CD1d Selectively Down Regulates the Expression of the Oxidized Phospholipid-Specific E06 IgM Natural Antibody in Ldlr−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains
2.2. Splenic and Peritoneal Cell Preparations
2.3. Measurement of Antibody Titer by ELISA
2.4. ELISPOT Assay
2.5. Flow Cytometry and Cell Sorting
3. Results
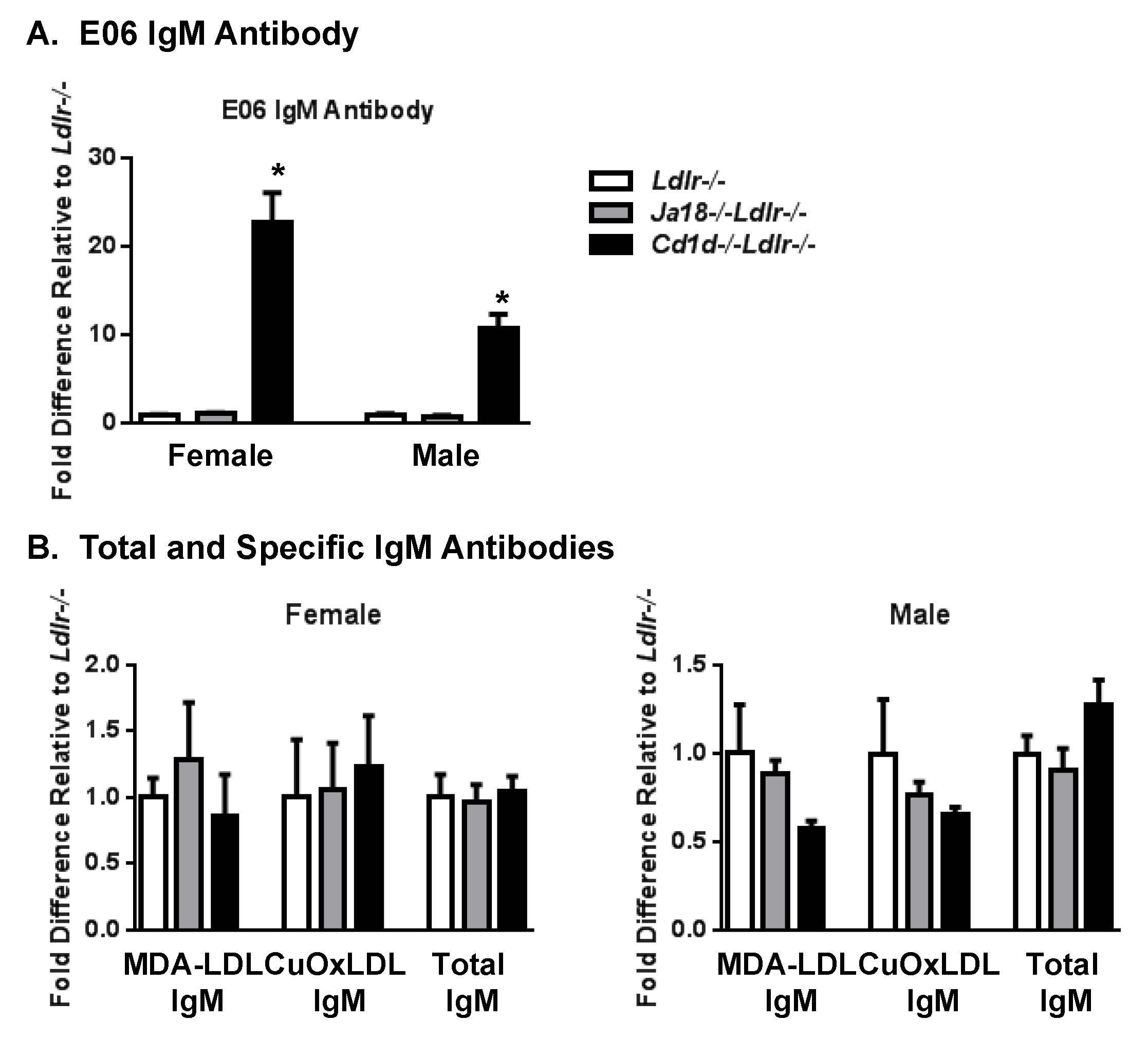
3.1. Increased Titer of E06 NAb in Cd1d−/−Ldlr−/− Mice
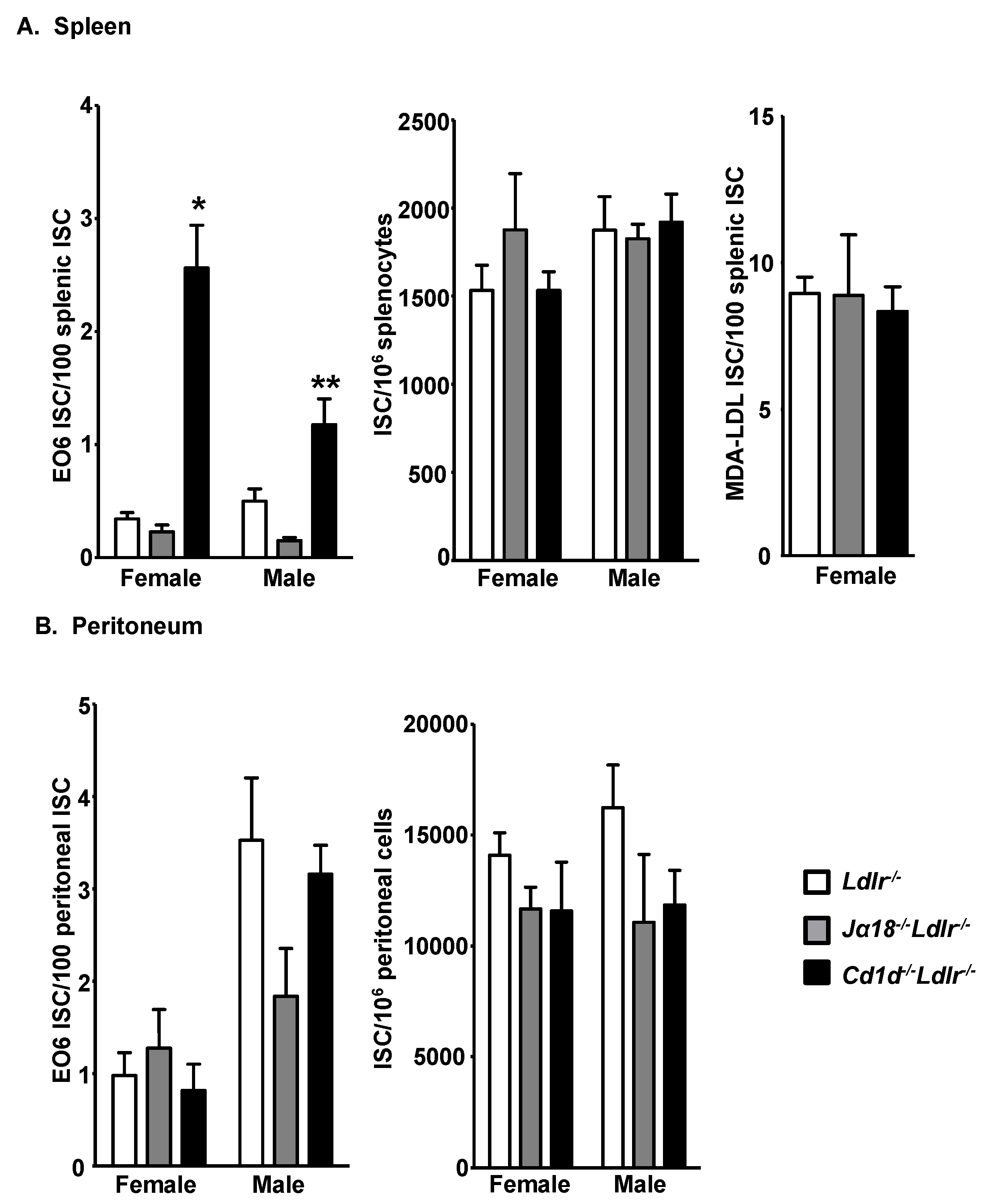
3.2. Increased E06 IgM-Secreting B-1 Cells in the Spleens of Cd1d−/−Ldlr−/− Mice
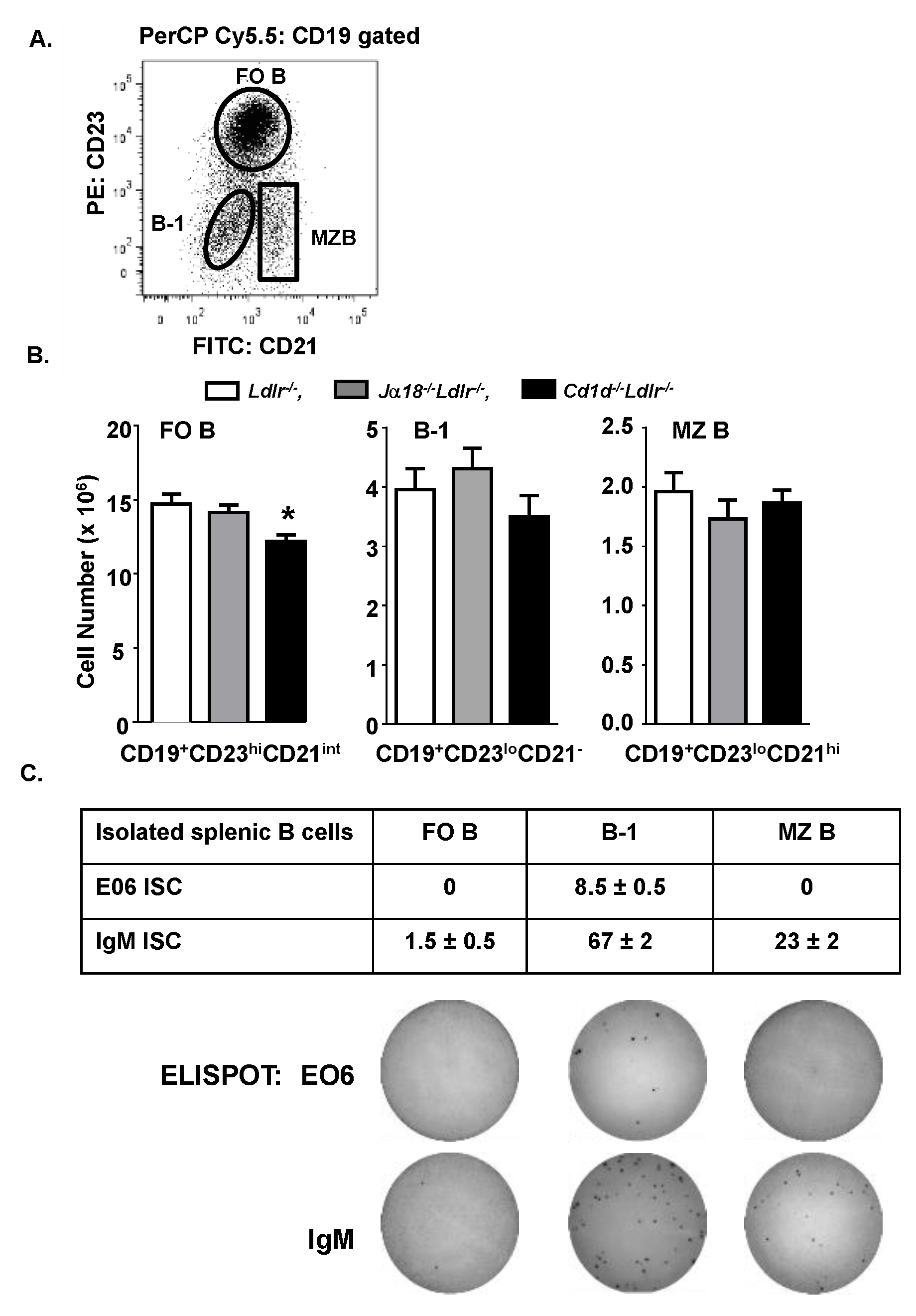
3.3. Total B-1 Cells Are Not Increased in the Spleen of Cd1d−/−Ldlr−/− Mice
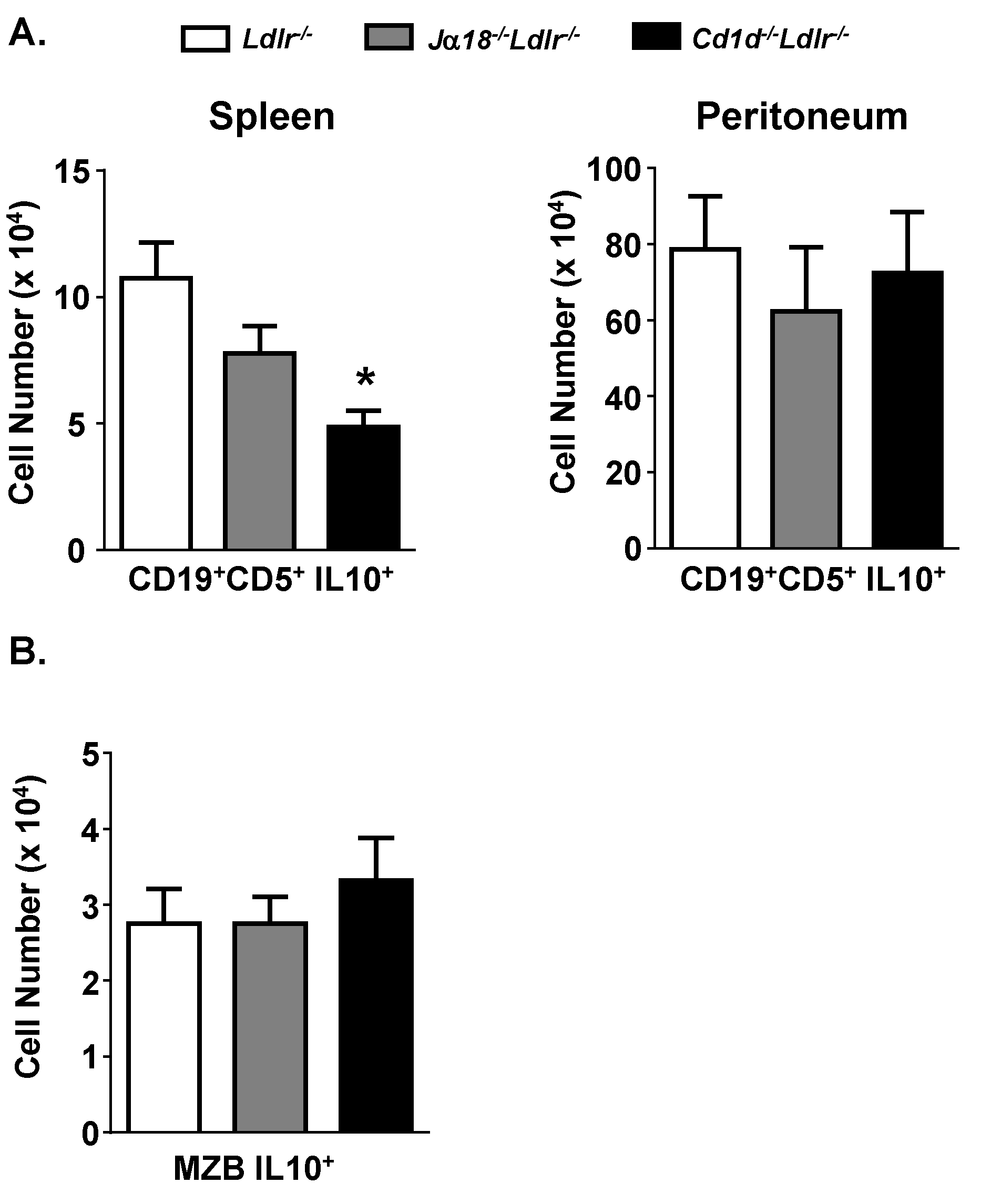
3.4. Reduced Number of Splenic CD19+CD5+IL-10+ Cells in Cd1d−/−Ldlr−/− Mice
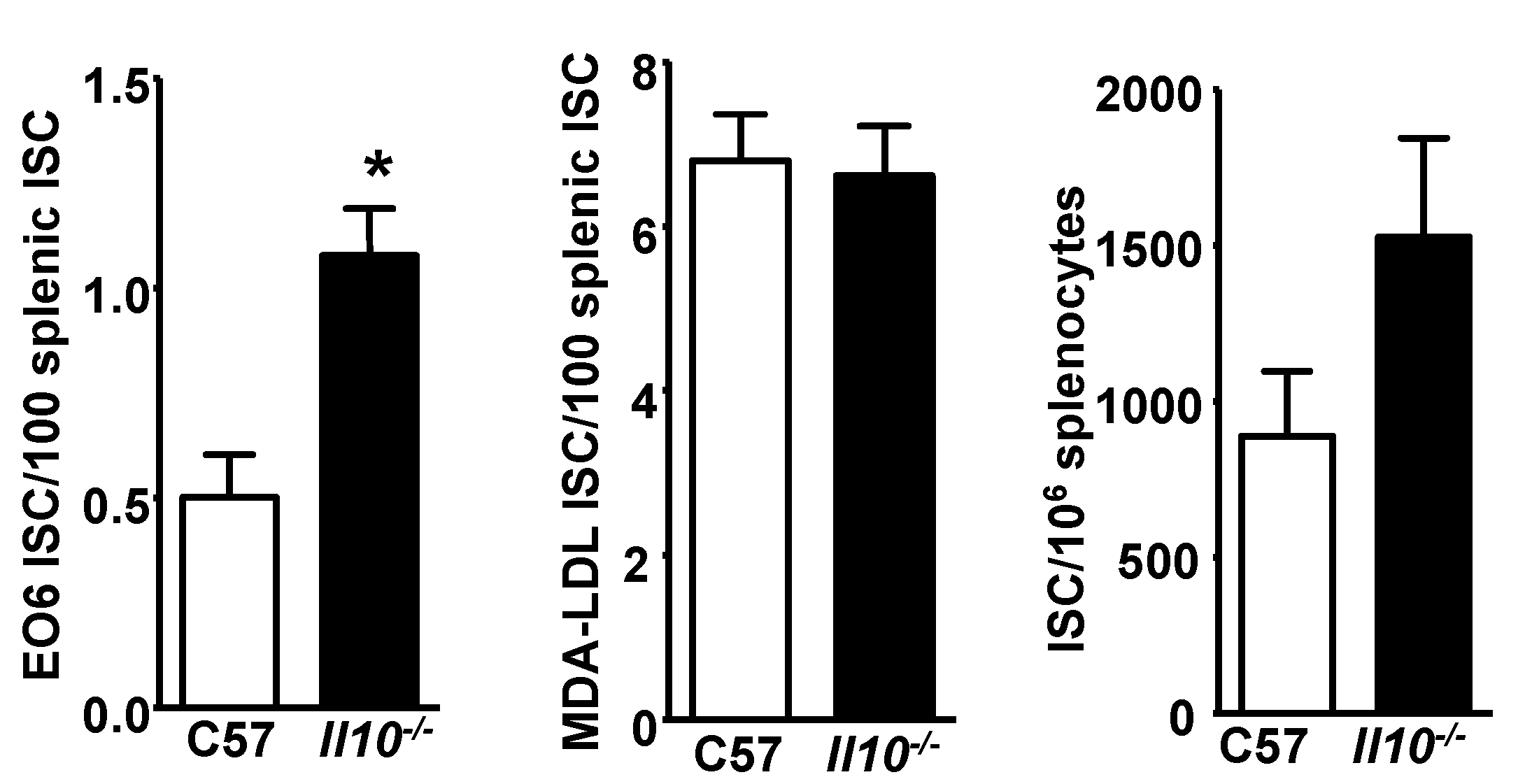
3.5. E06 ISC in the Spleen of Il10−/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gronwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Kearney, J.F. B1 cells: Similarities and differences with other B cell subsets. Curr. Opin. Immunol. 2001, 13, 195–201. [Google Scholar] [CrossRef]
- Baumgarth, N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Berland, R.; Wortis, H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002, 20, 253–300. [Google Scholar] [CrossRef]
- Chou, M.Y.; Fogelstrand, L.; Hartvigsen, K.; Hansen, L.F.; Woelkers, D.; Shaw, P.X.; Choi, J.; Perkmann, T.; Backhed, F.; Miller, Y.I.; et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Investig. 2009, 119, 1335–1349. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Gruber, S.; Binder, C.J. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front. Immunol. 2012, 3, 415. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Chen, Y.; Park, Y.B.; Patel, E.; Silverman, G.J. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J. Immunol. 2009, 182, 6031–6043. [Google Scholar] [CrossRef]
- Ogden, C.A.; Kowalewski, R.; Peng, Y.; Montenegro, V.; Elkon, K.B. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity 2005, 38, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Tangirala, R.K.; Miller, E.; Young, S.G.; Witztum, J.L. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Ord, V.A.; Plump, A.S.; Breslow, J.L.; Steinberg, D.; Witztum, J.L. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 1994, 14, 605–616. [Google Scholar] [CrossRef]
- Sage, A.P.; Tsiantoulas, D.; Binder, C.J.; Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 180–196. [Google Scholar] [CrossRef]
- Lewis, M.J.; Malik, T.H.; Ehrenstein, M.R.; Boyle, J.J.; Botto, M.; Haskard, D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009, 120, 417–426. [Google Scholar] [CrossRef]
- Binder, C.J.; Horkko, S.; Dewan, A.; Chang, M.K.; Kieu, E.P.; Goodyear, C.S.; Shaw, P.X.; Palinski, W.; Witztum, J.L.; Silverman, G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003, 9, 736–743. [Google Scholar] [CrossRef]
- Caligiuri, G.; Nicoletti, A.; Poirier, B.; Hansson, G.K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Investig. 2002, 109, 745–753. [Google Scholar] [CrossRef]
- Kyaw, T.; Tay, C.; Krishnamurthi, S.; Kanellakis, P.; Agrotis, A.; Tipping, P.; Bobik, A.; Toh, B.H. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011, 109, 830–840. [Google Scholar] [CrossRef]
- Rosenfeld, S.M.; Perry, H.M.; Gonen, A.; Prohaska, T.A.; Srikakulapu, P.; Grewal, S.; Das, D.; McSkimming, C.; Taylor, A.M.; Tsimikas, S.; et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ. Res. 2015, 117, e28–e39. [Google Scholar] [CrossRef]
- Palinski, W.; Horkko, S.; Miller, E.; Steinbrecher, U.P.; Powell, H.C.; Curtiss, L.K.; Witztum, J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Investig. 1996, 98, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Horkko, S.; Chang, M.K.; Curtiss, L.K.; Palinski, W.; Silverman, G.J.; Witztum, J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Investig. 2000, 105, 1731–1740. [Google Scholar] [CrossRef]
- Briles, D.E.; Forman, C.; Hudak, S.; Claflin, J.L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 1982, 156, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Binder, C.J.; Stavrakis, G.; Witztum, J.L.; Glimcher, L.H.; Lichtman, A.H. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA 2005, 102, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Maatta, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Boullier, A.; Friedman, P.; Harkewicz, R.; Hartvigsen, K.; Green, S.R.; Almazan, F.; Dennis, E.A.; Steinberg, D.; Witztum, J.L.; Quehenberger, O. Phosphocholine as a pattern recognition ligand for CD36. J. Lipid Res. 2005, 46, 969–976. [Google Scholar] [CrossRef]
- Gillotte-Taylor, K.; Boullier, A.; Witztum, J.L.; Steinberg, D.; Quehenberger, O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001, 42, 1474–1482. [Google Scholar]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Witztum, J.L.; Lichtman, A.H. The influence of innate and adaptive immune responses on atherosclerosis. Annu. Rev. Pathol. 2014, 9, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef]
- Kronenberg, M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu. Rev. Immunol. 2005, 23, 877–900. [Google Scholar] [CrossRef]
- Cohen, N.R.; Garg, S.; Brenner, M.B. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv. Immunol. 2009, 102, 1–94. [Google Scholar]
- Getz, G.S.; Reardon, C.A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 304–314. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiu, N.M.; Mandal, M.; Wang, N.; Wang, C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity 1997, 6, 459–467. [Google Scholar] [CrossRef]
- Mendiratta, S.K.; Martin, W.D.; Hong, S.; Boesteanu, A.; Joyce, S.; Van Kaer, L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 1997, 6, 469–477. [Google Scholar] [CrossRef]
- Cui, J.; Shin, T.; Kawano, T.; Sato, H.; Kondo, E.; Toura, I.; Kaneko, Y.; Koseki, H.; Kanno, M.; Taniguchi, M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 1997, 278, 1623–1626. [Google Scholar] [CrossRef]
- Bendelac, A.; Hunziker, R.D.; Lantz, O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 1996, 184, 1285–1293. [Google Scholar] [CrossRef]
- Binder, C.J.; Hartvigsen, K.; Chang, M.K.; Miller, M.; Broide, D.; Palinski, W.; Curtiss, L.K.; Corr, M.; Witztum, J.L. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Investig. 2004, 114, 427–437. [Google Scholar] [CrossRef]
- Bouaziz, J.D.; Yanaba, K.; Tedder, T.F. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol. Rev. 2008, 224, 201–214. [Google Scholar] [CrossRef]
- Sakaguchi, S. Regulatory T cells: History and perspective. Methods Mol. Biol. 2011, 707, 3–17. [Google Scholar] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F. B10 cells: A functionally defined regulatory B cell subset. J. Immunol. 2015, 194, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Solt, L.A.; Hastings, W.D.; Yang, K.; Gerstein, R.M.; Nikolajczyk, B.S.; Clarke, S.H.; Rothstein, T.L. Splenic and peritoneal B-1 cells differ in terms of transcriptional and proliferative features that separate peritoneal B-1 from splenic B-2 cells. Cell Immunol. 2001, 213, 62–71. [Google Scholar] [CrossRef]
- Lee, C.C.; Kung, J.T. Marginal zone B cell is a major source of Il-10 in Listeria monocytogenes susceptibility. J. Immunol. 2012, 189, 3319–3327. [Google Scholar] [CrossRef]
- Belperron, A.A.; Dailey, C.M.; Bockenstedt, L.K. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 2005, 174, 5681–5686. [Google Scholar] [CrossRef]
- Sindhava, V.; Woodman, M.E.; Stevenson, B.; Bondada, S. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS ONE 2010, 5, e11445. [Google Scholar] [CrossRef]
- Wermeling, F.; Lind, S.M.; Jordo, E.D.; Cardell, S.L.; Karlsson, M.C. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J. Exp. Med. 2010, 207, 943–952. [Google Scholar] [CrossRef]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-lipid antigen recognition by the gammadelta TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dieter, J.A.; Rothaeusler, K.; Luo, Z.; Baumgarth, N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 2012, 42, 120–129. [Google Scholar] [CrossRef]
- Yang, Y.; Tung, J.W.; Ghosn, E.E.; Herzenberg, L.A. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. USA 2007, 104, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Lipinski, M.J.; Oldham, S.N.; Garmey, J.C.; Campbell, K.A.; Skaflen, M.D.; Cutchins, A.; Lee, D.J.; Glover, D.K.; Kelly, K.A.; et al. B-cell aortic homing and atheroprotection depend on Id3. Circ. Res. 2012, 110, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Upadhye, A.; Srikakulapu, P.; Gonen, A.; Hendrikx, S.; Perry, H.M.; Nguyen, A.; McSkimming, C.; Marshall, M.A.; Garmey, J.C.; Taylor, A.M.; et al. Diversification and CXCR4-Dependent Establishment of the Bone Marrow B-1a Cell Pool Governs Atheroprotective IgM Production Linked to Human Coronary Atherosclerosis. Circ. Res. 2019, 125, e55–e70. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Harris, R.B.; Cyster, J.G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002, 16, 67–76. [Google Scholar] [CrossRef]
- Colgan, S.P.; Hershberg, R.M.; Furuta, G.T.; Blumberg, R.S. Ligation of intestinal epithelial CD1d induces bioactive IL-10: Critical role of the cytoplasmic tail in autocrine signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 13938–13943. [Google Scholar] [CrossRef]
- Olszak, T.; Neves, J.F.; Dowds, C.M.; Baker, K.; Glickman, J.; Davidson, N.O.; Lin, C.S.; Jobin, C.; Brand, S.; Sotlar, K.; et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 2014, 509, 497–502. [Google Scholar] [CrossRef]
- Pillai, S.; Cariappa, A.; Moran, S.T. Marginal zone B cells. Annu. Rev. Immunol. 2005, 23, 161–196. [Google Scholar] [CrossRef]
- Bendelac, A.; Bonneville, M.; Kearney, J.F. Autoreactivity by design: Innate B and T lymphocytes. Nat. Rev. Immunol. 2001, 1, 177–186. [Google Scholar] [CrossRef]
- Vincent, M.S.; Gumperz, J.E.; Brenner, M.B. Understanding the function of CD1-restricted T cells. Nat. Immunol. 2003, 4, 517–523. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, T.K.; VanderLaan, P.A.; Que, X.; Gonen, A.; Krishack, P.; Binder, C.J.; Witztum, J.L.; Getz, G.S.; Reardon, C.A. CD1d Selectively Down Regulates the Expression of the Oxidized Phospholipid-Specific E06 IgM Natural Antibody in Ldlr−/− Mice. Antibodies 2020, 9, 30. https://doi.org/10.3390/antib9030030
Biswas TK, VanderLaan PA, Que X, Gonen A, Krishack P, Binder CJ, Witztum JL, Getz GS, Reardon CA. CD1d Selectively Down Regulates the Expression of the Oxidized Phospholipid-Specific E06 IgM Natural Antibody in Ldlr−/− Mice. Antibodies. 2020; 9(3):30. https://doi.org/10.3390/antib9030030
Chicago/Turabian StyleBiswas, Tapan K., Paul A. VanderLaan, Xuchu Que, Ayelet Gonen, Paulette Krishack, Christoph J. Binder, Joseph L. Witztum, Godfrey S. Getz, and Catherine A. Reardon. 2020. "CD1d Selectively Down Regulates the Expression of the Oxidized Phospholipid-Specific E06 IgM Natural Antibody in Ldlr−/− Mice" Antibodies 9, no. 3: 30. https://doi.org/10.3390/antib9030030
APA StyleBiswas, T. K., VanderLaan, P. A., Que, X., Gonen, A., Krishack, P., Binder, C. J., Witztum, J. L., Getz, G. S., & Reardon, C. A. (2020). CD1d Selectively Down Regulates the Expression of the Oxidized Phospholipid-Specific E06 IgM Natural Antibody in Ldlr−/− Mice. Antibodies, 9(3), 30. https://doi.org/10.3390/antib9030030





