1. Introduction
The global market for pharmaceuticals is predicted to grow to
$1.5 trillion by 2021, with biologics such as monoclonal antibodies (mAbs), hormones, and therapeutic enzymes accounting for nearly 20% of the market share, based on current projections. With US sales rising from
$8.29 billion in 2005 to
$24.6 billion in 2012 [
1,
2]—coupled with the increase in regulatory agencies approvals of mAb treatments for different indications ranging from cancer to rheumatoid arthritis and Crohn’s disease [
3,
4], the growing market for mAbs has resulted in active development of these biological products. While several different mammalian cell expression systems can be used to synthesize mAb therapeutics, over half of all currently approved mAbs are produced in Chinese Hamster Ovary (CHO) cell lines. CHO cells are the preferred host for a large number of recombinant mAb therapeutics because of the ease of adapting them to suspension growth, and the availability of powerful gene amplification systems to improve specific productivity [
5]. However, more importantly, the post-translational modification machinery in CHO cells produces human-like structures in mAbs, thus ensuring biocompatibility.
One important post-translational modification in mAbs is N-glycosylation, a process by which an oligosaccharyltransferase complex in the endoplasmic reticulum adds a sugar substrate (glycan) to the Asn-X-Ser/Thr motif in the heavy chain of the mAb (where X is any amino acid other than Pro). As the mAb traverses the Golgi complex, the core glycan undergoes a series of non-template driven reactions that are mediated by the localized glycosyltransferase enzymes in the different Golgi compartments [
6,
7]. The result is a heterogeneous distribution of glycan residues or glycoforms, which affects the immunogenicity, effector functions, and the pharmacokinetic properties of the mAb, and consequently the final drug product quality [
8,
9,
10]. Thus, there is considerable motivation for manufacturers and regulators to understand, characterize, and, if necessary, modulate the glycoform distribution in mAbs during production, in order to maintain a consistent glycan profile [
11,
12,
13,
14]. However, manipulating the glycan distribution effectively requires (i) identifying the factors that can influence the glycan distribution; (ii) quantifying the degree to which these factors affect the concentration of the glycoform species; and (iii) using such information to design effective control systems.
Previous studies have demonstrated that protein glycosylation can be influenced by various factors [
15], such as pH [
16,
17], temperature [
18,
19,
20], dissolved oxygen [
21,
22], ammonia [
23], and media supplements such as nucleotide sugar precursors [
24] and manganese chloride (MnCl
2) [
25,
26,
27,
28]. Each study focused on an individual factor, establishing empirical relationships between the individual factor in question and the specific set of glycan species it affects. However, modulating the complete glycan distribution profile requires manipulating multiple input factors simultaneously, and to be effective, such action must be based on a thorough, holistic understanding of how these inputs individually and jointly affect various glycan species. Such process understanding can be generated systematically by using statistical design of experiments whereby input factors are judiciously varied simultaneously to generate data on the main and interaction effects they exert on all the output responses of interest. Such structural information indicates which inputs to manipulate, and by how much, in order to alter the relative concentrations of different glycan species appropriately. In most cases, however, the available inputs are fewer than the glycan species to be controlled, resulting in a system with insufficient degrees of freedom. Consequently, we must first answer a fundamental question: given a limited set of inputs, to what extent can we independently control the concentrations of all the desired glycan species? In other words, is the desired change in the glycan distribution achievable using the available inputs? We address this question using “controllability analysis”, by which we can determine quantitatively the extent to which the system is controllable. (Informally, a system is considered completely controllable if it is possible to drive the complete set of outputs from some initial value to any arbitrarily specified final, desired value, by manipulating the available set of inputs.) We have previously introduced, in [
29], the concept of output controllability, demonstrated how to use it to assess the controllability of the glycan reaction network using data generated from statistical design of experiments, and in [
30] we illustrated practical applicability by using it to identify in an experimental system, the glycan species whose concentrations can be controlled using such media supplements as MnCl
2, galactose and NH
4Cl as manipulated variables.
The role of different media supplements in modulating critical quality attributes of the mAb in general, and the glycan distribution profile in particular, has received considerable attention recently [
31]. Typically, supplements such as MnCl
2, which are known to affect the expression and activity of several glycosyltransferase enzymes, are added to the media at the start of the batch to alter the glycan distribution. However, over the course of the batch run, as the cells continue to grow and produce mAb molecules, it is clear that changes in the cellular availability of supplements will influence not just the antibody productivity but also the activity of the glycosyltransferase enzymes, thus affecting the final glycan distribution. Consequently, we postulate that it is possible to control the glycosylation profile in mAbs by introducing specific media supplements at different stages of cell growth. We postulate further that introducing a chelating agent to the media can alter the effect of MnCl
2 addition on the glycan distribution. Consequently, a chelating agent such as EDTA provides an additional degree of freedom for fine-tuning the effect of MnCl
2, the media supplement of primary interest. Although EDTA is toxic to cells and can titrate both Mn
++ and other bivalent ions in the medium that are necessary for cell growth and may be cofactors for other glycosyltransferase enzymes, it has been used in this study primarily to elucidate the effects of a dynamic addition of the two media supplements. (It is important to stress that the proposed time-dependent media supplementation is distinct from current fed-batch strategies where the objective is primarily to meet the nutrient demand in the culture, for the express purpose of enhancing cell growth and productivity—not to ensure that the product quality meets specific targets.) Specifically, we aim to identify the glycan species that can be controlled by adding MnCl
2 during lag, exponential, and stationary phases of cell growth, and to quantify the effect of such time-dependent MnCl
2 additions on the glycan distribution.
In this work, we use a mixed factorial experimental design to add MnCl2 and EDTA at various stages of cell growth and, via appropriate analysis of the resulting data, we quantify the effect of time-dependent media supplementation on the antibody productivity and the glycosylation profile in mAbs. Subsequently, we use controllability analysis to identify the glycan species whose relative percentages can be controlled effectively by introducing MnCl2 and EDTA to the media at different time points, and quantify the effect of these time-dependent additions. Overall, our results highlight the importance of taking into account the dynamic nature of media supplementation, and also provide concepts that can be exploited to develop new strategies for controlling the glycosylation profile in mAbs.
4. Discussion
While the addition of specific media supplements has been studied widely for its effect on the quality attributes of mAbs, such studies have been limited to the introduction of media supplements exclusively at the start of the culture, and the results, when quantitative, have yielded only isolated single factor relationships. The results from the current study show that introducing media supplements at different time points during cell culture does in fact have an effect on cell growth conditions and antibody glycosylation distribution, and the effects can be quantified globally and potentially used to design effective control schemes.
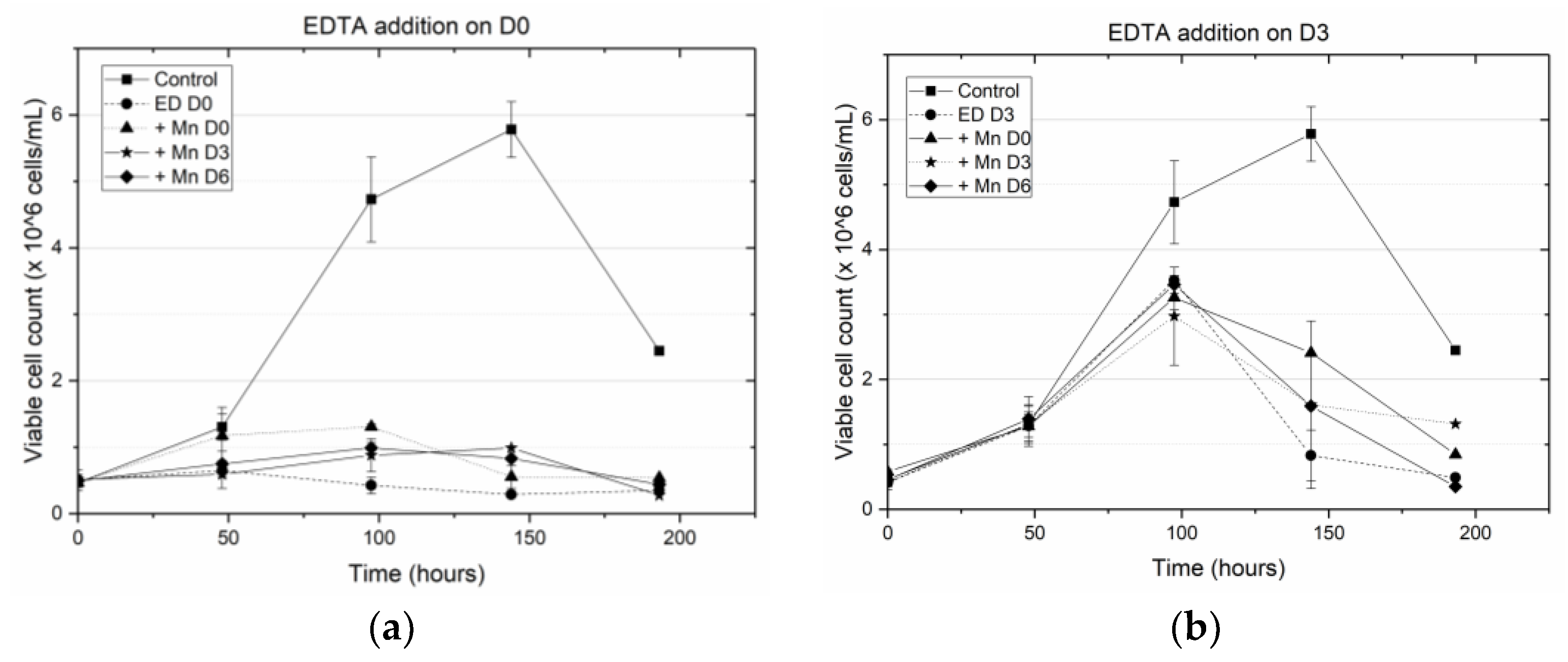
Specifically, we have shown that earlier addition of EDTA is detrimental to cell growth and results in a decrease in antibody titer. When EDTA was added on D0 (at the inoculation stage) the peak viable cell density (VCD) remained close to the seeding density, indicating a hampering of cell growth. This result is consistent with the well-known fact that EDTA is toxic to cells [
35]. The decline in the viable cell densities due to EDTA addition during the growth phase can also be attributed to the removal of such trace metals as Ca
++, Zn
++ etc. that are essential for cell survival, from the media (as a result of EDTA’s chelating effect). By contrast, when both EDTA and MnCl
2 were added on D0, the peak VCD increased two-fold. While this peak VCD is lower than the peak VCD of the control flask (5.78 × 10
6 cells/mL), the increased viability can be attributed to the presence of excess MnCl
2 titrating EDTA, resulting in reduced cytotoxicity. When EDTA was added on D3, the cells were in the middle of the growth phase and the addition of the cytotoxic EDTA hampered further growth, leading to a steep decline in the cell viability beyond D3 (
Figure 1b). By contrast, when MnCl
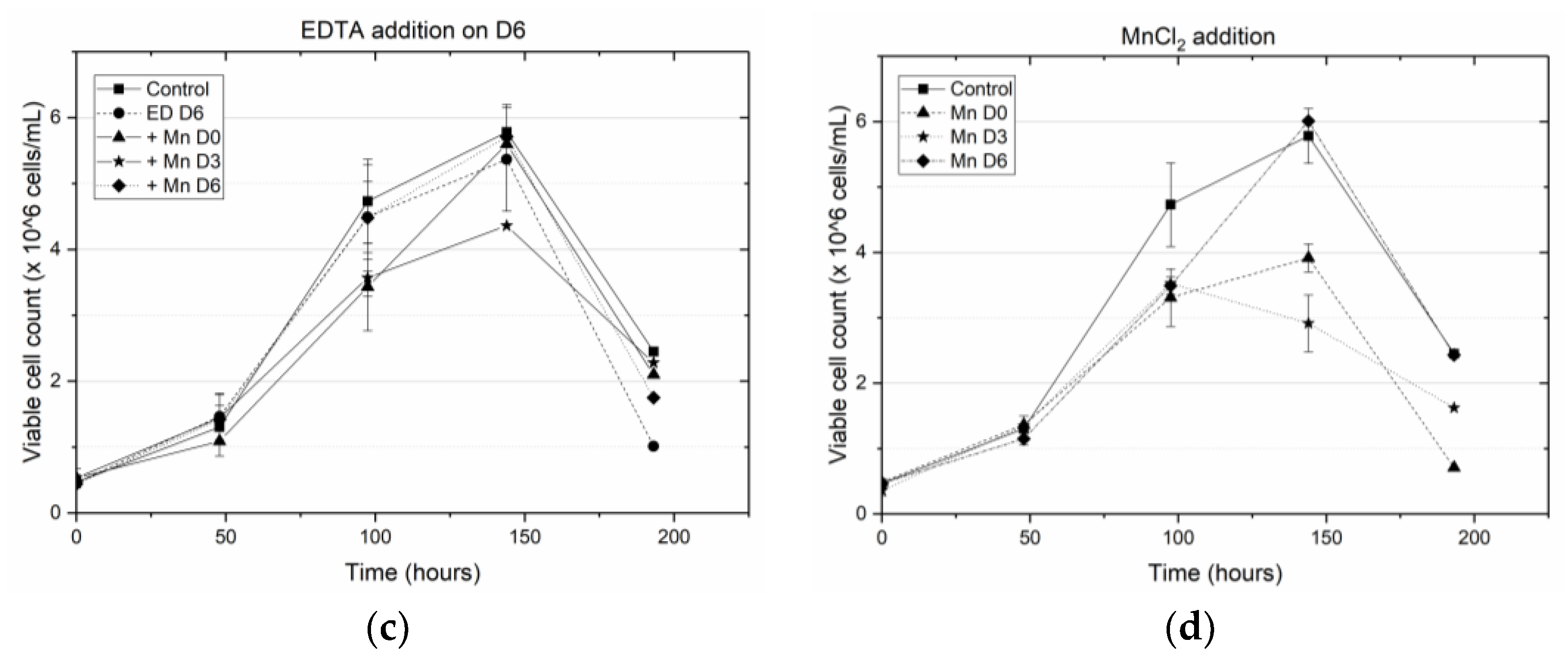
2 was added to the media (on D0 or D3) in the presence of EDTA, the cells did not experience a similar reduction in viability. When EDTA was added on D6, the cells were already at the end of the growth phase and hence the introduction of EDTA did not alter the cell viability. However, unlike the other cases where EDTA addition on D0 or D3 resulted in low titers, adding EDTA on D6 along with MnCl
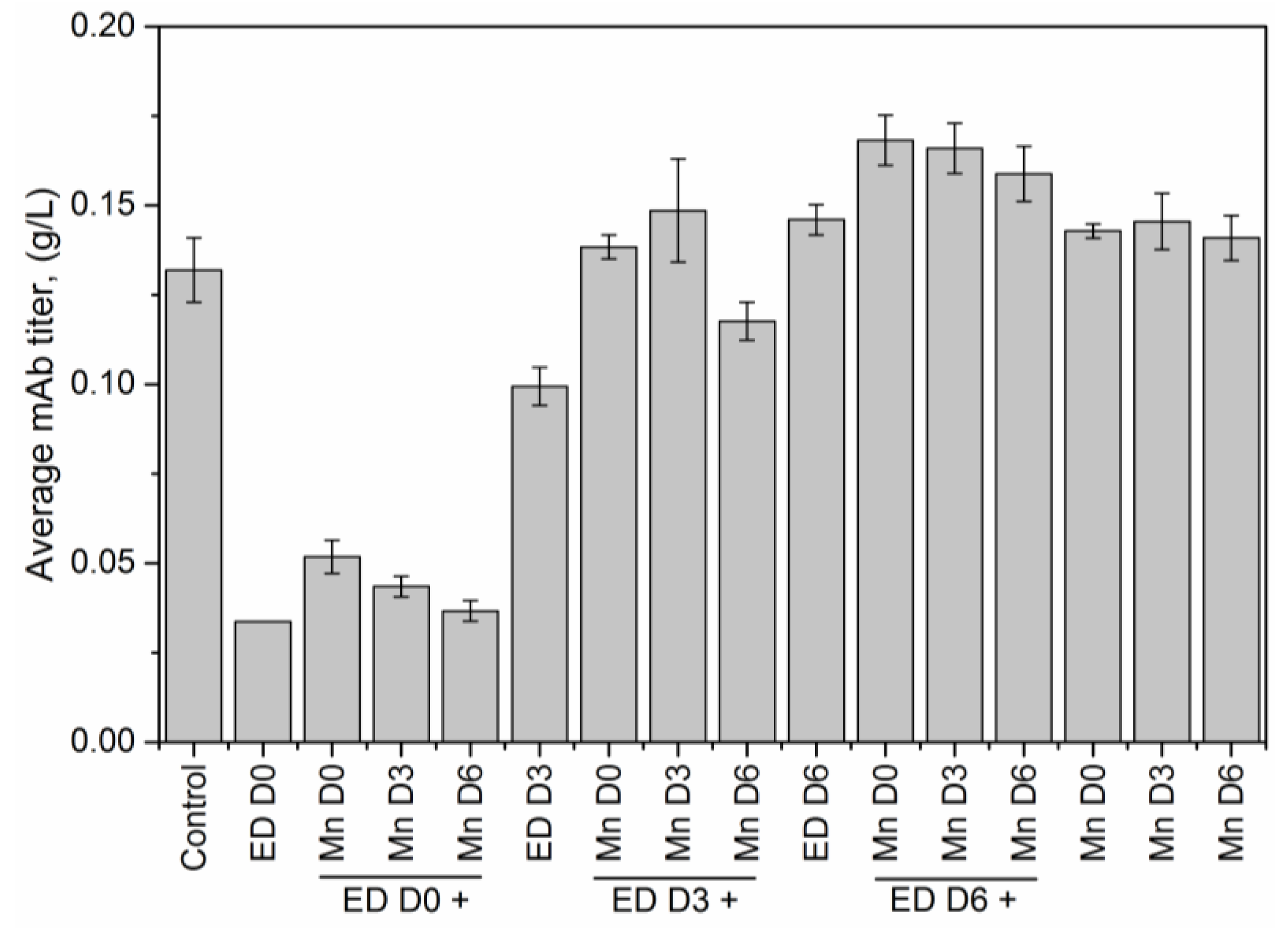
2 supplementation on D0, D3 or D6, resulted in end of run (EOR) titers higher than the titer value in the control flask. The increase in antibody titer in the presence of EDTA was observed by others as well [
36,
37] and is attributed to the inhibition of antibody reduction during cell lysis. Further, analyzing the EOR titer data using ANOVA shows that the factor coefficients for the concentration of EDTA, the concentration of MnCl
2, and time of addition of EDTA are all statistically significant (at a significance level of α = 0.05). The expected change in the EOR titer in response to a unit change in any of these factors is quantified by the magnitude of that factor coefficient, while the sign of the factor coefficient indicates the direction of change. Thus, for example, a unit positive step change in the concentration of EDTA (with a factor coefficient of −0.017) or time of addition of EDTA (factor coefficient −0.032) results in a decrease in EOR titer; a unit positive step change in the concentration of MnCl
2 (factor coefficient 0.008) causes an increase in titer. Quantifying the effect of these input factors on the EOR titer provides a rational basis for selecting which particular supplement to add, and how much of it to add, at a given stage of cell culture in order to maximize product yield. However, any media supplementation strategy must meet not just the desired specifications for final titer but also for product quality, i.e., to be effective, the implemented media supplementation strategy must not alter the glycan distributions significantly.
The EOR titer represents the total amount of antibody accumulated at the end of the batch, while the measured glycan distribution represents the relative amount of each individual glycan isoform that has accumulated over the duration of the batch. Now, the relative amount of individual glycan species is a function of antibody productivity and it changes over the course of the batch. In our case, the addition of different media supplements at different stages of batch cultures affected both viability and antibody titer; consequently, the observed change in the glycan distribution has been induced by dynamic media supplementation and changes in productivity. To develop a mechanistic understanding of the effect of dynamic media supplementation on the glycosylation profile, therefore, we first decouple the effect of antibody productivity on the glycan distribution from the overall change observed at the end of the batch. One such decoupling approach involves estimating the mass fractions of specific glycoforms produced at different stages of cell culture using the expression [
38]:
for
fi, the fraction of mAb glycoform
i produced in the time period [
t1,
t2] relative to the total amount of antibody secreted in the same period. However, we cannot use this expression for our purpose because we only measured EOR titer and final glycan distribution, not intermittent antibody titer or glycan concentration. Consequently, we propose an alternative metric-based solely on the final titer and glycan measurements.
To illustrate, consider the glycan distribution in the control flask and in the flask with MnCl
2 added on D6. In both flasks, the cell growth profile and antibody productivity will be the same until Day 6, when MnCl
2 is introduced to the latter flask. Thus, the amount of
ith glycoform fractions accumulated between Day 6 (D6) and the end of the run (EOR) for the two flasks can be written as:
Recognizing that the D6 values in Equations (5) and (6) above are identical, we can eliminate the intermittent time point by simple arithmetic manipulations and obtain the change in the accumulation of the
ith glycoform-based solely on EOR values, as:
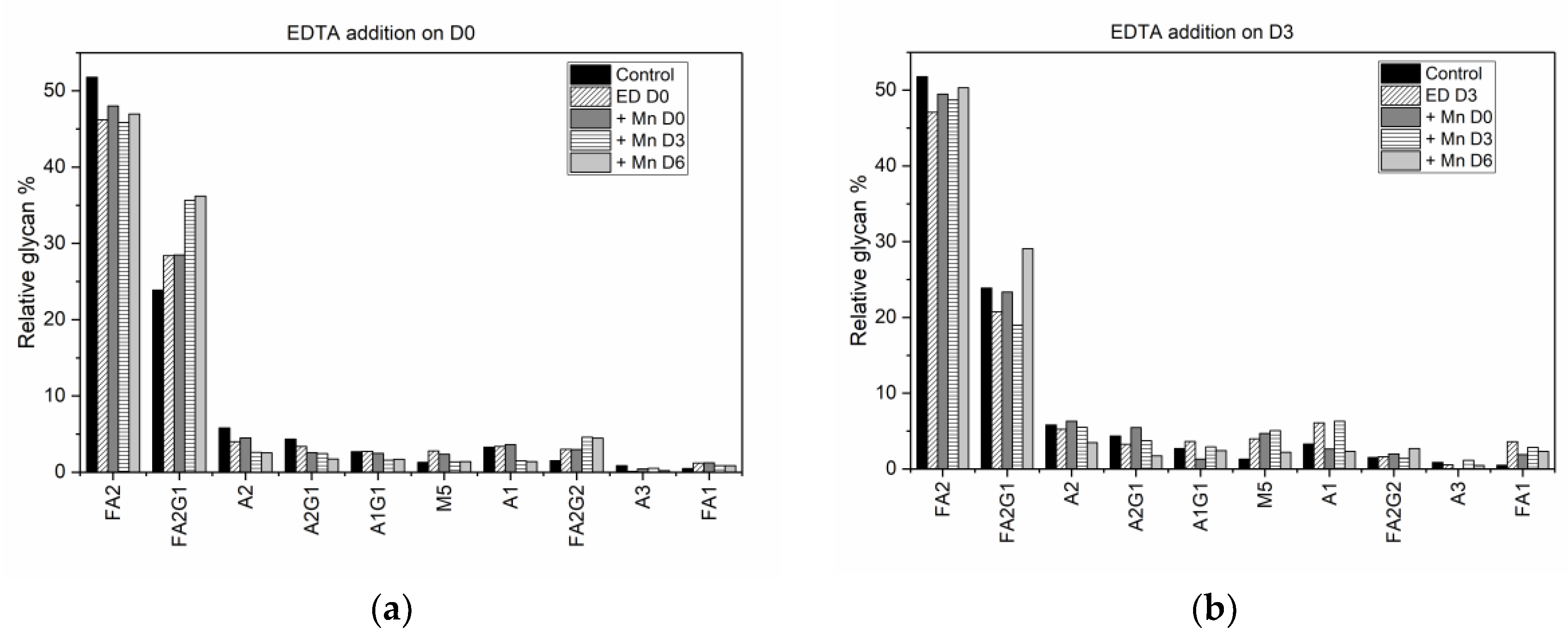
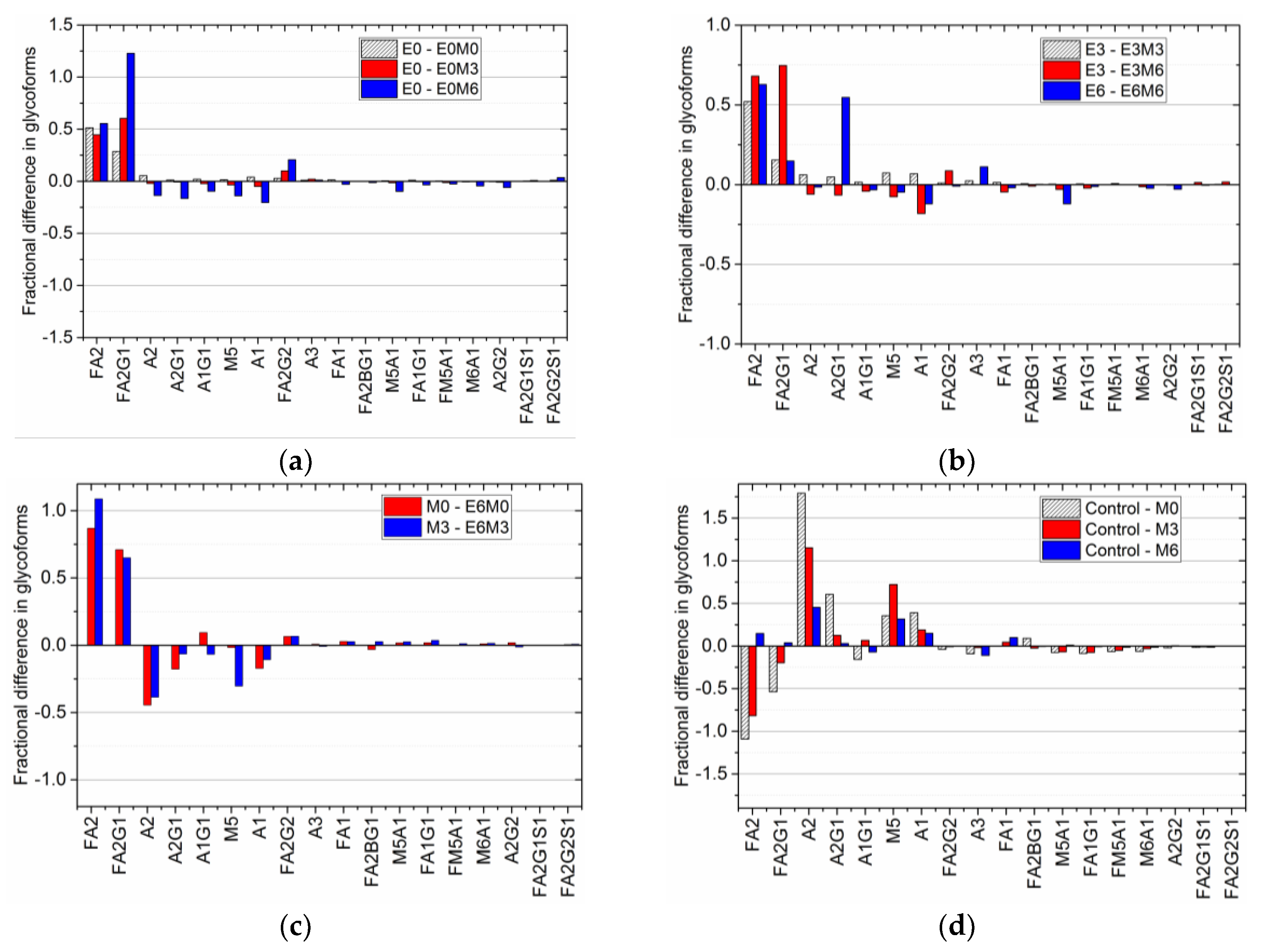
Thus, this fractional difference allows us to group together different experimental conditions with similar antibody titers, making it possible to compare final glycan distributions and hence quantify the effect of individual media supplements on the glycan profile appropriately. Such analyses extended to other experimental conditions produced the comparative fractional difference in the glycoform distribution shown in
Figure 5.
A comparison of the fractional difference in the glycan distribution in flasks where MnCl
2 is added to D0 EDTA supplemented flasks relative to the glycan distribution in D0 EDTA supplemented flasks (
Figure 5a), shows the following: an increase in the amount of FA2 (by nearly 50% in all cases), FA2G1 (by 29% during D0 supplementation, 60% during D3 supplementation, and 122% during D6 supplementation), and FA2G2 (by 3%, 10%, and 20% respectively), with a relative decrease in A2 and M5 by 2% and 4% when MnCl
2 is added on D3, and nearly 14% when MnCl
2 is added on D6. A similar trend is observed in the fractional difference in the glycan distribution in flasks where MnCl
2 is added on D3 and D6 to D3 EDTA supplemented flasks, and when MnCl
2 is added on D6 to D6 EDTA supplemented flasks (
Figure 5b). Again, we notice an increase in FA2, FA2G1 and FA2G2, with a decrease in A2, M5, and A2G1 observed only when MnCl
2 is added on day 6 after the addition of EDTA on D3. Previous studies have shown that adding MnCl
2 produces an upregulation of galactosyltransferase enzymes [
30], and subsequently in increased galactosylation [
26,
27]. Hence, the increase in the amount of FA2G1 and FA2G2 species can be attributed to the effect of late stage manganese addition on the galactosyltransferase enzyme.
Figure 5c shows fractional difference when EDTA is added on D6 after MnCl
2 has been added to the culture on D0 and D3. These differences are calculated relative to the glycan distribution observed due to the addition of MnCl
2 on D3 and D6, respectively. We note that the fractional difference in the fucosylated species FA2 and FA2G1 is positive when EDTA is added after MnCl
2 supplementation, indicating that the addition of EDTA increases the concentration of these species relative to their respective concentrations in MnCl
2 supplemented cultures. Also, a comparison of the fractional difference in the glycan distribution when MnCl
2 was introduced on D0, D3, or D6 relative to the glycan distribution in the control flask (
Figure 5d), shows that the relative concentrations of FA2 and FA2G1 species decreased in flasks with D0, while late stage addition of MnCl
2 did not have a significant effect on the overall glycan distribution. Taken together, our findings indicate that the latter addition of EDTA reverses the changes in the glycan distribution induced by MnCl
2.
Fractional difference analysis helps to identify which glycan species are altered as a result of the addition of specific media supplements, but not why those particular glycan species changed. To identify the kinetic mechanisms underlying the changes observed in the glycan distribution upon adding MnCl
2 to the media, we use an existing dynamic mathematical model of glycosylation [
39] to simulate the system response under control conditions and when MnCl
2 is added on D0, and obtain predictions of both the control and D0 glycan distributions (see
Supplementary Information section S4 for details). The simulation results show that changes in the glycan distribution due to the addition of MnCl
2 can be directly attributed to the changes in the reaction rates associated with the FucT enzyme.
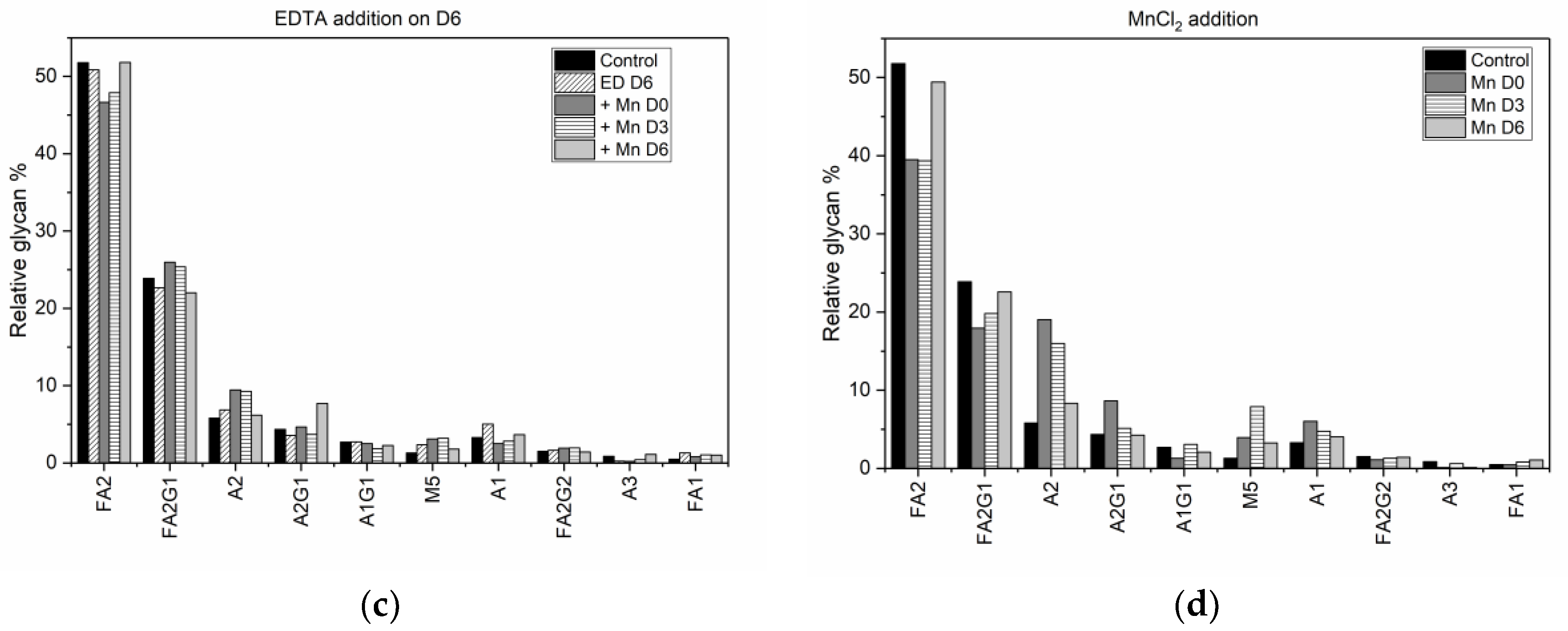
In addition to fractional difference analysis, we use the glycan indices computed for each experimental condition (
Table 2), as a metric for quantitative assessment of the change in the final glycosylation profile caused by the addition of different media supplements. Specifically, a comparison of the individual glycan indices for each condition against the corresponding values under control conditions allows us to establish, objectively, that altering the availability of MnCl
2 in the media using a chelating agent reverses the changes in the glycan distribution. While MnCl
2 addition on D0 resulted in a decrease in the fucosylation index from 79.9% in the control flask to 60.5%, we see that subsequent introduction of EDTA on either D0, D3, or D6 reversed that trend. Early stage addition of EDTA on D0 increased the fucosylation index to 82.46%, while adding EDTA on D3 and D6 resulted in fucosylation indices of 78.8%, and 76.5% respectively. Similarly, the decrease in the galactosylation index, upon adding MnCl
2 on D0, from 17.9% in the control flask to 15.8%, could be reversed by subsequently adding EDTA on D3. Adding MnCl
2 to the media on D3 reduced the fucosylation index to 61.9%, but if we then added EDTA on D6, the fucosylation index increased to 77.8%, which is comparable to the value of 79.9% in the control flask. It is important to note however, that the reversal in the glycan indices observed due to the addition of EDTA on D0 and D3 is achieved at the expense of reduced titer and reduced viability, as discussed above. The indication from our results is that changes in the glycan distribution due to MnCl
2 addition can be reversed only when EDTA is added to the media after MnCl
2 addition. Thus, the effect of MnCl
2 supplementation can be reversed, without decreasing productivity, by adding EDTA on D6 to MnCl
2 supplemented media, providing a means of ensuring higher productivity without altering glycan distribution.
Although such observations as these provide useful qualitative information about the system, they cannot be used to develop an automatic control strategy; that requires a quantitative representation (and hence understanding) of the effects of media supplementation on glycan distribution. Such quantitative representation can be obtained via formal analysis of the experimental data using analysis of variance (ANOVA) to generate the process gain matrix,
K, as described in Materials and Methods. Singular value decomposition of the gain matrix
K produces a (diagonal) matrix of singular values,
Σ, and two unitary matrices,
W and
VT. Together these three matrices provide a particularly insightful representation of the process information encapsulated in the gain matrix,
K: Equation (3) is transformed into a series of
n individual and independent equations (see [
29]) where, in each case, a linear combination of the original process input factors, with weighting coefficients from the matrix
W, now constituting an “input mode”,
μi, is connected through the associated singular value
σi, to the corresponding linear combination of the output glycans, (with weighting coefficients from the matrix
V), now constituting an output mode
ηi [
29]. Furthermore, as a result of this decoupling transformation, the magnitude of each singular value naturally quantifies the extent to which the output mode in question will change in response to a change in the corresponding input mode. Thus, the larger the value of
σi, the greater will be the change in the corresponding output mode
ηi as a result of changes in the input mode
μi, so that output modes associated with larger values of
σi will be more “controllable” than modes associated with smaller values of
σi.
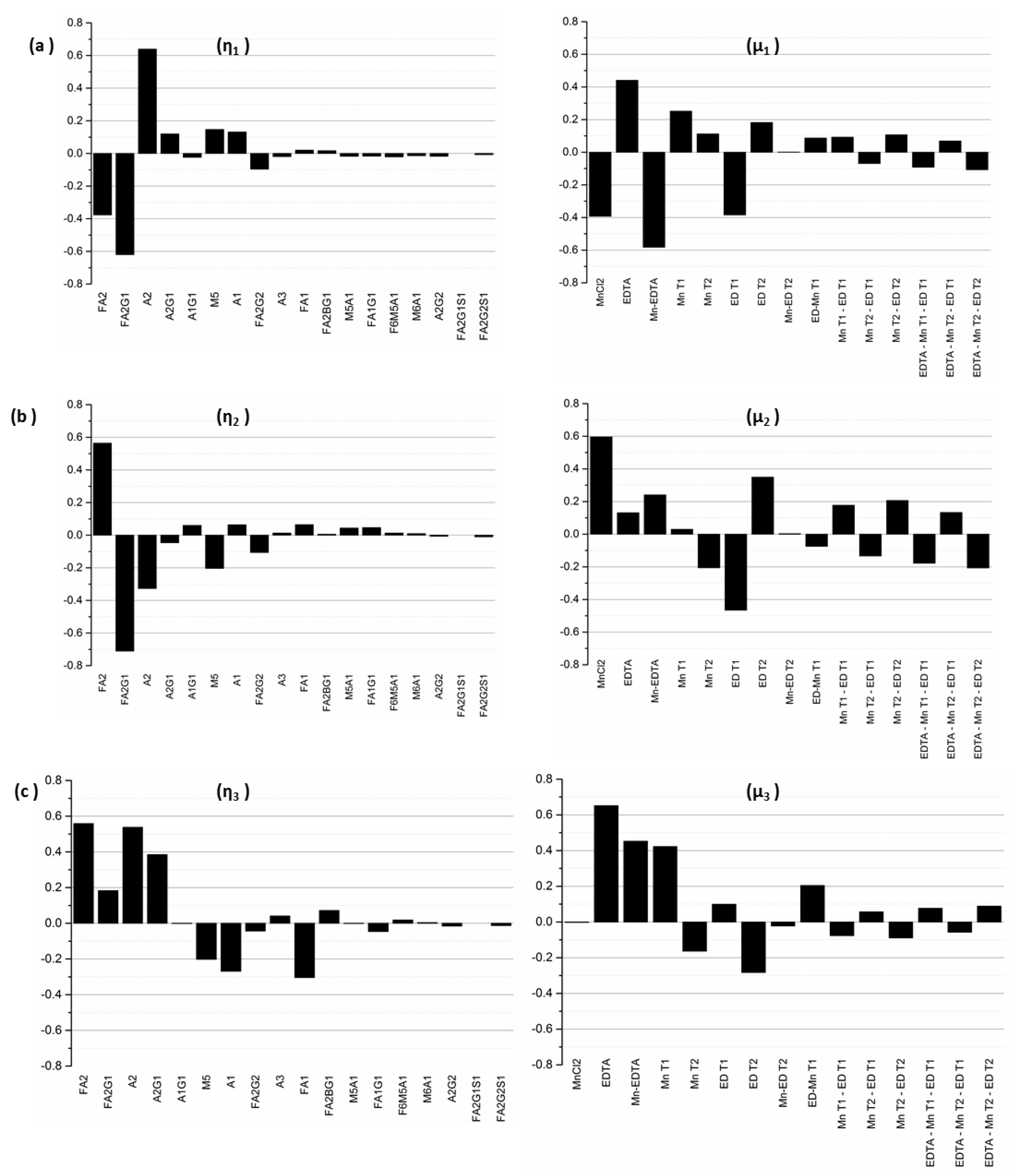
The first ten singular values (
σ1—σ10) for our experimental system are listed in
Table 3 in decreasing order of magnitude. As modes associated with singular values of smaller magnitude are less controllable, we limit our analysis only to those modes that are practically controllable; we do this by using a threshold cutoff value,
σ*, arbitrarily selected to be 0.50 in this example, thereby limiting our analysis to the first five singular values. From a process control perspective, modes associated with singular values below this threshold are considered to be of no practical importance since, for all intents and purposes, they are not controllable.
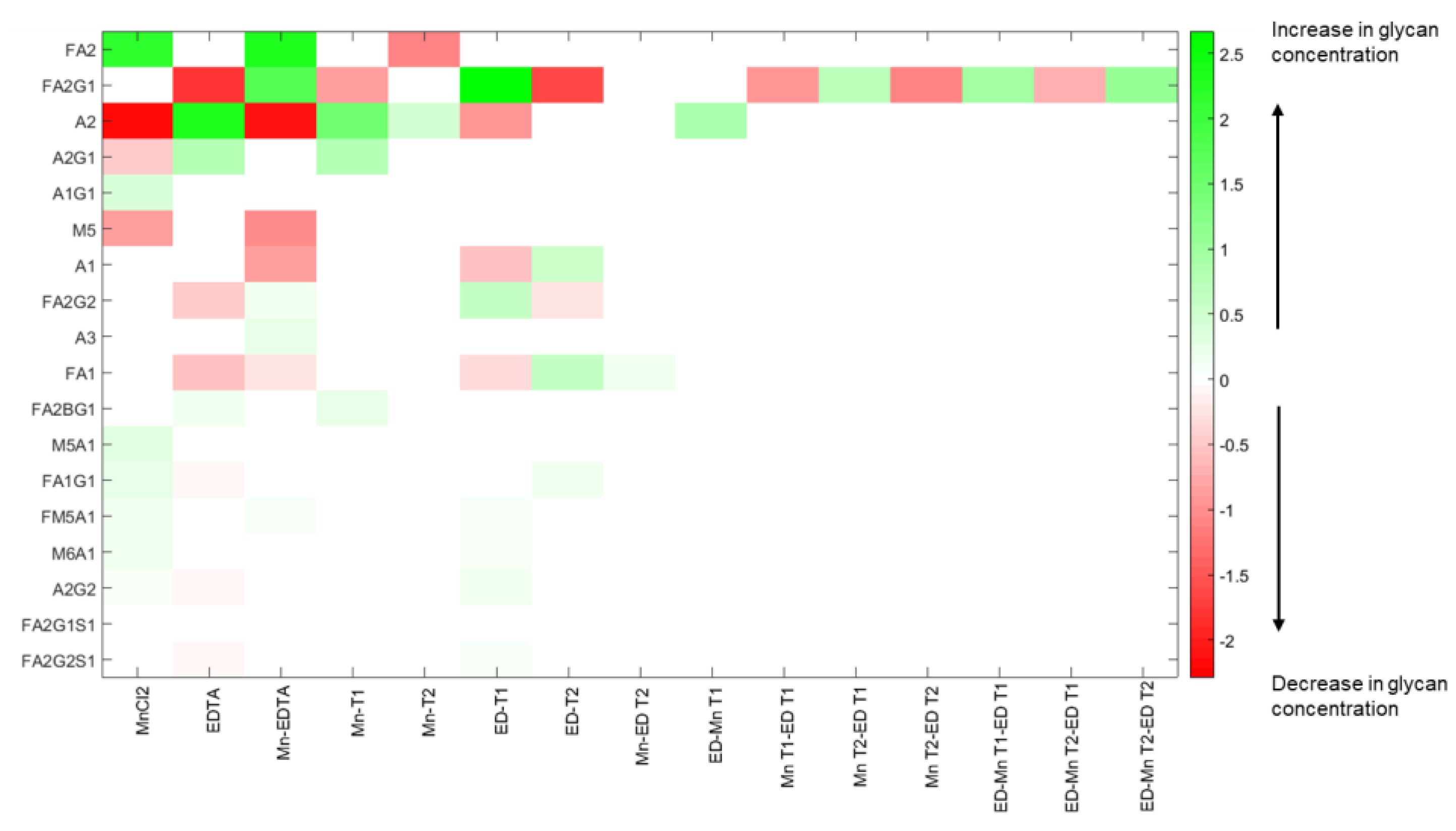
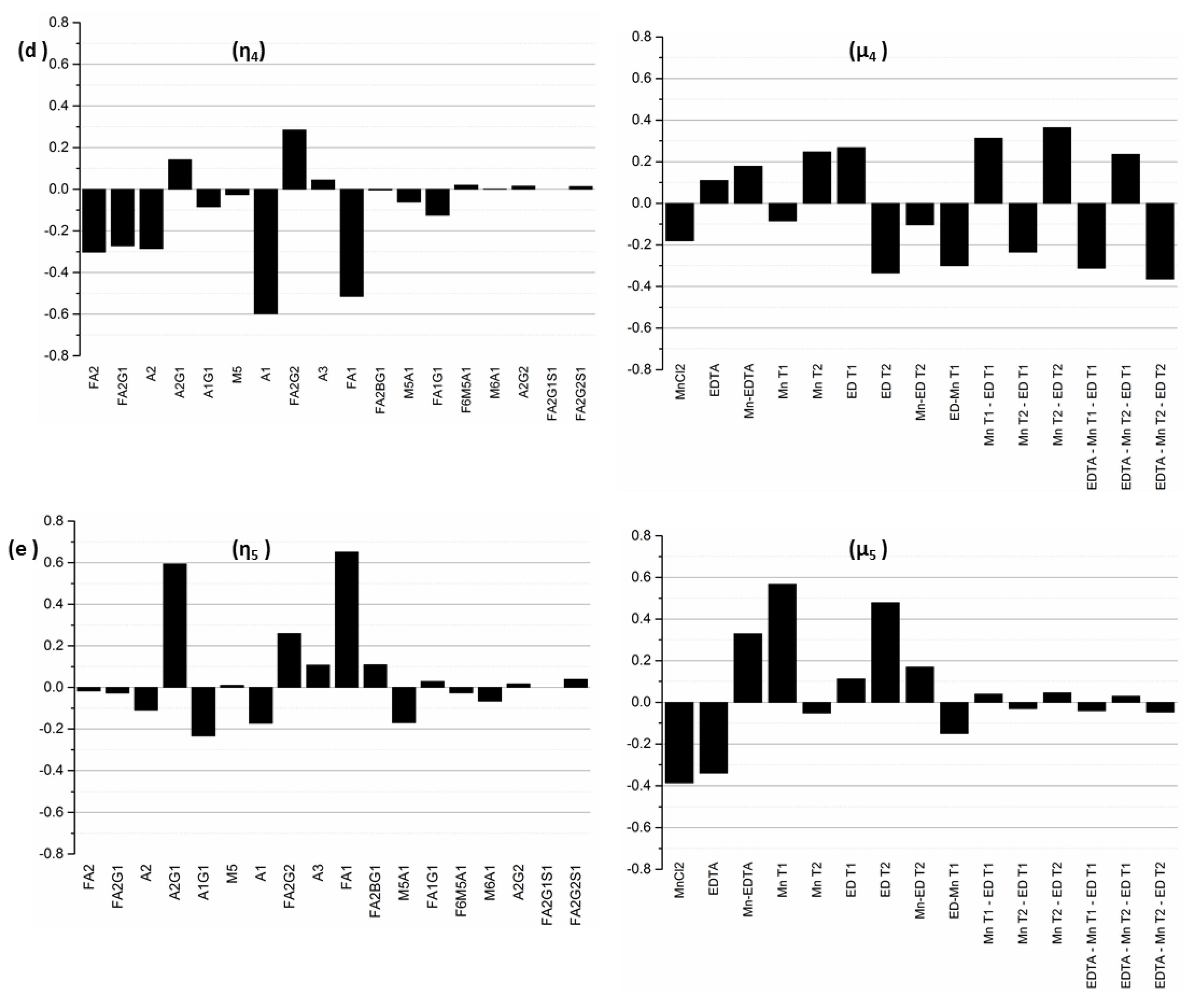
Next, since by definition, each input–output mode pair represents the linear combination of output glycan species that can be controlled by manipulating the specific input factors in the corresponding input mode, the coefficients of each output factor in the output modes and of each input factor in the input modes provide further information about the relative influences exerted by each original input factor on each output factor. Specifically, the coefficient of a particular output factor in a particular mode represents the magnitude by which the relative percentages of those particular glycans will change in response to a unit change in the input mode. On the other hand, the coefficient of a particular input factor in the associated input mode corresponds to the relative contribution of that input factor to the unit change the input mode in question. Thus, the inputs with the largest coefficients in an input mode represent the dominant factors and hence the largest contributors to the influence of that mode, while the output glycans with the largest coefficients in an output mode represent those species whose relative percentages will change the most under the influence of the input modes. The input-output mode pairs and their associated coefficients are shown in
Figure 6.
Because it is associated with the largest singular value of σ1 = 6.62, the first output mode η1 is the most controllable output mode. The value of σ1 represents the change in the overall output mode η1 resulting from a unit change in the input mode μ1. For this output mode, we note that the dominant glycan species are A2, with a coefficient of 0.64, followed by FA2G1 (with a coefficient of −0.62), FA2 (−0.38), M5 (0.14), and A1 (0.13), indicating that a unit positive change in the input mode μ1 will result in an increase in A2, M5, and A1, accompanied by a decrease in FA2G1 and FA2, each in the amount indicated by the identified coefficients. The biantennary species A2, with the largest coefficient, is the most controllable glycan in the first mode, followed by FA2G1 and FA2. The indicated coupling between the glycans A2, FA2G1, and FA2 makes sense because an increase in the afucosylated glycoforms occurs at the expense of the fucosylated forms, as our experimental results show. The associated input mode μ1 is a linear combination of different input factors representing the media supplements MnCl2 and EDTA as well as the times of their addition. Mode μ1 is primarily dominated by the interaction of MnCl2 and EDTA, and the concentrations of EDTA and MnCl2, with associated coefficients −0.58, 0.44, and −0.39 respectively, indicating that the addition of these two media supplements has opposing individual effects on output mode 1. Early stage addition of EDTA and MnCl2, denoted by the factors EDT1 and MnT1, with associated coefficients −0.38 and 0.25 respectively, also exert important influences on the first output mode. Based on the different elements that comprise the first input mode, this analysis indicates that one can control the glycans in output mode η1 by adjusting the concentrations of the two supplements at the early stages of the cell culture.
The next controllable output mode η2 is associated with the singular value σ2 = 3.68, and the linear combination of glycans represented by this mode is dominated by the glycan species FA2G1, FA2, A2, M5, and FA2G2. The coefficients associated with these glycans are −0.71, 0.56, −0.33, −0.20 and −0.11, respectively, indicating that a unit positive change in the input mode μ2 will cause a relative increase in FA2 while causing the other glycan species to decrease. The increase in FA2 coupled with the decrease in FA2G1 and FA2G2 indicates that perturbations to the input mode μ2 affect the galactosylated species particularly. Since the singular values are arranged in decreasing order, the influence of mode μ2 on output mode η2 is less than that of μ1 on mode η1. The largest coefficients in mode μ2 are associated with the input factors MnCl2 (with a coefficient of 0.6), the early stage addition of EDTA denoted by factors EDT1 (with a coefficient of −0.47) and EDT2 (0.35), and the interaction effect of MnCl2 and EDTA (0.24).
A unit positive change to the input mode μ3 causes the following changes in the relative concentrations of the glycan species that comprise output mode η3 (with σ3 = 2.21): A2, A2G1, FA2, and FA2G1 increase, and M5, A1, and FA1 decrease, simultaneously. This indicates that input mode μ3 can be used to increase the biantennary species, but at the expense of a (perfectly logical) concomitant decrease in the species that are upstream of these biantennary species. The input mode μ3 is dominated by EDTA (with associated coefficient 0.65), the interaction effect of MnCl2 and EDTA (with coefficient 0.45), and the early stage addition of MnCl2 denoted by the factor MnT1). In each of the three input modes, the coefficients associated with the interaction effect of MnCl2 and EDTA indicate the importance of this combination input factor in altering the concentrations of the glycan species associated with the respective modes.
The fourth and the fifth modes are less controllable, as they are associated with singular values of comparatively smaller magnitudes (σ4 = 0.80 and σ5 = 0.61). η4 is dominated by glycans A1, A2, FA1, FA2, FA2G1, and FA2G2, while η5 is dominated by FA1, A2G1, and FA2G2. The input mode μ4 is dominated by the interaction effects Mn T2-ED T2 and EDTA*Mn T2-ED T2 representing the interaction between the time of addition of the media supplements. By contrast, the predominant factors in mode μ5 are MnT1 and EDT2, which represent the addition of MnCl2 on D0 and EDTA on D3 respectively.
It is worth mentioning the following facts about the controllability analysis presented here: (i) even though it is a theoretical analysis, the process gain matrix, K (on which the analysis is-based), was obtained entirely from experimental data. The information it contains about how changes in the inputs (amount of supplement added, and time of addition) affect glycan species, come directly from our designed experiments; (ii) Notwithstanding, a follow-up independent experimental validation of the modal analysis predictions (which contributor to which mode is affected in what manner) will be valuable. Such a validation experimental study, which lies outside the intended scope of this current work, is slated for future work. That study’s focus will not only be to validate the controllability analysis results; it will also encompass the more consequential design and implementation of a control system to achieve effective control of glycan distribution during a batch.
For now, we note that the controllability analysis provides a data-based quantification of the effect that the addition of specified amounts of particular media supplements and the respective times of addition jointly have on the output glycan distribution at the end of the batch. As discussed above, introducing these media supplements dynamically also results in quantifiable changes in the antibody titer. Thus, the dynamic supplementation strategies discussed here present a challenging problem involving a trade-off between product yield and product quality. To be effective, a control strategy based on these considerations, must therefore be carefully designed to resolve these conflicts appropriately in order to optimize both the titer and the quality simultaneously. These matters will be addressed theoretically and experimentally in a follow up study.
In closing, we note that
- (1)
the relationships between time-dependent changes in media supplements and the corresponding changes in glycan distribution are (understandably) complex and not necessarily obvious or easily amenable to qualitative thinking; but
- (2)
controllability analysis via singular value decomposition, and the resulting input-output mode pairs determined specifically for our experimental system, using data from carefully designed experiments, have enabled us to identify which input factors are best manipulated, in order to effect changes in the relative percentage of specific glycan species;
- (3)
in addition, the coefficients in the equations representing the input and output modes allow us to quantify by how much we expect the glycan species to change in response to specific time-dependent media supplementation actions.