Stability Study of Etoricoxib a Selective Cyclooxygenase-2 Inhibitor by a New Single and Rapid Reversed Phase HPLC Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments
2.3. Samples Preparation for the Recovery Study
2.4. Samples Preparation for the Linearity Test
2.5. Samples Preparation for Robustness Test, Lower Limit of Detection (LLOD) and Lower Limit of Quantification (LLOQ)
2.6. Samples Preparation for Selectivity
2.7. Method Preparation of the Formula
2.8. Samples Preparation for Particle Size Analysis
3. Results and Discussion
3.1. HPLC Analytical Method Development
3.2. HPLC Analytical Method Validation
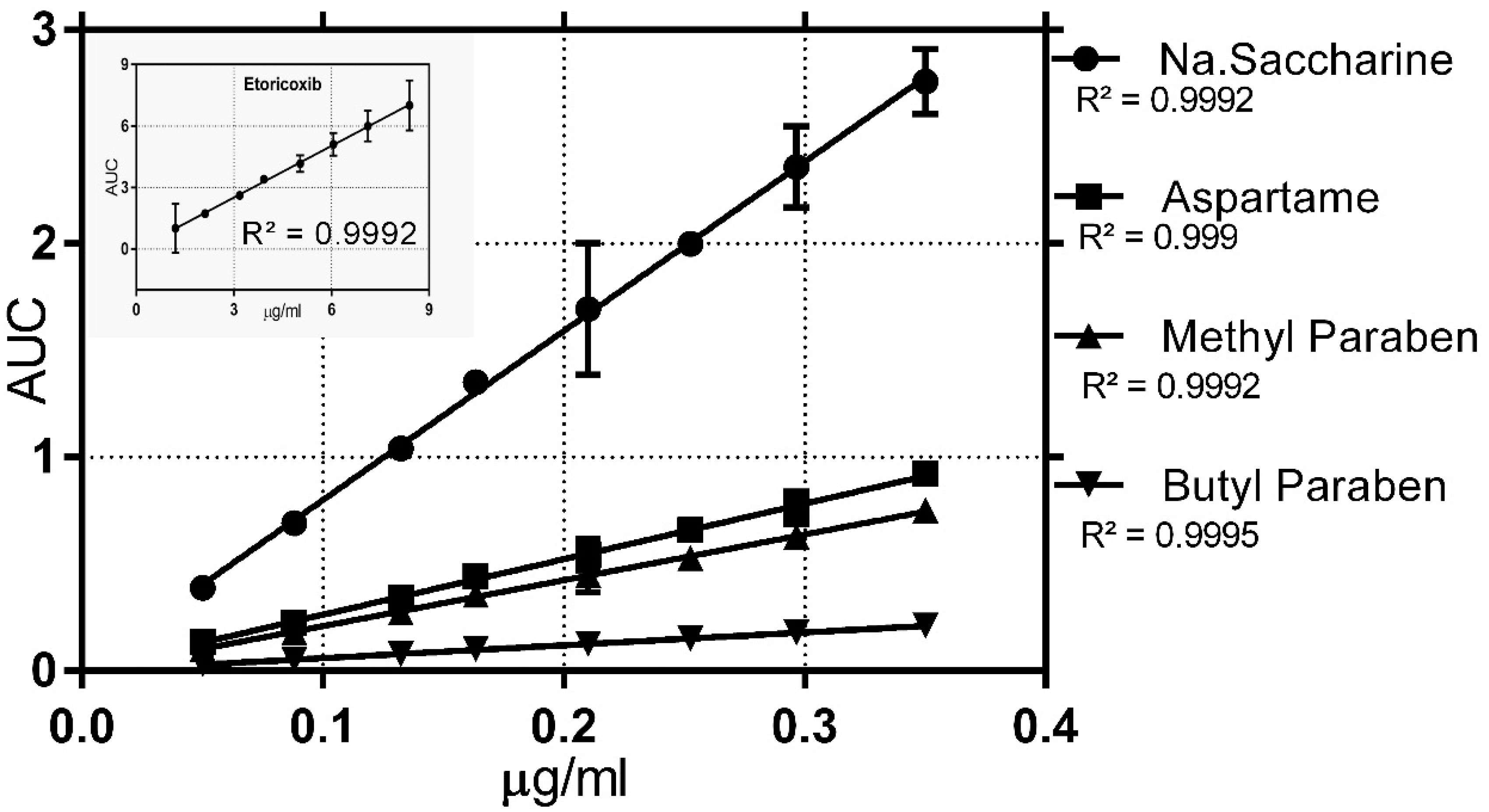
3.2.1. Linearity
3.2.2. Accuracy (Recovery)
3.2.3. Precision
3.2.4. Range
3.2.5. Specificity
3.2.6. LLOD and LLOQ
3.2.7. Robustness
3.3. Crystal Growth of Etoricoxib Suspensions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cochrane, D.; Jarvis, B.; Keating, G. Etoricoxib. Drugs 2002, 62, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Arfè, A.; Scotti, L.; Varas-Lorenzo, C.; Nicotra, F.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Bavry, A.A. Risk of stroke associated with nonsteroidal anti-inflammatory drugs. Vasc. Health Risk Manag. 2014, 10, 25–32. [Google Scholar] [PubMed]
- Moraes, B.M.; do Amaral, B.C.; Morimoto, M.S.; Vieira, L.G.; Perazzo, F.F.; Carvalho, J.C. Anti-inflammatory and analgesic actions of etoricoxib (an NSAID) combined with misoprostol. Inflammopharmacology 2007, 15, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Derry, S.; Moore, R.A. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Suhagia, B.N.; Patel, H.M.; Shah, S.A.; Rathod, I.; Parmar, V.K. Preparation and characterization of etoricoxib-polyethylene glycol 4000 plus polyvinylpyrrolidone K30 solid dispersions. Acta Pharm. 2006, 56, 285–298. [Google Scholar] [PubMed]
- Kulshreshtha, A.K.; Singh, O.N.; Wall, G.M. Pharmaceutical Suspensions: From Formulation Development to Manufacturing; Springer: New York, NY, USA, 2009. [Google Scholar]
- Aulton, M.E. Pharmaceutics: The Science of Dosage Form Design; hurchill Livingstone: London, UK, 2002. [Google Scholar]
- Clas, S.D.; Crocker, L.S.; McCauley, J.A.; Davies, I.; Dalton, C. Polymorphic, Amorphous and Hydrated Forms of 5-Chloro-60-methyl-3-[4-(methylsulfonyl)phenyl]-2,30-bipyridine. U.S. Patent 6,441,002, 27 August 2002. [Google Scholar]
- Dalton, C.R.; Clas, S.D.; Singh, J.; Khougaz, K.; Bilbeisi, R. Investigating the hydrate conversion propensity of different etoricoxib lots. J. Pharm. Sci. 2006, 95, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.S.H.; Shah, S.; Rathod, I. Determination of Etoricoxib in Pharmaceutical Formulations by HPLC Method. Indian J. Pharm. Sci. 2007, 69, 703–705. [Google Scholar] [CrossRef]
- Dalmora, S.L.; Mda, S.S.; da Silva, L.M.; Macedo, R.O.; Barth, T. Validation of a capillary zone electrophoresis method for the comparative determination of etoricoxib in pharmaceutical formulations. J. Sep. Sci. 2008, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Shah, S.J.; Patel, D.M.; Patel, N.M. Development and Validation of HPTLC Method for the Estimation of Etoricoxib. Indian J. Pharm. Sci. 2006, 68, 788–789. [Google Scholar] [CrossRef]
- Narajji, C.; Karvekar, M.D. Method development and validation for simultaneous estimation of Paracetamol and Etoricoxib in pharmaceutical dosage form by RP-HPLC method. Der Pharma Chem. 2011, 3, 7–12. [Google Scholar]
- Syal, P.K.; Sahoo, M.; Ingale, K.D.; Ingale, S.S.; Choudhari, V.P.; Kuchekar, B.S. Development and validation of a RP-HPLC-PDA method for simultaneous estimation of Drotaverine and Etoricoxib in tablet and its application for dissolution studies. Der Pharma Chem. 2010, 2, 93–102. [Google Scholar]
- Hartman, R.; Abrahim, A.; Clausen, A.; Mao, B.; Crocker, L.S.; Ge, Z. Development and Validation of an HPLC Method for the Impurity and Quantitative Analysis of Etoricoxib. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2551–2566. [Google Scholar] [CrossRef]
- Likhar, A.; Wadodkar, S.G.; Gupta, K.R. Application of Stability Indicating HPLC Method for Quantitative Determination of Etoricoxib and Paracetamol in Pharmaceutical Dosage Form. Eurasian J. Anal. Chem. 2010, 5, 218–226. [Google Scholar]
- Vora, D.N.; Kadav, A.A. Separation of Etoricoxib and Its Degradation Products in Drug Substance Using UPLC. Eurasian J. Anal. Chem. 2007, 2, 151–158. [Google Scholar]
- Shabir, G.A. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. Drug Dev. Ind. Pharm. 2003, 987, 57–66. [Google Scholar]
- Ermer, J. Validation in pharmaceutical analysis. Part I: An integrated approach. J. Pharm. Biomed. Anal. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology, Q2 (R1); ICH: Geneva, Switzerland, 2005; Volume 1. [Google Scholar]
- McCalley, D.V. Rationalization of retention and overloading behavior of basic compounds in reversed-phase HPLC using low ionic strength buffers suitable for mass spectrometric detection. Anal. Chem. 2003, 75, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [PubMed]
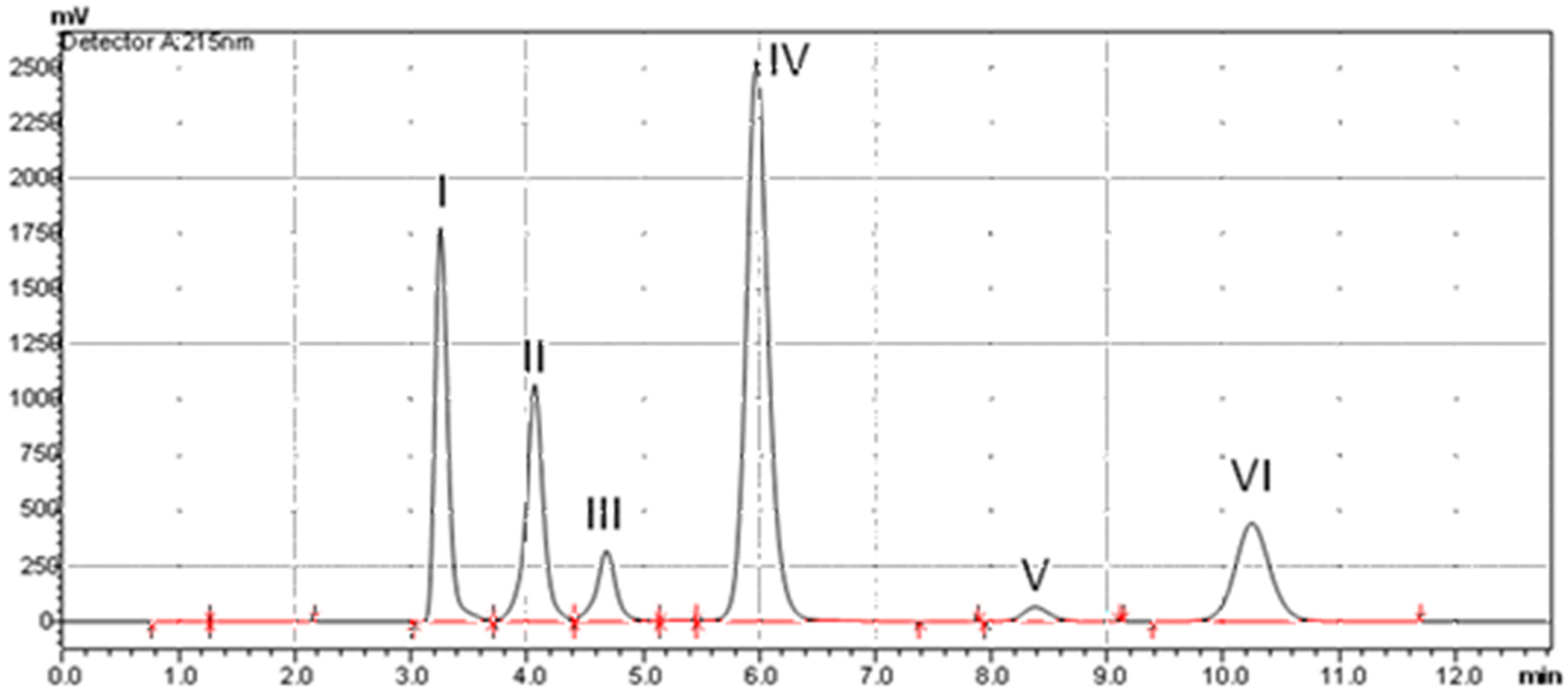
| Compound | Log P | pKa | Retention Time in Minutes |
|---|---|---|---|
| Sodium saccharin | −0.96 | 1.6 | 3.26 |
| Vanillin | 1.21–1.35 | 7.38 | 4.10 |
| Methyl Paraben | 1.91 | 8.87 | 4.69 |
| Etoricoxib | 2.79 | 4.60 | 5.98 |
| Butyl Paraben | 3.50 | 8.79 | 8.38 |
| Celecoxib | 3.90 | 10.70 | 10.25 |
| Analyte | LLOD (μg/mL) | LLOQ (μg/mL) | Conc. of Each Analyte in Sample Solution (μg/mL) |
|---|---|---|---|
| Sodium saccharin | 8.18 | 27.28 | 125 |
| Vanillin | 6.86 | 22.87 | 125 |
| Methyl Paraben | 3.34 | 11.13 | 32.5 |
| Etoricoxib | 5.08 | 16.94 | 300 |
| Butyl Paraben | 0.73 | 2.44 | 12.5 |
| Variable | Sodium Saccharin | Vanillin | Methyl Paraben | Etoricoxib | Butyl Paraben |
|---|---|---|---|---|---|
| Flow rate: 0.7–0.9 mL/min Optimum: (0.8 mL/min) | 3.30 ± 0.431 mL/min | 4.17 ± 0.532 mL/min | 4.72 ± 0.590 mL/min | 5.26 ± 0.746 mL/min | 8.38 ± 1.044 mL/min |
| Mobile Phase: 68/32–72/28 Optimum: 70/30 | 3.22 ± 0.037 mL/min | 4.03 ± 0.0774 min/min | 4.62 ± 0.149 min/min | 5.85 ± 0.400 min/min | 8.07 ± 0.895 mL/min |
| pH: 4–6.5 Optimum: pH 6.0 | 3.04 ± 0.058 mL/min | 4.09 ± 0.074 mL/min | 4.66 ± 0.066 mL/min | 5.87 ± 0.187 mL/min | 8.07 ± 0.370 mL/min |
| Items | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Optimum HPLC parameters | 1.25 | 0.95 | 1.72 | 3.43 | 2.49 |
| Flow rate: 0.8 mL/min | |||||
| Mobile phase: 70:30 | |||||
| pH: 6.0 | |||||
| Wavelength at 215 nm | |||||
| Flow rate 0.7 mL/min | 1.52 | 0.51 | 1.91 | 3.84 | 2.62 |
| Flow rate 0.9 mL/min | 1.35 | 0.7 | 1.39 | 2.78 | 1.91 |
| Mobile phase 68:32 | 1.31 | 1.02 | 1.93 | 3.71 | 3.11 |
| Mobile phase 72:28 | 1.25 | 0.86 | 1.35 | 2.55 | 1.54 |
| Wavelength at 213 nm | 1.36 | 0.79 | 1.57 | 3.14 | 2.62 |
| Wavelength at 217 nm | 1.24 | 0.79 | 1.79 | 3.37 | 2.47 |
| pH 4 | 1.39 | 0.72 | 1.37 | 2.79 | 1.57 |
| pH 5.5 | 1.22 | 0.88 | 1.44 | 2.57 | 1.66 |
| pH 6.5 | 1.01 | 1.03 | 1.62 | 2.88 | 2.63 |
| Sample | Method | D(0.1) *, μm | D(0.5) *, μm | D(0.9) *, μm |
|---|---|---|---|---|
| Etoricoxib, powder | Dry Dispersion | 0.81 | 3.1 | 10.1 |
| Suspension, initial | Wet Dispersion | 3.4 | 7.1 | 16.8 |
| Suspension, 48 h | Wet Dispersion | 3.9 | 10.0 | 62.1 |
| Suspension, 72 h | Wet Dispersion | 4.3 | 12.0 | 88.3 |
| Suspension, 96 h | Wet Dispersion | 10.1 | 52.5 | 113.4 |
| Suspension, 336 h | Wet Dispersion | 52.4 | 140.7 | 326.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzweiri, M.; Sallam, M.; Al-Zyoud, W.; Aiedeh, K. Stability Study of Etoricoxib a Selective Cyclooxygenase-2 Inhibitor by a New Single and Rapid Reversed Phase HPLC Method. Symmetry 2018, 10, 288. https://doi.org/10.3390/sym10070288
Alzweiri M, Sallam M, Al-Zyoud W, Aiedeh K. Stability Study of Etoricoxib a Selective Cyclooxygenase-2 Inhibitor by a New Single and Rapid Reversed Phase HPLC Method. Symmetry. 2018; 10(7):288. https://doi.org/10.3390/sym10070288
Chicago/Turabian StyleAlzweiri, Muhammad, Mariam Sallam, Walid Al-Zyoud, and Khaled Aiedeh. 2018. "Stability Study of Etoricoxib a Selective Cyclooxygenase-2 Inhibitor by a New Single and Rapid Reversed Phase HPLC Method" Symmetry 10, no. 7: 288. https://doi.org/10.3390/sym10070288
APA StyleAlzweiri, M., Sallam, M., Al-Zyoud, W., & Aiedeh, K. (2018). Stability Study of Etoricoxib a Selective Cyclooxygenase-2 Inhibitor by a New Single and Rapid Reversed Phase HPLC Method. Symmetry, 10(7), 288. https://doi.org/10.3390/sym10070288





