Investigating Human Torso Asymmetries: An Observational Longitudinal Study of Fluctuating and Directional Asymmetry in the Scoliotic Torso
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
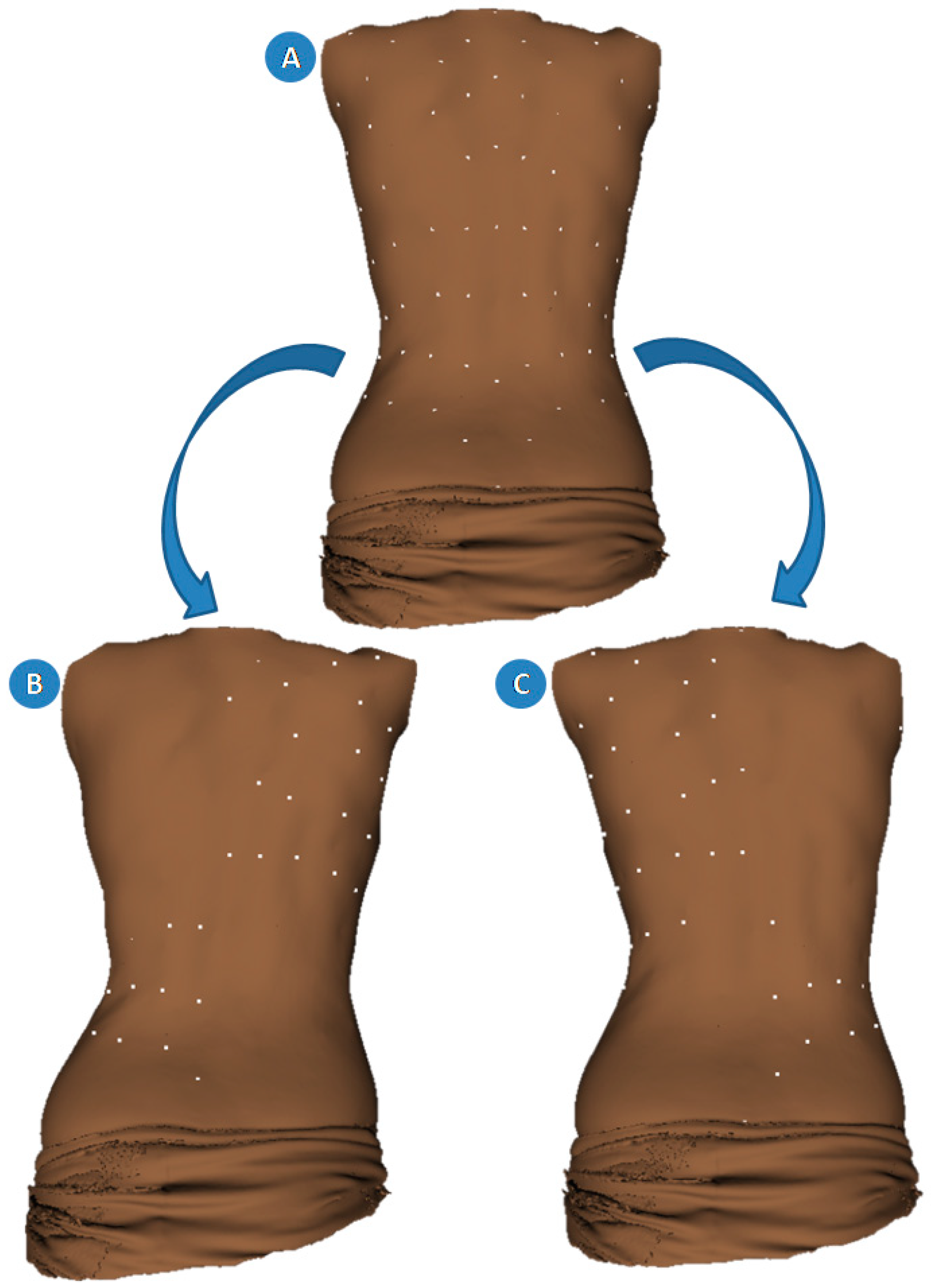
2.2. Geometric Morphometrics
3. Results
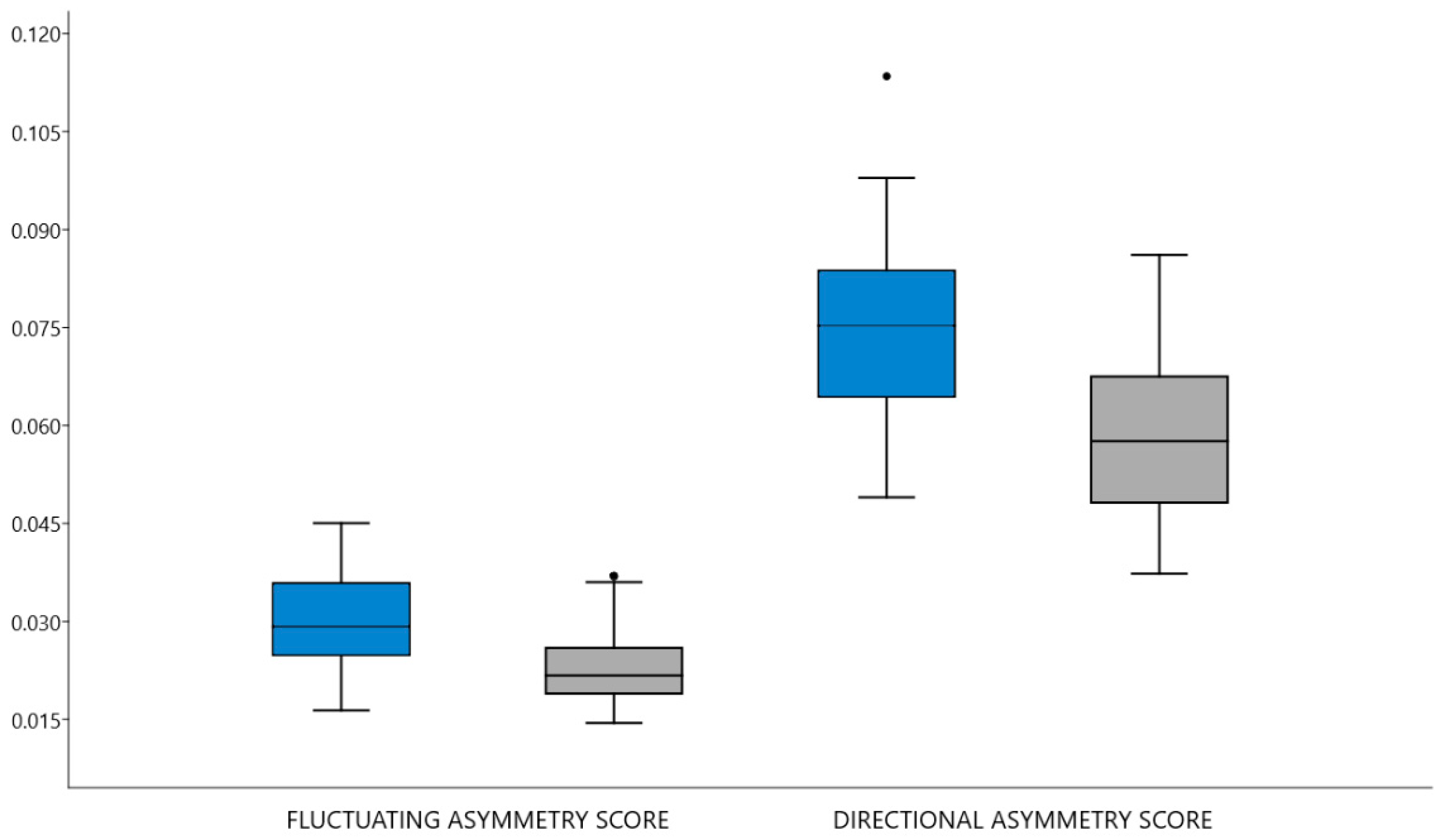
3.1. Hypothesis 1
3.2. Hypothesis 2
3.3. Objective 1
3.4. Objective 2
4. Discussion
- -
- Our data is limited to the surface of the human torso, and we did not have access to the three-dimensional shape of the spine. It is probable that the measure of the individual directional asymmetry in a dataset of three-dimensional coordinates digitized in each vertebra will show a higher correlation than the surface data did. It is accepted that the concordance between the spine shape and the surface asymmetry of the torso is not accurate enough [25].
- -
- Additionally, measuring directional asymmetry in a three-dimensional shape will introduce some noise derived from the transversal and sagittal planes, which do not interfere in the two-dimensional approach. To serve as an example, factors such as body fat tissue and breast development in females may confuse the output of three-dimensional directional asymmetry that we have measured in this research [11].
- -
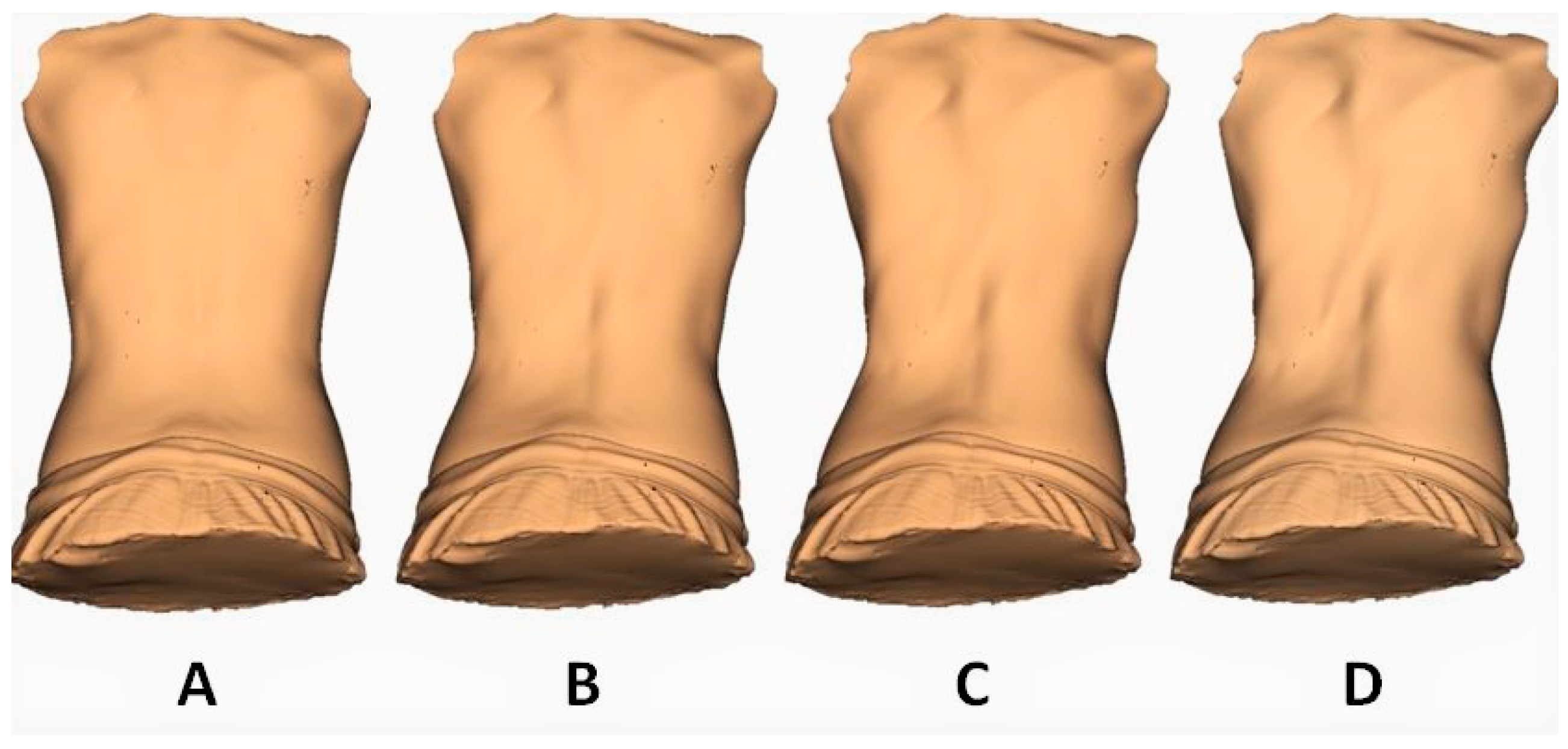
- We observed great concordance with our previous two-dimensional GMM findings [9]. The simulated increment of fluctuating asymmetry produced an opposite shape transformation, as can be appreciated in Figure 3 and Supplementary Video S1. In this case, the torso showed a very moderate inclination towards the left side in the thoracic area and towards the right side in the lumbar spine. What is relevant is that the increment of the rotation in both segments was the major trait observed.
- -
- On the other hand, as we have observed in our previous two-dimensional GMM research [9], the increment of the directional asymmetry effect deforms the torso following the most prevalent pattern described in AIS [18,29]. This increment of the right thoracic and the left lumbar convexities with a lesser effect of rotation in both segments can be observed in Figure 4 and Supplementary Video S2 (right rotation in the thoracic spine and left rotation in the lumbar spine).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekrami, O.; Claes, P.; White, J.D.; Zaidi, A.A.; Shriver, M.D.; Van Dongen, S. Measuring asymmetry from high-density 3D surface scans: An application to human faces. PLoS ONE 2018, 13, e0207895. [Google Scholar] [CrossRef] [PubMed]
- Chovalopoulou, M.-E.; Papageorgopoulou, C.; Bertsatos, A. Cranium asymmetry in a modern Greek population sample of known age and sex. Int. J. Legal. Med. 2017, 131, 803–812. [Google Scholar] [CrossRef]
- Tobolsky, V.A.; Kurki, H.K.; Stock, J.T. Patterns of directional asymmetry in the pelvis and pelvic canal. Am. J. Hum. Biol. Off. J. Hum. Biol. 2016, 28, 804–810. [Google Scholar] [CrossRef]
- Goldberg, C.J.; Dowling, F.E.; Fogarty, E.E.; Moore, D.P. Adolescent idiopathic scoliosis as developmental instability. Genetica 1995, 96, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.J.; Fogarty, E.E.; Moore, D.P.; Dowling, F.E. Scoliosis and developmental theory: Adolescent idiopathic scoliosis. Spine 1997, 22, 2228. [Google Scholar] [CrossRef] [PubMed]
- Benítez, H.A.; Lemic, D.; Villalobos-Leiva, A.; Bažok, R.; Órdenes-Claveria, R.; Pajač Živković, I.; Mikac, K.M. Breaking Symmetry: Fluctuating Asymmetry and Geometric Morphometrics as Tools for Evaluating Developmental Instability under Diverse Agroecosystems. Symmetry 2020, 12, 1789. [Google Scholar] [CrossRef]
- González-Ruiz, J.M.; Pérez-Núñez, M.I.; García-Alfaro, M.D.; Bastir, M. Geometric morphometrics of adolescent idiopathic scoliosis: A prospective observational study. Eur. Spine J. 2020, 30, 612–619. [Google Scholar] [CrossRef]
- Markow, T.A.; Wandler, K. Fluctuating dermatoglyphic asymmetry and the genetics of liability to schizophrenia. Psychiatry Res. 1986, 19, 323–328. [Google Scholar] [CrossRef]
- González-Ruiz, J.M.; Pérez-Núñez, M.I.; García-Alfaro, M.D.; Bastir, M. How can directional and fluctuating asymmetry help in the prognosis of scoliosis along the course of the condition? J. Anat. 2021. [Google Scholar] [CrossRef]
- Beyer, B.; Biteau, D.; Snoeck, O.; Dugailly, P.-M.; Bastir, M.; Feipel, V. Morphometric analysis of the costal facet of the thoracic vertebrae. Anat. Sci. Int. 2020, 95, 478–488. [Google Scholar] [CrossRef]
- Ghaneei, M.; Ekyalimpa, R.; Westover, L.; Parent, E.C.; Adeeb, S. Customized k-nearest neighbourhood analysis in the management of adolescent idiopathic scoliosis using 3D markerless asymmetry analysis. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Matusik, E.; Durmala, J.; Matusik, P. Association of Body Composition with Curve Severity in Children and Adolescents with Idiopathic Scoliosis (IS). Nutrients 2016, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Burwell, R.G.; Dangerfield, P.H.; Moulton, A.; Grivas, T.B. Adolescent idiopathic scoliosis (AIS), environment, exposome and epigenetics: A molecular perspective of postnatal normal spinal growth and the etiopathogenesis of AIS with consideration of a network approach and possible implications for medical therapy. Scoliosis 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Mínguez, M.F.; Buendía, M.; Cibrián, R.M.; Salvador, R.; Laguía, M.; Martín, A.; Gomar, F. Quantifier variables of the back surface deformity obtained with a noninvasive structured light method: Evaluation of their usefulness in idiopathic scoliosis diagnosis. Eur. Spine J. 2007, 16, 73–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Goldberg, C.J.; Dowling, F.E.; Fogarty, E.E. Left thoracic scoliosis configurations.Why so different? Spine 1994, 19, 1385–1389. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- Özener, B. Fluctuating and directional asymmetry in young human males: Effect of heavy working condition and socioeconomic status. Am. J. Phys. Anthropol. 2010, 143, 112–120. [Google Scholar] [CrossRef]
- Zaidi, Z.F. Body Asymmetries: Incidence, Etiology and Clinical Implications. Aust. J. Basic Appl. Sci. 2011, 5, 2157–2191. [Google Scholar]
- Macchi, C.; Biricolti, C.; Cappelli, L.; Galli, F.; Molino-Lova, R.; Cecchi, F.; Corigliano, A.; Miniati, B.; Conti, A.A.; Gulisano, M.; et al. Physiological asymmetry of trunk ranging and pelvis motility: An anatomo-functional study in 80 healthy subjects. Ital. J. Anat. Embryol. 2002, 107, 85–91. [Google Scholar]
- van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Trobisch, P.; Suess, O.; Schwab, F. Die idiopathische skoliose. Dtsch. Arztebl. 2010, 107, 875–884. [Google Scholar]
- Stokes, I.A.; Moreland, M.S. Concordance of back surface asymmetry and spine shape in idiopathic scoliosis. Spine 1989, 14, 73–78. [Google Scholar] [CrossRef]
- Levy, A.R.; Goldberg, M.S.; Mayo, N.E.; Hanley, J.A.; Poitras, B. Reducing the lifetime risk of cancer from spinal radiographs among people with adolescent idiopathic scoliosis. Spine 1996, 21, 1540–1547. [Google Scholar] [CrossRef]
- Knott, P.; Mardjetko, S.; Tager, D.; Hund, R.; Thompson, S. The influence of body mass index (BMI) on the reproducibility of surface topography measurements. Scoliosis 2012, 7 (Suppl. 1), O18. [Google Scholar] [CrossRef][Green Version]
- Grivas, T.B.; Burwell, R.G.; Dangerfield, P.H. Body mass index in relation to truncal asymmetry of healthy adolescents, a physiopathogenetic concept in common with idiopathic scoliosis: Summary of an electronic focus group debate of the IBSE. Scoliosis 2013, 8, 10. [Google Scholar] [CrossRef]
- Sangole, A.P.; Aubin, C.E.; Labelle, H.; Stokes, I.A.F.; Lenke, L.G.; Jackson, R.; Newton, P. Three-dimensional classification of thoracic scoliotic curves. Spine 2009, 34, 91–99. [Google Scholar] [CrossRef]
- Palmer, A.R. Waltzing with Asymmetry. Bioscience 1996, 46, 518–532. [Google Scholar] [CrossRef]
- Rothstock, S.; Weiss, H.R.; Krueger, D.; Paul, L. Clinical classification of scoliosis patients using machine learning and markerless 3D surface trunk data. Med. Biol. Eng. Comput. 2020, 58, 2953–2962. [Google Scholar] [CrossRef] [PubMed]

| AIS Group | Control Group | ||||
|---|---|---|---|---|---|
| Baseline Time (n = 24) | 6 Months (n = 19) | Baseline Time (n = 24) | 6 Months (n = 22) | ||
| Age (years) | 12.98 (2.11) | 13.26 (2.28) | 12.39 (2.46) | 13 (2.69) | |
| SEX 1 | Male (n = 2) Female (n = 22) | Male (n = 2) Female (n = 17) | Male (n = 13) Female (n = 11) | Male (n = 12) Female (n = 10) | |
| Height (cm) | p-value = 0.85 between groups | 158.12 (10.69) | 158.43 (11.11) | 156.49 (14.68) | 159.05 (14.31) |
| Weight (kg) | p-value = 0.46 between groups | 52.83 (10.89) | 49.32 (10.41) | 48.05 (14.62) | 49.53 (12.88) |
| Body fat (%) | p-value < 0.01 between groups 2 | 23.98 (6.19) | 21.82 (5.89) | 17.64 (5.07) | 17.46 (5.17) |
| BMI | p-value = 0.15 between groups | 20.41 (2.63) | 19.54 (2.31) | 19.22 (2.82) | 19.19 (2.37) |
| Cobb angle | 23.53 (9.02) | 23.36 (10.53) | Data not available due to ethical reasons | ||
| Overall Manova | p–Value | R^2 2 |
|---|---|---|
| Test on the dependent variable | ||
| COBB | 0.009 1 | 0.25 |
| Test on independent variables | ||
| DA | 0.926 | 0.16 |
| FA | 0.061 | 0.25 |
| BMI–Cobb Angle | BMI–FA Score | %Body Fat–Cobb Angle | %Body Fat–FA Score | |
|---|---|---|---|---|
| R 3 | 0.41024 | 0.30272 | 0.24722 | 0.25093 |
| R^2 2 | 0.16829 | 0.091639 | 0.061118 | 0.062968 |
| p-value | 0.0248 1 | 0.1032 | 0.1922 | 0.172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ruiz, J.M.; Pérez-Núñez, M.I.; García-Alfaro, M.D.; Bastir, M. Investigating Human Torso Asymmetries: An Observational Longitudinal Study of Fluctuating and Directional Asymmetry in the Scoliotic Torso. Symmetry 2021, 13, 1821. https://doi.org/10.3390/sym13101821
González-Ruiz JM, Pérez-Núñez MI, García-Alfaro MD, Bastir M. Investigating Human Torso Asymmetries: An Observational Longitudinal Study of Fluctuating and Directional Asymmetry in the Scoliotic Torso. Symmetry. 2021; 13(10):1821. https://doi.org/10.3390/sym13101821
Chicago/Turabian StyleGonzález-Ruiz, José María, María Isabel Pérez-Núñez, María Dolores García-Alfaro, and Markus Bastir. 2021. "Investigating Human Torso Asymmetries: An Observational Longitudinal Study of Fluctuating and Directional Asymmetry in the Scoliotic Torso" Symmetry 13, no. 10: 1821. https://doi.org/10.3390/sym13101821
APA StyleGonzález-Ruiz, J. M., Pérez-Núñez, M. I., García-Alfaro, M. D., & Bastir, M. (2021). Investigating Human Torso Asymmetries: An Observational Longitudinal Study of Fluctuating and Directional Asymmetry in the Scoliotic Torso. Symmetry, 13(10), 1821. https://doi.org/10.3390/sym13101821






