Histological Evaluation of a New Beta-Tricalcium Phosphate/Hydroxyapatite/Poly (1-Lactide-Co-Caprolactone) Composite Biomaterial in the Inflammatory Process and Repair of Critical Bone Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bony Defects
2.2. Preparation for Histological Analysis
2.3. Histological Analysis
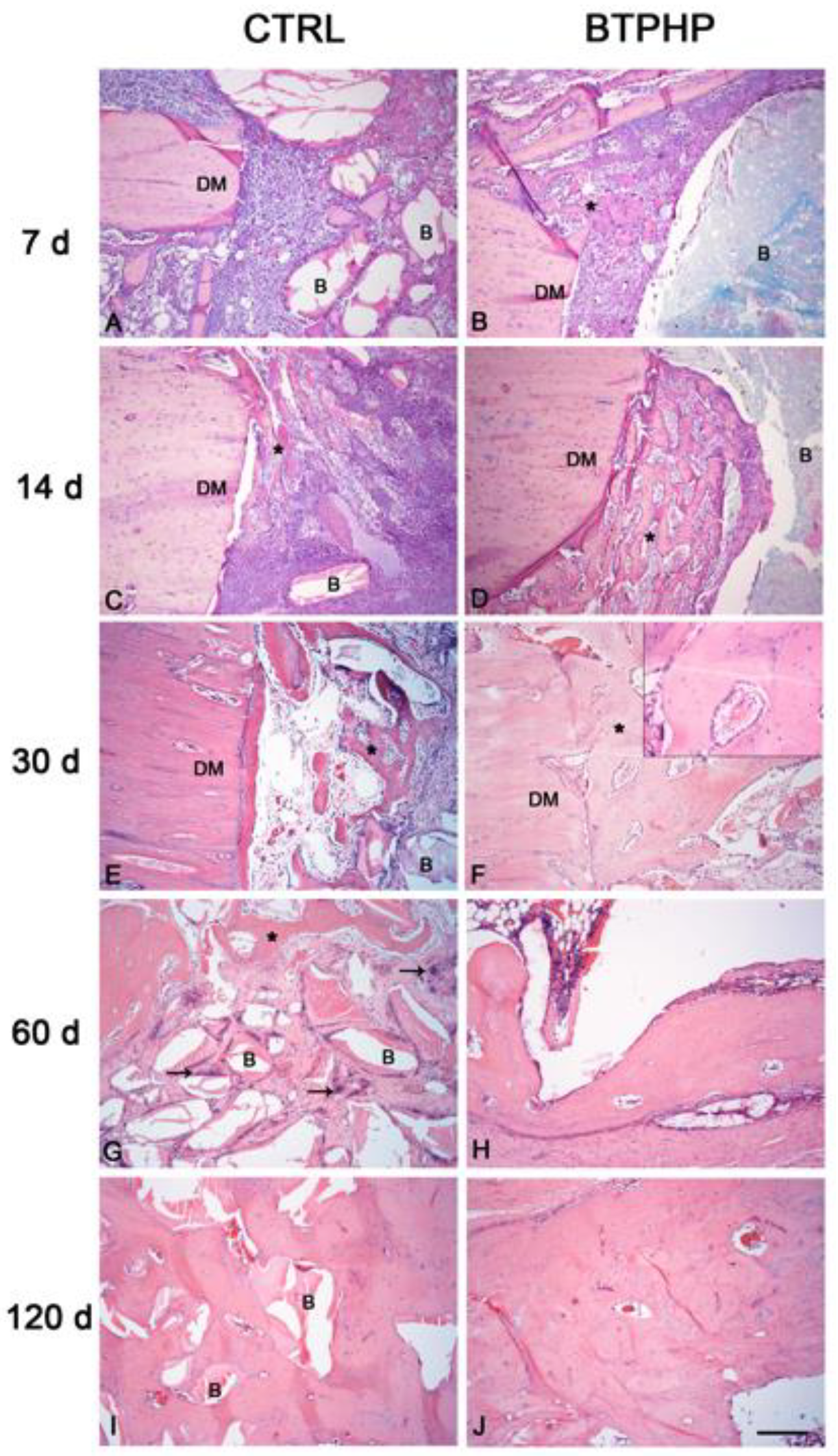
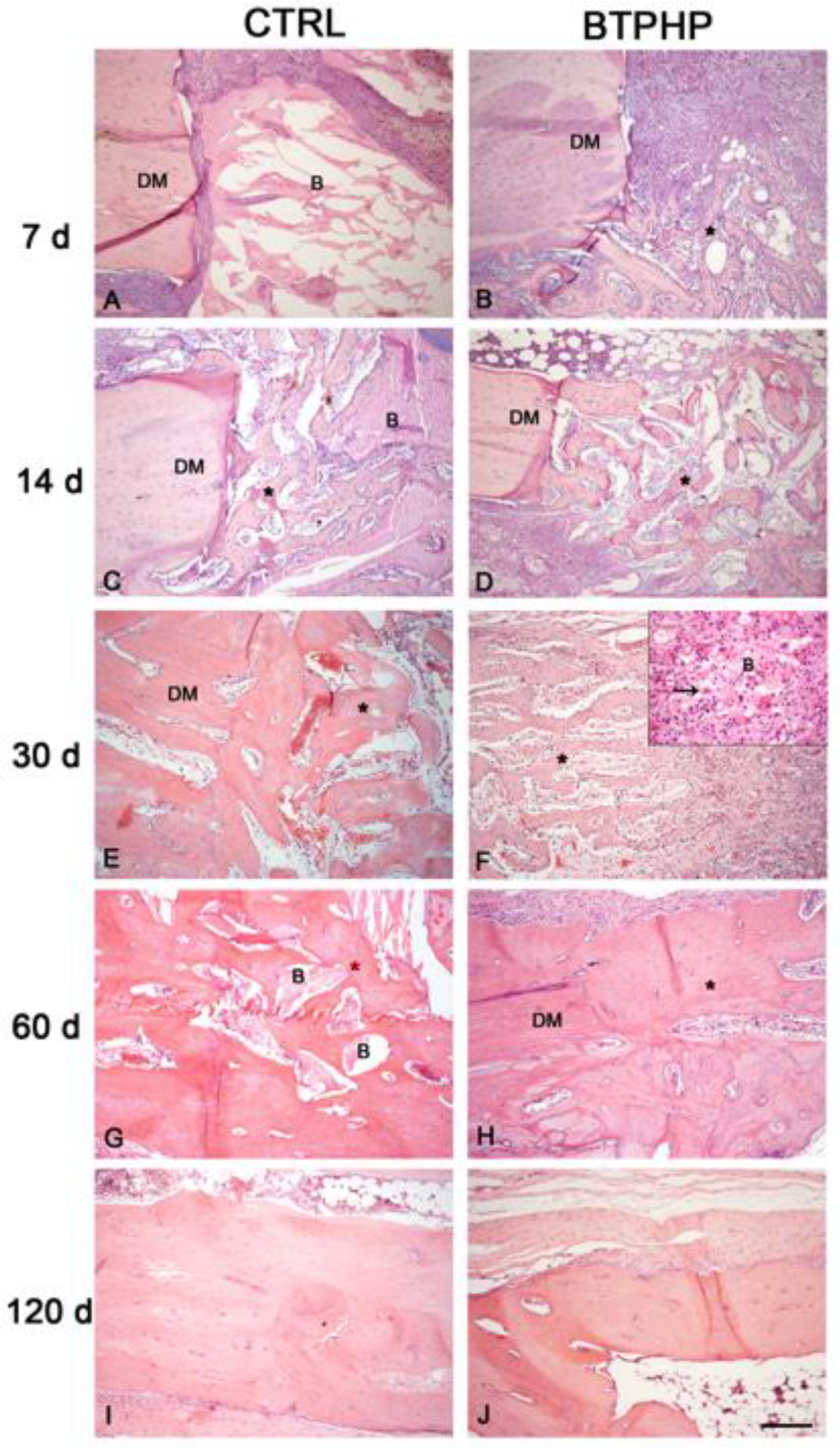
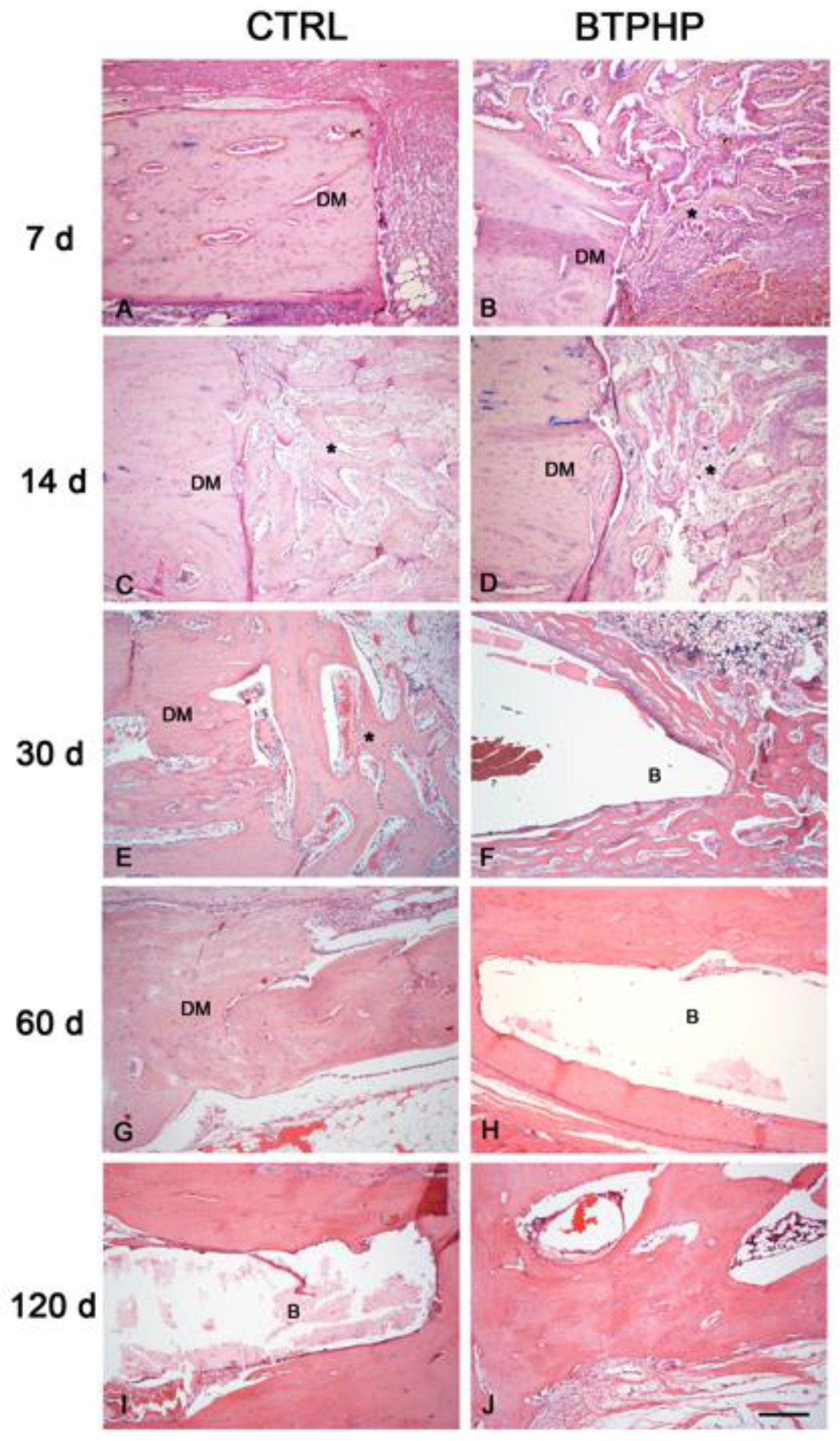
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used indental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; de Buitrago, J.G.; Padial-Molina, M.; Fernández-Barbero, J.E.; Ata-Ali, J.; O Valle, F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin. Oral Implants Res. 2018, 29, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials e from space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Borzabadi-Farahani, A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J. Cranio-Maxillofac. Surg. 2014, 42, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration: A review of ceramics and polymers. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, J.A.; Koup, R. Localized maxillary ridge augmentation with a block allograft for dental implant placement: Case reports. Implant Dent. 2003, 12, 217–226. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Wang, F.; Wang, Z. Maxillary sinus floor augmentation and dental implant placement using dentin matrix protein-1 gene-modified boné marrow stromal cells mixed with deproteinized boving bone: A comparative study in beagles. Arch. Oral Biol. 2016, 64, 102–108. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Mesa, F.; Carranza, N.; Juodzbalys, G.; Aguilar, M.; O’Valle, F. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2013, 15, 858–866. [Google Scholar] [CrossRef]
- Froum, S.J.; Wallace, S.S.; Elian, N.; Cho, S.C.; Tarnow, D.P. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: Histomorphometry at 26 to 32 weeks after grafting. Int. J. Periodontics Restor. Dent. 2006, 26, 543–551. [Google Scholar]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implants Res. 2012, 23, 263–273. [Google Scholar] [CrossRef]
- Orsini, G.; Traini, T.; Scarano, A.; Degidi, M.; Perrotti, V.; Piccirilli, M.; Piattelli, A. Maxillary sinus augmentation with Bio-Oss® particles: A light, scanning, and transmission electron microscopy study in man. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implants Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, B.O.; Yu, S.J. Clinical evaluation of a biphasic calcium phosphate grafting material in the treatment of human periodontal intrabony defects. J. Periodontal Implant Sci. 2012, 42, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catros, S.; Guillemot, F.; Lebraud, E.; Chanseau, C.; Perez, S.; Bareille, R.; Amédeé, J.; Fricain, J.C. Physico-chemical and biological properties of a nano-hydroxyapatite powder synthesized at room temperature. IRBM 2010, 31, 226–233. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Da Cruz, A.C.; Pochapski, M.T.; Daher, J.B.; da Silva, J.C.; Pilatti, G.L.; Santos, F.A. Physico-chemical characterization and biocompatibility evaluation of hydroxyapatites. J. Oral Sci. 2006, 48, 219–226. [Google Scholar] [CrossRef]
- Rossi, F.; Santoro, M.; Perale, G. Polymeric scaffolds as stem cell carriers in bone repair. J. Tissue Eng. Regen. Med. 2015, 9, 1093–1119. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Dekhtya, Y.; Dvornichenko, M.V.; Karlov, A.V.; Khlusov, I.A.; Polyaka, N.; Sammons, R.; Zaytsev, K.V. Electrically Functionalized Hydroxyapatite and Calcium Phosphate Surfaces to Enhance Immobilization and Proliferation of Osteoblasts In Vitro and Modulate Osteogenesis In Vivo. IFMBE Proc. 2009, 25, 245–248. [Google Scholar]
- Kabaso, D.; Gongadze, E.; Perutková, S.; Matschegewski, C.; Kralj-Iglic, V.; Beck, U.; van Rienen, U.; Iglic, A. Mechanics and electrostatics of the interactions between osteoblasts and titanium surface. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.M.; de Carvalho, V.R.; Govone, J.S.; Hernandes, A.C.; da Cruz, N.C. Effects of negatively and positively charged Ti metal surfaces on ceramic coating adhesion and cell response. J. Mater. Sci. Mater. Med. 2017, 28, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J. Periodontal Res. 2018, 53, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Tadjoedin, E.S.; de Lange, G.L.; Bronckers, A.L.; Lyaruu, D.M.; Burger, E.H. Deproteinized cancellous bovine bone (Bio-Oss) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J. Clin. Periodontol. 2003, 30, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.F.; Amaral, J.B.D.; Santos, É.B.D.; Martinez, E.F.; Montalli, V.A.M.; Junqueira, J.L.C.; Araújo, V.C.; Napimoga, M.H. Presence of Cells in Fresh-Frozen Allogeneic Bone Grafts from Different Tissue Banks. Braz. Dent. J. 2017, 28, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfleisch, R.; Jung, F.J. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- De Lacerda, P.E.; Pelegrine, A.A.; Teixeira, M.L.; Montalli, V.A.; Rodrigues, H.; Napimoga, M.H. Homologous transplantation with fresh frozen bone for dental implant placement can induce HLA sensitization: A preliminary study. Cell Tissue Bank 2016, 17, 465–472. [Google Scholar] [CrossRef]
- Asikainen, A.J.; Pelto, M.; Noponen, J.; Kellomäki, M.; Pihlajamäki, H.; Lindqvist, C.; Suuronen, R. In vivo degradation of poly (DTE carbonate) membranes. Analysis of the tissue reactions and mechanical properties. J. Mater. Sci. Mater. Med. 2008, 19, 53–58. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Zhang, X.; Chang, J.; Dai, K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater 2018, 66, 81–92. [Google Scholar] [CrossRef]
- Li, S.H.; De Wijn, J.R.; Layrolle, P.; de Groot, K. Synthesis of macroporous hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 2002, 61, 109–120. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Li, X.; Li, S.; Zhou, Z.; Zhang, Y.; Feng, Q.L.; Yu, B. In vitro biocompatibility and osteoblast differentiation of an injectable chitosan/nano-hydroxyapatite/collagen scaffold. J. Nanomater 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Pielichowska, K.; Blazewicz, S. Bioactive polymer/hydroxyapatite (nano) composites for bone tissue regeneration. In Advances in Polymer Science; Abe, A., Dušek, K., Kobayashi, S., Eds.; Springer: Berlin, Germany, 2010; Volume 232, pp. 97–207. [Google Scholar]
- Dey, R.E.; Wimpenny, I.; Gough, J.E.; Watts, D.C.; Budd, P.M. Poly (vinylphosphonic acid-co-acrylic acid) hydrogels: The effect of copolymer composition on osteoblast adhesion and proliferation. J. Biomed. Mater. Res. A 2018, 106, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Kao, R.T.; Nares, S.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Rosen, P.S. Periodontal regeneration—Intrabony defects: Practical applications from the AAP regeneration workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int. J. Oral Maxillofac. Implants 1999, 14, 835–840. [Google Scholar]
- Sartori, S.; Silvestri, M.; Forni, F.; Icaro Cornaglia, A.; Tesei, P.; Cattaneo, V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin. Oral Implants Res. 2003, 14, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Vignoletti, F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent. Mater. 2015, 31, 640–647. [Google Scholar] [CrossRef]
- Trisi, P.; Rao, W.; Rebaudi, A.; Fiore, P. Histologic effect of pure-phase beta-tricalcium phosphate on bone regeneration in human artificial jawbone defects. Int. J. Periodontics Restor. Dent. 2003, 23, 69–77. [Google Scholar]
- Jensen, S.S.; Broggini, N.; Hjorting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef]
- Artzi, Z.; Weinreb, M.; Givol, N.; Rohrer, M.D.; Nemcovsky, C.E.; Prasad, H.S.; Tal, H. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int. J. Oral Maxillofac. Implants 2004, 19, 357–368. [Google Scholar]
- Schwarz, F.; Herten, M.; Ferrari, D.; Wieland, M.; Schmitz, L.; Engelhardt, E.; Becker, J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic®) or a collagen-coated natural bone mineral (BioOss Collagen®): An immunohistochemical study in dogs. Int. J. Oral Maxillofac. Surg. 2007, 36, 1198–1206. [Google Scholar] [CrossRef]
- Pezzatini, S.; Solito, R.; Morbidelli, L.; Lamponi, S.; Boanini, E.; Bigi, A.; Ziche, M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. A 2006, 76, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, D.A.; Mashoof, A.A.; Novak, J.; Strates, B.S.; McGuire, M.H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J. Biomed. Mater. Res. 1994, 28, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means ofbarrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Matsuo, M.; Todoki, K.; Ozono, S.; Fukuoka, S.; Tsuzuki, H.; Nakamura, M.; Tomihata, K.; Shimamoto, T.; Ikada, Y. Alveolar bone regeneration using absorbable poly (L-lactide-co-epsilon-caprolactone)/beta-tricalcium phosphate membrane and gelatin sponge incorporating basic fibroblast growth factor. Int. J. Oral Maxillofac. Surg. 2008, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Leknes, K.N.; Pedersen, T.O.; Xing, Z.; Sun, Y.; Lie, S.A.; Finne-Wistrand, A.; Mustafa, K. Cell seeding density is a critical determinant for copolymer scaffolds-induced bone regeneration. J. Biomed. Mater. Res. A 2015, 103, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Zwahlen, R.; Weber, F.E.; Molenberg, A.; van Lenthe, G.H.; Hammerle, C.H. Evaluation of an in situ formed synthetic hydrogel as a biodegradable membrane for guided bone regeneration. Clin. Oral Implants Res. 2006, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, E.F.; Rodrigues, A.E.A.; Teixeira, L.N.; Esposito, A.R.; Cabrera, W.I.R.; Demasi, A.P.D.; Passador-Santos, F. Histological Evaluation of a New Beta-Tricalcium Phosphate/Hydroxyapatite/Poly (1-Lactide-Co-Caprolactone) Composite Biomaterial in the Inflammatory Process and Repair of Critical Bone Defects. Symmetry 2019, 11, 1356. https://doi.org/10.3390/sym11111356
Martinez EF, Rodrigues AEA, Teixeira LN, Esposito AR, Cabrera WIR, Demasi APD, Passador-Santos F. Histological Evaluation of a New Beta-Tricalcium Phosphate/Hydroxyapatite/Poly (1-Lactide-Co-Caprolactone) Composite Biomaterial in the Inflammatory Process and Repair of Critical Bone Defects. Symmetry. 2019; 11(11):1356. https://doi.org/10.3390/sym11111356
Chicago/Turabian StyleMartinez, Elizabeth Ferreira, Ana Elisa Amaro Rodrigues, Lucas Novaes Teixeira, Andrea Rodrigues Esposito, Walter Israel Rojas Cabrera, Ana Paula Dias Demasi, and Fabricio Passador-Santos. 2019. "Histological Evaluation of a New Beta-Tricalcium Phosphate/Hydroxyapatite/Poly (1-Lactide-Co-Caprolactone) Composite Biomaterial in the Inflammatory Process and Repair of Critical Bone Defects" Symmetry 11, no. 11: 1356. https://doi.org/10.3390/sym11111356
APA StyleMartinez, E. F., Rodrigues, A. E. A., Teixeira, L. N., Esposito, A. R., Cabrera, W. I. R., Demasi, A. P. D., & Passador-Santos, F. (2019). Histological Evaluation of a New Beta-Tricalcium Phosphate/Hydroxyapatite/Poly (1-Lactide-Co-Caprolactone) Composite Biomaterial in the Inflammatory Process and Repair of Critical Bone Defects. Symmetry, 11(11), 1356. https://doi.org/10.3390/sym11111356




