUs and GPUs. Front. Neuroinform. 2014, 8, 24. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.J.; Turner, R. Movement-Related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Griffanti, L.; Douaud, G.; Bijsterbosch, J.; Evangelisti, S.; Alfaro-Almagro, F.; Glasser, M.F.; Duff, E.P.; Fitzgibbon, S.; Westphal, R.; Carone, D.; et al. Hand classification of fMRI ICA noise components. NeuroImage 2017, 154, 188–205. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- Knyazev, G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Kounios, J.; Parrish, T.; Beeman, M. A Brain Mechanism for Facilitation of Insight by Positive Affect. J. Cogn. Neurosci. 2008, 21, 415–432. [Google Scholar] [CrossRef]
- Brown, S.; Martinez, M.J.; Parsons, L.M. Music and language side by side in the brain: A PET study of the generation of melodies and sentences. Eur. J. Neurosci. 2006, 23, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Simard, F.; Monchi, O. Differences between patterns of brain activity associated with semantics and those linked with phonological processing diminish with age. PLoS ONE 2014, 9, e99710. [Google Scholar] [CrossRef] [PubMed]
- Tik, M.; Sladky, R.; Luft, C.D.B.; Willinger, D.; Hoffmann, A.; Banissy, M.J.; Bhattacharya, J.; Windischberger, C. Ultra-high-field fMRI insights on insight: Neural correlates of the Aha!-moment. Hum. Brain Mapp. 2018, 39, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Lou, H.C.; Gross, J.; Biermann-Ruben, K.; Kjaer, T.W.; Alfons, S. Coherence in consciousness: Paralimbic gamma synchrony of self-reference links conscious experiences. Hum. Brain Mapp. 2009, 31, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Meador, K.J.; Ray, P.G.; Echauz, J.R.; Loring, D.W.; Vachtsevanos, G.J. Gamma coherence and conscious perception. Neurology 2002, 59, 847–854. [Google Scholar] [CrossRef]
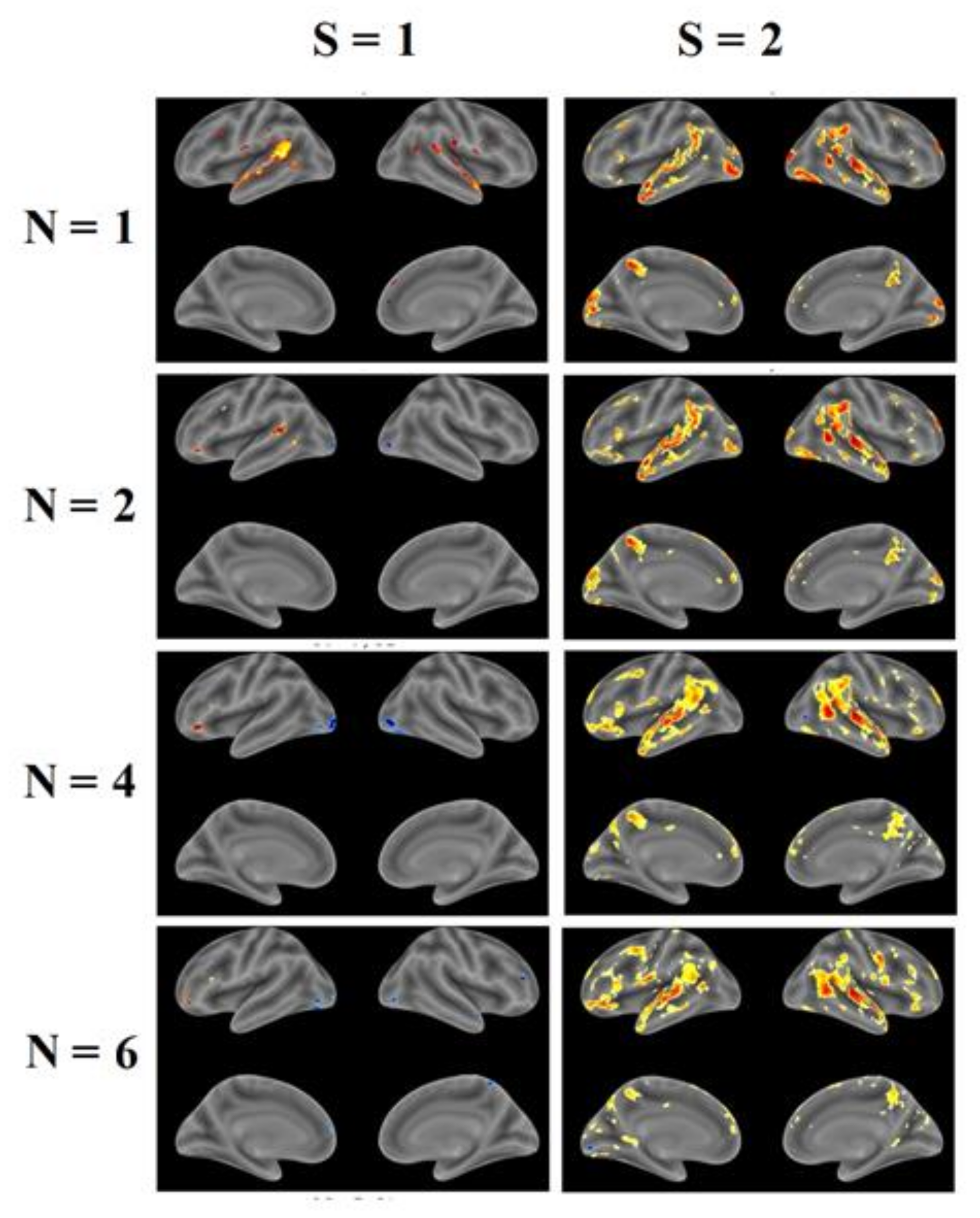
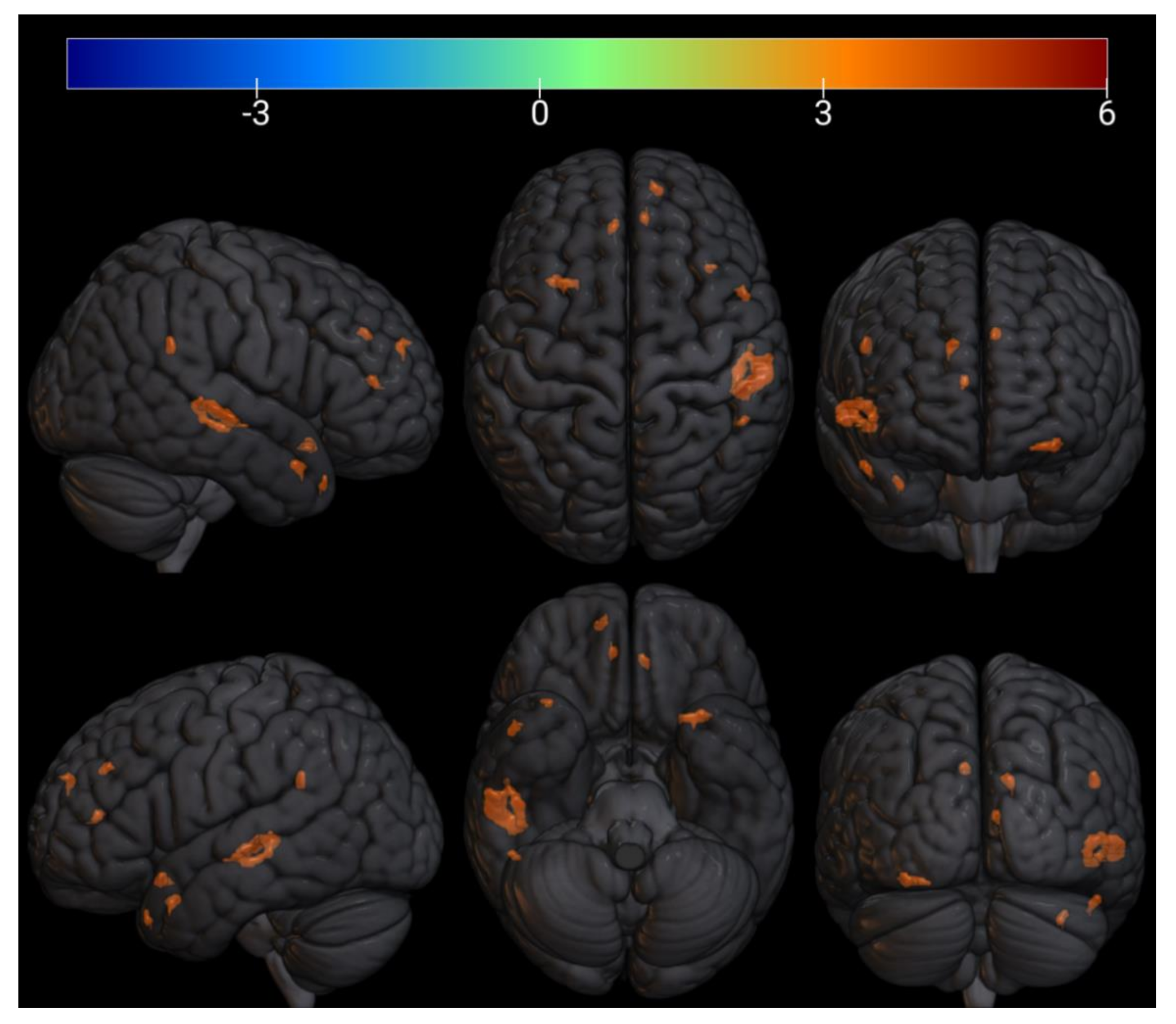
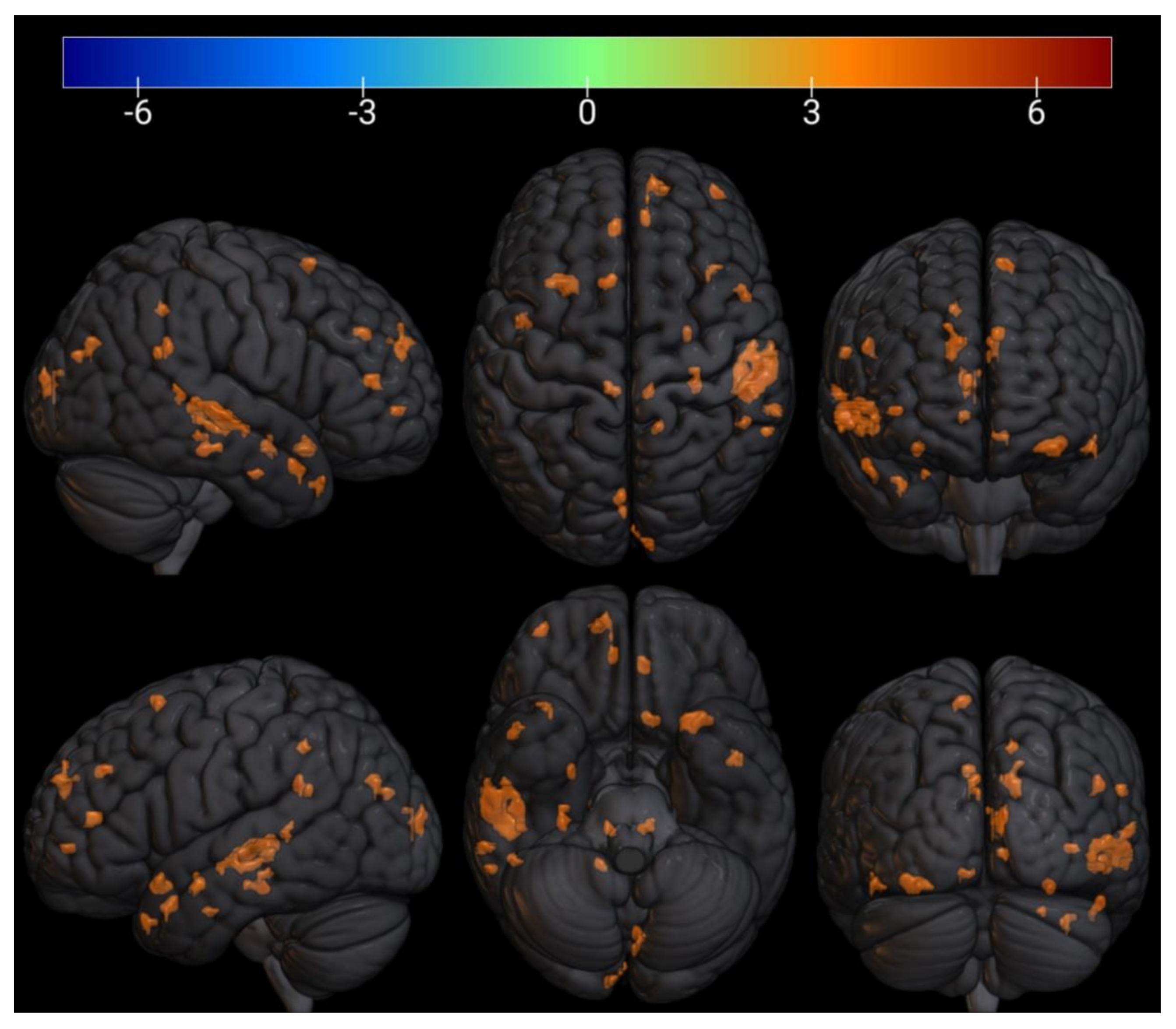
| № Cluster | N Active Voxels in Cluster | Anatomical ROI | N Active Voxels in ROI | Peak MNI-Coordinates, mm | Peak T-Value | Peak p-Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 1 | 34 | TP r (Temporal Pole) | 34 | 54 | 10 | −24 | 5.4 | 0.0002 |
| 2 | 9 | aPaHC r (Parahippocampal Gyrus) | 1 | 28 | −6 | −28 | 5.8 | 0.0001 |
| Hippocampus r | 8 | |||||||
| 3 | 11 | pPaHC r (Parahippocampal Gyrus) | 11 | 30 | −30 | −18 | 7.2 | 2.65 × 10−5 |
| 4 | 36 | IC l (Insular Cortex) | 6 | −34 | 16 | −16 | 5.7 | 0.0001 |
| FOrb l (Frontal Orbital Cortex) | 30 | |||||||
| 5 | 4 | TP r (Temporal Pole) | 4 | 40 | 16 | −20 | 4.8 | 0.0005 |
| 6 | 3 | PP l (Planum Polare) | 3 | −46 | 0 | −16 | 4.4 | 0.0008 |
| 7 | 6 | Brainstem | 6 | −8 | −32 | −12 | 5.5 | 0.0002 |
| 8 | 173 | pSTG r (Superior Temporal Gyrus) | 88 | 60 | −20 | −4 | 6.5 | 5.26 × 10−5 |
| pMTG r (Middle Temporal Gyrus) | 85 | |||||||
| 9 | 7 | Thalamus r | 1 | 12 | −32 | −4 | 6.6 | 5.25 × 10−5 |
| Brainstem | 6 | |||||||
| 10 | 10 | FP r (Frontal Pole) | 10 | 38 | 56 | −2 | 5.1 | 0.0003 |
| 11 | 11 | toMTG r (Middle Temporal Gyrus) | 5 | 62 | −40 | 4 | 5.3 | 0.0003 |
| pSMG r (Supramarginal Gyrus) | 6 | |||||||
| 12 | 10 | OP r (Occipital Pole) | 10 | 10 | −98 | 14 | 5.5 | 0.0002 |
| 13 | 10 | PaCiG l (Paracingulate Gyrus) | 8 | −14 | 46 | 6 | 4.9 | 0.0004 |
| AC (Cingulate Gyrus) | 2 | |||||||
| 14 | 3 | ICC l (Intracalcarine Cortex) | 3 | −16 | −74 | 10 | 4.9 | 0.0004 |
| 15 | 16 | PaCiG r (Paracingulate Gyrus) | 7 | 10 | 42 | 12 | 4.8 | 0.0005 |
| AC (Cingulate Gyrus) | 9 | |||||||
| 16 | 6 | OP r (Occipital Pole) | 6 | 6 | −94 | 14 | 5 | 0.0004 |
| 17 | 28 | FP r (Frontal Pole) | 28 | 28 | 52 | 20 | 5 | 0.0004 |
| 18 | 17 | AG r (Angular Gyrus) | 17 | 62 | −48 | 24 | 4.4 | 0.0008 |
| 19 | 14 | PaCiG l (Paracingulate Gyrus) | 14 | −6 | 40 | 32 | 5.9 | 0.0001 |
| 20 | 4 | Precuneous (Precuneous Cortex) | 4 | 16 | −46 | 42 | 5.1 | 0.0003 |
| 21 | 7 | SFG l (Superior Frontal Gyrus) | 7 | −10 | 16 | 60 | 4.9 | 0.0004 |
| 22 | 5 | FOrb r (Frontal Orbital Cortex) | 5 | 26 | 30 | −14 | −5.1 | 0.0003 |
| 23 | 7 | PreCG l (Precentral Gyrus) | 7 | −6 | −26 | 58 | −5.8 | 0.0001 |
| № Cluster | N Active Voxels in Cluster | Anatomical ROI | N Active Voxels in ROI | Peak MNI-Coordinates, mm | Peak T-Value | Peak p-Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 1 | 27 | TP r (Temporal Pole) | 27 | 38 | 20 | −38 | 5.4 | 0.0002 |
| 2 | 3 | Cereb1 l (Cerebellum Crus1) | 3 | −36 | −60 | −36 | 4.9 | 0.0004 |
| 3 | 28 | TP r (Temporal Pole) | 28 | 54 | 8 | −24 | 5.5 | 0.0002 |
| 4 | 6 | Cereb1 l (Cerebellum Crus1) | 6 | −14 | −74 | −28 | 4.7 | 0.0005 |
| 5 | 11 | aPaHC r (Parahippocampal Gyrus) | 2 | 28 | −6 | −28 | 6.7 | 4.69 × 10−5 |
| Hippocampus r | 9 | |||||||
| 6 | 6 | aPaHC r (Parahippocampal Gyrus) | 1 | 30 | −14 | −26 | 6.1 | 8.75 × 10−5 |
| Hippocampus r | 5 | |||||||
| 7 | 7 | aPaHC l (Parahippocampal Gyrus) | 2 | −28 | −4 | −26 | 5.3 | 0.0002 |
| Amygdala l | 5 | |||||||
| 8 | 7 | Hippocampus l | 6 | −24 | −8 | −24 | 5.1 | 0.0003 |
| Amygdala l | 1 | |||||||
| 9 | 6 | pPaHC l (Parahippocampal Gyrus) | 6 | −20 | −24 | −20 | 5 | 0.0004 |
| 10 | 20 | aSTG l (Superior Temporal Gyrus) | 3 | −46 | −2 | −16 | 6 | 0.0001 |
| PP l (Planum Polare) | 17 | |||||||
| 11 | 32 | pPaHC r (Parahippocampal Gyrus) | 19 | 30 | −28 | −16 | 7.6 | 1.74 × 10−5 |
| pTFusC r(Temporal Fusiform Cortex) | 2 | |||||||
| Hippocampus r | 11 | |||||||
| 12 | 9 | pMTG r (Middle Temporal Gyrus) | 9 | 60 | −10 | −20 | 5.9 | 0.0001 |
| 13 | 64 | IC l (Insular Cortex) | 13 | −32 | 16 | −18 | 6.3 | 7.09 × 10−5 |
| FOrb l (Frontal Orbital Cortex) | 51 | |||||||
| 14 | 9 | TP r (Temporal Pole) | 9 | 46 | 14 | −18 | 5 | 0.0004 |
| 15 | 18 | Brainstem | 17 | −8 | −32 | −12 | 6.4 | 5.92 × 10−5 |
| Cereb45 l (Cerebellum 4 5) | 1 | −8 | −32 | −12 | ||||
| 16 | 8 | Hippocampus l | 8 | −24 | −26 | −12 | 5.9 | 0.0001 |
| 17 | 6 | aSTG r (Superior Temporal Gyrus) | 5 | 60 | 0 | −16 | 4.4 | 0.0008 |
| aMTG r (Middle Temporal Gyrus) | 1 | |||||||
| 18 | 3 | Brainstem | 3 | 10 | −28 | −12 | 5 | 0.0004 |
| 19 | 240 | pSTG r (Superior Temporal Gyrus) | 122 | 60 | −24 | −2 | 7.2 | 2.58 × 10−5 |
| pMTG r (Middle Temporal Gyrus) | 118 | |||||||
| 20 | 10 | Thalamus r | 2 | 12 | −32 | −4 | 6.9 | 3.63 × 10−5 |
| Brainstem | 8 | 12 | −32 | −4 | ||||
| 21 | 5 | LG l (Lingual Gyrus) | 5 | −26 | −60 | −2 | 5.5 | 0.0002 |
| 22 | 17 | FP r (Frontal Pole) | 17 | 38 | 56 | −2 | 6 | 0.0001 |
| 23 | 39 | OP r (Occipital Pole) | 39 | 10 | −98 | 14 | 6.7 | 4.27 × 10−5 |
| 24 | 22 | toMTG r (Middle Temporal Gyrus) | 9 | 62 | −40 | 4 | 5.6 | 0.0002 |
| pSMG r (Supramarginal Gyrus) | 13 | 62 | −40 | 4 | ||||
| 25 | 5 | toMTG r (Middle Temporal Gyrus) | 4 | 50 | −38 | 4 | 4.8 | 0.0005 |
| pSMG r (Supramarginal Gyrus) | 1 | |||||||
| 26 | 9 | ICC l (Intracalcarine Cortex) | 9 | −14 | −74 | 8 | 6 | 5.91 × 10−5 |
| 27 | 7 | PaCiG l (Paracingulate Gyrus) | 7 | −14 | 46 | 6 | 5.5 | 0.0002 |
| 28 | 26 | PaCiG r (Paracingulate Gyrus) | 13 | 10 | 42 | 12 | 5.1 | 0.0003 |
| AC (Cingulate Gyrus) | 13 | 10 | 42 | 12 | ||||
| 29 | 5 | AC (Cingulate Gyrus) | 5 | −4 | 36 | 18 | 5 | 0.0003 |
| 30 | 11 | FP r (Frontal Pole) | 11 | 26 | 54 | 20 | 5.3 | 0.0002 |
| 31 | 9 | Cuneal r (Cuneal Cortex) | 2 | 8 | −88 | 22 | 4.8 | 0.0005 |
| OP r (Occipital Pole) | 7 | |||||||
| 32 | 37 | Precuneous (Precuneous Cortex) | 3 | −4 | −76 | 28 | 5.9 | 0.0001 |
| Cuneal l (Cuneal Cortex) | 34 | −4 | −76 | |||||
| 33 | 21 | pSMG r (Supramarginal Gyrus) | 1 | 50 | −46 | 26 | 7.2 | 2.54 × 10−5 |
| AG r (Angular Gyrus) | 20 | 50 | −46 | 26 | ||||
| 34 | 7 | FP l (Frontal Pole) | 5 | −20 | 44 | 22 | 4.8 | 0.0005 |
| 35 | 51 | FP r (Frontal Pole) | 50 | 14 | 54 | 26 | 6.5 | 5.62 × 10−5 |
| SFG r (Superior Frontal Gyrus) | 1 | 14 | 54 | 26 | ||||
| 36 | 15 | AG r (Angular Gyrus) | 15 | 62 | −50 | 22 | 4.6 | 0.0006 |
| 37 | 24 | SFG l (Superior Frontal Gyrus) | 1 | −6 | 40 | 32 | 5.9 | 0.0001 |
| PaCiG l (Paracingulate Gyrus) | 23 | −6 | 40 | 32 | ||||
| 38 | 3 | sLOC r (Lateral Occipital Cortex) | 3 | 44 | −64 | 34 | 4.5 | 0.0008 |
| 39 | 14 | Precuneous (Precuneous Cortex) | 14 | 16 | −46 | 42 | 5.3 | 0.0003 |
| 40 | 9 | SFG r (Superior Frontal Gyrus) | 6 | 26 | 28 | 48 | 4.6 | 0.0006 |
| MidFG r (Middle Frontal Gyrus) | 3 | 26 | 28 | 48 | ||||
| 41 | 26 | SFG l (Superior Frontal Gyrus) | 26 | −10 | 14 | 62 | 6 | 9.75 × 10−5 |
| 42 | 10 | SFG r (Superior Frontal Gyrus) | 10 | 12 | 18 | 68 | 4.6 | 0.0006 |
| 43 | 8 | PreCG l (Precentral Gyrus) | 8 | −6 | −26 | 58 | −7.8 | 0.00001 |
| 44 | 4 | PostCG r (Postcentral Gyrus) | 4 | 16 | −32 | 74 | −4.5 | 0.0008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyazev, G.G.; Ushakov, V.L.; Orlov, V.A.; Malakhov, D.G.; Kartashov, S.I.; Savostyanov, A.N.; Bocharov, A.V.; Velichkovsky, B.M. EEG and fMRI Correlates of Insight: A Pilot Study. Symmetry 2021, 13, 330. https://doi.org/10.3390/sym13020330
Knyazev GG, Ushakov VL, Orlov VA, Malakhov DG, Kartashov SI, Savostyanov AN, Bocharov AV, Velichkovsky BM. EEG and fMRI Correlates of Insight: A Pilot Study. Symmetry. 2021; 13(2):330. https://doi.org/10.3390/sym13020330
Chicago/Turabian StyleKnyazev, Gennady G., Vadim L. Ushakov, Vyacheslav A. Orlov, Denis G. Malakhov, Sergey I. Kartashov, Alexander N. Savostyanov, Andrey V. Bocharov, and Boris M. Velichkovsky. 2021. "EEG and fMRI Correlates of Insight: A Pilot Study" Symmetry 13, no. 2: 330. https://doi.org/10.3390/sym13020330
APA StyleKnyazev, G. G., Ushakov, V. L., Orlov, V. A., Malakhov, D. G., Kartashov, S. I., Savostyanov, A. N., Bocharov, A. V., & Velichkovsky, B. M. (2021). EEG and fMRI Correlates of Insight: A Pilot Study. Symmetry, 13(2), 330. https://doi.org/10.3390/sym13020330







