CFD Simulation of Airflow Dynamics During Cough Based on CT-Scanned Respiratory Airway Geometries
Abstract
1. Introduction
2. Materials and Methods
2.1. Governing Equation
2.2. Turbulent Model
2.3. Boundary Condition
2.4. Numerical Modelling
3. Validation
4. Results and discussion
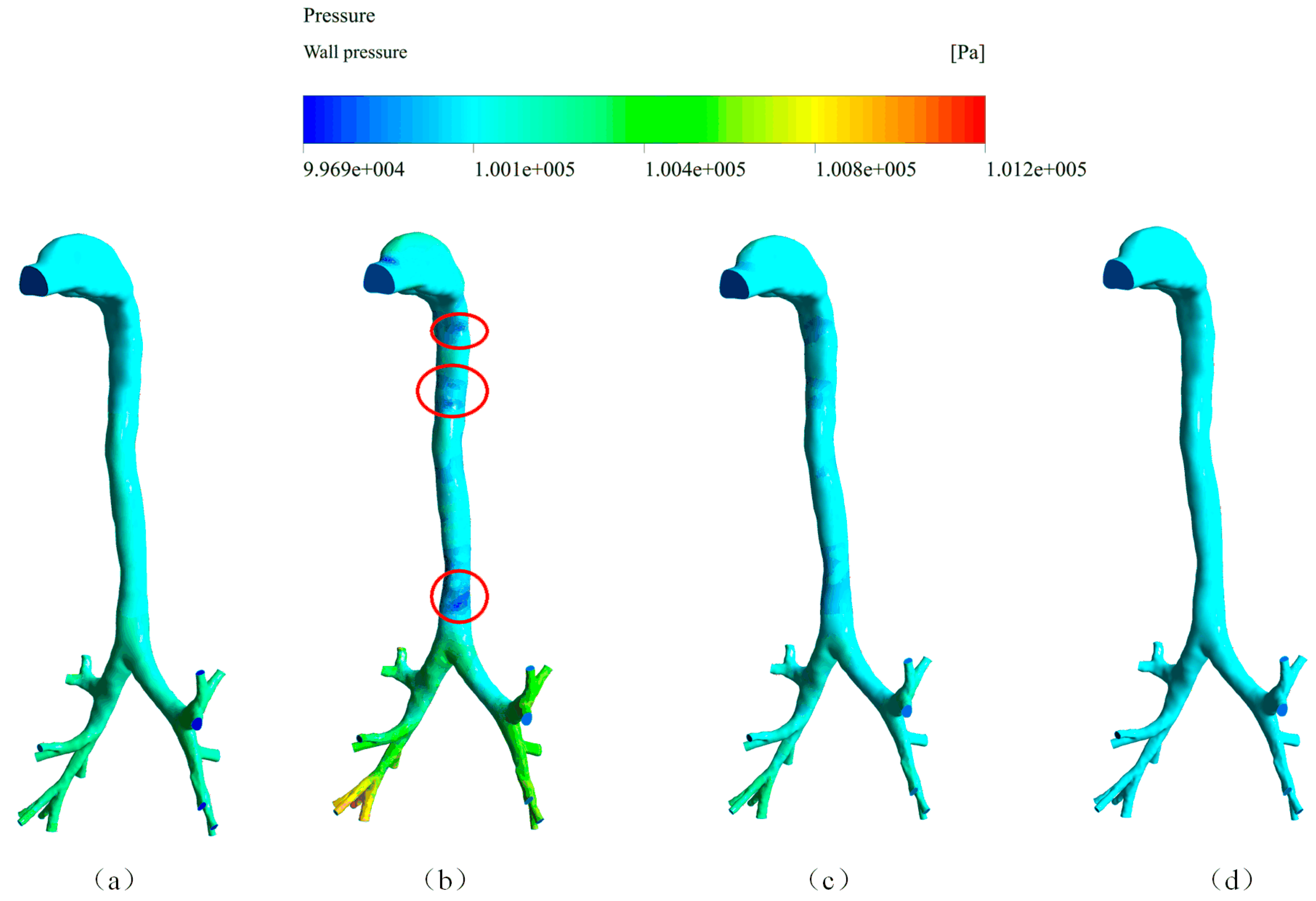
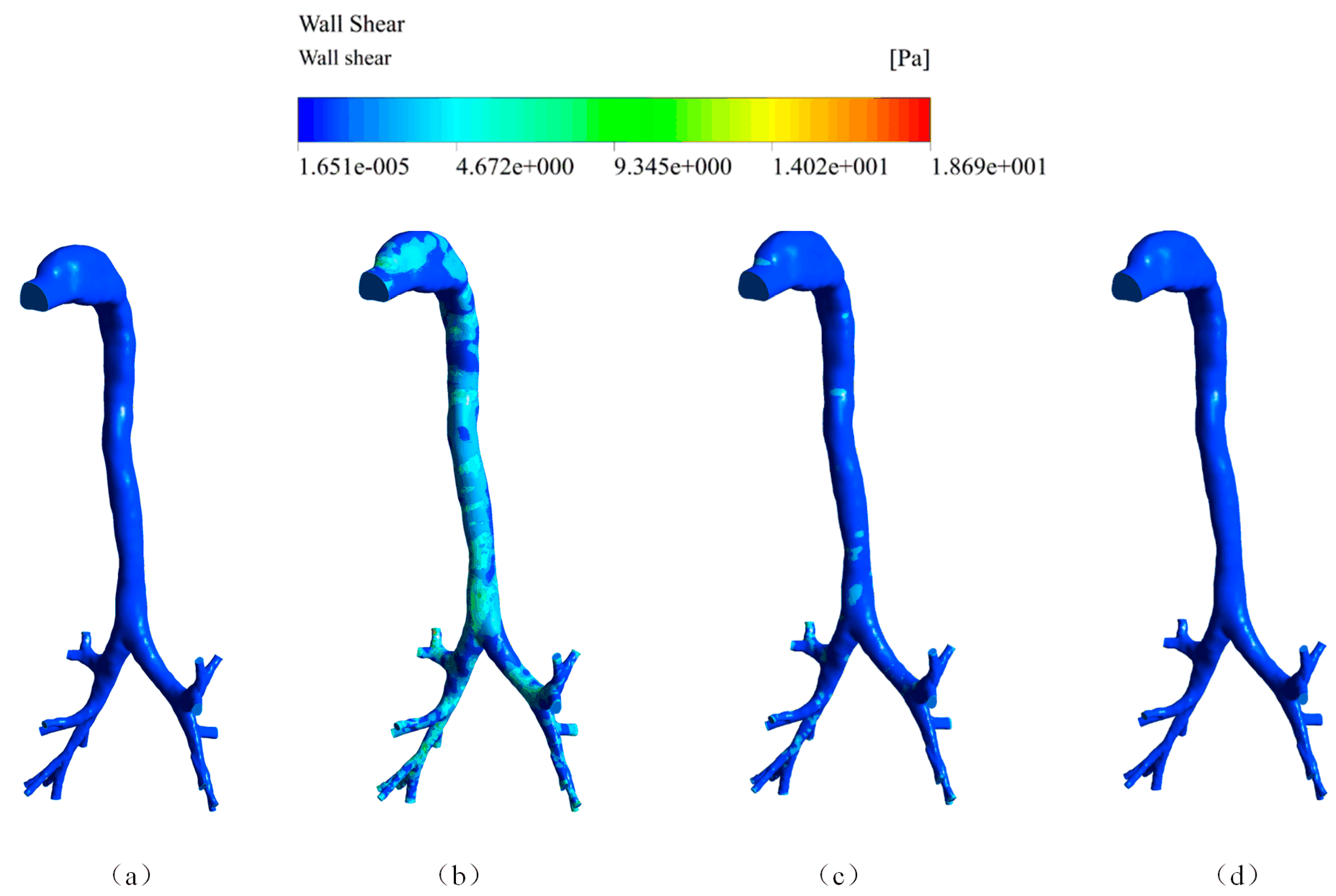
4.1. Air Velocity, Wall Pressure, and Wall Shear Stress of the Cough Process
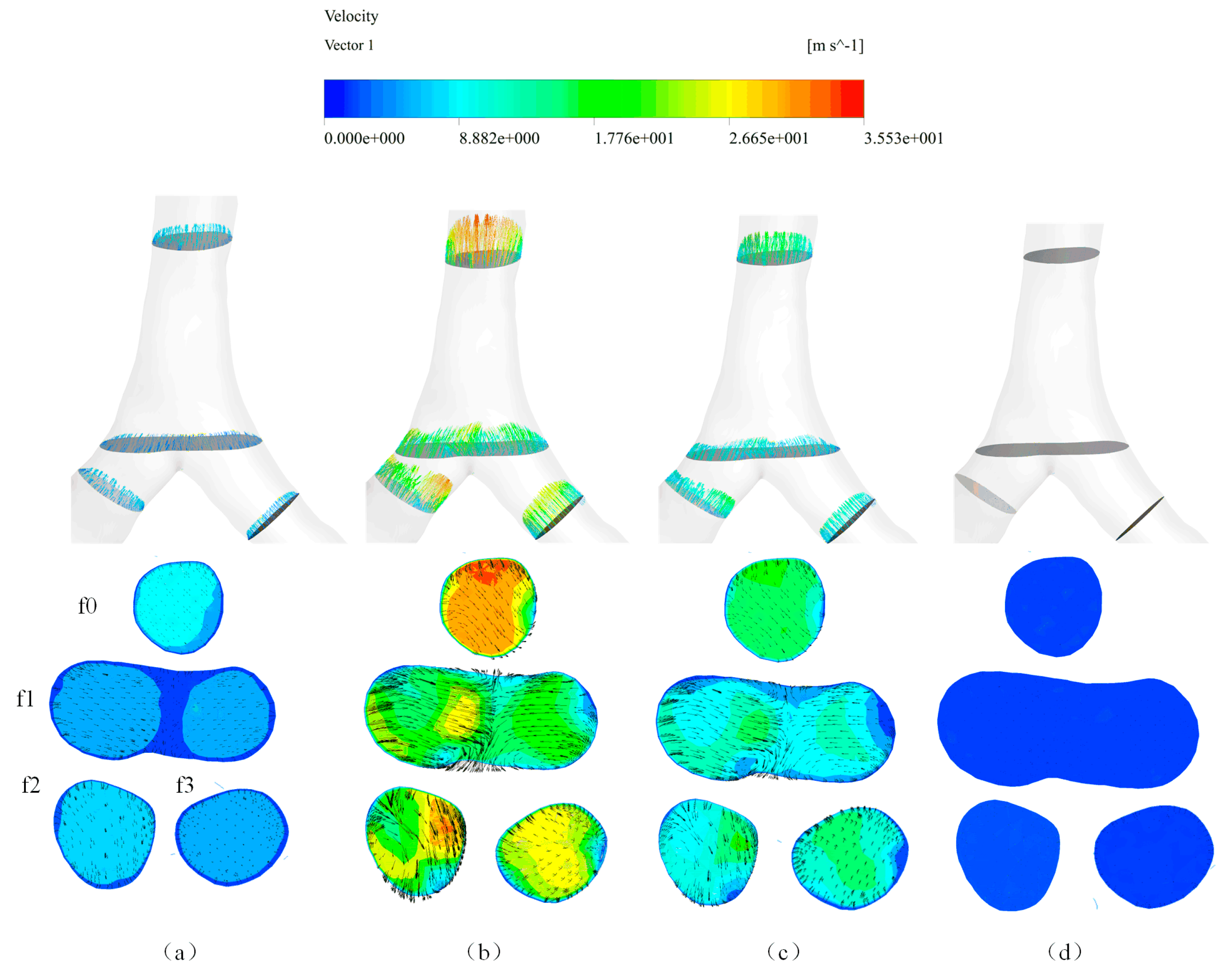
4.2. Local Flow Properties
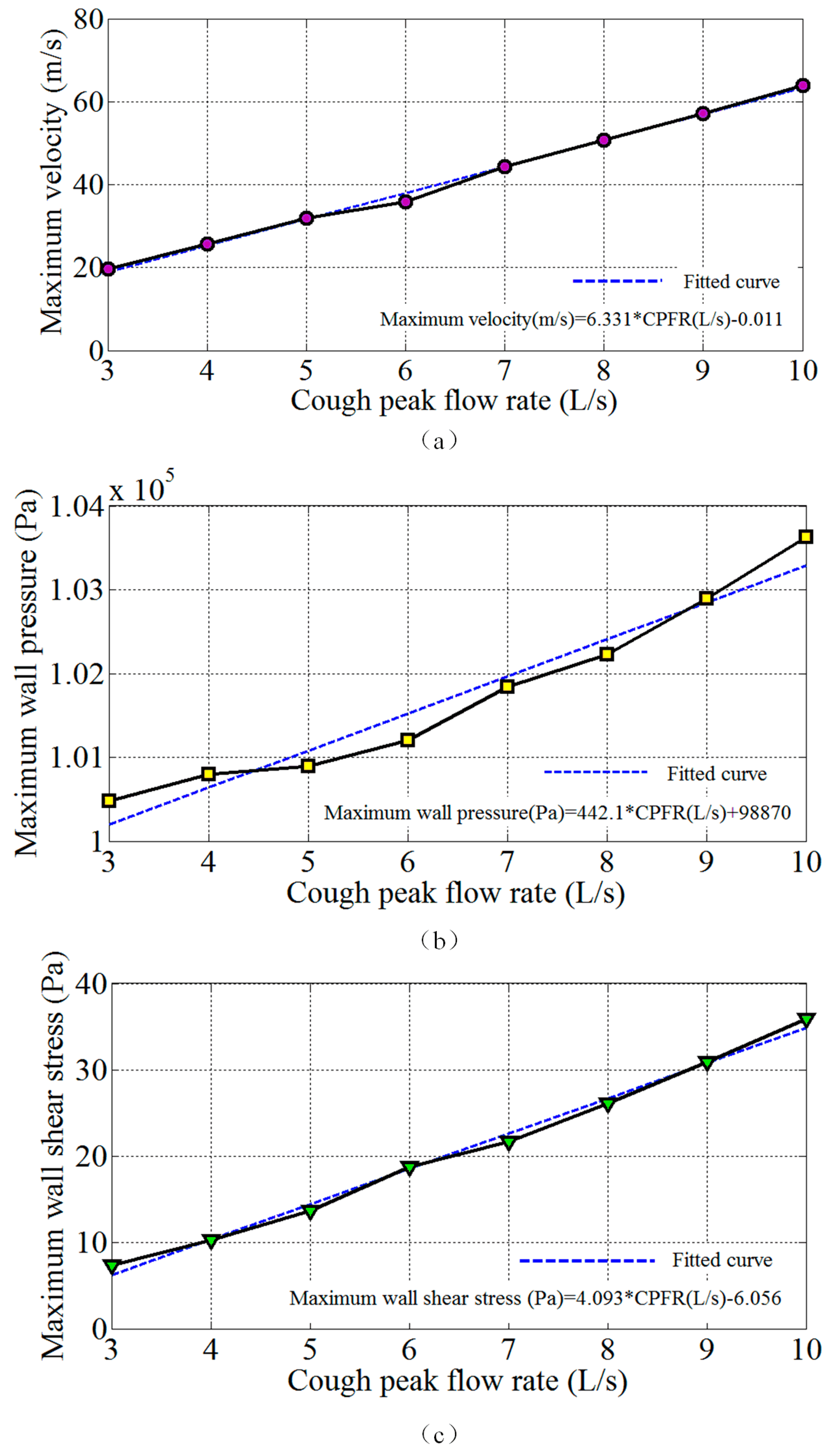
4.3. Influence of the Cough Peak Flow Rate on Airflow Dynamics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| CT | Computerized tomography |
| CFD | Computational fluid dynamics |
| CPFR | Cough peak flow rate |
| MRI | Magnetic resonance imaging |
| QUICK | Quadratic upwind interpolation |
| SST | Shear stress transport |
| SIMPLE | Semi-implicit method |
References
- Dubuis, E.; Wortley, M.A.; Grace, M.S.; Maher, S.A.; Adcock, J.J.; Birrell, M.A.; Belvisi, M.G. Theophylline inhibits the cough reflex through a novel mechanism of action. J. Allergy Clin. Immunol. 2014, 133, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.A.; Federico, L. Cough motor mechanisms. Respir. Physiol. Neurobiol. 2006, 152, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, B.H.; Kuruppumullage, D.N.; Carnaby, G.; Crary, M.; Lehman, J.; Ilegbusi, O.J. Computational modelling of cough function and airway penetrant behavior in patients with disorders of laryngeal function. Laryngoscope Investig. Otolaryngol. 2017, 2, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Vertigan, A.E.; Kapela, S.M.; Franke, I.; Gibson, P.G. The effect of a vocal loading test on cough and phonation in patients with chronic cough. J. Voice 2017, 31, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.B. Forced expiratory technique, directed cough, and autogenic drainage. Respir. Care 2007, 52, 1210–1223. [Google Scholar] [PubMed]
- Gobert, F.; Yonis, H.; Tapponnier, R.; Fernandez, R.; Labaune, M.A.; Burle, J.F.; Vincent, B.; Cleyet, M.; Richard, J.C.; Guérin, C. Predicting extubation outcome by cough peak flow measured using a built-in ventilator flow meter. Respir. Care 2017, 62, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Tzani, P.; Chiesa, S.; Aiello, M.; Scarascia, A.; Catellani, C.; Elia, D.; Marangio, E.; Chetta, A. The value of cough peak flow in the assessment of cough efficacy in neuromuscular patients. A cross sectional study. Eur. J. Phys. Rehabil. Med. 2014, 50, 427–432. [Google Scholar] [PubMed]
- Yang, S.; Lee, G.W.; Chen, C.M.; Wu, C.C.; Yu, K.P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol. Med. 2007, 20, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Y.; Chwang, A.T.; Ho, P.L.; Seto, W.H. How far droplets can move in indoor environments-revisiting the Wells evaporation-falling curve. Indoor Air 2007, 17, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, K.P.; Martyny, J.W.; Fulton, K.E.; Orme, I.M.; Cave, D.M.; Heifets, L.B. Cough-generated aerosols of mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 2004, 169, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.H.; Wan, M.P.; Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.L.; Steve, C.; Li, Y.; Xie, X.; et al. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol. Sci. 2009, 40, 122–133. [Google Scholar] [CrossRef]
- Hersen, G.; Moularat, S.; Robine, E.; Géhin, E.; Corbet, S.; Vabret, A.; Freymuth, F. Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean (Weinh) 2010, 36, 572–577. [Google Scholar] [CrossRef]
- Morawska, L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2010, 16, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Kato, S.; Yang, J.H. Study on transport characteristics of saliva droplets produced by coughing in a calm indoor environment. Build. Environ. 2006, 41, 1691–1702. [Google Scholar] [CrossRef]
- Singh, P.; Mahajan, R.P.; Murty, G.E.; Aitkenhead, A.R. Relationship of peak flow rate and peak velocity time during voluntarv coughing. Br. J. Anaesth. 1995, 74, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.P.; Singh, P.; Murty, G.E.; Aitkenhead, A.R. Relationship between expired lung volume, peak flow rate and peak velocity time during a voluntary cough manoeuvre. Br. J. Anaesth. 1994, 72, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Flow dynamics and characterization of a cough. Indoor Air 2009, 19, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, G.; Murugappan, S.; Mihaescu, M.; Kalra, M.; Khosla, S.; Gutmark, E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J. Biomech. 2009, 42, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, L.T.; Longest, P.W. Validating CFD predictions of highly localized aerosol deposition in airway models: In vitro data and effects of surface properties. J. Aerosol. Sci. 2013, 59, 6–21. [Google Scholar] [CrossRef]
- de Rochefort, L.; Vial, L.; Fodil, R.; Maître, X.; Louis, B.; Isabey, D.; Caillibotte, G.; Thiriet, M.; Bittoun, J.; Durand, E.; et al. In vitro validation of computational fluid dynamic simulation in human proximal airways with hyperpolarized 3he magnetic resonance phase-contrast velocimetry. J. Appl. Physiol. 2007, 102, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kleinstreuer, C. Airflow structures and nano-particle deposition in a human upper airway model. J. Comput. Phys. 2004, 198, 178–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, C.S.; Kleinstreuer, C. Flow structure and particle transport in a triple bifurcation airway model. J. Fluids Eng. 2001, 123, 320–330. [Google Scholar] [CrossRef]
- van Erbruggen, E.; Hirsch, C.; Paiva, M. Anatomically based three-dimensional model of airways to simulate flow and particle transport using computational fluid dynamics. J. Appl. Physiol. 2005, 98, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Suárez, E.; Concheiro, M.; Porteiro, J. Airflow simulation and particulate deposition of a breathing cycle in an extrathoracic model. J. Biomech. 2012, 45, S3. [Google Scholar] [CrossRef]
- Paz, C.; Suárez, E.; Vence, J. CFD transient simulation of the cough clearance process using an Eulerian wall film model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Suárez, E.; Parga, O.; Vence, J. Glottis effects on the cough clearance process simulated with a cfd dynamic mesh and eulerian wall film model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhang, B.; Teng, Y.; Li, J.; Yue, Y.; Kang, Y.; Qian, W. Transient dynamics simulation of airflow in a ct-scanned human airway tree: more or fewer terminal bronchi? Comput. Math. Methods Med. 2017, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Liu, Y. Modeling the bifurcating flow in a ct-scanned human lung airway. J. Biomech. 2008, 41, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; So, R.M.; Zhang, C.H. Modeling the bifurcating flow in a human lung airway. J. Biomech. 2002, 35, 465–473. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Laminar-to-turbulent fluid-particle flows in a human airway model. Int. J. Multiph. Flow 2003, 29, 271–289. [Google Scholar] [CrossRef]





| Right Main Bronchus | Current CFD Simulation | Measurement (Rochefort (2007)) |
|---|---|---|
| A (cm2) | 4.75 | 5.10 |
| Q (L/s) | 0.139 | 0.140 ± 0.011 |
| Location | 3 L/s | 4 L/s | 5 L/s | 6 L/s | 7 L/s | 8 L/s | 9 L/s | 10 L/s |
|---|---|---|---|---|---|---|---|---|
| Left main bronchus | 46.19% | 46.14% | 46.12% | 46.06% | 46.12% | 46.08% | 46.11% | 46.07% |
| Right main bronchus | 53.81% | 53.86% | 53.88% | 53.94% | 53.88% | 53.92% | 53.89% | 53.93% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, G.; Li, X.; Wang, Y.; Lin, M.; Zeng, Y.; Yang, X.; Yang, Y.; Gan, Z. CFD Simulation of Airflow Dynamics During Cough Based on CT-Scanned Respiratory Airway Geometries. Symmetry 2018, 10, 595. https://doi.org/10.3390/sym10110595
Kou G, Li X, Wang Y, Lin M, Zeng Y, Yang X, Yang Y, Gan Z. CFD Simulation of Airflow Dynamics During Cough Based on CT-Scanned Respiratory Airway Geometries. Symmetry. 2018; 10(11):595. https://doi.org/10.3390/sym10110595
Chicago/Turabian StyleKou, Guiyue, Xinghu Li, Yan Wang, Mouyou Lin, Yuping Zeng, Xiaopin Yang, Yanyan Yang, and Zhimei Gan. 2018. "CFD Simulation of Airflow Dynamics During Cough Based on CT-Scanned Respiratory Airway Geometries" Symmetry 10, no. 11: 595. https://doi.org/10.3390/sym10110595
APA StyleKou, G., Li, X., Wang, Y., Lin, M., Zeng, Y., Yang, X., Yang, Y., & Gan, Z. (2018). CFD Simulation of Airflow Dynamics During Cough Based on CT-Scanned Respiratory Airway Geometries. Symmetry, 10(11), 595. https://doi.org/10.3390/sym10110595




