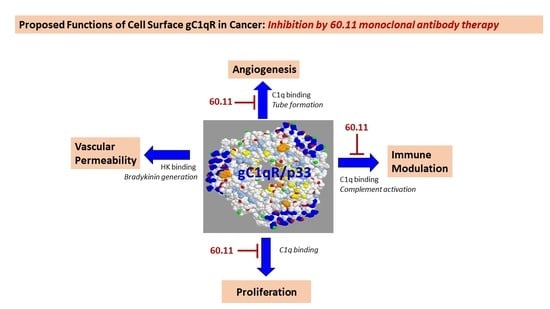
Anti gC1qR/p32/HABP1 Antibody Therapy Decreases Tumor Growth in an Orthotopic Murine Xenotransplant Model of Triple Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibody Production
2.2. Murine Xenotransplantation Model
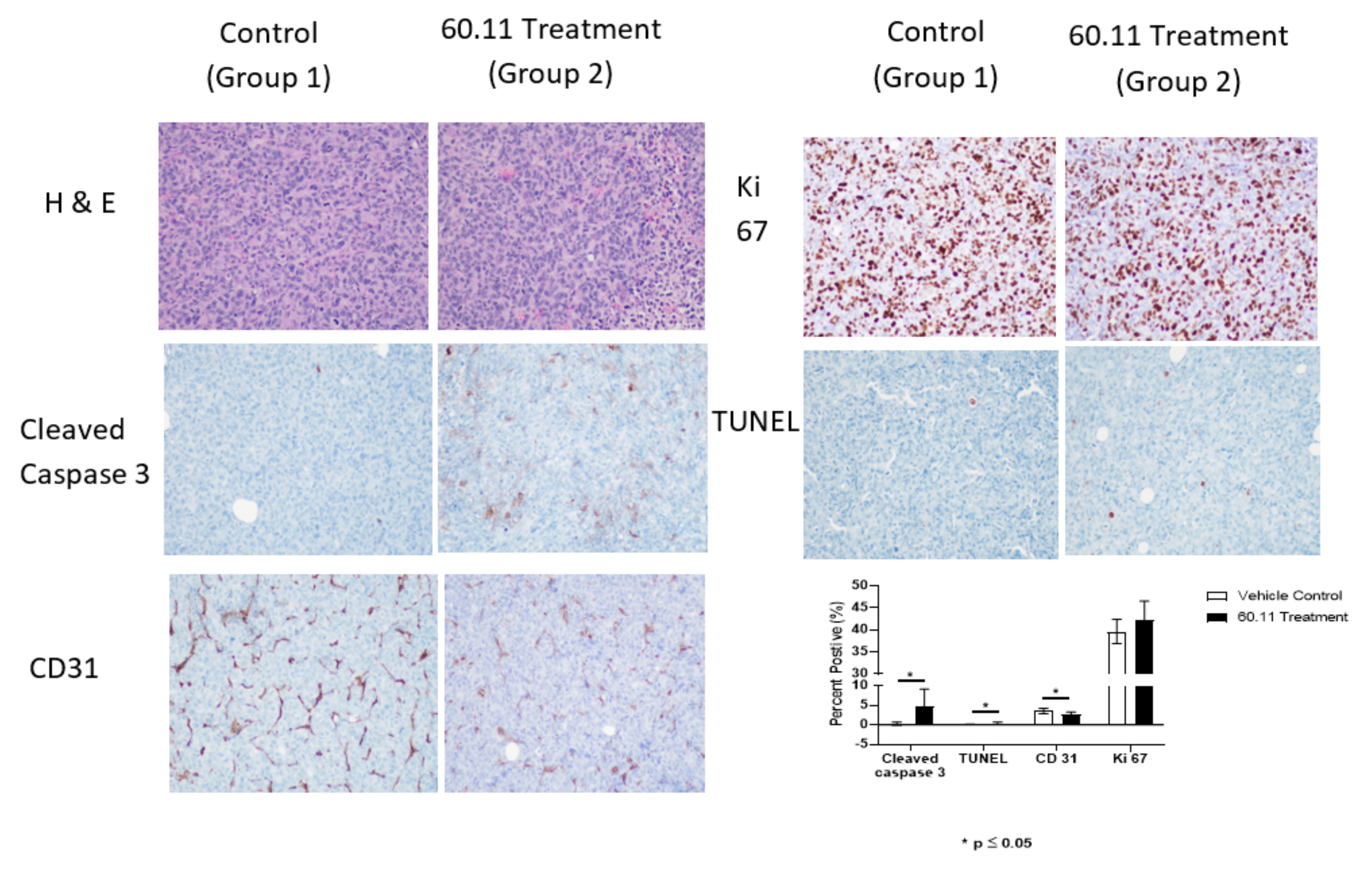
2.3. Immunohistochemical Analysis
2.3.1. Ki 67 Immunostaining
2.3.2. Cleaved Caspase 3 Immunostaining
2.3.3. TUNEL Immunostaining
2.3.4. CD31 Immunostaining
2.4. Quantitative Analysis of Target Staining
3. Results
4. Discussion
5. Patents
Author Contributions
Funding
Conflicts of Interest
Appendix A
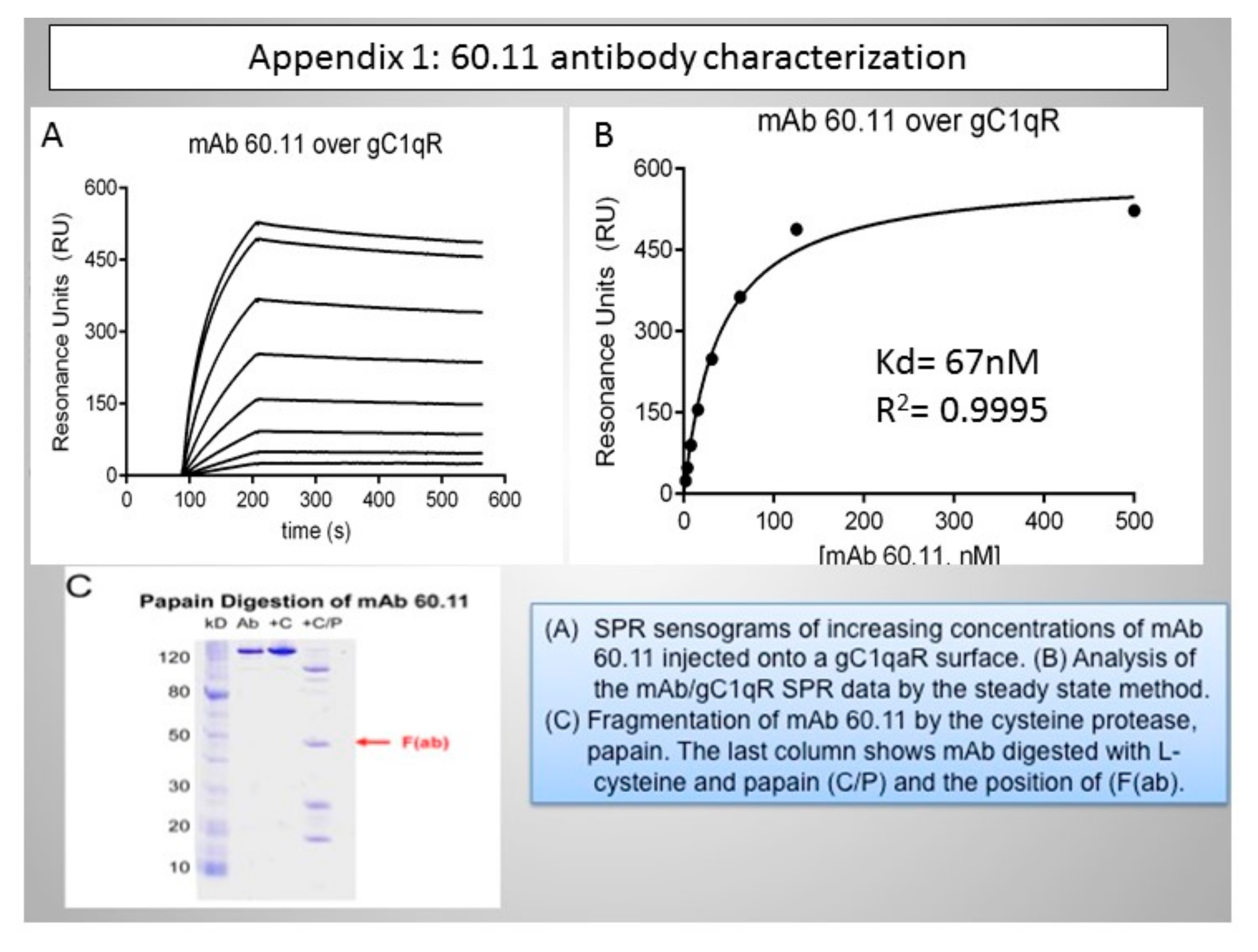
Appendix B
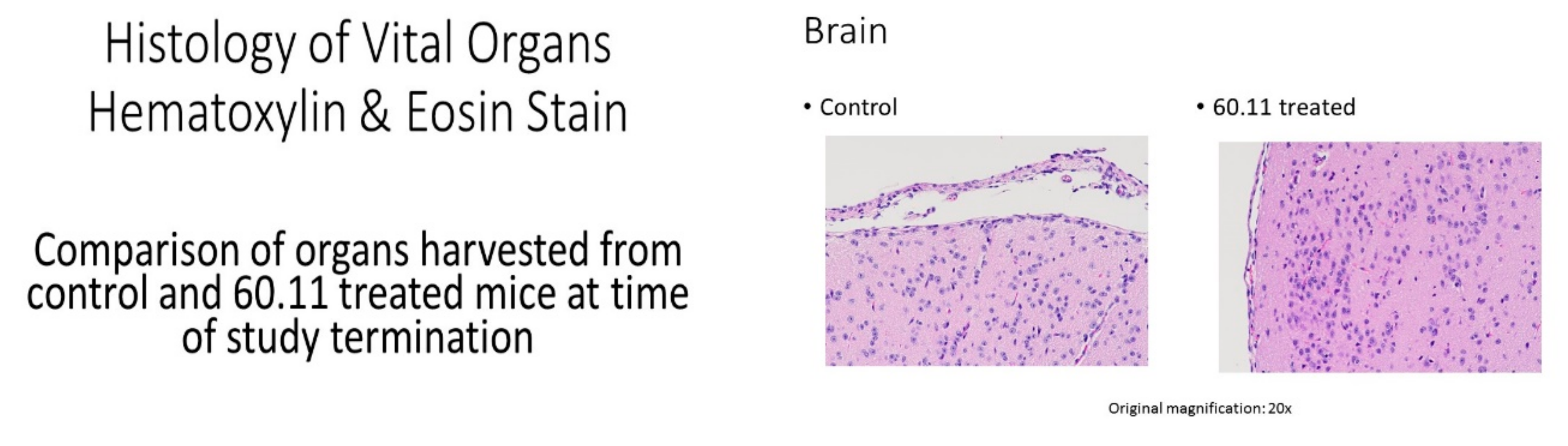
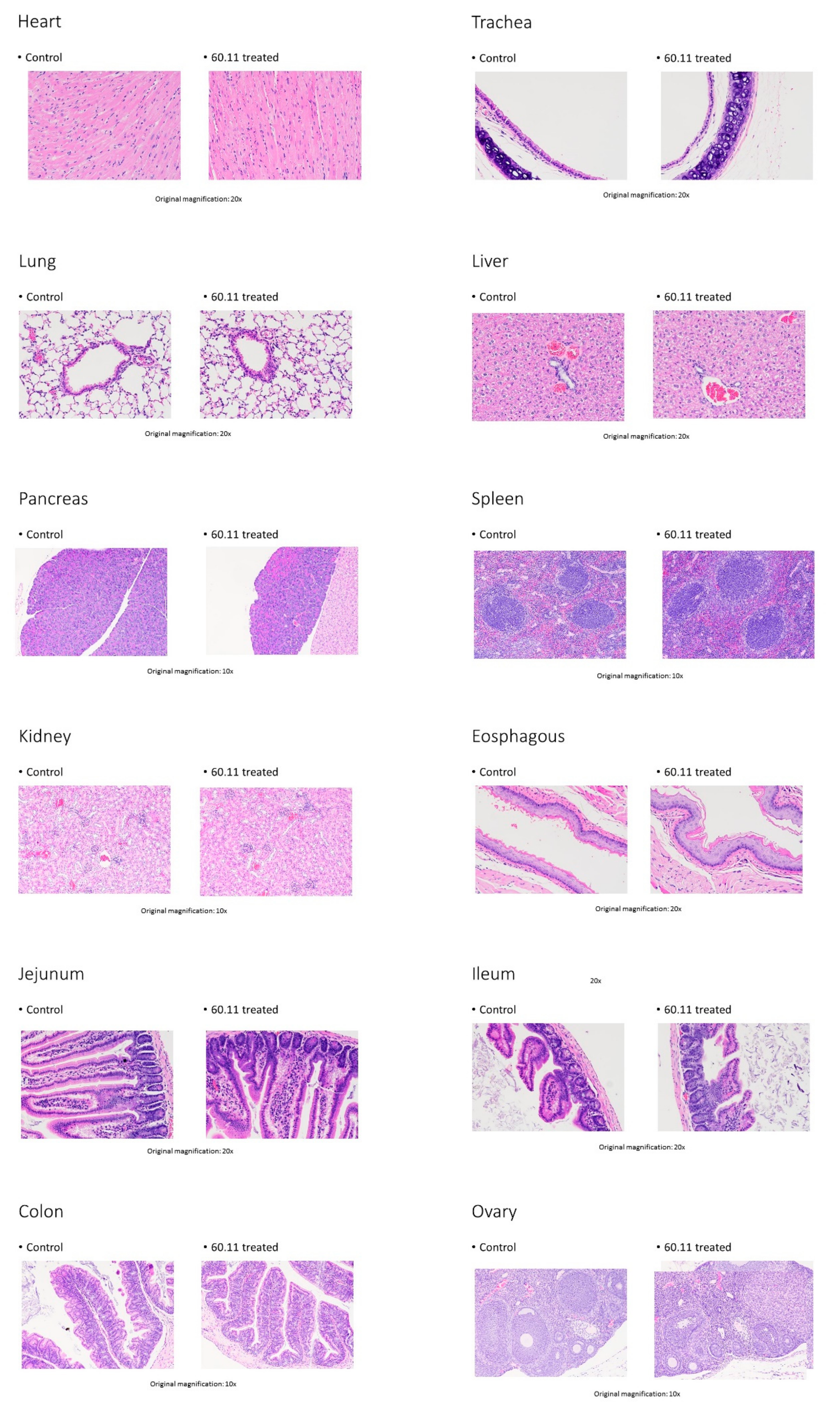
References
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Broglio, K.; Esteva, F.J.; Yang, W.; Kau, S.W.; Islam, R.; Albarracin, C.; Yu, T.K.; Green, M.; Hortobagyi, G.N.; et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann. Oncol. 2009, 20, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Dianan, A.; Franzese, E.; Centonze, S.; Carlino, F.; Della Corte, C.M.; Ventriglia, J.; Petrillo, A.; De Vita, F.; Alfano, R.; Ciardiello, F.; et al. Triple-negative breast cancers: Systematic review of the literature on molecular and clinical features with a focus on treatment with innovative drugs. Curr. Oncol. Rep. 2018, 20, 76. [Google Scholar] [CrossRef]
- Tray, N.; Adams, S.; Esteva, F.J. Antibody-drug conjugates in triple negative breast cancer. Future Oncol. 2018, 14, 2651–2661. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Rafail, S. The dual role of complement in cancer and its implication in anti-tumor therapy. Ann. Transl. Med. 2016, 4, 265. [Google Scholar] [CrossRef]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q acts in the tumor microenvironment as a cancer-promoting factor independently of complement activation. Nature Commun. 2016, 7, 10346. [Google Scholar] [CrossRef]
- Bossi, F.; Tripodo, C.; Rizzi, L.; Bulla, R.; Agostinis, C.; Guarnotta, C.; Munaut, C.; Baldassarre, G.; Papa, G.; Zorzet, S.; et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc. Natl. Acad. Sci. USA 2014, 111, 4209–4214. [Google Scholar] [CrossRef]
- Ghebrehiwet, B.; Hosszu, K.K.; Valentino, A.; Peerschke, E.I.B. The C1q family of proteins: Insights into the emerging non-traditional functions. Front. Immunol. 2012, 3, 52. [Google Scholar] [CrossRef]
- Peerschke, E.I.B.; Ghebrehiwet, B. cC1qR/CR and gC1qR/p33: Observations in cancer. Mol. Immunol. 2014, 61, 100–109. [Google Scholar] [CrossRef]
- Saha, P.; Datta, K. Multifunction, multicompartmental hyaluronan-binding protein 1 (HABP1/p32/gC1qR: Implication in cancer progression and metastasis. Oncotarget 2018, 9, 10784–10807. [Google Scholar] [CrossRef] [PubMed]
- Kandov, E.; Kaur, A.; Kishore, U.; Ji, P.; Williams, J.; Peerschke, E.I.B.; Ghebrehiwet, B. C1q and C1q receptors (gC1qR and cC1qR) as potential novel targets for therapy against breast cancer. Cur. Trends Immunol. 2018, 19, 59–76. [Google Scholar]
- Ghebrehiwet, B.; Lim, B.L.; Peerschke, E.I.; Willis, A.C.; Reid, K.B. Isolation, cDNA cloning, and overexpression of a 33-kDa cell surface glycoprotein that binds to the globular “heads” of C1q. J. Exp. Med. 1994, 179, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, D.B.; Stortchevoi, A.; Boosalis, M.; Ashfaq, R.; Ghebrehiwet, B.; Peerschke, E.I.; Calvo, F.; Guillaume, T. Receptor for the globular heads of C1q [gC1q-R, p33, hyaluronan binding protein) is preferentially expressed by adenocarcinoma cells. Int. J. Cancer 2004, 110, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Dembitzer, F.R.; Kinoshita, Y.; Burstein, D.; Phelps, R.G.; Beasley, M.B.; Garcia, R.; Harpaz, N.; Jaffer, S.; Thung, S.N.; Unger, P.D.; et al. gC1qR expression in normal and pathologic human tissues: Differential expression in tissues of epithelial and mesenchymal origin. J. Histochem. Cytochem. 2012, 60, 467–474. [Google Scholar] [CrossRef]
- Chen, Y.B.; Jiang, C.T.; Zhang, G.Q.; Wang, J.S.; Pang, D. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J. Surg. Oncol. 2009, 100, 382–386. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, H.; Liu, J.; Chen, Y.; Xie, J.; Zhao, Y.; Pang, D. Increased breast cancer risk with HABP1/p32/gC1qR genetic polymorphism rs2285747 and its upregulation in northern Chinese women. Oncotarget 2017, 8, 13932–13941. [Google Scholar] [CrossRef][Green Version]
- Amamoto, R.; Yagi, M.; Song, Y.; Oda, Y.; Tsuneyoshi, M.; Naito, S.; Yokomizo, A.; Kuroiwa, K.; Tokunaga, S.; Kato, S.; et al. Mitochondrial p32/C1QBP is highly expressed in prostate cancer and is associated with shorter prostate-specific antigen relapse time after radical prostatectomy. Cancer Sci. 2011, 102, 639–647. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J. Significance of hyaluronan binding protein [HABP1/P32/gC1qR] expression in advanced serous ovarian cancer patients. Exp. Mol. Pathol. 2013, 94, 210–215. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Yu, G.; Wang, J. Overexpression of HABP1 correlated with clinicopathological characteristics and unfavorable prognosis in endometrial cancer. Tumour Biol. 2015, 36, 1299–1306. [Google Scholar] [CrossRef]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondria/cell surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008, 68, 7210–7218. [Google Scholar] [CrossRef] [PubMed]
- Paasonen, L.; Sharma, S.; Braun, G.B.; Katamraju, V.R.; Chung, T.D.Y.; She, Z.; Sugahara, K.N.; Yliperttula, M.; Wu, B.; Pellecchia, M.; et al. New p32/gC1qR ligands for targeted drug delivery. Chembiochemistry 2016, 17, 570–575. [Google Scholar] [CrossRef]
- Ghebrehiwet, B.; Jesty, J.; Peerschke, E.I.B. gC1qR/p33: Structure-function predictions from the crystal structure. Immunobiology 2002, 205, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Lee, C.H.; Lee, H. Mouse models of breast cancer in preclinical research. Lab. Anim. Res. 2018, 34, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-C.; Hwang, H.-J.; An, H.-T.; Lee, H.; Partk, J.-S.; Hong, J.; Ko, J.; Kim, C.; Lee, J.-S.; Ko, Y.-G. Antibody neutralization of cell-surface gC1qR/HABP1/SF2-p32 prevents lamellipodia formation and tumorigenesis. Oncotarget 2016, 7, 49972–49985. [Google Scholar] [CrossRef]
- McGee, A.M.; Douglas, D.L.; Liang, Y.; Hyder, S.M.; Baines, C.P. The mitochondrial protein C1qbp promotes cell proliferation, migration and resistance to cell death. Cell Cycle 2011, 10, 4119–4127. [Google Scholar] [CrossRef]
- Ghebrehiwet, B.; Lu, P.D.; Zhang, W.; Lim, B.-L.; Eggleton, P.; Leigh, L.E.A.; Reid, K.B.M.; Peerschke, E.I.B. Identification of functional domins on gC1q-R, a cell surface protein, which binds to the globular heads of C1q, using monoclonal antibodies and synthetic peptides. Hybridoma 1996, 15, 333–344. [Google Scholar] [CrossRef]
- Ghebrehiwet, B.; Geisbrecht, B.V.; Xu, X.; Savitt, A.G.; Peerschke, E.I.B. The C1q receptors: Focus on gC1qR [C1qBP, p32, HABP-1]. Semin. Immunol. 2019, 45, 101338. [Google Scholar] [CrossRef]
- Lim, B.L.; White, R.A.; Hummel, G.S.; Mak, S.C.; Schwaeble, W.J.; Reid, K.B.M.; Peerschke, E.I.B.; Ghebrehiwet, B. Characterization of the murine gene for gC1q-BP [gC1q-R], a novel cell protein that binds the globular heads of C1q, vitronectin, high molecular weight kinogen and factor XII. Gene 1998, 209, 229–237. [Google Scholar] [CrossRef]
- Lynch, N.J.; Reid, K.B.M.; van den Berg, R.H.; Daha, M.R.; Leigh, L.E.A.; Lim, B.L.; Ghebrehiwet, B.; Schwaeble, W.J. The murine homologues of gC1qBP, a 33kDa protein that binds to the globular ‘heads’ of C1q. FEBS Lett. 1997, 418, 111–114. [Google Scholar] [CrossRef]
- Savitt, A.G.; Mena-Taboada, P.; Monsalve, G.; Benach, J.L. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection and therapy. Clin. Vacc. Immunol. 2009, 16, 414–422. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/STAT3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.P.; Chang, Q.; Mao, N.; Daly, L.A.; Vogel, R.; Chan, T.; Liu, S.H.; Bournazou, E.; Schori, E.; Zhang, H.; et al. JAK2 inhibition sensitizes resistant EGFR-mutant lung adenocarcinoma to tyrosine kinase inhibitors. Cancer 2016, 9, 421–445. [Google Scholar] [CrossRef] [PubMed]
- Moroz, M.A.; Kochetkov, T.; Cai, S.; Wu, J.; Shamis, M.; Nair, J.; de Stanchina, E.; Serganova, I.; Schwartz, G.K.; Banerjee, D.; et al. Imaging colon cancer response following treatment with AZD1152: A preclinical analysis of [18F]Fluoro-2-deoxuglucose and 3′-deoxy-3′-[18F]Fluorothymidine imaging. Clin. Cancer Res. 2011, 17, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Peerschke, E.I.B.; Bayer, A.S.; Ghebrehiwet, B.; Xiong, Y.Q. gC1qR/p33 blockade reduces Staphylococcus aureus colonization of target tissues in an animal model of infective endocarditis. Infect. Immun. 2006, 74, 4418–4423. [Google Scholar] [CrossRef]
- Rodrik-Outmezguine, V.S.; Okaniwa, M.; Yao, Z.; Novotny, C.J.; McWhirter, C.; Banaji, A.; Won, H.; Wong, W.; Berger, M.; de Stanchina, E.; et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016, 534, 272–276. [Google Scholar] [CrossRef]
- Shaffer, D.R.; Viale, A.; Ishiwata, R.; Leversha, M.; Olgac, S.; Manova, K.; Satagopan, J.; Scher, H.; Koff, A. Evidence for a p27 tumor suppressive function independent of its role regulating cell proliferation in the prostate. Proc. Natl. Acad. Sci. USA 2005, 102, 210–215. [Google Scholar] [CrossRef]
- Peerschke, E.I.B.; Reid, K.B.M.; Ghebrehiwet, B. Identification of a novel 33-kDa C1q binding site on human blood platelets. J. Immunol. 1994, 152, 5896–5901. [Google Scholar]
- Eggleton, P.; Ghebrehiwet, B.; Sastry, K.N.; Coburn, J.P.; Zaner, K.S.; Reid, K.B.M.; Tauber, A.I. Identification of a gC1q-bindin protein [gC1q-R] on the surface of human neutrophils. Subcellular localization and binding properties in comparison with cC1q-R. J. Clin. Investig. 1995, 95, 1569–1578. [Google Scholar] [CrossRef]
- Kuna, P.; Iyer, M.; Peerschke, E.I.; Kaplan, A.P.; Reid, K.B.; Ghebrehiwet, B. Human C1q induces eosinophil migration. Clin. Immunol. Immunopathol. 1996, 81, 48–54. [Google Scholar] [CrossRef]
- Steinberger, P.; Szekeres, A.; Wille, S.; Stockl, J.; Selenko, N.; Prager, E.; Staffler, G.; Madic, O.; Stockinger, H.; Knapp, W. Identification of human CD93 as the phagocytic C1q receptor [C1qRP] by expression cloning. J. Leukoc. Biol. 2002, 71, 33–140. [Google Scholar]
- Vegh, Z.; Goyarts, E.C.; Rozengarten, K.; Mazumder, A.; Ghebrehiwet, B. Maturation-dependent expression of C1q-binding proteins on the cell surface of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2003, 3, 345–357. [Google Scholar] [CrossRef]
- O’Connell, D.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildririm, E.; Starpoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar]
- Ghebrehiwet, B.; Peerschke, E.I.B. Structure and function of gC1qR: A multiligand binding cellular protein. Immunobiology 1998, 199, 225–238. [Google Scholar] [CrossRef]
- Peterson, K.L.; Zhang, W.; Lu, P.D.; Keilbaugh, S.A.; Peerschke, E.I.; Ghebrehiwet, B. The C1q-binding cell membrane proteins cC1qR and gC1qR are released from activated cells: Subcellular distribution and immunochemical characterization. Clin. Immunol. Immunopathol. 1997, 84, 17–26. [Google Scholar] [CrossRef]
- Sanchez-Martin, D.; Cuesta, A.M.; Fogal, V.; Ruoslahti, E.; Alvarez-Vallin, L. The multicompartmental p32/gC1qR as a new target for antibody-based tumor targeting strategies. J. Biol. Chem. 2011, 286, 5197–5203. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Loveland, B.E.; Cebon, J. Cancer exploiting complement: A clue or an exception? Nat. Immunol. 2008, 9, 1205–1206. [Google Scholar] [CrossRef]
- Winslow, S.; Leandersson, K.; Edsjo, A.; Larssen, C. Prognostic stromal gene signatures in breast cancer. Breast Cancer Res. 2015, 17, 23. [Google Scholar] [CrossRef]
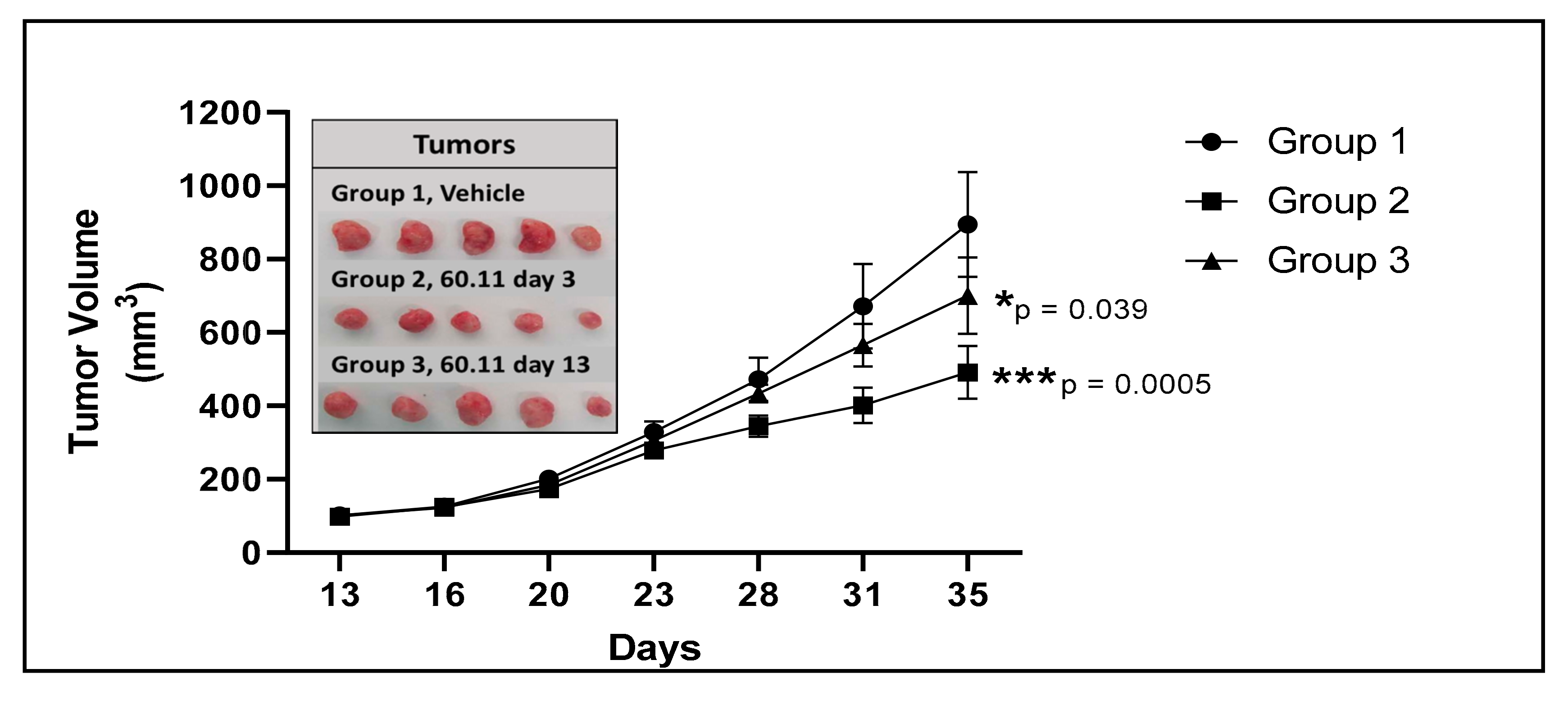
| Vehicle | 60.11 Treatment (Group 2) | 60.11 Treatment (Group 3) | |
|---|---|---|---|
| Tumor Volume (mm3) | 894 ± 143 | 401 ± 48 | 700 ± 104 |
| (p = 8.34 × 10−5) | (p = 0.040) | ||
| Mouse Weight (g) | 24.80 ± 2.16 | 25.00 ± 2.00 | 23.60 ± 1.67 |
| (p = 0.883) | (p = 0.356) | ||
| Serum 60.11 (μg/mL) | undetectable | 52 ± 40 | 49 ± 25 |
| (median 34; range 31–124) | (median 47; range 28–89) |
| Treatment Groups | Reference Values * | ||
|---|---|---|---|
| Cell Count | Vehicle Control (Group 1) | 60.11 Treatment (Group2) | |
| RBC (1012/L) | 9.77 ± 0.05 | 9.68 ± 0.41 (p = 0.779) | 7.4–10.1 |
| Hgb (g/dL) | 15.95 ± 0.81 | 15.58 ± 0.54 (p = 0.441) | 13.2–18.0 |
| Platelets (109/L) | 769 ± 133 | 778 ± 137 (p = 0.923) | 659–1372 |
| WBC (109/L) | 7.38 ± 3.50 | 5.09 ± 1.40 (p = 0.005) | 2.1–11.3 |
| Neutrophils (109/L) | 2.10 ± 0.93 | 1.42 ± 0.44 (p = 0.064) | 0.4–2.1 |
| Lymphocytes (109/L) | 4.98 ± 2.54 | 3.46 ± 1.07 (p = 0.002) | 0.7–9.3 |
| Monocytes (109/L) | 0.20 ± 0.10 | 0.106 ± 0.052 (p = 0.42) | 0.01–0.43 |
| Eosinophils (109/L) | 0.090 ± 0.24 | 0.094 ± 0.017 (p = 7 × 10−5) | 0–0.4 |
| Basophils (109/L) | 0.010 ± 0.005 | 0.006 ± 0.005 (p = 2 × 10−5) | 0–0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peerschke, E.I.; Stanchina, E.d.; Chang, Q.; Manova-Todorova, K.; Barlas, A.; Savitt, A.G.; Geisbrecht, B.V.; Ghebrehiwet, B. Anti gC1qR/p32/HABP1 Antibody Therapy Decreases Tumor Growth in an Orthotopic Murine Xenotransplant Model of Triple Negative Breast Cancer. Antibodies 2020, 9, 51. https://doi.org/10.3390/antib9040051
Peerschke EI, Stanchina Ed, Chang Q, Manova-Todorova K, Barlas A, Savitt AG, Geisbrecht BV, Ghebrehiwet B. Anti gC1qR/p32/HABP1 Antibody Therapy Decreases Tumor Growth in an Orthotopic Murine Xenotransplant Model of Triple Negative Breast Cancer. Antibodies. 2020; 9(4):51. https://doi.org/10.3390/antib9040051
Chicago/Turabian StylePeerschke, Ellinor I., Elisa de Stanchina, Qing Chang, Katia Manova-Todorova, Afsar Barlas, Anne G. Savitt, Brian V. Geisbrecht, and Berhane Ghebrehiwet. 2020. "Anti gC1qR/p32/HABP1 Antibody Therapy Decreases Tumor Growth in an Orthotopic Murine Xenotransplant Model of Triple Negative Breast Cancer" Antibodies 9, no. 4: 51. https://doi.org/10.3390/antib9040051
APA StylePeerschke, E. I., Stanchina, E. d., Chang, Q., Manova-Todorova, K., Barlas, A., Savitt, A. G., Geisbrecht, B. V., & Ghebrehiwet, B. (2020). Anti gC1qR/p32/HABP1 Antibody Therapy Decreases Tumor Growth in an Orthotopic Murine Xenotransplant Model of Triple Negative Breast Cancer. Antibodies, 9(4), 51. https://doi.org/10.3390/antib9040051