Impact of Acetylated and Non-Acetylated Fucose Analogues on IgG Glycosylation
Abstract
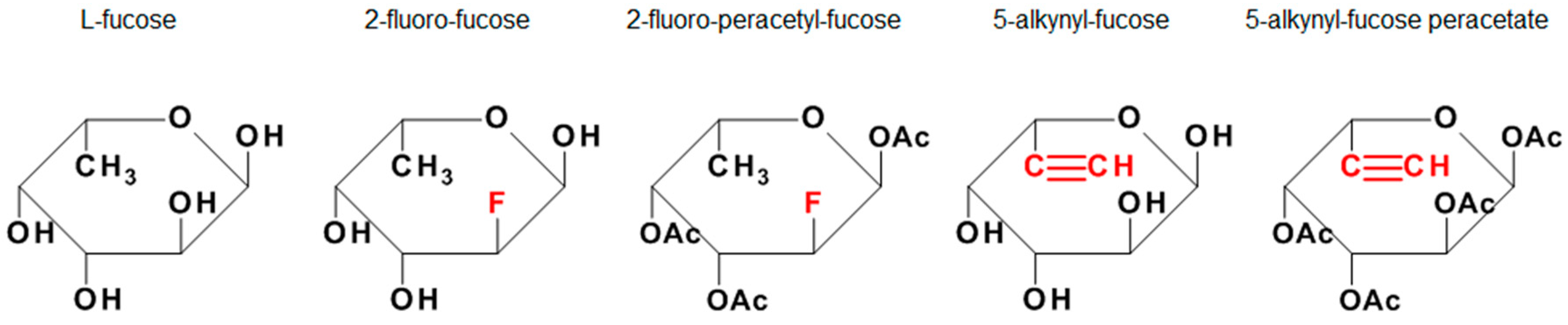
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Line
2.2. Cell Culture Media and Process Conditions
2.3. Antibody Purification and Analysis of the Glycosylation Pattern
2.4. Statistical Analysis
3. Results
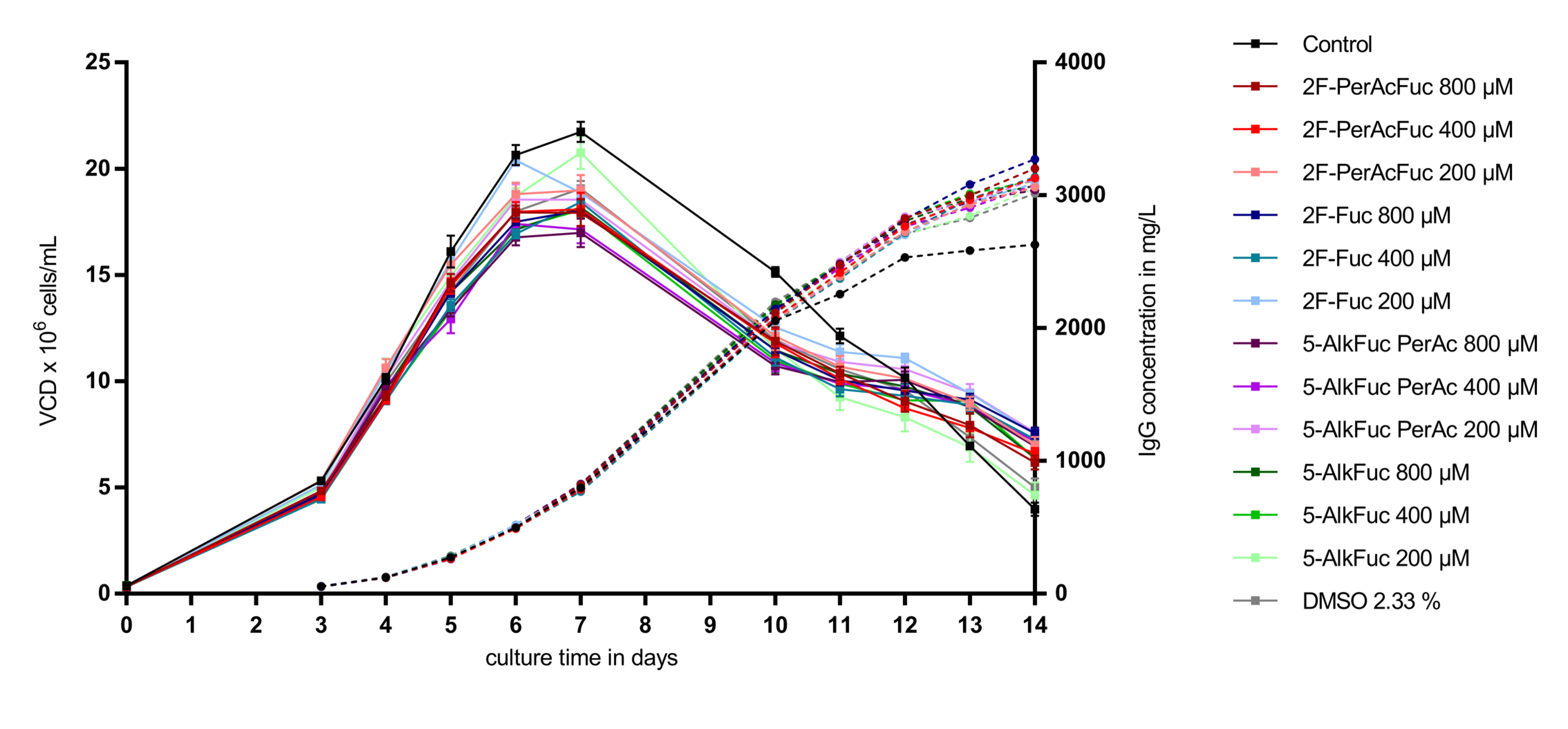
3.1. Cell Performance
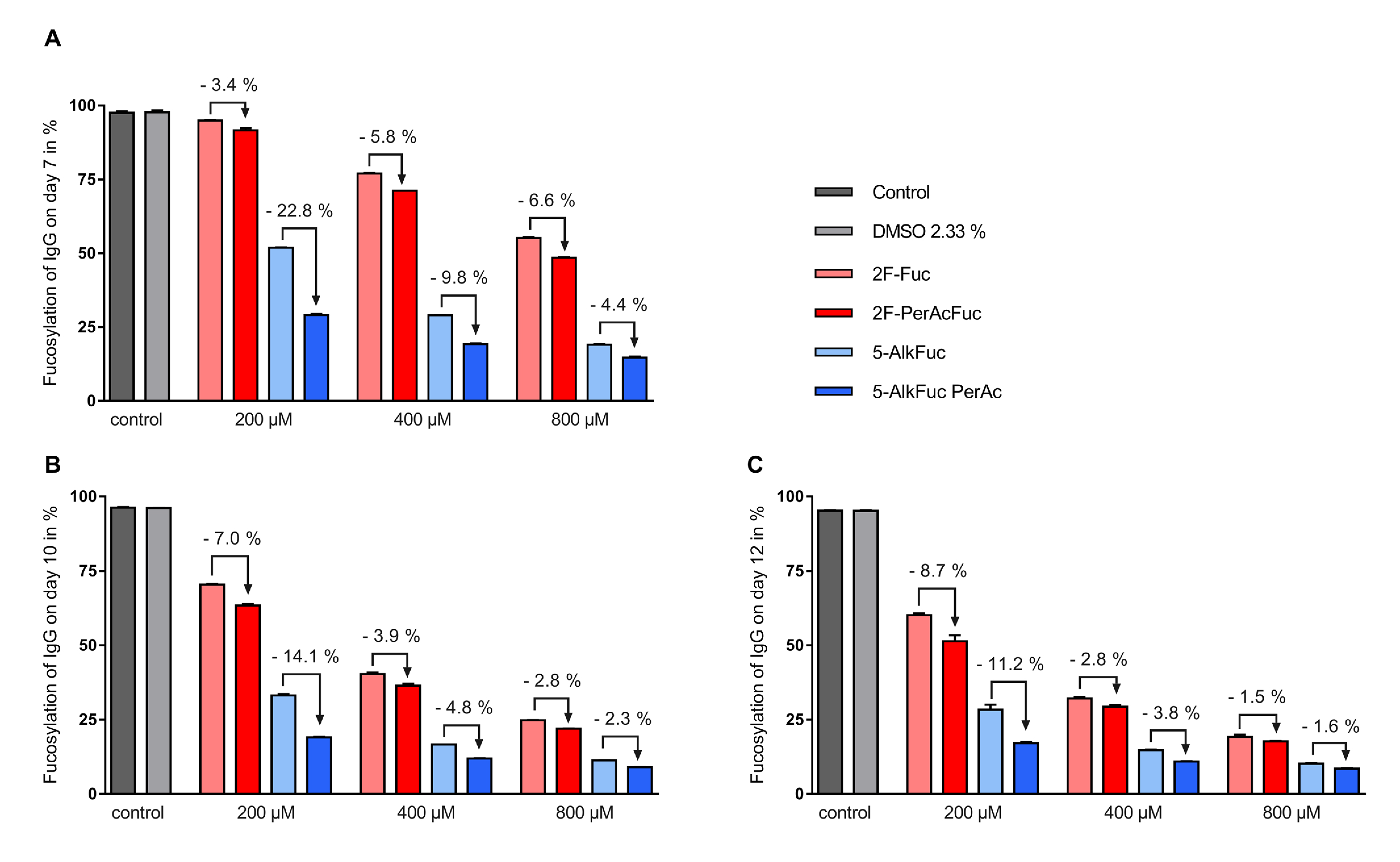
3.2. Glycosylation Profile
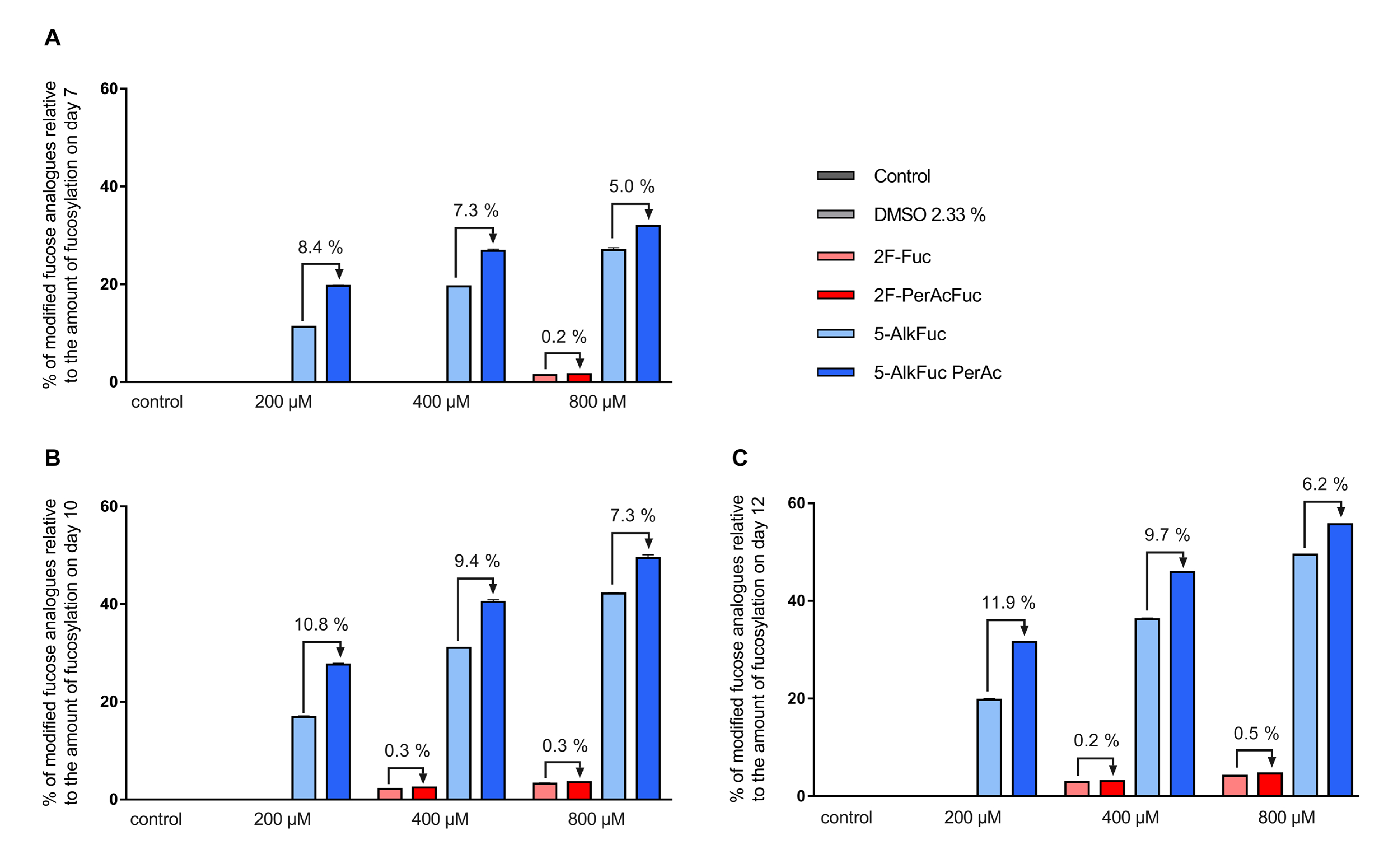
3.3. Incorporation of Fucose Analogues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| 2-deoxy-2-fluorofucose | 2F-Fuc |
| 2F-Peracetyl-fucose | 2F-PerAcFuc |
| 5-alkynylfucose | 5-AlkFuc |
| 5-alkynylfucose peracetylated | 5-AlkFuc PerAc |
| 6-fluorofucose | 6F-Fuc |
| antibody-dependent cellular cytotoxicity | ADCC |
| chinese hamster ovary cells | CHO cells |
| critical quality attribute | cQA |
| dimethyl sulfoxide | DMSO |
| Fucosyltransferase 8 | FUT8 |
| Guanosine diphosphate | GDP |
| GDP-4-keto-6-deoxymannose 3,5-epimerase-4-reductase | FX |
| GDP-mannose 4,6-dehydratase | GMD |
| monoclonal antibodies | mAbs |
| N-acetylglucosamine | GlcNAc |
| N-acetylmannosamine | ManNAc |
| standard error of the mean | SEM |
| ultra-performance liquid chromatography coupled to a mass spectrometer | UPLC-MS |
| viable cell density | VCD |
References
- Jayapal, K.P.; Wlaschin, K.F.; Hu, W.S.; Yap, M.G.S. Recombinant protein therapeutics from CHO Cells—20 years and counting. Chem. Eng. Prog. 2007, 103, 40–47. [Google Scholar]
- Yang, Z.; Halim, A.; Narimatsu, Y.; Jitendra Joshi, H.; Steentoft, C.; Schjoldager, K.T.; Alder Schulz, M.; Sealover, N.R.; Kayser, K.J.; Paul Bennett, E.; et al. The GalNAc-type O-Glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol. Cell. Proteomics 2014, 13, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Abès, R.; Teillaud, J.-L. Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals 2010, 3, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Wu, D. Advances in Therapeutic Fc Engineering—Modulation of IgG-Associated Effector Functions and Serum Half-life. Front. Immunol. 2016, 7, 580. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Durocher, Y.; Butler, M. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 2009, 20, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Niwa, R.; Satoh, M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des. Dev. Ther. 2009, 3, 7–16. [Google Scholar] [CrossRef]
- Dekkers, G.; Plomp, R.; Koeleman, C.A.M.; Visser, R.; von Horsten, H.H.; Sandig, V.; Rispens, T.; Wuhrer, M.; Vidarsson, G. Multi-level glyco-engineering techniques to generate IgG with defined Fc-glycans. Nature 2016, 6, 36964. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T.; et al. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell 2018, 9, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef]
- Burkart, M.D.; Vincent, S.P.; Duffels, A.; Murray, B.W.; Ley, S.V.; Wong, C.H. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: Useful mechanistic probes for glycosyltransferases. Bioorg. Med. Chem. 2000, 8, 1937–1946. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Fritz, T.A.; Taylor, W.H.; Esko, J.D. Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Gal beta 1→4GlcNAc beta-O-naphthalenemethanol. Proc. Natl. Acad. Sci. USA 1995, 92, 3323–3327. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Jeffrey, S.C.; Sussman, D.; Benjamin, D.R.; Toki, B.; Burke, P.J. Methods and Compositions for Making Antibodies and Antibody Derivatives with Reduced Core Fucosylation. Patent WO 2009135181, 5 November 2009. [Google Scholar]
- Okeley, N.M.; Alley, S.C.; Anderson, M.E.; Boursalian, T.E.; Burke, P.J.; Emmerton, K.M.; Jeffrey, S.C.; Klussman, K.; Law, C.L.; Sussman, D.; et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 5404–5409. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Nakano, M.; Yamaguchi, Y.; Nakajima, K.; Oka, R.; Sato, K.; Ren, C.T.; Hsu, T.L.; Wong, C.H.; Taniguchi, N. An Alkynyl-Fucose Halts Hepatoma Cell Migration and Invasion by Inhibiting GDP-Fucose-Synthesizing Enzyme FX, TSTA3. Cell Chem. Biol. 2017, 24, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.L.; Hanson, S.R.; Kishikawa, K.; Wang, S.K.; Sawa, M.; Wong, C.H. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc. Natl. Acad. Sci. USA 2007, 104, 2614–2619. [Google Scholar] [CrossRef]
- Ramsden, M.J.; Blake, F.S.R.; Fey, N.J. The effect of acetylation on the mechanical properties, hydrophobicity, and dimensional stability ofPinus sylvestris. Wood Sci. Technol. 1997, 31, 97–104. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L. Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Marathe, D.D.; Buffone, A.; Chandrasekaran, E.V.; Xue, J.; Locke, R.D.; Nasirikenari, M.; Lau, J.T.Y.; Matta, K.L.; Neelamegham, S. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 2010, 115, 1303–1312. [Google Scholar] [CrossRef]
- Malicdan, M.C.; Noguchi, S.; Tokutomi, T.; Goto, Y.; Nonaka, I.; Hayashi, Y.K.; Nishino, I. Peracetylated N-acetylmannosamine, a synthetic sugar molecule, efficiently rescues muscle phenotype and biochemical defects in mouse model of sialic acid-deficient myopathy. J. Biol. Chem. 2012, 287, 2689–2705. [Google Scholar] [CrossRef]
- Okeley, N.M.; Toki, B.E.; Zhang, X.; Jeffrey, S.C.; Burke, P.J.; Alley, S.C.; Senter, P.D. Metabolic Engineering of Monoclonal Antibody Carbohydrates for Antibody–Drug Conjugation. Bioconj. Chem. 2013, 24, 1650–1655. [Google Scholar] [CrossRef]
- Jones, M.B.; Teng, H.; Rhee, J.K.; Lahar, N.; Baskaran, G.; Yarema, K.J. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol. Bioeng. 2004, 85, 394–405. [Google Scholar] [CrossRef]
- Gilormini, P.A.; Lion, C.; Vicogne, D.; Levade, T.; Potelle, S.; Mariller, C.; Guerardel, Y.; Biot, C.; Foulquier, F. A sequential bioorthogonal dual strategy: ManNAl and SiaNAl as distinct tools to unravel sialic acid metabolic pathways. Chem. Commun. 2016, 52, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Zaro, B.W.; Chuh, K.N.; Pratt, M.R. Chemical reporter for visualizing metabolic cross-talk between carbohydrate metabolism and protein modification. ACS Chem. Biol. 2014, 9, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S. Inositol transport proteins. FEBS Lett. 2015, 589, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Hazama, A.; Kwon, H.M.; Loo, D.D.F.; Handler, J.S.; Wright, E.M. Kinetics and specificity of the renal Na+/myo-inositol cotransporter expressed in Xenopus Oocytes. J. Membr. Biol. 1995, 143, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chinese Hamster Genome Database. Available online: http://www.chogenome.org (accessed on 28 December 2018).
- Hossler, P.; Chumsae, C.; Racicot, C.; Ouellette, D.; Ibraghimov, A.; Serna, D.; Mora, A.; McDermott, S.; Labkovsky, B.; Scesney, S.; et al. Arabinosylation of recombinant human immunoglobulin-based protein therapeutics. mAbs 2017, 9, 715–734. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.C.; Scott, N.E.; John, A.; White, J.M.; Goddard-Borger, E.D. Synthesis and use of 6,6,6-trifluoro-L-fucose to block core-fucosylation in hybridoma cell lines. Carbohydr. Res. 2018, 465, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Funayama, S.; Shogomori, H.; Nakano, M.; Nakajima, K.; Oka, R.; Kitazume, S.; Yamaguchi, Y.; Sano, M.; Korekane, H.; et al. High-Sensitivity and Low-Toxicity Fucose Probe for Glycan Imaging and Biomarker Discovery. Cell Chem. Biol. 2016, 23, 782–792. [Google Scholar] [CrossRef]
- Allen, J.G.; Mujacic, M.; Frohn, M.J.; Pickrell, A.J.; Kodama, P.; Bagal, D.; San Miguel, T.; Sickmier, E.A.; Osgood, S.; Swietlow, A.; et al. Facile Modulation of Antibody Fucosylation with Small Molecule Fucostatin Inhibitors and Cocrystal Structure with GDP-Mannose 4,6-Dehydratase. ACS Chem. Biol. 2016, 11, 2734–2743. [Google Scholar] [CrossRef]
- Tu, Z.; Lin, Y.N.; Lin, C.H. Development of fucosyltransferase and fucosidase inhibitors. Chem. Soc. Rev. 2013, 42, 4459–4475. [Google Scholar] [CrossRef]
| Control | 2.33% DMSO | 200 µM 2F-Fuc | 200 µM 2F-Fuc PerAc | 400 µM 2F-Fuc | 400 µM 2F-Fuc PerAc | 800 µM 2F-Fuc | 800 µM 2F-Fuc PerAc | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name ‡ | Structure † | D7 (n = 5) | D10 (n = 6) | D12 (n = 6) | D7 (n = 3) | D10 (n = 3) | D12 (n = 3) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 4) | D10 (n = 2) | D12 (n = 5) |
| G0F | 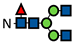 | 80.4 ± 2.1 | 77.5 ± 1.0 | 77.0 ± 0.9 | 81.8 ± 1.6 | 78.8 ± 1.1 | 77.1 ± 0.1 | 80.9 ± 0.7 | 57.7 ± 0.2 | 49.0 ± 0.2 | 78.5 ± 0.4 | 52.1 ± 0.3 | 42.0 ± 1.9 | 65.7 ± 0.1 | 33.1± 0.04 | 26.3 ± 0.1 | 59.2 ± 1.1 | 29.7 ± 0.3 | 23.7 ± 0.5 | 46.4 ± 0.1 | 20.5 ± 0.5 | 15.6 ± 0.7 | 38.6 ± 2.8 | 17.9 ± 0.5 | 13.1 ± 1.5 |
| G0F * | 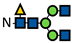 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.9 ± 0.03 | 0.9 ± 0.03 | - | 0.9 ± 0.05 | 0.9 ± 0.01 | 0.8 ± 0.01 | 0.8 ± 0.06 | 0.8 ± 0.01 | 0.8 ± 0.02 | 0.8 ± 0.03 | 0.8 ± 0.1 |
| G1F | 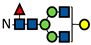 | 15.6 ± 2.5 | 16.4 ± 1.4 | 15.5 ± 1.2 | 14.2 ± 2.0 | 14.9 ± 1.3 | 14.9 ± 0.8 | 12.3 ± 0.5 | 10.5 ± 0.1 | 8.7 ± 0.6 | 11.5 ± 0.8 | 9.3 ± 0.1 | 7.4 ± 0.8 | 9.9 ± 0.4 | 5.7 ± 0.2 | 4.4 ± 0.4 | 9.2 ± 0.6 | 5.3 ± 0.2 | 4.3 ± 0.4 | 7.4 ± 0.1 | 3.2 ± 0.7 | 2.8 ± 0.3 | 7.4 ± 1.0 | 3.0 ± 0.4 | 2.7 ± 0.8 |
| G1F * | 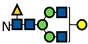 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| G0F-N | 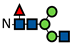 | 0.9 ± 0.1 | 1.4 ± 0.3 | 1.8 ± 0.2 | 0.8 ± 0.2 | 1.5 ± 0.4 | 2.1 ± 0.2 | 0.7 ± 0.02 | 1.2 ± 0.01 | 1.3 ± 0.01 | 0.7 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.01 | 0.8 ± 0.1 | - | - | 0.8 | - | - | - | - | - | - | - | - |
| G0F *-N | 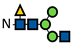 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| G1F-N |  | - | 0.3 ± 0.1 | 0.4 ± 0.04 | - | 0.3 ± 0.1 | 0.4 ± 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| G2F | 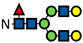 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.03 | 0.6 ± 0.1 | 0.7 ± 0.01 | 0.6 ± 0.01 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.02 | 0.5 ± 0.03 | 0.4 ± 0.02 | 0.3 ± 0.02 | 0.5 ± 0.01 | 0.4 ± 0.01 | 0.3 ± 0.01 | 0.4 ± 0.04 | 0.3 ± 0.02 | - | 0.4 ± 0.1 | 0.3 ± 0.02 | - |
| G0 | 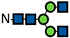 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.04 | 0.7 ± 0.1 | 0.8 ± 0.1 | 3.8 ± 0.1 | 23.4 ± 0.5 | 30.8 ± 0.6 | 6.0 ± 0.05 | 29.1 ± 0.1 | 36.3 ± 0.7 | 20.2 ± 0.5 | 48.7 ± 0.6 | 54.6 ± 0.7 | 25.5 ± 0.6 | 52.1 ± 1.4 | 56.9 ± 1.2 | 39.4 ± 0.03 | 62.1 ± 0.4 | 64.0 ± 1.3 | 46.0 ± 1.0 | 64.0 ± 0.8 | 68.0 ± 2.5 |
| G1 | 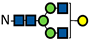 | - | - | - | - | - | - | 0.3 ± 0.01 | 2.6 ± 0.03 | 3.4 ± 0.1 | 0.4 ± 0.02 | 3.3 ± 0.1 | 3.9 ± 0.4 | 1.6 ± 0.02 | 5.6 ± 0.04 | 6.2 ± 0.3 | 2.2 ± 0.1 | 6.1 ± 0.04 | 6.6 ± 0.3 | 3.6 ± 0.04 | 6.9 ± 0.1 | 7.4 ± 0.5 | 5.0 ± 0.9 | 7.4 ± 0.08 | 8.8 ± 1.1 |
| G2 |  | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.21 | - | 0.2 ± 0.01 | 0.2 ± 0.03 | - | 0.2 ± 0.01 | 0.2 ± 0.1 |
| Man5 | 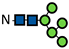 | 0.7 ± 0.2 | 1.8 ± 0.4 | 3.0 ± 0.3 | 0.7 ± 0.1 | 2.1 ± 0.4 | 3.2 ± 0.3 | 0.8 ± 0.1 | 2.3 ± 0.1 | 3.4 ± 0.1 | 0.8 ± 0.01 | 2.3 ± 0.1 | 3.4 ± 0.3 | 0.8 ± 0.05 | 2.4 ± 0.1 | 3.6 ± 0.2 | 0.9 ± 0.01 | 2.4 ± 0.05 | 3.6 ± 0.2 | 0.9 ± 0.02 | 2.5 ± 0.01 | 3.4 ± 0.2 | 0.9 ± 0.04 | 2.5 ± 0.04 | 3.5 ± 0.2 |
| G0-N | 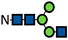 | 0.2 ± 0.03 | 0.2 ± 0.2 | 0.5 ± 0.1 | 0.3 | 0.3 ± 0.3 | 0.4 ± 0.1 | 0.3 | 1.2 ± 0.05 | 2.1 ± 0.3 | 0.3 ± 0.2 | 1.4 ± 0.2 | 3.4 ± 1.7 | 0.4 ± 0.2 | 2.0 ± 0.1 | 2.9 ± 0.3 | 0.5 ± 0.03 | 2.1 ± 0.01 | 2.9 ± 0.4 | 0.8 ± 0.1 | 2.4 ± 0.04 | 3.7 ± 1.0 | 0.8 ± 0.1 | 2.5 ± 0.1 | 2.8 ± 0.5 |
 represents N-acetylglucosamine (GlcNAc);
represents N-acetylglucosamine (GlcNAc);  represents mannose;
represents mannose;  represents fucose;
represents fucose;  represents fucose analogues;
represents fucose analogues;  represents galactose. ‡ respective fucose-analogue is indicated with *. The Glycan species were identified through the mass. The percentages of various glycans were calculated according to the relative area of each glycan species as detected using RapiFluor™ (Waters) as labelling reagent. Data are presented as mean values ± SEM and without SEM means that the peaks were only detected in one replicate.
represents galactose. ‡ respective fucose-analogue is indicated with *. The Glycan species were identified through the mass. The percentages of various glycans were calculated according to the relative area of each glycan species as detected using RapiFluor™ (Waters) as labelling reagent. Data are presented as mean values ± SEM and without SEM means that the peaks were only detected in one replicate.| Control | 2.33% DMSO | 200 µM 5-AlkFuc | 200 µM 5-AlkFuc PerAc | 400 µM 5-AlkFuc | 400 µM 5-AlkFuc PerAc | 800 µM 5-AlkFuc | 800 µM 5-AlkFuc PerAc | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name ‡ | Structure † | D7 (n = 5) | D10 (n = 6) | D12 (n = 6) | D7 (n = 3) | D10 (n = 3) | D12 (n = 3) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n = 2) | D10 (n = 2) | D12 (n = 2) | D7 (n =2) | D10 (n = 2) | D12 (n =2) |
| G0F |  | 80.4 ± 2.1 | 77.5 ± 1.0 | 77.0 ± 0.9 | 81.8 ± 1.6 | 78.8 ± 1.1 | 77.1 ± 0.1 | 37.9 ± 0.02 | 21.8 ± 0.5 | 17.8 ± 1.3 | 19.4 ± 0.2 | 11.2 ± 0.2 | 9.4 ± 0.3 | 19.4 ± 0.1 | 9.4 ± 0.2 | 7.6 ± 0.1 | 11.4 ± 0.1 | 5.8 ± 0.1 | 4.8 ± 0.1 | 11.5 ± 0.01 | 5.2 ± 0.2 | 4.1 ± 0.1 | 8.0 ± 0.1 | 3.7 ± 0.1 | 3.0 ± 0.01 |
| G0F * |  | - | - | - | - | - | - | 4.9 ± 0.1 | 4.7 ± 0.01 | 4.7 ± 0.2 | 5.0 ± 0.01 | 4.5 ± 0.02 | 4.6 ± 0.1 | 5.0 ± 0.03 | 4.4 ± 0.01 | 4.6 ± 0.02 | 4.5 ± 0.02 | 4.1 ± 0.05 | 4.3 ± 0.01 | 4.5 ± 0.01 | 4.0 ± 0.03 | 4.3 ± 0.04 | 4.1 ± 0.03 | 3.8 ± 0.03 | 4.1 ± 0.04 |
| G1F |  | 15.6 ± 2.5 | 16.4 ± 1.4 | 15.5 ± 1.2 | 14.2 ± 2.0 | 14.9 ± 1.3 | 14.9 ± 0.8 | 7.2 ± 0.2 | 4.9 ± 0.1 | 4.1 ± 0.7 | 3.5 ± 0.6 | 2.3 ± 0.1 | 2.0 ± 0.2 | 3.5 ± 0.4 | 2.0 ± 0.01 | 1.6 ± 0.2 | 2.7 ± 0.1 | 1.3 ± 0.01 | 1.1 ± 0.03 | 2.4 ± 0.34 | 1.2 ± 0.02 | 1.0 ± 0.1 | 2.0 ± 0.3 | 0.9 ± 0.03 | 0.8 ± 0.1 |
| G1F * |  | - | - | - | - | - | - | 0.9 ± 0.04 | 0.9 ± 0.01 | 0.9 ± 0.1 | 0.7 ± 0.04 | 0.7 ± 0.01 | 0.8 ± 0.1 | 0.7 ± 0.02 | 0.7 ± 0.01 | 0.7 ± 0.04 | 0.7 ± 0.2 | 0.7 ± 0.01 | 0.7 ± 0.1 | 0.6 ± 0.04 | 0.8 ± 0.04 | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.03 | 0.7 ± 0.02 |
| G0F-N |  | 0.9 ± 0.1 | 1.4 ± 0.3 | 1.8 ± 0.2 | 0.8 ± 0.2 | 1.5 ± 0.4 | 2.1 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.03 | 0.7 ± 0.02 | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.3 | 0.3 ± 0.1 | - | 0.2 ± 0.01 | - | - | - | - | - | - | - | - | - |
| G0F *-N |  | - | - | - | - | - | - | - | - | 0.3 ± 0.2 | - | - | 0.2 | - | - | 0.2 ± 0.01 | - | - | - | - | - | - | - | - | - |
| G1F-N |  | - | 0.3 ± 0.1 | 0.4 ± 0.04 | - | 0.3 ± 0.1 | 0.4 ± 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| G2F |  | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.03 | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.2 ± 0.03 | 0.2 | - | - | 0.2 | - | - | - | - | - | - | 0.29 | - | - | - | - |
| G0 |  | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.04 | 0.7 ± 0.1 | 0.8 ± 0.1 | 41.8 ± 0.4 | 53.7 ± 0.3 | 54.2 ± 2.2 | 62.8 ± 0.9 | 66.6 ± 0.2 | 66.9 ± 0.2 | 63.1 ± 0.6 | 69.2 ± 0.3 | 70.0 ± 0.6 | 70.6 ± 1.0 | 73.8 ± 0.2 | 73.7 ± 0.2 | 71.7 ± 0.7 | 73.6 ± 0.6 | 74.1 ± 0.9 | 74.7 ± 0.5 | 75.9 ± 0.7 | 75.6 ± 0.4 |
| G1 |  | - | - | - | - | - | - | 4.3 ± 0.1 | 6.7 ± 0.1 | 6.5 ± 0.7 | 6.1 ± 0.1 | 7.7 ± 0.1 | 7.6 ± 0.6 | 5.9 ± 0.03 | 8.2 ± 0.03 | 7.9 ± 0.6 | 6.9 ± 0.1 | 8.4 ± 0.1 | 8.1 ± 0.3 | 7.0 ± 0.1 | 8.7 ± 0.2 | 8.6 ± 0.5 | 7.2 ± 0.4 | 8.7 ± 0.3 | 8.4 ± 0.5 |
| G2 |  | - | - | - | - | - | - | - | - | 0.2 | 0.2 | 0.3 ± 0.01 | 0.3 ± 0.03 | - | 0.3 ± 0.01 | 0.3 ± 0.1 | 0.2 | 0.3 ± 0.01 | 0.3 ± 0.01 | - | 0.3 ± 0.02 | 0.3 ± 0.1 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.03 |
| Man5 |  | 0.7 ± 0.2 | 1.8 ± 0.4 | 3.0 ± 0.3 | 0.7 ± 0.1 | 2.1 ± 0.4 | 3.2 ± 0.3 | 0.8 ± 0.04 | 2.2 ± 0.1 | 3.1 ± 0.4 | 0.9 ± 0.04 | 2.3 ± 0.1 | 3.4 ± 0.3 | 0.8 ± 0.01 | 2.3 ± 0.01 | 3.4 ± 0.2 | 1.0 ± 0.03 | 2.4 ± 0.01 | 3.4 ± 0.2 | 0.9 ± 0.01 | 2.5 ± 0.04 | 3.5 ± 0.2 | 1.0 ± 0.03 | 2.5 ± 0.02 | 3.6 ± 0.2 |
| G0-N |  | 0.2 ± 0.03 | 0.2 ± 0.2 | 0.5 ± 0.1 | 0.3 | 0.3 ± 0.3 | 0.4 ± 0.1 | 0.9 ± 0.1 | 2.2 ± 0.1 | 3.5 ± 1.5 | 1.0 ± 0.01 | 2.4 ± 0.1 | 1.0 ± 0.01 | 1.0 ± 0.1 | 2.4 ± 0.04 | 3.2 ± 0.4 | 1.0 ± 0.1 | 2.5 ± 0.02 | 3.2 ± 0.3 | 1.0 ± 0.02 | 2.4 ± 0.03 | 3.2 ± 0.4 | 3.3 ± 0.6 | 2.6 ± 0.1 | 3.3 ± 0.3 |
 represents GlcNAc;
represents GlcNAc;  represents mannose;
represents mannose;  represents fucose;
represents fucose;  represents fucose analogues;
represents fucose analogues;  represents galactose. ‡ respective fucose-analogue is indicated with *. The Glycan species were identified through the mass. The percentages of various glycans were calculated according to the relative area of each glycan species as detected using RapiFluor™ as labelling reagent. Data are presented as mean values ± SEM and without SEM means that the peaks were only detected in one replicate.
represents galactose. ‡ respective fucose-analogue is indicated with *. The Glycan species were identified through the mass. The percentages of various glycans were calculated according to the relative area of each glycan species as detected using RapiFluor™ as labelling reagent. Data are presented as mean values ± SEM and without SEM means that the peaks were only detected in one replicate.© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, M.; Ehret, J.; Kolmar, H.; Zimmer, A. Impact of Acetylated and Non-Acetylated Fucose Analogues on IgG Glycosylation. Antibodies 2019, 8, 9. https://doi.org/10.3390/antib8010009
Zimmermann M, Ehret J, Kolmar H, Zimmer A. Impact of Acetylated and Non-Acetylated Fucose Analogues on IgG Glycosylation. Antibodies. 2019; 8(1):9. https://doi.org/10.3390/antib8010009
Chicago/Turabian StyleZimmermann, Martina, Janike Ehret, Harald Kolmar, and Aline Zimmer. 2019. "Impact of Acetylated and Non-Acetylated Fucose Analogues on IgG Glycosylation" Antibodies 8, no. 1: 9. https://doi.org/10.3390/antib8010009
APA StyleZimmermann, M., Ehret, J., Kolmar, H., & Zimmer, A. (2019). Impact of Acetylated and Non-Acetylated Fucose Analogues on IgG Glycosylation. Antibodies, 8(1), 9. https://doi.org/10.3390/antib8010009





