1. Introduction
It has been maintained for a long time that functional lateralization is a prerogative of human beings [
1,
2]. More recent studies, however, showed that asymmetries and functional lateralization are present in both vertebrates and invertebrates, related to different types of functions, such as foraging, prey recognition, visual discrimination [
1,
3,
4,
5,
6]. Certainly, the most important species for a possible comparison with human beings about functional lateralization are non-human primates. In monkeys, right hemisphere preference related to orofacial asymmetries for species-specific vocalizations have been reported. Furthermore, it has been found a left hemisphere activation for the discrimination of species-specific vocal calls [
1]. Nonetheless, it is well known that in these species, unilateral hemispheric lesions/inactivations do not usually knock-out high-order functions, while inactivations of primary sensory and motor cortices produce a contralateral deficit.
At the behavioral level, humans show a clear hand dominance [
7]. Indeed, while both hands can perform similar motor acts or gestures, in the great majority of human beings there is a preference for performing a certain number of skilled acts with one hand, such as in tool use. In other mammals, generally speaking, there is no indication of such a strong dominance [
8]. If one considers the primate lineage, in monkeys, although it is possible to see a hand preference when, for example, the animal reaches and grasps an object presented centrally in front of it, there is no demonstration of specific skills mastered by one hand with respect to the other. In apes, it is possible to observe a hand dominance, but it seems that the preferred hand depends, in the same individual, on the type of task [
5,
9].
These behavioral observations go in parallel with neuroanatomical data. In fact, in monkeys there is no evidence of anatomical differences between the two hemispheres. In apes, however, structural neuroimaging demonstrated some asymmetries, with the left frontal and temporal cortex more developed, in the majority of animals tested, than the right one [
10,
11], resembling the clear asymmetry present in humans, where inferior frontal cortex and left planum temporale, in right-handed people, are larger than their right counterpart. Another clear asymmetry is present in apes in the left precentral cortex, at the level of hand representation of the primary motor cortex, that shows a knob-like shape, also typical of human hand motor field [
12].
Interestingly, a series of studies in apes investigated handedness in motor tasks with and without communicative meaning, such as throwing, tool-use or gestural communication, and correlated hand preference with neuroanatomical asymmetries. Altogether, the results show that right handedness in gestures correlates with left inferior frontal gyrus and planum temporale asymmetries, but not with precentral asymmetries. The latter, on the other hand, correlates with non-communicative right handedness [
12,
13,
14].
As already mentioned, in mammals, and in particular in monkeys, the lesion of one hemisphere usually elicits, when present, only a contralateral deficit. In humans there are well known syndromes due to the lesion of one hemisphere, such as apraxia, aphasia and neglect [
15,
16,
17,
18,
19]. Therefore, it seems that in evolution, the achievement of a clear neuroanatomical and functional lateralization appeared in the primate lineage, starting with some example of not complete dominance in apes, and becoming consolidated only in the human species. This does not mean that the cortical areas of non-human primates perform only a contralateral control. Indeed, many non-primary cortical areas in monkeys are endowed with bilateral connections, thus exerting a complex role in the perception of sensory stimuli or in the control of motor acts.
In this review, we will mainly focus on cortical areas involved in sensory-motor integration, namely posterior parietal and agranular frontal cortices. We will first consider the monkey model, describing the functional properties of neurons of parieto-frontal circuits in terms of unilateral or bilateral control. We will concentrate, in particular, on the properties of the mirror neuron system (MNS). Then, we will consider the evidence on the mirror neuron mechanism in humans, and we will try to describe studies in which MNS activation is related to unilateral or bilateral control. Finally, we will discuss how the MNS is subjected to plastic changes following specific unilateral brain damage and how this can impact on the rehabilitation outcome.
2. Parieto-Premotor Circuits Involved in Sensory-Motor Transformations and in the Pragmatic Description of Space and Objects
Primary sensory and motor cortices have very specific roles, often involving fine discrimination or discrete movement control, respectively. For example, primary somatosensory areas play a strong role in precise tactile stimulus localization and discriminative acuity, with particular reference to distal body parts. It is obvious that this capacity must be limited to contralateral body parts, otherwise the individual would be confused about the site of stimulation. Similarly, the primary motor cortex (MI) controls discrete contralateral movements, because only in this way individuals are able to perform skilled motor acts. In these areas, neurons related to both body sides would be inappropriate, because the probability of a conflict between symmetric body parts would be very high.
The scenario changes completely when high-level sensory or motor functions are considered. In this case, it is necessary that neurons involved in these functions possess more complex properties, bilateral representation included. The secondary somatosensory cortex (SII) is a good example of this concept. In this area, directly connected to primary somatosensory cortex (SI), there are quite different neuronal properties. First of all, neuronal receptive fields (RFs) are much larger than those of SI neurons, being not appropriate anymore to signal the precise localization of the applied stimulus. Secondly, most neurons have bilateral RFs, very likely due to the strong reciprocal connections between the left and right SII [
20]. Very similar considerations can be made for high order visual areas. In these latter areas there is a hierarchical increase of the size of RFs and of the complexity of the elaboration. At the same time in these areas, differently from V1, callosal connections convey information on wide portions of the opposite visual hemifield [
21].
Concerning the motor cortex, high order motor areas are more complex than MI and their involvement in movement control is not related to very limited body parts. Furthermore, the callosal connections of these areas allow them to interact with the corresponding areas of the contralateral hemisphere.
In the last 30 years the picture on the organization and functioning of the cortical motor system, in its wider meaning, became richer and more complete than before, when the knowledge on motor control was mostly focused on primary motor cortex.
The new neuroanatomical and neurophysiological concepts, derived from monkey studies [
22,
23], can be so summarized:
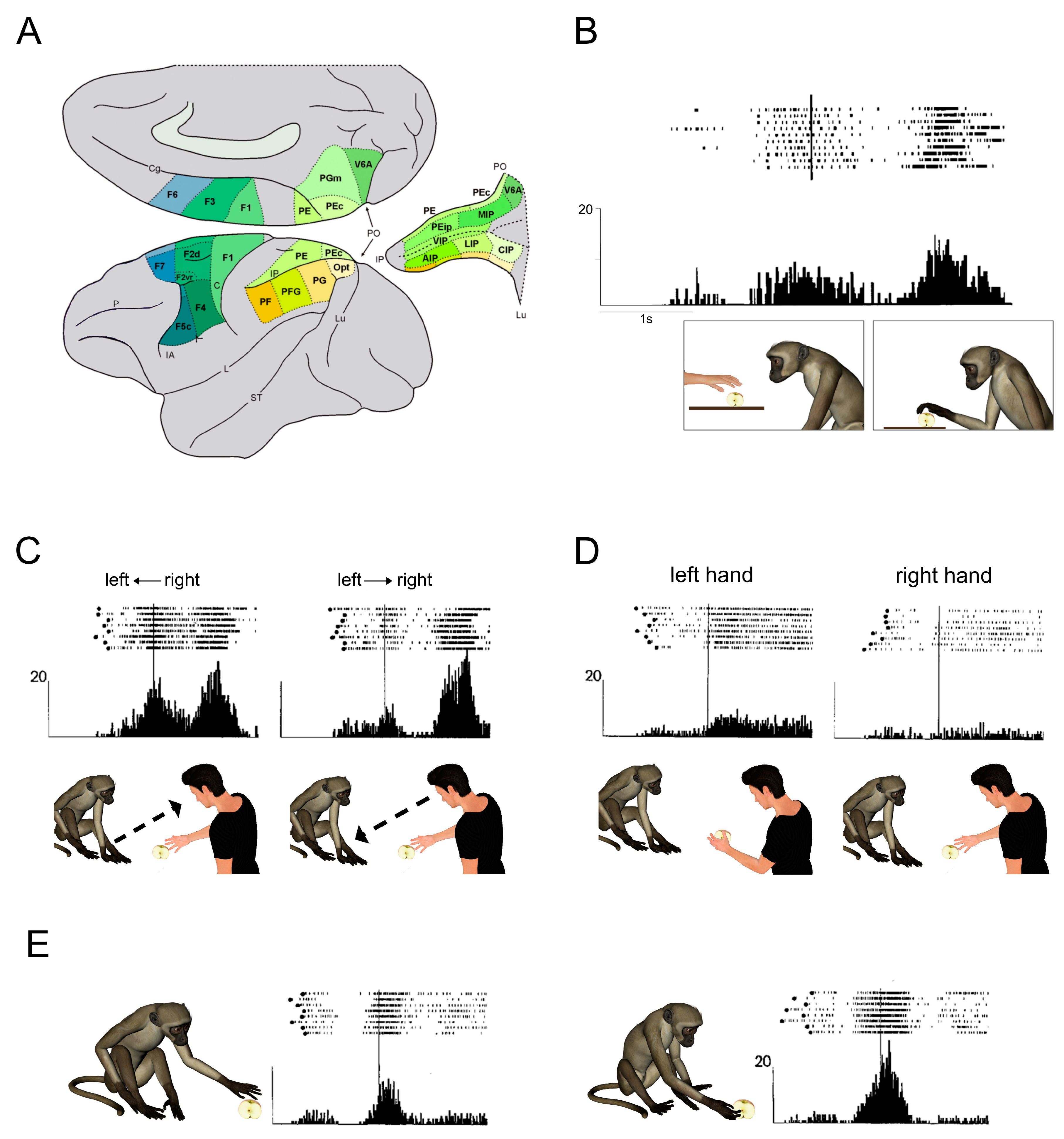
The agranular motor cortex (so defined on the basis of the lack of the fourth layer) is not just limited to two areas (Brodmann’s areas 4 and 6) but is composed by at least seven areas, one corresponding to area 4/MI, the other six forming area 6/premotor cortex) (See
Figure 1A). Each of these areas contains a partial somatotopic organization, except MI and the Supplementary Motor Area, that contain a whole map of the body;
Neurons of the motor cortex encode primarily the goal of motor acts. Within this functional framework, MI has the role of transforming the more complex acts coded by premotor areas in discrete movements;
Superior and inferior parietal cortex, corresponding to cytoarchitectonic Brodmann’s areas 5 and 7, is also constituted by a mosaic of areas, several of them are also contained inside the intraparietal sulcus (
Figure 1A).
Reciprocal connections link single areas of posterior parietal cortex with single areas of motor cortex, thus forming dedicated circuits;
The areas of posterior parietal cortex are not simply “associative areas” related to polymodal processing, as previously maintained, but are directly involved in the coding of motor acts, sharing this property with premotor neurons, and, together, contain a gross somatotopic subdivision;
The parieto-premotor circuits, based on the properties mentioned above, constitute the anatomical scaffolds for the organization of actions performed with different effectors, such as reaching, grasping and oculomotion;
From these concepts it can be concluded that frontal agranular and posterior parietal cortex form together the cortical motor system. Of course, the frontal node is closer to the motor output, but action organization is the result of the integrated work of the two nodes. Furthermore, thanks to the strong callosal connections of both posterior parietal and premotor areas, this organization is not simply limited to the control of the contralateral hemibody/hemispace, as it will be described below.
3. The F5-AIP Circuit and Its Role in Pragmatic Coding
Monkey area F5, located in the most rostral part of the ventral premotor cortex, is one of the most studied areas of the motor cortex. It is endowed with a limited corticospinal projection. Indeed, electrical microstimulation shows that this area is not highly excitable but it is possible to elicit from it hand and mouth movements. Single neuron recordings confirm that this area contains a hand and mouth somatotopy [
31]. The most important property is that its neurons code the goal of motor acts, such as grasping, manipulating, breaking, holding [
28]. This type of coding can be very abstract—in fact, neurons of the premotor cortex activate when grasping is performed with different body parts, such as the left hand, the right hand, the mouth or even a tool [
28,
32]. Other, more numerous, neurons respond only during grasping with the hand. In both categories, many neurons discharge during execution of specific types of grip, such as precision grip, finger prehension or whole hand prehension. Interestingly, both categories of neurons respond during execution of grasping with both the left or the right hand, and often the neuron response is not different when either hand is used. Only few neurons showed exclusively contralateral activation. Thus, the high functional complexity of these neurons goes together with bilateral control. Less than half of the F5 neurons can be activated also by somatosensory, both tactile and proprioceptive, stimuli. Interestingly, neurons discharging during grasping with the left and right hand, also had passive responses on both hands, suggesting a strong integration between sensory information and related motor reactions. Altogether, these data suggest that area F5 has a high order role in motor control, not only with respect to its anatomofunctional position in the motor hierarchy, but also for its possibility to control both hands. This is very similar to high level pragmatic control present in humans, with the relevant difference that in the monkey there is no apparent specialization of one hemisphere with respect to the other.
Area F5, and specifically its portion buried inside the posterior arcuate bank (F5p) entertains a specific, reciprocal connection with the anterior intraparietal area (AIP), located in the anterior part of the lateral bank of inferior parietal lobule [
22]. This area, representing the posterior node of the grasping circuit, shares several properties with area F5. Differently from F5, it contains neurons (called visual dominant) responding only to the visual presentation of 3D objects of specific shape and size [
33]. This property is considered the basis for the visuomotor transformation allowing the individual to adapt the hand configuration to the object features. In most of the investigations of AIP neuron activity, the task consisted in grasping different types of object with one hand. In a recent paper [
34], Michaels and Scherberger trained monkeys to perform a task consisting in grasping of a handle, in which different cues instructed the animal to use two different types of grips with two different orientation, while they recorded F5 and AIP from both areas. Notably, the monkeys executed the task with their right and left hand. The data show that in both areas there were many neurons responding equally for both hands, but about 20% showed also a hand preference. Interestingly, at the population level, while in both areas there was a higher percentage of neurons responding better during grasping execution with the contralateral hand, during the instruction and memory period only in F5 there was a preference for the contralateral hand, while in AIP the percentage of neurons controlling the two hands were equally represented.
Interesting additional information about hand laterality is given by inactivation experiments in AIP and F5. The inactivation of AIP with muscimol (a GABA-agonist) produces a contralateral impairment of hand shaping during grasping [
35]. However, the ipsilateral hand has not been tested. The inactivation of area F5 produces a marked impairment in the hand contralateral to the inactivation site, when using small muscimol concentration, while the impairment becomes bilateral when the dose is increased [
36]. The deficits observed in both AIP and F5 are not related to movement execution, but to visuomotor transformation. In fact, when the monkey touched the object, it was able to grasp it on the basis of proprioceptive and tactile information, showing that motor execution is still possible, but based on somatomotor transformation. The inactivation findings confirm the relevance of bilateral, high order, hand control performed by F5 neurons. On the other hand, as in the study of Fogassi and coworkers, as only the left hemisphere was inactive, there was no chance to assess a possible difference between the two hemispheres.
In addition to the visuomotor deficit, F5 inactivation also produces an alteration in the perception of the contralateral tactile and peripersonal space. This deficit consists in the reduced or absent motor reaction to tactile stimuli applied to the contralateral mouth and to visual stimuli introduced near the contralateral mouth, arm or body. Furthermore, at the bilateral presentation of food, the monkey prefers to take the ipsilateral one. When presented with a kind of pegboard, the monkey began to first grasp the raisins located in the ipsilateral half, independently of the hand that was used. Thus, while in most tasks processing was limited to the contralateral side both in sensory and motor terms, in the latter case the contralateral spatial deficit influences both hands.
4. The Contribution of F5-PFG Circuit to Pragmatic Coding
Differently from area F5p, the exposed part of area F5 (F5c) is strongly connected with the PFG area [
37], located in the convexity of the inferior parietal lobule, just lateral to AIP. In a general electrophysiological mapping study of the inferior parietal lobule convexity [
38] it has been shown that area PFG contains neurons that, like those of F5 and AIP, are involved in grasping motor acts, and neurons activated by tactile stimuli applied to the forelimb to visual stimuli introduced in the peripersonal space near the forelimb and to object presentation. While there is no clear report on the laterality of hand control in grasping neurons, fifty percent of tactile RFS, usually large, are bilateral. The percentage of bilateral RFs of visual peripersonal neurons is even higher, and the response to objects are usually space independent. These findings, in terms of laterality, are similar to older studies involving a higher number of monkeys, in which, however, the neuronal properties were studied in area 7b, that encompasses both area PFG and PF [
39].
Interestingly, lesions of area 7b cause impairment in grasping and a kind of neglect or extinction of the contralateral space [
40,
41]. Although these findings are in favor of a clear contralateral control, some pilot studies from our lab suggest that this could depend on the complexity of the task. A series of experiments [
42,
43] demonstrated that motor neurons of area PFG have a strong role in coding action intention. Muscimol inactivation of this area during monkey performance of two types of intentional grasping actions with both hands shows not only a deficit of the hand contralateral to the inactivation, but also a misuse of the ipsilateral hand [
44]. This impairment has been interpreted in terms of a coordinated activity of right and left inferior parietal lobules underlying context-based selection of the neuronal repertoire necessary for the organization of intentional actions. Although, in the presence of a unilateral inactivation, the intact contralateral PFG would prevail, thus leading to perform the action with the arm ipsilateral to the injected hemisphere. Nonetheless, action performance would be influenced by the inactivation.
5. The F4-VIP Circuit and Space Coding
Area F4 is located in the caudalmost part of ventral premotor cortex, posterior to area F5 and anterior to area F1 (primary motor cortex). It is electrically excitable and its stimulation elicits arm, neck and facial movements, very often in combination [
31]. This latter property suggests that this area, differently from F1, controls complex movement synergies, such as, for example, the coordination of neck, shoulder and lip movements. This finding is reinforced by the neuronal response obtained during active movements [
31,
45]. Neurons in F4 activate during goal-directed motor acts, as in F5. The represented effective motor acts are related to reaching, bringing to the mouth, neck and trunk orientation and to facial and mouth acts. The coded motor acts are bilateral in most cases. Furthermore, about 30% of motor neurons discharge when two or more of these movements are associated, such as, bringing to the mouth and mouth opening, or reaching plus neck and trunk orienting [
31]. The peculiarity of area F4 neurons is, however, that of responding to pure tactile or to tactile and visual, stimuli [
31,
45,
46,
47]. Tactile RFs, generally large, are on the same body parts involved in active movements. Although RFs on various parts of the face are predominant, there are also RFs on the arm and trunk. About 10 percent of tactile RFs combine two or more body parts. Other neurons respond to proprioceptive stimulation, predominantly from the arm or trunk. Although most neurons have contralateral RFs, about 30 percent of them were bilateral. The tactile RFs constitute the scaffold on which bimodal, visual and tactile, neurons are built. In fact, neurons of this category have the same RFs locations of purely tactile neurons, and, in addition, respond to the introduction, and even better to the approach of 3D objects to the neuron tactile RFs. The visual response begins when the stimulus is within reaching distance; because of this, the RFs of these neurons have been named peripersonal. The most interesting property is that these visual RFs do not shift when the monkey shifts its eye position. This brought us to conclude that these neurons code space in a somatocentered frame of reference [
45]. This means not only that there are visual RFs for everybody part represented in F4, but also that when the tactile RF is unilateral also the visual RF is unilateral, and when the tactile RF is bilateral, the same is true for the corresponding visual RF. Interestingly, when considering F4 motor neurons, when they have also somatosensory and/or visual properties, the coded motor act is spatially corresponding to the sensory RF. So, for example, a neuron responding during reaching in the upper space has tactile RF on the upper face and visual RF in the upper space; a neuron that is active while bringing to the mouth has a tactile RF on the lips and a visual RF around the lips [
28]. All these notions converge, leading to the proposal that bimodal neurons code peripersonal space in motor terms, in other words their responses would represent potential motor acts in different space sectors. Although it is reasonable that most of these neurons privilege contralateral space, there is a considerable (at least 30 percent) number of neurons coding bilateral space. Thus, on the one hand this area facilitates the choice of motor acts in the contralateral space, so that the subsequent command for motor execution by F1 (to which F4 is strongly connected) is sent only to the contralateral half of the body. On the other hand, F4 represents motorically a bilateral space in which both arms can theoretically act individually or synergically, or the mouth/face can perform symmetrical motor acts.
Area F4 is strongly connected with the ventral intraparietal area (VIP) [
22,
48]. VIP is not electrically excitable, however, using different stimulation parameters, it has been shown that its microstimulation elicits face, arm and neck movements [
49], the same as those elicited by F4 stimulation with classical parameters, further confirming their strong anatomofunctional link. Since VIP receives a strong visual input from the middle temporal area (MT) many of its neurons are visual, responding very well to stimulus motion. However, in VIP there are also neurons with both tactile and visual responses [
50], elicited, differently from F4, by 2D bars presented on a tangent screen. As in F4, in each neuron the spatial location of visual RFs, mostly related to the face/head but, in a lower percent, to the arm, corresponds to the location of the tactile RF. The majority of these neurons are driven by contralateral stimuli, but there is also a consistent percentage of bilateral or ipsilateral RFs. A small subset of bimodal neurons also responds to stimuli approached to the tactile RF. In these latter neurons the visual RFs are anchored to the tactile ones and independent of eye position [
51].
6. Parieto-Premotor Circuit Involved in Recognition and Understanding of Others’ Actions
Area F5c contains neurons coding goal directed motor acts performed with the hand and/or with the mouth [
28,
52]. Furthermore, in this area, much more than in area F5p, there are visuomotor neurons that become active not only during execution of motor acts, but also during monkey observation of the same motor act done by another, either a human experimenter or another monkey [
30,
53]. Due to these properties, they have been called “mirror neurons” [
29,
30] (
Figure 1B). The most effective observed motor act is grasping, followed in percentage by other motor acts such as manipulating, placing and hands interaction. More than half of the mirror neurons respond to only one observed motor act, while more than one-third respond equally well to two observed motor acts (e.g., grasping/placing, grasping/holding, etc.). Importantly, these neurons do not respond to object presentation or pantomimes of the observed motor act without the object. Thus, these neurons respond to hand-object interaction, that is to the goal of the observed motor act. The motor response more effective in triggering mirror neurons was grasping mainly with the hand, but also with the mouth. On the motor side, the bilateral control of these neurons has not been studied systematically, however it is reasonable to hypothesize that most of them should be bilateral, since their motor properties are undistinguishable from those of purely motor F5 neurons.
On the basis of an analysis on the match between their visual and motor discharge, these neurons have been classified in “strictly congruent” neurons (about 30%) in which both the goal of the motor act, and the way in which it is executed, correspond and are “broadly congruent” (about 60%), in which the goal corresponded but the motor response could be more specific than the visual one. Due to this congruence, mirror neurons have been considered to play a major role in understanding others’ actions.
The neural mechanism enabling an individual to understand others’ actions is complex, because on the one hand it implies the comprehension of an abstract meaning, the goal, starting from a visual input concerning biological motion, on the other hand includes also the decoding of the details of the observed motor acts. The goal of a motor act is recognized independently of several variables, such as species, size, distance, perspective, direction, handedness, and on the other hand, the recognition of these variables is very important, because it can be crucial for social interaction. Furthermore, there is also the issue of the symmetry and of unilateral/bilateral properties. To date, there is no evidence of a difference between left and right hemisphere in the percentage of mirror neurons. Since F5 purely motor neurons do not show significant differences between the two hemispheres, it is unlikely that mirror neurons do not follow this rule. Furthermore, when neurons of area F5 of both hemispheres were recorded in the same monkey, no significant difference in the presence of mirror neurons between the two hemispheres was noted. However, some details of observed motor act could be coded in a lateralized way as described below.
6.1. Direction of the Motor Act
When a motor act (e.g., grasping of an object) was performed by the experimenter from left to right or from right to left (monkey side), 65% of the recorded mirror neurons showed a directional preference, most preferring the direction toward the recorded side. In some cases, in addition to the directional preference, there was also a preference for the side of the space in which the motor act was executed (for example, directional preference from right to left, with higher activity when the act was performed in the left space than in the right space) (
Figure 1C). Therefore, the two preferences could be combined.
6.2. Hand Preference
When the experimenter executed the motor act in the central space, in front of the monkey, using either the left or the right hand, 35 percent of the neurons showed a differential discharge, most preferring the hand ipsilateral to the recorded hemisphere, a lower number preferring the contralateral hand (
Figure 1D).
Figure 1E shows the neuron’s discharge when the monkey grasps an object with the right than with the left hand. Note that when two individuals stay in front of each other, the right hand of the acting individual corresponds to the left hand of the observing one. One could speculate that the two types of preferences correspond to what psychologists call “mirror imitation” and “anatomical imitation”, respectively. Note that in the cases in which it was demonstrated a neuron hand preference and the motor act was performed centrally and in both left and right visual hemifields, the absence of a spatial preference confirmed that the differential response was only due to hand preference.
While all the above-described properties mainly concern hand mirror neurons, in another study the properties of mouth mirror neurons have been studied, in a more lateral part of area F5c where mouth-related motor neurons predominate [
52]. Mouth mirror neurons have the same visual properties of hand mirror neurons, i.e., they respond to the observation of mouth motor acts, such as grasping, breaking or sucking similar to the acts motorically coded by the same neurons. Interestingly, although the act was directed frontally, or toward the left or the right side, the discharge did not change. It is noteworthy that, although it is possible to make lateralized movement of the mouth, mouth motor control is much more bilateral (as anatomical output) than the control of the hand.
As already described, area F5c has strong reciprocal connections with area PFG of the inferior parietal lobule. This area, whose motor neurons are related to hand motor acts, also contains mirror neurons [
38,
42]. Their properties are very similar to those found in F5, with neurons responding to grasping acts, alone or in combination, representing the majority of mirror responses. It is noteworthy that 15% of neurons that respond visually to only one motor act are selected for “two-hands interaction”, meaning that they respond when the monkey observes two hands (belonging to the same or to two different experimenters) interacting together with food, not when the experimenter uses only one hand. Although the discharge of most PFG mirror neurons is independent from several variables such as distance, hand preference, type of object, etc., some of them, as in area F5c, show a different response with respect to the direction or the side of the space in which the motor act is performed.
7. The Human Mirror Neuron System
The presence of a mirror matching mechanism similar to that discovered in monkeys was reported in a pioneering transcranial magnetic stimulation (TMS) study [
54], showing how the motor cortex is activated during action observation. The experiment consisted of delivering a TMS pulse while the subjects observed the experimenter grasping a certain object. Motor-evoked potentials (MEPs) were recorded from the muscles of the subject’s contralateral hand. The results of this first study showed that during action observation there was a significant enhancement of the hand MEPs, even if the subject’s state is passive. The same muscle pattern activation was observed when subjects performed the observed action. Interestingly, the same hand muscles were also activated during the observation of intransitive gestures, suggesting that in humans, unlike in monkeys, the motor system also activates during the observation of non-goal-directed actions, i.e., simple movements without objects.
For ethical reasons, in humans it is not possible to record the activity of single neurons, except in those patients undergoing surgery for treatment of epilepsy. In these, usually, recordings are made at the level of the mesial part of both hemispheres, so that it is difficult to obtain information about the neural activity of areas homologous of those containing mirror neurons in the monkey, such as the inferior parietal lobule and the ventral premotor cortex. The only study reporting single neurons recordings in human epileptic patients during action observation and execution was performed by Mukamel et al. [
55], in which the authors reported single neurons of supplementary motor cortex and hippocampus discharging during action observation and execution. These results, although not focused on cortical mirror areas homologous to those described in monkeys, confirm directly that a mirror matching mechanism exists also in humans.
Most of the available knowledge about the organization and functions of the mirror neuron system in humans derives from brain imaging experiments (e.g., Positron Emission Tomography [PET], functional MRI [fMRI]), which allow one to localize cortical areas involved in action observation and to study the overlap of these areas with the sensorimotor areas recruited during the performance of the same actions.
Among these experiments, the first studies showing brain activation during action observation in humans employed Positron Emission Tomography (PET). These studies reported an activation of the superior temporal cortex, the inferior parietal lobule (IPL), the ventral premotor cortex (PMv) and the inferior frontal gyrus (IFG) [
56,
57]. A long series of neuroimaging experiments confirmed these first data, as recently reported in some meta-analyses [
58,
59,
60]. See
Figure 2A for a visualization of the areas typically involved during both action observation and execution.
Generally, studies on the observation and execution of actions in humans have mainly investigated objects’ grasping. These studies provided evidence for the representation of the action goal in the premotor and parietal areas. In particular, a series of fMRI studies explored the role of the parietal and premotor areas during the observation of actions performed by different agents such as human or robotic effectors. Gazzola et al. [
62] investigated the brain activations while a group of healthy volunteers observed object grasping performed by an industrial robotic arm or a human actor. The results showed that, regardless of the shape and kinematics of the observed action, the mirror system was recruited in both experimental conditions, suggesting that this system codes of the goal of the observed motor act, beyond the physical features of the agent. Other evidence about the recruitment of the mirror system for the processing of the goal of motor acts in humans derives from the study of aplasic patients observing actions performed with the hands or feet. In these patients, Gazzola et al. [
63] have found that the mirror system activates also when patients observe actions that they can never perform (e.g., hand actions), but the purpose of which they can achieve using a different effector (e.g., mouth or foot).
Another important aspect is that premotor and parietal nodes of the mirror system code the observed motor acts in a somatotopic way. In a pioneering study, Buccino et al. [
64] demonstrated that when subjects observe motor acts performed with the mouth, the hand and the foot there is an activation of different sectors of both premotor and parietal cortex, organized in a somatotopic fashion, with some overlap between adjacent fields.
More recent fMRI studies used, in addition to standard univariate analysis, advanced techniques such as analysis on un-smoothed data and multivariate pattern analyses, allow the contribution of multiple cortical and subcortical areas to be highlighted for both the observation and execution of actions. Gazzola and Keysers [
65] used single-subject un-smoothed fMRI data to investigate the contribution of other areas involved during the observation and execution of grasping actions performed with the hand, mouth and foot, showing that voxels shared between both conditions were localized, in addition to the classical mirror areas, also in the dorsal premotor cortex (PMd), middle cingulate cortex (MCC), superior parietal lobule (SPL) and middle temporal cortex and cerebellum. According with the author interpretation, the activations related to the areas outside the classic circuit are related to the sensory prediction of the observed action in terms of postural adjustment and correction to improve motor performance.
Other data about the involvement of other cortical areas within the extended mirror system are related to the observation of proximal arm movements, isolated from the distal component of hand grasping. Only a few studies specifically addressed this issue (i.e., [
66]), reporting that observation and execution of pure reaching movement activate a dorso-medial circuit including the convexity of the SPL and the PMd. Furthermore, a study specifically investigated functional activations during the observation of proximal and axial movements, reporting that axial movements activate more the supplementary motor cortex, while proximal movements activate the dorsal sector of the premotor cortex [
67].
Very recent studies have further improved the knowledge about the extended mirror circuit by describing the contribution of some subcortical areas involved both during the observation and the execution of specific grasping and manipulative actions. The cerebellum, for example, has reciprocal connections with parietal and premotor areas, that are part of the classical mirror system. In addition, it has been shown that specific sectors of the putamen, a nucleus of basal ganglia, also receive projections from the main nodes of the cortical mirror system (i.e., AIP, PFG and PMv) [
68]. Altogether, these data indicate the possible contribution of subcortical structures within the mirror system. In humans, very recent fMRI studies [
69,
70] demonstrated that the cerebellum and basal ganglia are recruited during the performance of grip force tasks and during the observation of grasping and more complex object manipulation actions. Moreover, Ge et al. [
71] demonstrated the engagement of putamen during the observation of grasping recorded form different perspectives, suggesting that the subcortical nuclei combine the action observed with the internal simulation of similar actions.
8. Symmetry and Asymmetry during the Observation of Unimanual Actions
In humans the symmetry of shared brain activations during the observation and execution of actions made by others is still a controversial issue. Several studies, although not focused on the laterality problem, reported a left lateralized activation of the parieto-premotor circuit in normal volunteer adults during the observation-execution of unimanual actions. In the majority of cases this may be due to the employed paradigm, in fact in most studies right-handed subjects were required to observe a right hand performing object grasping or intransitive acts. However, it is necessary to consider several factors that may play an important role in the processing of the observed actions and that can modulate motor resonance in the observer brain. Some of these factors consist in the stimuli and/or the paradigm used, for example: the type of motor act; the type of effector; the identity of the effector (left/right); the perspective from which the action is observed.
Concerning the observed motor acts, a classical distinction is found between the two parallel modules of reaching and grasping described in the monkey [
72,
73,
74], with reaching movements processed within the dorso-dorsal circuit and grasping movements within the dorso-ventral circuit [
33,
38]. A similar distinction has been shown in humans, where reaching movements typically activate a dorsal-most circuit (SPL and PMd), whereas grasping movements mainly activate a ventral-most circuit (anterior IPS and PMv). The majority of studies on the MNS in humans was focused on hand grasping or simple hand movements [
59]. These studies have repeatedly shown that all areas involved during action observation showed bilateral activations when subjects were required to passively observe a grasping act [
62,
64,
65]. In some cases, it has been reported a greater activation (e.g., peak intensity) of the left hemisphere, although some clusters were present in both hemispheres. Concerning studies on the observation of reaching acts, the available studies reported a very different effect of laterality as compared to grasping acts. For example, Filimon and colleagues [
66] showed that observation of reaching performed with the right hand produced stronger activations in the left hemisphere, the dorsal premotor cortex showing the strongest lateralization. Visual areas and the posterior sector of the superior temporal sulcus, with respect to premotor cortex, showed a more bilateral pattern of activation. One can argue that the results about the premotor activation reported by Filimon and colleagues may be related to eye-movements, rather than MNS activation for reaching. However, the sector usually activated in humans during eye movements is more lateral and posterior with respect to premotor activation. Furthermore, the asymmetry of activation during reaching speaks against this argument. In fact, if subjects had moved their eyes one could expect more bilateral and symmetric activation of dorsal premotor cortex.
The other possible effect that may have a role in lateralization is related to the observed effector. Concerning this issue, the majority of studies investigated the MNS activation during observation of hand-actions, while few studies reported shared activations during observation and execution of mouth end/or foot actions. Buccino et al. [
64] showed a trend toward a somatotopic organization both in premotor and parietal MNS areas during observation of motor acts performed with different body parts. During observation of mouth actions, authors found activations of PMv and IFG in both hemispheres, and of area BA45 in the right hemisphere. Activations in the parietal lobe were bilateral. Thus, overall, there is no evidence supporting a clear lateralization due to the observed effector, but a more symmetric distribution of activation in both the parietal and premotor cortex.
A recent study by Biagi et al. [
75] explored the neural response of normal subjects to the observation of actions done with the left and the right hand. The results showed a higher activation of left/right anterior IPS and inferior temporal gyrus for the observation of the right/left hand, respectively. This is particularly relevant because it suggests that some nodes of the MNS, can reflect not only the goal of observed motor acts, but also some of their details, such as the identity of the effector used. These data have also been corroborated by another study by Cabinio et al. [
76] specifically addressing the issue of the symmetry of brain activation in right-handed and left-handed individuals during observation and execution of actions performed with their dominant or non-dominant hand. Interestingly, the authors found that in right-handed participants during the observation/execution of actions with dominant hand there was a strong lateralization of the activation to the left hemisphere, including both parietal and premotor MNS areas. On the contrary, when actions were performed by the non-dominant hand, a more bilateral pattern was revealed, with the additional activation of the left IFG. Differently from right-handed subjects, left-handed individuals showed more bilateral activations, during observation of actions performed with either hand. Altogether, the studies of Biagi et al. [
75] and Cabinio et al. [
76] suggest a possible differential contribution of the two hemispheres during the processing of observed and executed actions based on the identity of the specific effector involved in the action.
9. Observation of Complex Actions and Sequences
Experience of a certain motor skill is important in modulating the MNS response to observation of actions requiring that skill, both in terms of intensity of activation and of lateralization of the recruited areas. Evidence in this field comes from studies of specific populations of experts (e.g., dancers, elite sportsmen) who are skilled in specific actions. For example, Calvo-Merino and coworkers [
77] asked classical dancers, experts of capoeira and people naive to dancing to observe videos of either capoeira or classical dance steps. The results showed that, although both groups of experts had higher activation of the MNS than naïve subjects, by comparing the two groups of experts, observation of capoeira produced stronger activation in capoeira dancers, while observation of classical dance elicited higher activation in ballet dancers. Concerning the symmetry of this differential response, the activation of some clusters was strongly lateralized to the left hemisphere, such as the premotor areas (PMv and PMd), while the parietal activation (in particular the IPS) was more symmetrically distributed.
The results of studies on experts have also been corroborated by further investigations in normal subjects without particular hand skills, observing simple and complex actions performed by other individuals. For example, in the aforementioned study of Biagi and colleagues [
75], the authors found that the activation of the anterior IPS in the left hemisphere during observation of the contralateral hand was higher during the observation of complex with respect to simple actions performed with both the right and the left hand, while the activation of the right IPS for this complex vs simple comparison is higher only during observation of the contralateral hand. This suggests that the parietal cortex of both hemispheres is involved in the encoding of the complexity of the observed action, but the left hemisphere seems to express a kind of dominance. This dominance is in line with the classical praxic role attributed to parietal cortex, as also corroborated by the higher percentage of apraxic patients with a left parietal lesion [
15,
16].
Considering that motor resonance for a given action is also modulated by the subject’s personal motor repertoire, one could hypothesize that the brain of a naïve subject observing a hand action performed by another naive individual or by an expert, tends to resonate more strongly during the observation of the naive one, due to the similarity of their motor repertoire. According with this hypothesis, Errante and Fogassi [
61] found that brain activation of healthy naïve subjects was stronger when they observed a non-expert model than that elicited by observation of actions performed by an actor expert in object manipulation. The activation included a dorsal parieto-premotor circuit (SPL and PMd) (
Figure 2B,C). Similar to the results of Calvo-Merino et al. [
77] and Biagi et al. [
75], the differential activation of PMd was strongly lateralized to left dominant hemisphere, while that of SPL and IPS was more equally distributed in both hemispheres. Thus, when the effect of motor repertoire is considered, it tends to be more lateralized to the left dominant hemisphere in right-handed people, although more evident in the premotor cortex.
10. Functional Lateralization and Reorganization of the Sensorimotor System after Brain Damage
Studies in animal species showed that the effect of a lesion on the reorganization of the sensorimotor system depends on the developmental period in which the lesion occurred, according with the Kennard principle “the younger the age at insult, the better the outcome” [
78]. However, it has been demonstrated that this principle is not always correct [
79]. Many factors can play an important role in the reorganization of sensorimotor functions in terms of functional lateralization and symmetry of the recruited areas that try to compensate for the lost function. Some of these factors, besides the age at which the lesion occurred are, for example, lesion localization and size, laterality, sex, role of the environment and species considered. For example, in rodents it appears that rich environment and tactile stimulation at an early age are associated with better recovery [
79,
80].
On the one hand, the complex view emerging from animal studies indicates that several environmental factors play a key role in the improvement of ability to compensate the effects of an early lesion. On the other hand, however, some general principles have been reported in this field: (a) bilateral lesions show lower functional plasticity and worse recovery with respect to unilateral ones; (b) lesions involving large volume of gray/white matter, although unilateral, tend to recover less and worse than small lesions; (c) when compared to motor functions, cognitive abilities show a better improvement in terms of recovery [
79].
An interesting perspective come from studies on the effect of early brain lesions (first, second or third trimester of pregnancy) on sensorimotor reorganization of pediatric patients with cerebral palsy (CP), consisting in a group of neurodevelopmental disorders of movement and posture, showing also, very often, epilepsy, secondary musculoskeletal problems, sensory and cognitive deficits [
81]. The most common form consists in unilateral cerebral palsy (UCP) or hemiplegia, which involves in particular one side of the body and also impairs bimanual motor control. The main clinical characteristic of hemiplegia is the reduction of the complexity of the motor repertoire of the affected hemi-side due. Brain lesions most frequently involve periventricular white matter (PWM) [
82], or cortical gray matter, basal ganglia and thalamus (21%) [
82,
83,
84].
Concerning UCP, there are two main types of lesions: (a) unilateral periventricular hemorrhagic infarction (“preterm” lesions) [
85,
86] and (b) focal cortical–subcortical infarction, covering the cortical sector supplied by the middle cerebral artery (“term” lesions). Evidence suggests that important phases of sensorimotor reorganization occur during the first year of life but, in the same period, also maladaptive forms of reorganization may occur [
87]. In typical development in humans, transient ipsilateral corticospinal projections are predominantly eliminated when maturity is reached, leaving only a small percentage [
88,
89,
90], as shown by focal TMS delivered on the motor cortex of neonates, which elicits both ipsilateral and contralateral MEPs with similar properties. This indicates a bilateral innervation of the spinal cord from motor areas of both hemispheres [
90]. Longitudinal studies on healthy infants show a consistent withdrawal of ipsilateral corticospinal projections, while the contralateral projections are enhanced [
89,
90]. Several studies in animal models and humans demonstrated that such process depends also on the activity of environment and of experience [
91,
92,
93,
94].
In congenital brain damage leading to UCP, different types of asymmetrical brain reorganization can be observed. In children with small perinatal lesions, the main mechanism for a new connection of the brain with spinal cord consists in a reorganization that occurs in the primary motor cortex (M1) or non-primary motor areas of ipsilesional cortex [
86,
95,
96]. This reorganization pattern is similar to that occurring in adult stroke patients [
97]. In other cases of UCP children, where lesions are larger, a different mechanism can also be observed [
85,
97,
98], in which fast conducting ipsilateral projections from the undamaged cortex persist (contralesional reorganization), allowing the undamaged cortex to directly control both upper limbs [
99,
100]. It has also been demonstrated that ipsilesional reorganization, as compared to contralesional reorganization, is significantly associated to better functional outcome, assessed with functional scales [
101].
Sgandurra and colleagues [
102] performed a combined fMRI and a TMS investigation on a group of 12 patients with UCP, to explore the reorganization of the sensorimotor system and the brain activations during the observation of simple and complex actions consisting in object manipulation. According to the type of lesion, the authors classify the patients into three groups: (a) malformative lesions (Type I); (b) white matter damage (Type II); and connatal stroke (Type III). In addition, based on a TMS experiment, children were classified on the basis of contralesional (CL) and ipsilesional (IL) reorganization. All tested patients with type I lesions showed a contralateral pattern of reorganization, while children with the other two types of lesion were more heterogeneous in terms of reorganization. Interestingly, the results of the fMRI investigation revealed higher lateralized activations, including also the classical MNS areas, in Type I patients, during the observation of actions performed by others. In addition, the lateralization index was negatively related to performance on clinical scales. Altogether these data suggest that (1) the MNS can be activated in UCP patients, similarly to TD children; (2) the type of reorganization and, as a consequence, the different involvement of the two hemispheres during the process of recovery, is the main factor explaining the degree of functional outcome.
A later fMRI study carried out by Errante et al. [
103] extended these results demonstrating that UCP children with right hemiplegia show a higher activation of the MNS when they observe the paretic hand of another patient performing a grasping action, in comparison to the observation of the hand of a healthy individual. On the contrary, the authors found that healthy right-handed control children that observe the same two hand models (the pathological and the healthy one) have comparable activation of the MNS. Concerning the lateralization, the activation patterns in the inferior parietal cortex and premotor cortex were largely symmetrical in UCP, differently from TD children, where it was evident a higher lateralization toward the hemisphere contralateral to the observed hand. This indicates that, despite the presence of the lesion, some spared areas of the lesioned hemisphere can take part to compensate for the lost function. However, some patients showed the activation of the MNS only in the contralesional hemisphere, probably depending on a contralesional reorganization. A possible interpretation of this finding is that the spared hemisphere controls both upper limbs thanks to the presence of ipsilateral projections from the undamaged cortex. The intensity of activation of UCP children is in line with that reported by Sgandurra et al. [
102]. In fact, patients with ipsilesional reorganization showed, overall, a higher MNS activation with respect to patients with contralesional reorganization. Note that Sgandurra et al. report that patients with this latter type of reorganization generally have lower scores on clinical functional scales.
During action observation, in the motor cortex there is an active inhibition that is necessary to avoid the immediate reproduction of the observed action. An interesting clinical observation is that UCP patients with poor motor performance and contralesional reorganization show “mirror movements” frequently (involuntary movements produced by one hand during the execution of voluntary movements with the opposite hand). These movements are highly indicative of the reorganization of voluntary hand control in the preserved hemisphere, which becomes able to guide both hands. On the contrary, patients with ipsilesional reorganization show a better capacity to inhibit mirror movements. A possible explanation of these unnecessary movements is that the two hemispheres play a different role during the inhibition process. When the ipsilesional hemisphere maintains the capacity to control the contralateral limb, mirror movements can be still inhibited, while when the contralesional hemisphere controls both upper limbs, this function is more frequently lost. This interpretation is also in line with microstimulation studies in monkeys showing that the ventral premotor cortex tends to excite the primary motor cortex of the same hemisphere and to inhibit that of the opposite one [
104].
To date, several models of intervention are available to promote adaptive brain plasticity in UCP patients [
105]. Recently, it has been proposed that the systematic use of action observation followed by imitation (AOT—Action Observation Therapy) is an effective way to enhance motor experience and promote upper limb recovery in neurological patients and children with UCP [
106,
107]. AOT is a rehabilitation therapy that lasts about 2–4 weeks (3–5 days a week), based on the observation of daily actions (e.g., grasping a key and inserting it into a hole), immediately followed by their imitation.
Sgandurra and colleagues [
108] carried out a randomized controlled trial (RCT) to demonstrate the AOT efficacy to improve the recovery of upper limb in UCP children. The authors enrolled two groups of homogeneous UCP patients with mild-to-moderate hand impairment; the experimental group had to observe videos of unimanual or bimanual goal-directed actions and then reproduce them, while the control group had to perform the same actions, after observing videos devoid of any action content. After training, the experimental group showed a greater improvement than the control one, measured with a functional scale (AHA), which emphasized the role of the two hands in the daily life activities.
Very recently, Sgandurra and colleagues [
109] performed a pilot study on eight UCP children to test the efficacy of AOT combining a clinical and neuroimaging approach to assess also brain plasticity changes after the rehabilitation. The results demonstrated an improvement of motor performance of patients receiving the AOT (assessed with clinical scale AHA) as compared to the control group. Very interestingly, after the therapy there was a more bilateral activation of the MNS in the experimental group. This is in line with the studies described above, indicating that a bilateral activation of the MNS is associated to a better functional recovery. Besides the reorganization of the MNS, there was also a higher lateralized activation of the sensorimotor network in the experimental group, as compared to the control one, in particular at the level of the primary motor area, the premotor cortex, the IFG and the IPL. Thus, it is plausible that brain plasticity after the AOT has multiple effects on the sensorimotor network, inducing greater lateralization of sensory and motor functions, and producing bilateral activity of the MNS during the observation of actions performed by others.
11. Conclusions
The studies reported in this review show, first of all, that the parieto-frontal circuits underlying sensorimotor transformation for reaching and grasping in non-human primates do not show clear-cut lateralization, although one hemisphere (either right or left) can prevail during action execution. Secondly, when considering the MNS, functional lateralization can be revealed, depending on the specific task that subjects are required to perform within the experimental context. The evidence presented in this review suggests that the two hemispheres play a complementary role during the organization of voluntary movement, and the mechanisms responsible for motor execution are also used for understanding other’s behavior. Functional changes in terms of lateralization of the recruited areas can also be referred to situations in which motor learning is achieved by observation/imitation paradigms, which involve the MNS.
Future studies should go deeper in the investigation of the functional lateralization of the MNS also including left-handed subjects, both during pure action observation and during observation-based motor learning. Furthermore, from a translational perspective, it may be important to combine several techniques, for example TMS and fMRI, to verify the specific effects of AOT on the motor reorganization in hemiplegic and diplegic patients. Finally, a systematic meta-analysis could be useful to highlight the possible functional lateralization of the MSN, by including not only brain imaging data, but also the electrophysiological findings recently described in literature.