Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2
Abstract
1. Introduction
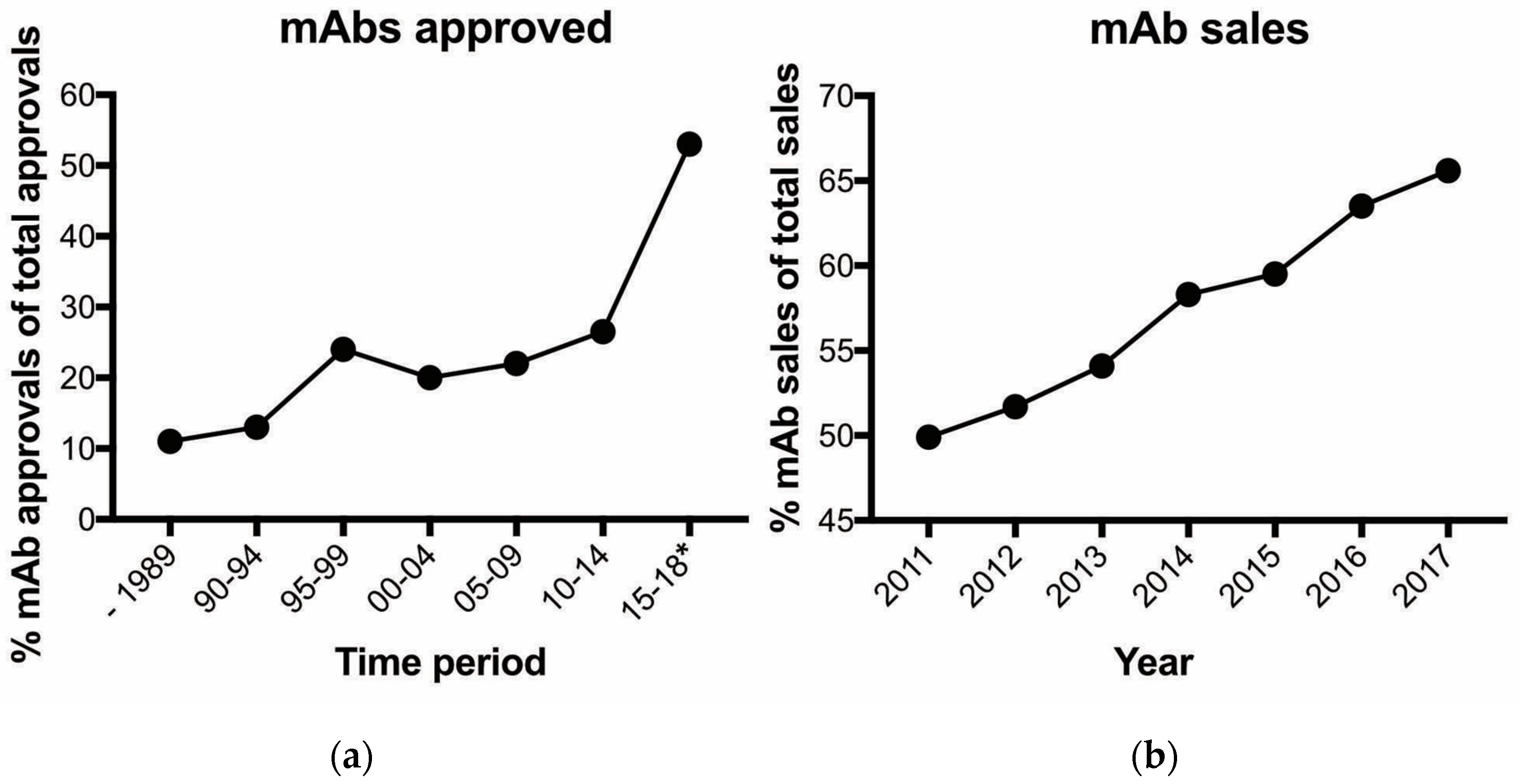
2. Antibodies and Antibody-Based Drugs Are Expensive but Are They Cost-Effective?
3. The HER2 System for Describing the Challenges of Antibodies Accessing the Intracellular Space
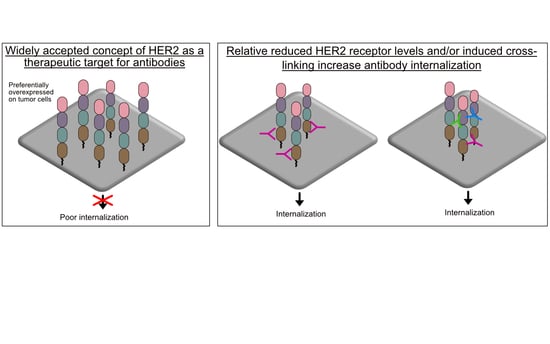
3.1. Antibody HER2 Has Limited Internalization
3.2. Antibody HER2 Is Efficiently Internalized and Is Processed in Lysosomes
- Diessner et al. showed that trastuzumab could internalize in HER2 low expressing cells [54]. Primary tumor cells from metastatic breast cancer patients were cell sorted into a putative cancer stem cell (CSC) population with a CD44 high/CD24 low/HER2 low phenotype [55]. Trastuzumab, directly labeled with a pH-sensitive dye, showed that >50% of the HER2-bound antibody internalized into acidic intracellular vesicles in these HER2 low breast CSCs. In contrast, <2% of trastuzumab internalized in CD44 high/CD24 high/HER2 high non-CSCs. The increased internalization in CSCs was associated with improved cytotoxicity when treated with T-DM1. In the same study, HER2 low expressing cell lines such as MCF7 were also susceptible to T-DM1. The enhanced trastuzumab and T-DM1 internalization was linked to autophagy, a regulated catabolic process that involves the degradation of a cell’s own components via the lysosome [56]. Because autophagy is known to recycle cellular components and, thus, enabling maintenance of cells, especially stem cells, the authors proposed that low HER2 expression was mainly due to internalization via autophagy. This work also slightly contradicts Ram et al., as the results suggested that cell origin is relevant for HER2 internalization.
- De Goeij et al. studied a novel panel of anti-HER2 mAbs for internalization in several HER2-positive cell lines [57]. It was revealed that, especially in HER2 low A431 and Colo205 cells, antibodies that did not interfere with HER2 heterodimerization induced effective internalization. In contrast to the results from Valabrega et al. (Section 3.1), this study suggested that the formation of HER2/ErbB heterodimers enhances antibody internalization and, importantly, heterodimer formation was more frequent in cells with low HER2 expression.
- Fehling-Kaschek et al. revealed that internalization did not occur in SKBR3 cells when HER2 was located in areas where the membrane was smooth [58]. When located in areas where the membrane was ruffled, trastuzumab was able to efficiently internalize. Interestingly, there were higher HER2 densities on membrane ruffles than in the flat regions. It is unclear whether the membrane topology is ruffled or smooth in additional cell types that express low levels of HER2. This most likely indicates that membrane ruffling activity may be an important aspect for internalization. Membrane ruffling is known to occur at distinct cellular zones undergoing rapid reorganization of the plasma membrane [59]. Interestingly, membrane ruffling was observed in EGFR-positive cells when stimulated with EGF [60]. In addition, membrane ruffling has been associated with cancer cell migration and invasion [61,62].
3.3. How Does the HER2 Internalization Profile Impact ADCs?
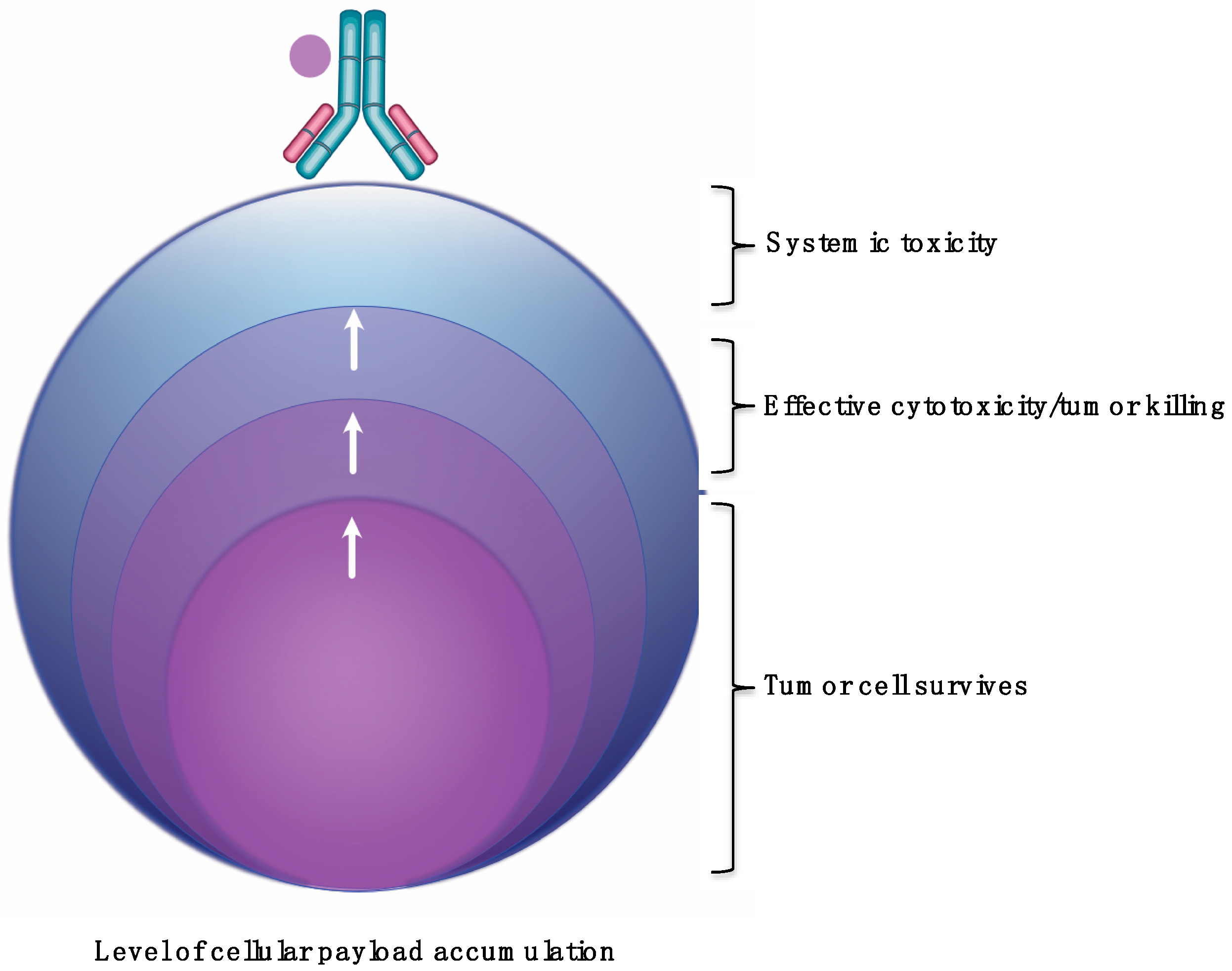
4. ADC Drug Cellular Accumulation and Impact on Cytotoxicity and Tumor Killing
- Linker type: There are contrasting findings between Caculitan et al. and Erickson et al. Both the T-SPP-DM1 and T-VC-MMAE result in a ‘traceless’ release of the drug. However, Erickson et al. showed that T-DM1 accumulated 1.5- and 3.3-fold more DM1 metabolites in BT474EEI and MCF7 cells, respectively, relative to T-SPP-DM1. Accordingly, T-DM1 had an increased cytotoxic potency by 5- and 3.5-fold over T-SPP-DM1 in BT474EEI and MCF7 cells, respectively. This demonstrated that an ADC with a non-cleavable linker accumulated better than a reducible linker. However, Caculitan et al. showed the opposite. The T-vc-MMAE accumulated 3.8-fold more drug in KPL4 cells than T-vc(R)-MMAE. Accordingly, T-vc-MMAE had an increased cytotoxic potency by a factor of 2.1.
- HER2 expression: It is unclear why T-DM1 accumulates the same level of DM1 metabolites in MCF7 HER2 Low as SKBR3 HER2 high cells.
- Resistant cells: Sauveur et al. demonstrated that actually the resistant OE19TR cell line accumulated more DM1 metabolites (albeit only a 28% increase) than the parental OE19 cells. However, T-DM1 still killed OE19 cells with an increased potency by a factor of 15 over the resistant OE19TR cells. The resistant OE19TCR cells accumulated 39% less DM1 metabolites than in parental OE19 cells. Accordingly, T-DM1 had an increased cytotoxic potency in OE19 cells by 19.6-fold over the OE19TCR cells.
- All the studies that investigated cellular payload accumulation were trastuzumab-based ADCs or T-DM1. There is no information on other anti-HER2 ADCs.
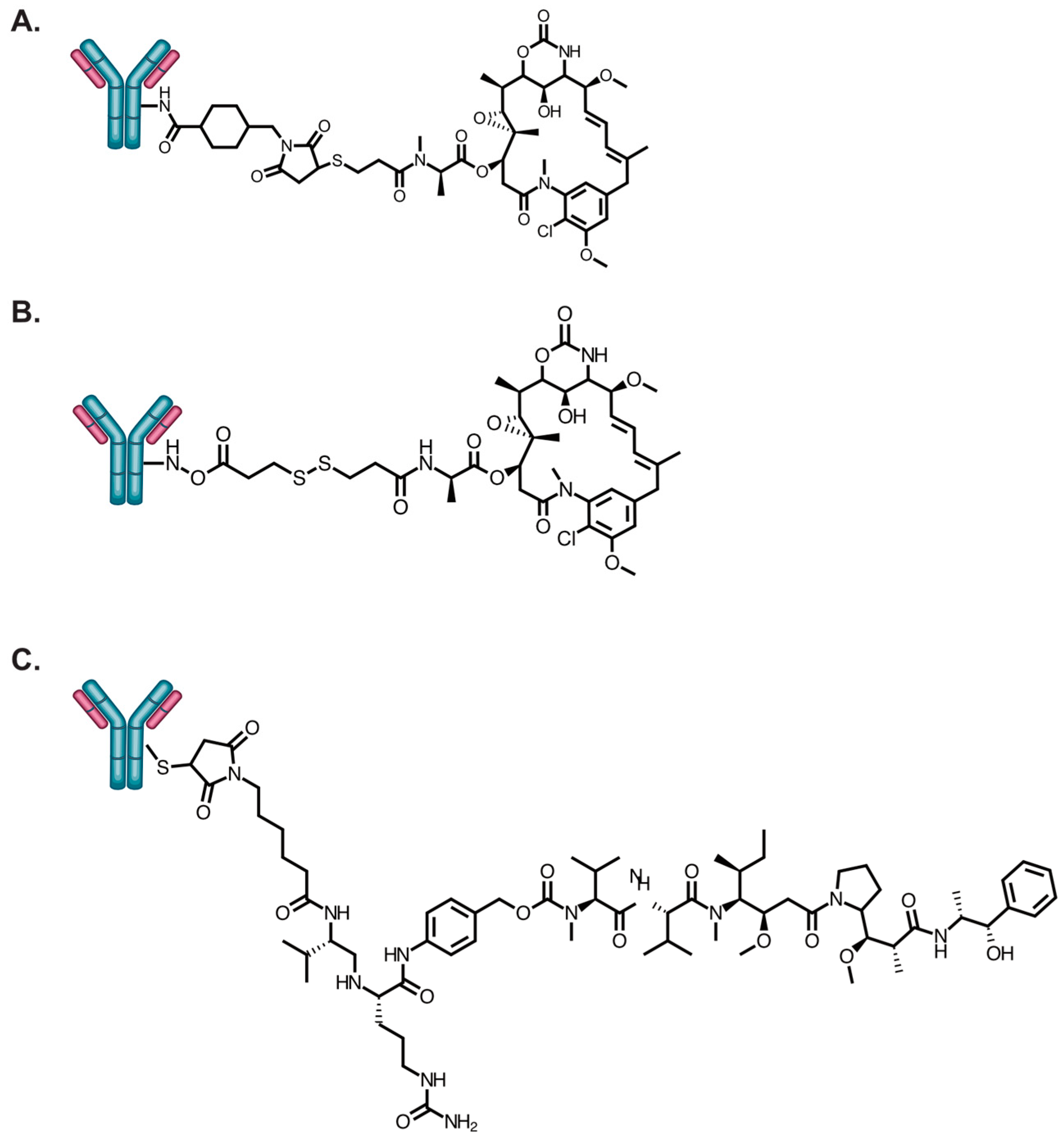
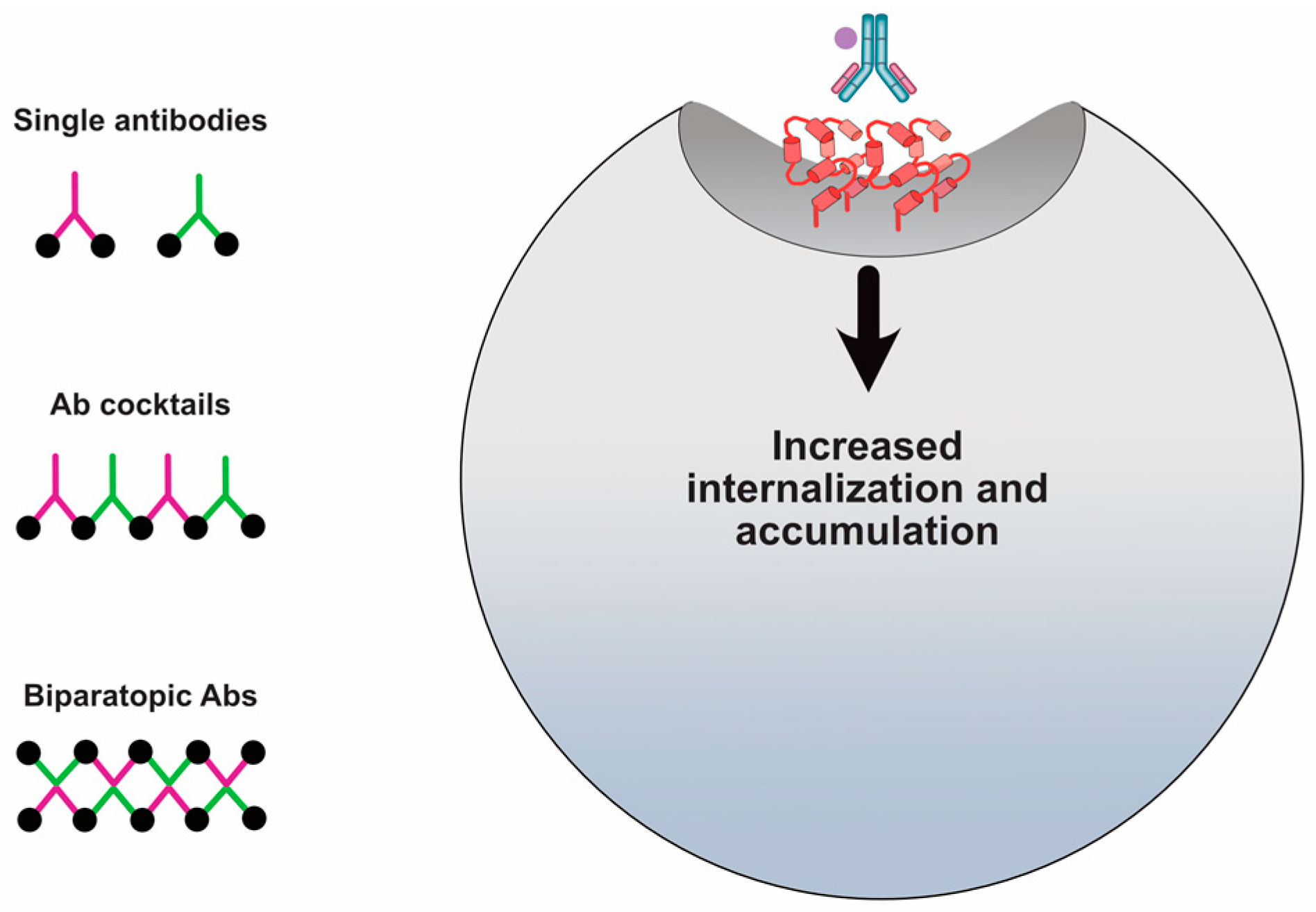
5. Approaches to Improve HER2-Specific Antibody Internalization and Accumulation
5.1. Combination Treatment with Molecular Inhibitors
5.2. Receptor Crosslinking
- (A)
- Trastuzumab/T-DM1 plus pertuzumab cocktails
- (B)
- Other anti-HER2 antibody cocktails
- (C)
- Bispecific antibodies/ADCs
- (D)
- Biparatopic ADCs
- (E)
- Avidin/streptavidin-biotin system
5.3. Membrane Traversal Technologies
5.4. Recommendations for Future Internalization and Accumulation Studies
- The conjugation of dyes directly to the antibodies/ADCs under investigation that only become fluorescent upon internalization into the acidic environment of endosomes and lysosomes is an effective approach to measure lysosomal processing efficiency. Riedl et al. and Nath et al. both published well-described methods and dyes for anti-HER2 ADCs and antibodies, respectively [67,77]. This approach removes necessary washing and cell-stripping steps used by studies in this review. If possible, one could consider the strategy by Lee et al., which incorporated fluorescence resonance energy transfer dyes into the linker connecting the antibody to the drug [136]. Using this approach, one can track both the antibody and payload inside cells.
- Incorporating LC–MS/MS to quantify absolute drug metabolite concentration (nmol/L) in tumor cells. One might also consider acid washing the surface of the cells prior to lysing, so that only intracellular concentrations can be determined. This way, it would be possible to accurately ascertain cellular accumulation levels, especially in cells with poor HER2 internalization and high levels of HER2 expression on the cell surface.
- Evaluate intracellular drug concentrations or antibody internalization in a minimum of three cells lines that have high, intermediate, and low HER2 expression levels and correlate with the impact on cytotoxicity.
- Compare cellular accumulation and cytotoxicity relationship with tumor accumulation and tumor killing to determine whether there is a correlation between in vitro and in vivo.
- Consider the time points used for evaluating internalization/accumulation and cytotoxicity, so that they can be directly associated based on time.
- Consider measuring the expression levels of EFGR, HER3, and HER4 and whether changes in internalization occur, including in the presence of activating ligands.
6. Future
7. Conclusions
8. Patents
Funding
Acknowledgments
Conflicts of Interest
References
- The Global Use of Medicine in 2019 and Outlook to 2023. Available online: Iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (accessed on 28 March 2020).
- Biopharmaceuticals—Market Analysis, Trends, and Forecasts (October 2019). Available online: Researchandmarkets.com/reports/3301135/biopharmaceuticals-market-analysis-trends-and#pos-1 (accessed on 29 March 2020).
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hangin‘ fruit. Nat. Rev. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.W.; Neumann, P.J. The cost-effectiveness of biopharmaceuticals: A look at the evidence. MAbs 2012, 4, 281–288. [Google Scholar] [CrossRef]
- Top 200 Medicines Annual Report 2018: Blockbusters Thriving Despite Tumultuous Climate. Available online: Pharmalive.com/top-200-medicines-annual-report-2018-blockbusters-thriving-despite-tumultuous-climate/ (accessed on 30 March 2020).
- Chambers, J.D.; Thorat, T.; Pyo, J.; Chenoweth, M.; Neumann, P.J. Despite high costs, specialty drugs may offer value for money comparable to that of traditional drugs. Health Aff. (Millwood) 2014, 33, 1751–1760. [Google Scholar] [CrossRef][Green Version]
- Fojo, T.; Grady, C. How much is life worth: Cetuximab, non-small cell lung cancer, and the $440 billion question. J. Natl. Cancer Inst. 2009, 101, 1044–1048. [Google Scholar] [CrossRef]
- NICE Shoots Down Roche’s Tecentriq in Tough-to-Treat Breast Cancer. Available online: Fiercepharma.com/pharma/roche-s-tecentriq-knocked-back-by-nice-despite-fda-eu-approval-rare-breast-cancer (accessed on 24 October 2019).
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody-drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Casi, G.; Neri, D. Antibody-drug conjugates: Basic concepts, examples and future perspectives. J. Control. Release 2012, 161, 422–428. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Squires, H.; Stevenson, M.; Simpson, E.; Harvey, R.; Stevens, J. Trastuzumab Emtansine for Treating HER2-Positive, Unresectable, Locally Advanced or Metastatic Breast Cancer After Treatment with Trastuzumab and a Taxane: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2016, 34, 673–680. [Google Scholar] [CrossRef]
- NICE. Advanced Breast Cancer: Diagnosis and Treatment. Available online: Nice.org.uk/guidance/cg81 (accessed on 1 June 2020).
- Gradishar, W.; Salerno, K.E. NCCN Guidelines Update: Breast Cancer. J. Natl. Compr. Canc. Netw. 2016, 14 (Suppl. 5), 641–644. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.B.; Gonzalez-Martin, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; King-Kallimanis, B.L.; Theoret, M.R.; Ibrahim, A.; et al. FDA Approval Summary: Ado-trastuzumab emtansine for the adjuvant treatment of HER2-positive early breast cancer. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Pollock, A. FDA Approves a New Drug for Advanced Breast Cancer. The New York Times. Available online: Nytimes.com/2013/02/23/business/fda-approves-breast-cancer-drug.html (accessed on 30 March 2020).
- The Lancet. Trastuzumab emtansine and cost-based decision making. Lancet 2017, 389, 2. [Google Scholar] [CrossRef]
- Dillon, A. Recommendations based on value, not cost. Lancet 2017, 389, 1102. [Google Scholar] [CrossRef]
- Le, Q.A.; Bae, Y.H.; Kang, J.H. Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2): Positive advanced breast cancer. Breast Cancer Res. Treat. 2016, 159, 565–573. [Google Scholar] [CrossRef]
- Breast Cancer Drug that Can Extend Lives Approved for NHS Use. The Guardian. Available online: Theguardian.com/society/2017/jun/15/breast-cancer-drug-kadcyla-approved-for-nhs-use (accessed on 25 February 2020).
- pCODR Expert Review Committee (pERC) Final Recommendation. 2014. Available online: Cadth.ca/sites/default/files/pcodr/pcodr-kadcyla-mbc.fn-rec.pdf (accessed on 12 March 2020).
- Trastuzumab Emtansine, Injections, 100 mg vial and 160 mg vial, Kadcyla, Roche Products Pty Ltd. 2014. Available online: Pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2014-03/trastuzumab-psd-03-2014.pdf (accessed on 30 April 2020).
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tusurtani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Blankenship, K. Enhertu. FiercePharma. Available online: Fiercepharma.com/special-report/1-enhertu (accessed on 2 June 2020).
- NICE. Trastuzumab Deruxtecan for Treating HER2-Positive Unresectable or Metastatic Breast Cancer after 2 or More Anti-HER2 Therapies [ID2697]. Available online: Nice.org.uk/guidance/indevelopment/gid-ta10582 (accessed on 2 June 2020).
- Bertelsen, V.; Stang, E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes 2014, 4, 424–446. [Google Scholar] [CrossRef]
- Onsum, M.D.; Geretti, E.; Paragas, V.; Kudla, A.J.; Moulis, S.P.; Luus, L.; Wickham, T.J.; McDonagh, C.F.; MacBeath, G.; Hendriks, B.S. Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am. J. Pathol. 2013, 183, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Broglio, K.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J. Clin. Oncol. 2010, 28, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Pauletti, G.; Dandekar, S.; Rong, H.; Ramos, L.; Peng, H.; Seshadri, R.; Slamon, D.J. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J. Clin. Oncol. 2000, 18, 3651–3664. [Google Scholar] [CrossRef]
- Yan, M.; Achwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Li, G.; Guo, J.; Shen, B.Q.; Yadav, D.B.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol. Cancer Ther. 2018, 17, 1441–1453. [Google Scholar] [CrossRef]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.D.; De Maziere, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Agazie, Y.M. The signaling and transformation potency of the overexpressed HER2 protein is dependent on the normally-expressed EGFR. Cell Signal. 2012, 24, 140–150. [Google Scholar] [CrossRef]
- Hommelgaard, A.M.; Lerdrup, M.; van Deurs, B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol. Biol. Cell 2004, 15, 1557–1567. [Google Scholar] [CrossRef]
- Longva, K.E.; Pedersen, N.M.; Haslekas, C.; Stang, E.; Madshus, I.H. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int. J. Cancer 2005, 116, 359–367. [Google Scholar] [CrossRef]
- Paris, L.; Podo, F.; Spadaro, F.; Abalsamo, L.; Pisanu, M.E.; Ricci, A.; Cecchietti, S.; Altabella, L.; Buoncervello, M.; Lozneanu, L.; et al. Phosphatidylcholine-specific phospholipase C inhibition reduces HER2-overexpression, cell proliferation and in vivo tumor growth in a highly tumorigenic ovarian cancer model. Oncotarget 2017, 8, 55022–55038. [Google Scholar] [CrossRef]
- Pedersen, M.W.; Jacobsen, H.J.; Koefoed, K.; Dahlman, A.; Kjaer, I.; Poulsen, T.T.; Meijer, P.J.; Nielsen, L.S.; Horak, I.D.; Lantto, J.; et al. Targeting Three Distinct HER2 Domains with a Recombinant Antibody Mixture Overcomes Trastuzumab Resistance. Mol. Cancer Ther. 2015, 14, 669–680. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar]
- Valabrega, G.; Montemurro, F.; Sarotto, I.; Petrelli, A.; Rubini, P.; Tacchetti, C.; Aglietta, M.; Comoglio, P.M.; Giordano, D. TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene 2005, 24, 3002–3010. [Google Scholar] [CrossRef]
- Ram, S.; Kim, D.; Ober, R.J.; Ward, E.S. The level of HER2 expression is a predictor of antibody-HER2 trafficking behavior in cancer cells. MAbs 2014, 6, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.S.; Martinez, C.; Vaccaro, C.; Zhou, J.; Tang, Q.; Ober, R.J. From sorting endosomes to exocytosis: Association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol. Biol. Cell 2005, 16, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Diessner, J.; Bruttel, V.; Stein, R.G.; Horn, E.; Hausler, S.F.; Dietl, J.; Honig, A.; Wishhusen, J. Targeting of preexisting and induced breast cancer stem cells with trastuzumab and trastuzumab emtansine (T-DM1). Cell Death Dis. 2014, 5, e1149. [Google Scholar] [CrossRef] [PubMed]
- Reim, F.; Dombrowski, Y.; Ritter, C.; Buttmann, M.; Huasler, S.; Ossadnik, M.; Krockenberger, M.; Beier, D.; Beier, C.P.; Dietl, J.; et al. Immunoselection of breast and ovarian cancer cells with trastuzumab and natural killer cells: Selective escape of CD44high/CD24low/HER2low breast cancer stem cells. Cancer Res. 2009, 69, 8058–8066. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- de Goeij, B.E.; Peipp, M.; de Haij, S.; van den Brink, E.N.; Kellner, C.; Riedl, T.; ke Jong, R.; Vink, T.; Strumane, K.; Bleeker, W.K.; et al. HER2 monoclonal antibodies that do not interfere with receptor heterodimerization-mediated signaling induce effective internalization and represent valuable components for rational antibody-drug conjugate design. MAbs 2014, 6, 392–402. [Google Scholar] [CrossRef]
- Fehling-Kaschek, M.; Peckys, D.B.; Kaschek, D.; Timmer, J.; Jonge, N. Mathematical modeling of drug-induced receptor internalization in the HER2-positive SKBR3 breast cancer cell-line. Sci. Rep. 2019, 9, 12709. [Google Scholar] [CrossRef]
- Mahankali, M.; Peng, H.J.; Cox, D.; Gomez-Cambronero, J. The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association. Cell Signal. 2011, 23, 1291–1298. [Google Scholar] [CrossRef]
- Sorkin, A. Internalization of the epidermal growth factor receptor: Role in signalling. Biochem. Soc. Trans. 2001, 29 Pt 4, 480–484. [Google Scholar] [CrossRef]
- Srinivasan, D.; Plattner, R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006, 66, 5648–5655. [Google Scholar] [CrossRef]
- van Larebeke, N.A.; Bracke, M.E.; Mareel, M.M. Invasive epithelial cells show more fast plasma membrane movements than related or parental non-invasive cells. Cytometry 1992, 13, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bjorkelund, H.; Gedda, L.; Barta, P.; Malmqvist, M.; Andersson, K. Gefitinib induces epidermal growth factor receptor dimers which alters the interaction characteristics with (1)(2)(5)I-EGF. PLoS ONE 2011, 6, e24739. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, T.; Truesdell, P.; Craig, A.W. Endophilin A2 promotes HER2 internalization and sensitivity to trastuzumab-based therapy in HER2-positive breast cancers. Breast Cancer Res. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Cortese, K.; Howes, M.T.; Lundmark, R.; Tagliatti, E.; Bagnato, P.; Petrelli, A.; Bono, M.; McMahon, H.T.; Parton, R.G.; Tacchetti, C. The HSP90 inhibitor geldanamycin perturbs endosomal structure and drives recycling ErbB2 and transferrin to modified MVBs/lysosomal compartments. Mol. Biol. Cell 2013, 24, 129–144. [Google Scholar] [CrossRef]
- Harwerth, I.M.; Wels, W.; Schlegel, J.; Muller, M.; Hynes, N.E. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br. J. Cancer 1993, 68, 1140–1145. [Google Scholar] [CrossRef]
- Nath, N.; Godat, B.; Zimprich, C.; Dwight, S.J.; Corona, C.; McDougall, M.; Urh, M. Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. J. Immunol. Methods 2016, 431, 11–21. [Google Scholar] [CrossRef]
- Sabbaghi, M.; Gil-Gomez, G.; Guardia, C.; Servitja, S.; Arpi, O.; Garcia-Alonso, S.; Menendez, S.; Arumi-Uria, M.; Serrano, L.; Salido, M.; et al. Defective Cyclin B1 Induction in Trastuzumab-emtansine (T-DM1) Acquired Resistance in HER2-positive Breast Cancer. Clin. Cancer Res. 2017, 23, 7006–7019. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.K.; Lewis-Phillips, G.D.; Leipold, D.D.; Provenzano, C.A.; Mai, E.; Johnson, H.A.; Gunter, B.; Audette, C.A.; Gupta, M.; Pinkas, J.; et al. The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Mol. Cancer Ther. 2012, 11, 1133–1142. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl.) 2010, 4, 35–41. [Google Scholar] [CrossRef]
- de Goeij, B.E.; Vink, T.; Ten Napel, H.; Breij, E.C.; Satijn, D.; Wubbolts, R.; Miao, D.; Parren, P.W. Efficient Payload Delivery by a Bispecific Antibody-Drug Conjugate Targeting HER2 and CD63. Mol. Cancer Ther. 2016, 15, 2688–2697. [Google Scholar] [CrossRef]
- Lewis, G.D.; Figari, I.; Fendly, B.; Wong, W.L.; Carter, P.; Gorman, C.; Shepard, H.M. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother. 1993, 37, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, J.; Otsuki, T.; Tang, C.K.; Kurosumi, M.; Yamamoto, S.; Tanaka, K.; Mochizuki, M.; Nakamura, H.; Sonoo, H. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br. J. Cancer 1999, 79, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Caculitan, N.G.; Dela Cruz Chuh, J.; Ma, Y.; Zhang, D.; Kozak, K.R.; Liu, Y.; Pillow, T.H.; Sadowsky, J.; Cheung, T.K.; Phung, Q.Q.; et al. Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody-Drug Conjugates. Cancer Res. 2017, 77, 7027–7037. [Google Scholar] [CrossRef] [PubMed]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.; Bezabeh, B.Z.; Leming, R.L.; Kimasi, N.; et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef]
- Riedl, T.; van Boxtel, E.; Bosch, M.; Parren, P.W.; Gerritsen, A.F. High-Throughput Screening for Internalizing Antibodies by Homogeneous Fluorescence Imaging of a pH-Activated Probe. J. Biomol. Screen. 2016, 21, 12–23. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Anghel, A.; Desai, A.J.; Ayala, R.; Luo, T.; Safran, B.; Fejzo, M.S.; Hecht, J.R.; Slamon, D.J.; Finn, R.S. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin. Cancer Res. 2010, 16, 1509–1519. [Google Scholar] [CrossRef]
- Sauveur, J.; Matera, E.L.; Chettab, K.; Valet, P.; Guitton, J.; Savina, A.; Dumontet, C. Esophageal cancer cells resistant to T-DM1 display alterations in cell adhesion and the prostaglandin pathway. Oncotarget 2018, 9, 21141–21155. [Google Scholar] [CrossRef]
- Kasprzyk, P.G.; Song, S.U.; DiFiore, P.P.; King, C.R. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992, 52, 2771–2776. [Google Scholar]
- Hurwitz, E.; Stancoviski, I.; Sela, M.; Yarden, Y. Suppression and promotion of tumor growth by monoclonal antibodies to ErbB-2 differentially correlate with cellular uptake. Proc. Natl. Acad. Sci. USA 1995, 92, 3353–3357. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xu, Y.; Yang, Y.; Chen, X.; Quan, H.; Lou, L. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in human epidermal growth factor receptor 2-positive gastric cancer cells. Cancer Sci. 2017, 108, 1458–1468. [Google Scholar] [CrossRef]
- Hughes, J.B.; Berger, C.; Rodland, M.S.; Hasmann, M.; Stang, E.; Madshus, I.H. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol. Cancer Ther. 2009, 8, 1885–1892. [Google Scholar] [CrossRef]
- Hughes, J.B.; Rodland, M.S.; Hasmann, M.; Madshus, I.H.; Stang, E. Pertuzumab Increases 17-AAG-Induced Degradation of ErbB2, and This Effect Is Further Increased by Combining Pertuzumab with Trastuzumab. Pharmaceuticals 2012, 5, 674–689. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, P.M.; Weiss, D.; Guardino, E.; Girish, S.; Sliwkowski, M.X. Trastuzumab emtansine: A unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin. Cancer Res. 2011, 17, 6437–6447. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blattler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Cilliers, C.; Menezes, B.; Nessler, I.; Linderman, J.; Thurber, G.M. Improved Tumor Penetration and Single-Cell Targeting of Antibody-Drug Conjugates Increases Anticancer Efficacy and Host Survival. Cancer Res. 2018, 78, 758–768. [Google Scholar] [CrossRef]
- Kulkarni, C.; Finley, J.E.; Bessire, A.J.; Zhong, X.; Musto, S.; Graziani, E.I. Development of Fluorophore-Labeled Thailanstatin Antibody-Drug Conjugates for Cellular Trafficking Studies. Bioconjug. Chem. 2017, 28, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Jacob, A.P.; Gurgel, J.L.; Tometsko, M.E.; Rock, S.M.; Patel, S.K.; Milburn, R.R.; Siu, S.; Ragan, S.P.; Rock, D.A.; et al. SLC46A3 Is Required to Transport Catabolites of Noncleavable Antibody Maytansine Conjugates from the Lysosome to the Cytoplasm. Cancer Res. 2015, 75, 5329–5340. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Gao, M.; Fu, L.; Li, Y.; Quan, H.; Lou, L. STAT3 activation confers trastuzumab-emtansine (T-DM1) resistance in HER2-positive breast cancer. Cancer Sci. 2018, 109, 3305–3315. [Google Scholar] [CrossRef]
- Uppal, H.; Doudement, E.; Mahapatra, K.; Darbonne, W.C.; Bumbaca, D.; Shen, B.Q.; Du, X.; Saad, O.; Bowles, K.; Olsen, S.; et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin. Cancer Res. 2015, 21, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Doronina, S.O.; Mendelsohn, B.A.; Bovee, T.D.; Cerveny, C.G.; Alley, S.C.; Meyer, D.L.; Oflazoglu, E.; Toki, B.E.; Sanderson, R.J.; Zabrinski, R.F.; et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: Effects of linker technology on efficacy and toxicity. Bioconjug. Chem. 2006, 17, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.V.; Audette, C.A.; Mayo, M.F.; Jones, G.E.; Doherty, H.; Maloney, E.K.; Erickson, H.K.; Sun, X.; Wilhelm, S.; Ab, O.; et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010, 70, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; LoRusso, P.; Miller, K.D.; Modi, S.; Yardley, D.; Rodriguez, G.; Guardino, E.; Lu, M.; Zheng, M.; Girish, S.; et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 2012, 30, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Dirix, L.; Kocsis, J.; Bianchi, G.V.; Lu, J.; Vinholes, J.; Guardino, E.; Song, C.; Tong, B.; Ng, V.; et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2013, 31, 1157–1163. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.F.; Khojasteh, S.C.; Ma, Y.; Pillow, T.H.; Sadowsky, J.D.; Su, D.; Kozak, K.R.; Xu, K.; Polson, A.G.; et al. Intratumoral Payload Concentration Correlates with the Activity of Antibody-Drug Conjugates. Mol. Cancer Ther. 2018, 17, 677–685. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Xu, W.; Neckers, L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003, 3, 213–217. [Google Scholar] [CrossRef]
- Tikhomirov, O.; Carpenter, G. Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J. Biol. Chem. 2000, 275, 26625–26631. [Google Scholar] [CrossRef]
- Austin, C.D.; Wen, X.; Gazzard, L.; Nelson, C.; Scheller, R.H.; Scales, S.J. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. USA 2005, 102, 17987–17992. [Google Scholar] [CrossRef]
- Garg, G.; Khandelwal, A.; Blagg, B.S. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv. Cancer Res. 2016, 129, 51–88. [Google Scholar] [PubMed]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; Cecchetti, S.; Spadaro, F.; Abalsamo, L.; Lugini, L.; Pisanu, M.E.; Iorio, E.; Natali, P.G.; Ramoni, C.; Podo, F. Inhibition of phosphatidylcholine-specific phospholipase C downregulates HER2 overexpression on plasma membrane of breast cancer cells. Breast Cancer Res. 2010, 12, R27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.B.; Neil, J.R.; Abramovitch, S.; Yarden, Y.; White, F.M.; Lauffenburger, D.A.; Wittrup, K.D. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13252–13257. [Google Scholar] [CrossRef]
- Nami, B.; Maadi, H.; Wang, Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers 2018, 10, 342. [Google Scholar] [CrossRef]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Takai, N.; Jain, A.; Kawamata, N.; Popoviciu, L.M.; Said, J.W.; Whittaker, S.; Miyakawa, I.; Agus, D.B.; Koeffler, H.P. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer 2005, 104, 2701–2708. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, X.; Bai, Y.; McBride, H.J.; Huang, X. Cryo-EM Structure of HER2-trastuzumab-pertuzumab complex. PLoS ONE 2019, 14, e0216095. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), S9–S15. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Yuan, L.; Garrett, J.T. HER3 signaling and targeted therapy in cancer. Oncol. Rev. 2018, 12, 355. [Google Scholar] [CrossRef]
- Wang, J.; Yin, J.; Yang, Q.; Ding, F.; Chen, X.; Li, B.; Tian, X. Human epidermal growth factor receptor 4 (HER4) is a favorable prognostic marker of breast cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 76693–76703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Memon, A.A.; Sorensen, B.S.; Melgard, P.; Fokdal, L.; Thykjaer, T.; Nexo, E. Expression of HER3, HER4 and their ligand heregulin-4 is associated with better survival in bladder cancer patients. Br. J. Cancer 2004, 91, 2034–2041. [Google Scholar] [CrossRef]
- Miller, K.D.; Dieras, V.; Harbeck, N.; Andre, F.; Mahtani, R.L.; Gianni, L.; Albain, K.S.; Crivellari, D.; Fang, L.; Michelson, G.; et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J. Clin. Oncol. 2014, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.B.; Burris, H.A.; et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2-positive advanced breast cancer: Final results from MARIANNE. Cancer 2019, 125, 3974–3984. [Google Scholar] [CrossRef]
- Spiridon, C.I.; Ghetie, M.A.; Uhr, J.; Marches, R.; Li, J.L.; Shen, G.L.; Vitetta, E.S. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin. Cancer Res. 2002, 8, 1720–1730. [Google Scholar]
- Ben-Kasus, T.; Schechter, B.; Lavi, S.; Yarden, Y.; Sela, M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3294–3299. [Google Scholar] [CrossRef]
- Varghese, B.; Barriere, H.; Carbone, C.J.; Banerjee, A.; Swaminathan, G.; Plotnikov, A.; Xu, P.; Peng, J.; Goffin, V.; Lukacs, G.L.; et al. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol. Cell Biol. 2008, 28, 5275–5287. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Brack, S.; Attinger-Toller, I.; Schade, B.; Mourlane, F.; Klupsch, K.; Woods, R.; Hachemi, H.; von der Bey, U.; Koenig-Friedrich, S.; Bertschinger, J.; et al. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol. Cancer Ther. 2014, 13, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Moody, P.R.; Sayers, D.J.; Magnusson, J.P.; Alexander, C.; Borri, P.; Watson, P.; Jones, A.T. Receptor Crosslinking: A General Method to Trigger Internalization and Lysosomal Targeting of Therapeutic Receptor:Ligand Complexes. Mol. Ther. 2015, 23, 1888–1898. [Google Scholar] [CrossRef]
- Zhu, W.; Okollie, B.; Artemov, D. Controlled internalization of Her-2/ neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 2007, 6, 1960–1966. [Google Scholar] [CrossRef]
- Jarver, P.; Langel, U. The use of cell-penetrating peptides as a tool for gene regulation. Drug Discov. Today 2004, 9, 395–402. [Google Scholar] [CrossRef]
- Niesner, U.; Halin, C.; Lozzi, L.; Gunthert, M.; Neri, P.; Wunderli-Allenspach, H.; Zardi, L.; Neri, D. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug. Chem. 2002, 13, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.; Kayser, V. Synthesis and Enhanced Cellular Uptake In Vitro of Anti-HER2 Multifunctional Gold Nanoparticles. Cancers 2019, 11, 870. [Google Scholar] [CrossRef]
- Guo, K.; Hawkins, R.; Wu, B. Engineering a cell-penetrating anti-HER2 monoclonal antibody for efficient delivery of gold nanoparticles into cancer cells to enhance X-ray cancer radiation therapy. J. Young Investig. 2020, 38, 13–22. [Google Scholar]
- Beaudoin, S.; Barok, M.; Charbonneau, M.; Dubois, C.; Joensuu, H.; Tai, L.H.; Leyton, J.V. CellAccumulator (ACCUM): A novel technology that enhances trastuzumab-emtansine cellular accumulation and cytotoxic effectiveness. In Proceedings of the EACR-AACR-SIC Special Conference 2017, Firenze Fiera, Florence, Italy, 24–27 June 2017. Book Abstract 314. [Google Scholar]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Shivanna, V.; Kim, Y.; Chang, K.O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology 2014, 456–457, 268–278. [Google Scholar] [CrossRef]
- Shivanna, V.; Kim, Y.; Chang, K.O. Ceramide formation mediated by acid sphingomyelinase facilitates endosomal escape of caliciviruses. Virology 2015, 483, 218–228. [Google Scholar] [CrossRef]
- Contreras, F.X.; Villar, A.V.; Alonso, A.; Goni, F.M. Ceramide-induced transbilayer (flip-flop) lipid movement in membranes. Methods Mol. Biol. 2009, 462, 155–165. [Google Scholar]
- Samanta, S.; Stiban, J.; Maugel, T.K.; Colombini, M. Visualization of ceramide channels by transmission electron microscopy. Biochim. Biophys. Acta 2011, 1808, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Chalouni, C.; Doll, S.; Nalle, S.C.; Darwish, M.; Tsai, S.P.; Kozak, K.R.; Del-Rosario, G.; Yu, S.F.; Erickson, H.; et al. FRET Reagent Reveals the Intracellular Processing of Peptide-Linked Antibody-Drug Conjugates. Bioconjug. Chem. 2018, 29, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Chen, Z.; Yang, T.; Yang, D.; Liu, Q.; Du, K.; Li, B.; Wang, Z.; Li, S.; et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnol. 2017, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Goldenberg, D.M. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 2011, 17, 3157–3169. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. (Tokyo) 2019, 67, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Ahu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur. J. Med. Chem. 2019, 183, 111682. [Google Scholar] [CrossRef]
- Nagai, Y.; Oitate, M.; Shiozawa, H.; Ando, O. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica 2019, 49, 1086–1096. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Source 1 | Tumor Type 2 | HER2 Expression 3 | Internalization Studies | Accumulation Studies |
|---|---|---|---|---|---|
| Breast cancer | |||||
| SKBR3 | PE | AC | High [52,63] | [44,46,47,49,51,52,58,64,65,66,67,68] | [69] |
| AU565 | PE | AC | High [70] | [57] | |
| BT474 | PT | IDC | High [52] | [44,49,52] | [69] |
| MCF7 | PE | IDC | Low [70] | [44,54,67] | [69] |
| HCC1419 | PT | Duc.Ca | High [52] | [52,68] | |
| HCC1954 | PT | Duc.Ca | High [71] | [52,64,68] | |
| HCC2185 | PE | MLCa | Low [52] | [52] | |
| HCC202 | PT | Duc.Ca | [49] | ||
| BT483 | PT | IDC | Intermediate [52] | [52] | |
| CSC4 | PE | IDC | Low [54] | [54] | |
| MDA-MB-175-VII | PE | IDC | Low [72] | [49] | |
| ZR75-1 | PE | AC | Intermediate [52] | [52] | |
| KPL4 | PE | AC | High/Int. [73] | [74] | |
| T47D | Low [75] | [75,76] | |||
| Prostate cancer | |||||
| LNCap | M | PC | Low [52] | [52] | |
| LAPC-4 | M | PC | Low [52] | [52] | |
| C42B | M | PC | Low [52] | [52] | |
| Ovarian, gastric, colorectal cancers and melanoma | |||||
| SKOV3 | AF | OC | Intermediate [48,71] | [47,48,66,77] | |
| SKOV3.ip | AF | OC | High [48,71] | [48] | |
| OE19 | PT | OC | High [78] | [79] | |
| N87 | M | GC | High [80] | [49,80,81] | [82] |
| Colo205 | PT | AC | Low [57] | [57] | |
| A431 | PT | SC | Low [63] | [57,77] | |
| Non-tumor model systems | |||||
| Porcine aortic endothelial cells | [83,84] | ||||
| Study | LC–MS/MS Method | Cell and ADC | Cell Incubation Parameters |
|---|---|---|---|
| Erickson et al. [69] | Converted from radioactivity counts | BT474EEI, SKBR3, and MCF7 cells in T75 flasks (107 cells). T-[3H]DM1 or T-SPP-[3H]DM1. | 20–40 nmol/L ADC pulsed for 30 min. Media replaced with fresh media and sampled at 24 h. |
| Caculitan et al. [74] | Calibration curve | KPL4 and KPL4-cathepsin B knocked down cells in 96-well plates. T-MMAE with protease-sensitive vc linker. | 0–10 μg/mL ADC for 24 h. |
| Caculitan et al. [74] | Calibration curve | KPL4 and KPL4-cathepsin B knocked down cells in T75 flasks. T-MMAE with vc linker or vc isomer that is protease-resistant (R). | 1 h pulse. Media replaced with fresh media and sampled at 4 h or 24 h. ADC concentration not reported. |
| Sauveur et al. [79] | Calibration curve | OE19 and OE19-resistant cells exposed to T-DM1. Cell format not reported. | 5 nmol/L for 24 h. |
| Wang et al. [82] | Calibration curve | N87 and N87-KR cells exposed to T-DM1 in 6-well plates. | 10 μg/mL for 24 h. |
| Uppal et al. [92] | Calibration curve | 750,000 donor cells separated into hematopoietic stem cells, immature and mature megakaryocyte populations. | 6.25 μg/mL of T-DM1 for 24 h. |
| Non-Cleavable Trastuzumab ADCs | ||||
|---|---|---|---|---|
| Cells | ADC | T-DM1 Sensitive | Accumulation (nmol/L) | IC50 (nmol/L) |
| KPL4 | T-vc(R)-MMAE | Yes | 125 | 0.42 |
| SKBR3 | T-DM1 | Yes | 500 | 0.0073 |
| BT474EEI | T-DM1 | Yes | 300 | 0.04 |
| MCF7 | T-DM1 | Yes | 500 | 0.02 |
| OE19 | T-DM1 | Yes | 4.1 1 | 0.05 |
| OE19TR | T-DM1 | No | 5.7 1 | 0.73 |
| OE19TCR | T-DM1 | No | 2.5 1 | 0.98 |
| N87 | T-DM1 | Yes | 4.9 1 | 0.2 |
| N87KR | T-DM1 | No | 3.2 1 | 12.5 |
| Premature megakaryocytes 2 | T-DM1 | Yes | 8.2 | 20 |
| Cleavable/reducible trastuzumab ADCs | ||||
| KPL4 | T-vc-MMAE | Yes | 475 | 0.2 |
| BT474EEI | T-SPP-DM1 | Yes | 200 | 0.2 |
| MCF7 | T-SPP-DM1 | Yes | 150 | 0.07 |
| Domains | Internalization | Relative HER2 Degradation 2 |
|---|---|---|
| I and II | No | No |
| II and IV | No | Weak |
| I and IV | Yes | Strong |
| I, II, and IV | Yes | Strong |
| Trastuzumab + Pertuzumab | Yes | Weak |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyton, J.V. Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2. Antibodies 2020, 9, 32. https://doi.org/10.3390/antib9030032
Leyton JV. Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2. Antibodies. 2020; 9(3):32. https://doi.org/10.3390/antib9030032
Chicago/Turabian StyleLeyton, Jeffrey V. 2020. "Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2" Antibodies 9, no. 3: 32. https://doi.org/10.3390/antib9030032
APA StyleLeyton, J. V. (2020). Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2. Antibodies, 9(3), 32. https://doi.org/10.3390/antib9030032