Epitope Specificity of Anti-Citrullinated Protein Antibodies
Abstract
:1. Introduction to Anti-Citrullinated Protein Antibodies
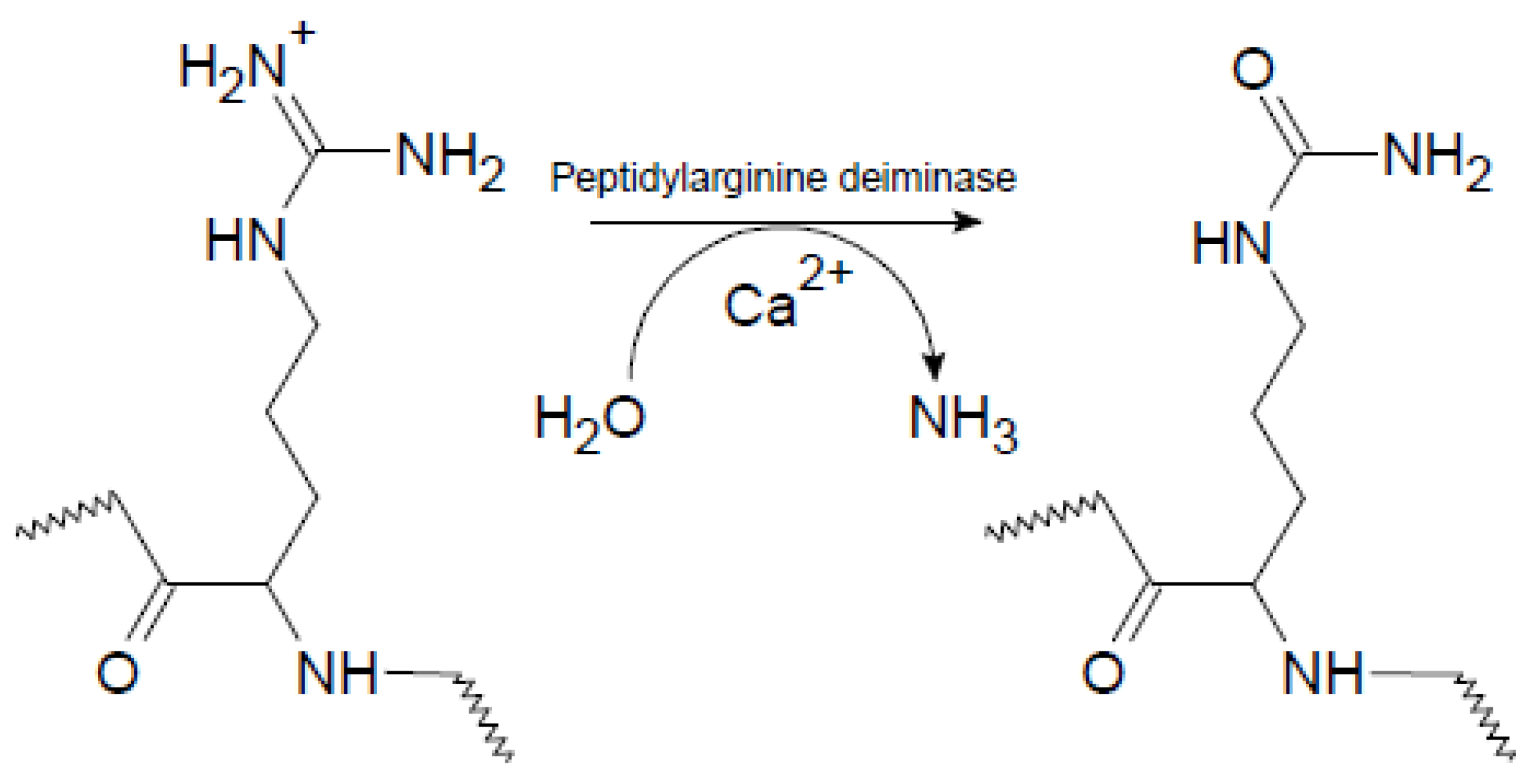
1.1. Citrullination of Proteins by Peptidylarginine Deiminases
1.2. Physiological Functions of Citrullination
1.3. Characteristics of Anti-Citrullinated Protein Antibodies
1.4. Pathogenesis of Rheumatoid Arthritis
1.5. Rheumatoid Arthritis Risk Factors
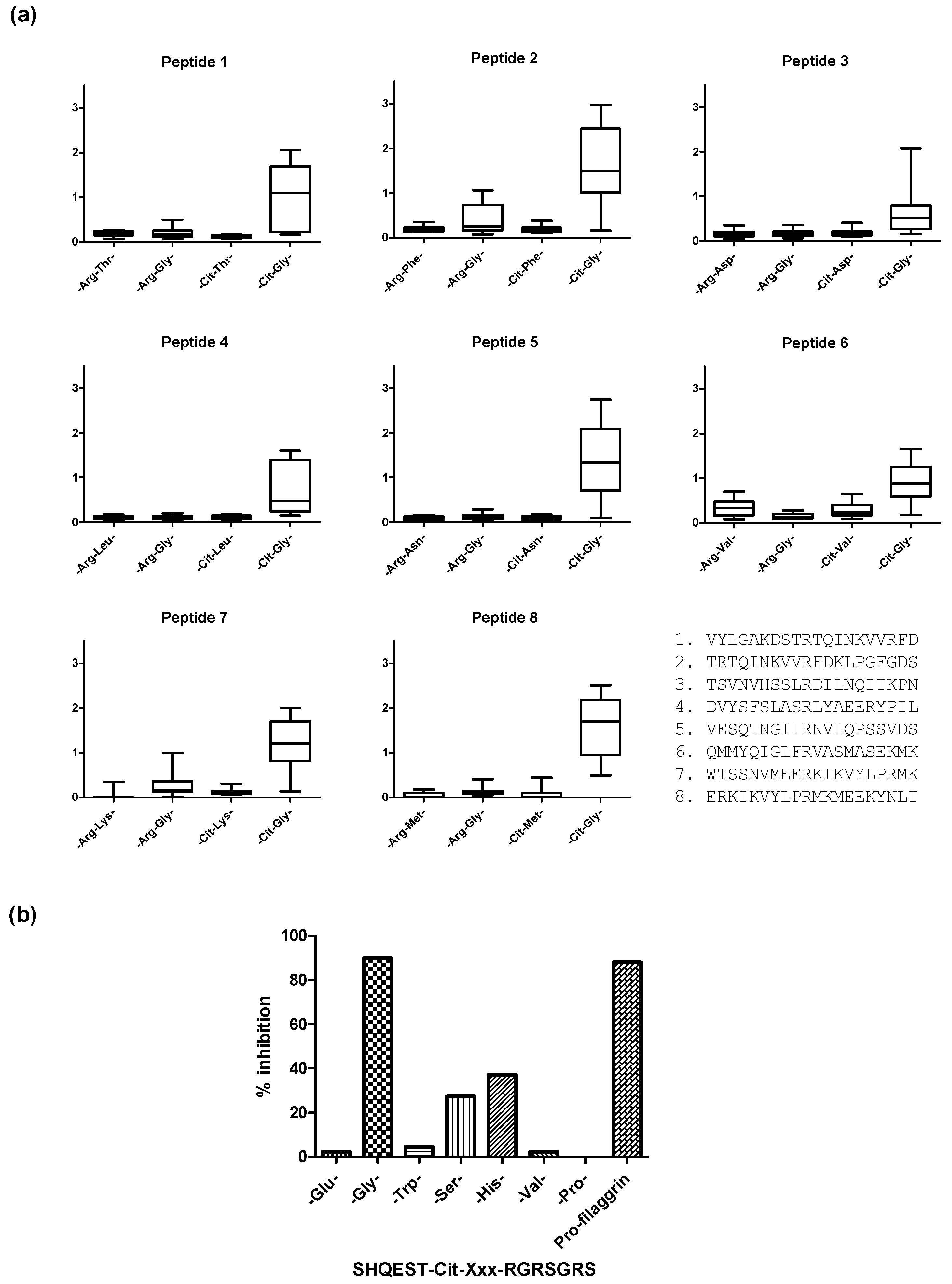
2. Citrullinated Epitope Characteristics
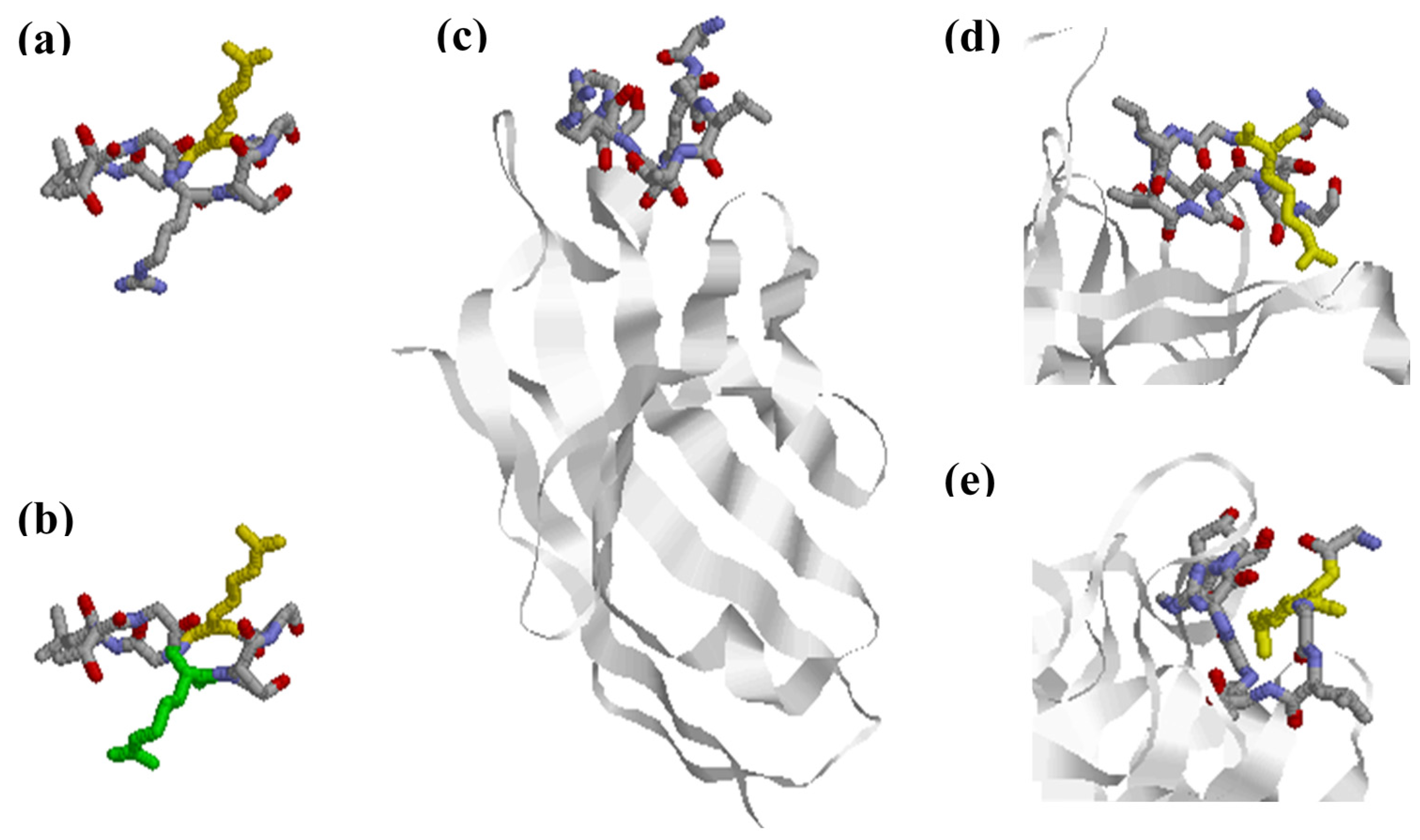
3. Citrullinated Epitope Structures
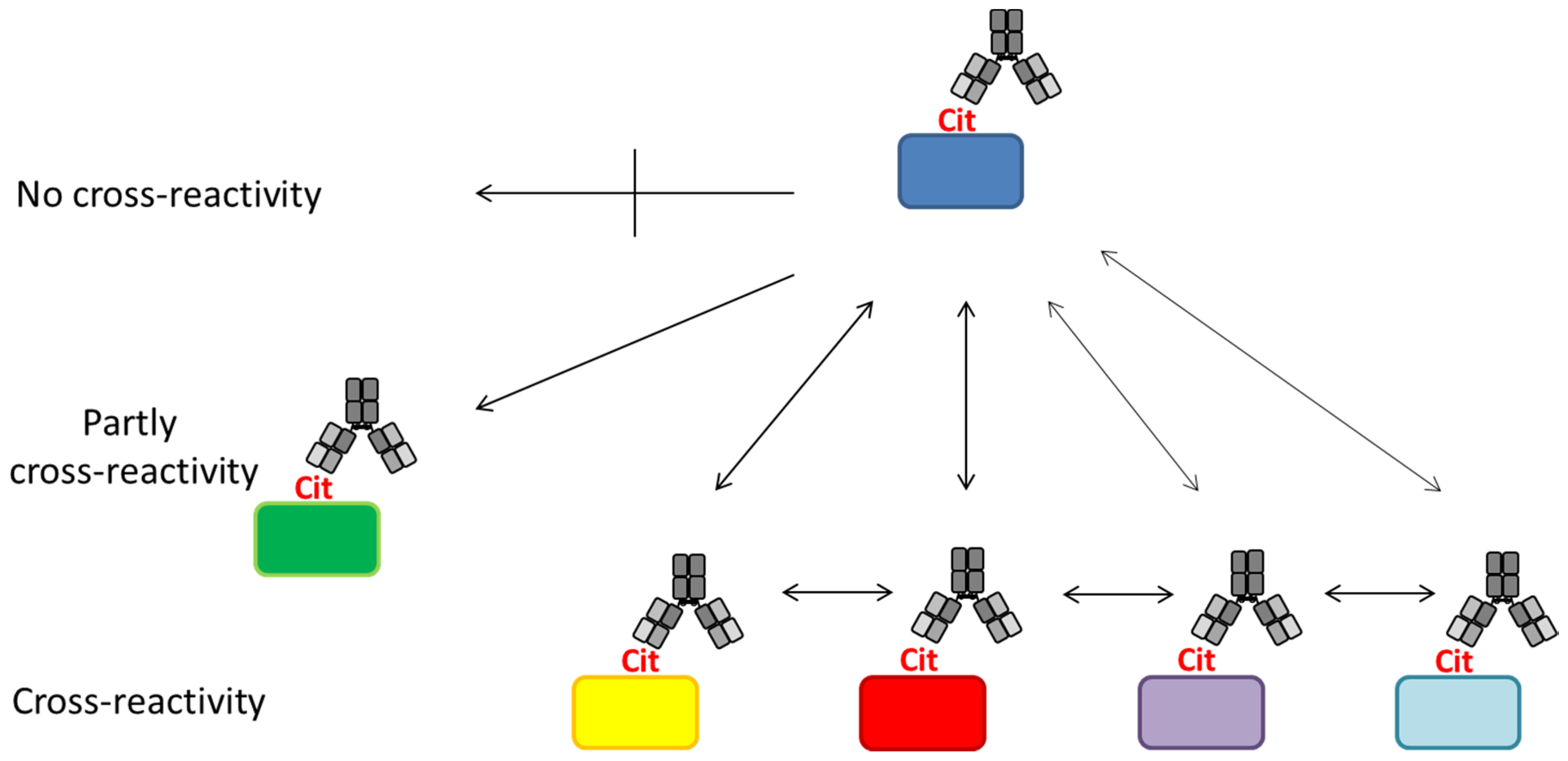
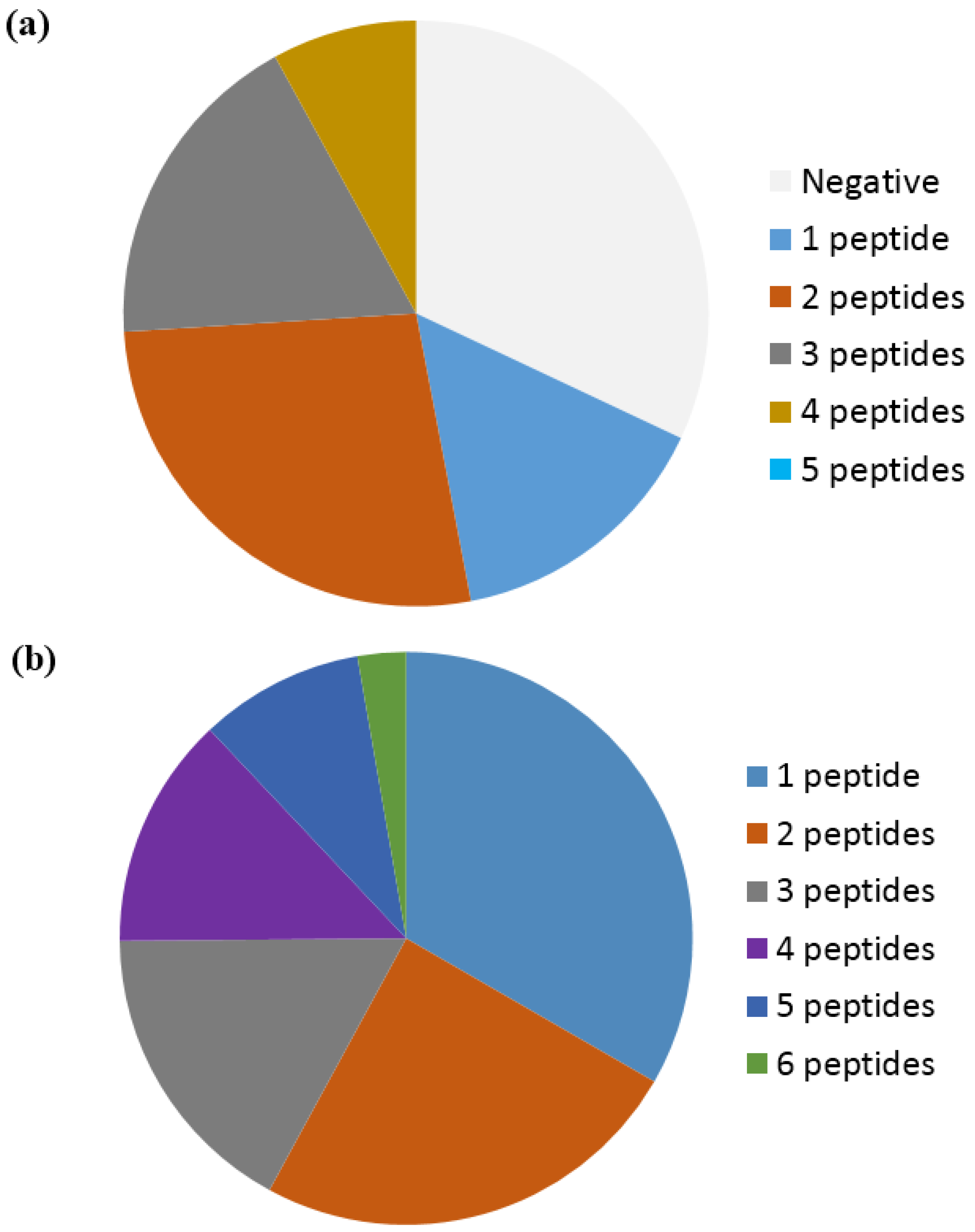
4. Anti-Citrullinated Protein Antibody Specificities
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Alamanos, Y.; Drosos, A.A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev 2005, 4, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Girbal-Neuhauser, E.; Durieux, J.J.; Arnaud, M.; Dalbon, P.; Sebbag, M.; Vincent, C.; Simon, M.; Senshu, T.; Masson-Bessiere, C.; Jolivet-Reynaud, C.; et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999, 162, 585–594. [Google Scholar] [PubMed]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bedford, M.T.; Fast, W. Discovery of peptidylarginine deiminase-4 substrates by protein array: Antagonistic citrullination and methylation of human ribosomal protein S2. Mol. Biosyst. 2011, 7, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Tarcsa, E.; Marekov, L.N.; Mei, G.; Melino, G.; Lee, S.C.; Steinert, P.M. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J. Biol. Chem. 1996, 271, 30709–30716. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Takahara, H.; Nishi, Y.; Sugawara, K.; Sato, C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J. Biol. Chem. 1989, 264, 18119–18127. [Google Scholar] [PubMed]
- Luban, S.; Li, Z.G. Citrullinated peptide and its relevance to rheumatoid arthritis: An update. Int. J. Rheum. Dis. 2010, 13, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Moeez, S.; John, P.; Bhatti, A. Anti-citrullinated protein antibodies: Role in pathogenesis of RA and potential as a diagnostic tool. Rheumatol. Int. 2013, 33, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, A. Apoptosis of rheumatoid arthritis fibroblast-like synoviocytes: Possible roles of nitric oxide and the thioredoxin 1. Mediat. Inflamm. 2013, 2013, 953462. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamada, R.; Chang, X.; Tokuhiro, S.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Nakayama-Hamada, M.; Kawaida, R.; Ono, M.; et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 2003, 34, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Radstake, T.R.; van der Heijden, A.; van Mansum, M.A.; Dieteren, C.; de Rooij, D.J.; Barrera, P.; Zendman, A.J.; van Venrooij, W.J. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann. Rheum. Dis. 2004, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.M.; van, S.D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rantapaa-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Pi, X.; Holoshitz, J. The rheumatoid arthritis shared epitope triggers innate immune signaling via cell surface calreticulin. J. Immunol. 2007, 179, 6359–6367. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Cheng, A.; Pumpens, P.; Michalak, M.; Holoshitz, J. Identification of the rheumatoid arthritis shared epitope binding site on calreticulin. PLoS ONE 2010, 5, e11703. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Cline, E.N.; Haug, T.S.; Fox, D.A.; Holoshitz, J. Citrullinated calreticulin potentiates rheumatoid arthritis shared epitope signaling. Arthritis Rheum. 2013, 65, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Holoshitz, J.; de Almeida, D.E.; Ling, S. A role for calreticulin in the pathogenesis of rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2010, 1209, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Vitale, A.M.; Leijten, F.P.; Strik, A.M.; Koonen-Reemst, A.M.; Yurttas, P.; Robben, T.J.; Coonrod, S.; Gossen, J.A. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol. Cell. Endocrinol. 2007, 273, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, L.B.; Nguyen, T.A.; Moscarello, M.A. The developmental expression and activity of peptidylarginine deiminase in the mouse. Neurosci. Lett. 1999, 266, 161–164. [Google Scholar] [CrossRef]
- Rogers, G.; Winter, B.; McLaughlan, C.; Powell, B.; Nesci, T. Peptidylarginine deiminase of the hair follicle: Characterization, localization, and function in keratinizing tissues. J. Investig. Dermatol. 1997, 108, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Hagiwara, T.; Yamada, M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J. Biol. Chem. 2002, 277, 49562–49568. [Google Scholar] [CrossRef] [PubMed]
- Denis, H.; Deplus, R.; Putmans, P.; Yamada, M.; Metivier, R.; Fuks, F. Functional connection between deimination and deacetylation of histones. Mol. Cell. Biol. 2009, 29, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Z.; Takahara, H. Estrogen-enhanced peptidylarginine deiminase type IV gene (PADI4) expression in MCF-7 cells is mediated by estrogen receptor-alpha-promoted transfactors activator protein-1, nuclear factor-Y, and Sp1. Mol. Endocrinol. 2007, 21, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fast, W. Citrullination of inhibitor of growth 4 (ING4) by peptidylarginine deminase 4 (PAD4) disrupts the interaction between ING4 and p53. J. Biol. Chem. 2011, 286, 17069–17078. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, H.; Zhang, Z.; Li, M.; Luo, Y.; Thompson, P.R.; Gilmour, D.S.; Wang, Y. Regulation of p53 target gene expression by peptidylarginine deiminase 4. Mol. Cell. Biol. 2008, 28, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Liao, Y.F.; Chang, W.H.; Liu, C.C.; Hsieh, M.C.; Hsu, P.C.; Tsay, G.J.; Hung, H.C. Overexpression of peptidylarginine deiminase IV features in apoptosis of haematopoietic cells. Apoptosis 2006, 11, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Ueda, K.; Nakagawa, H.; Yoshida, N.; Nakamura, Y.; Matsuda, K. Regulation of protein Citrullination through p53/PADI4 network in DNA damage response. Cancer Res. 2009, 69, 8761–8769. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, P.; Venters, B.J.; Zheng, S.; Thompson, P.R.; Pugh, B.F.; Wang, Y. Histone Arg modifications and p53 regulate the expression of OKL38, a mediator of apoptosis. J. Biol. Chem. 2008, 283, 20060–20068. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Joo, M.; Abdolrasulnia, R.; Young, D.G.; Choi, I.; Ware, L.B.; Blackwell, T.S.; Christman, B.W. Peptidylarginine deiminase 2 suppresses inhibitory {kappa}B kinase activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Biol. Chem. 2010, 285, 39655–39662. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Loos, T.; Mortier, A.; Gouwy, M.; Ronsse, I.; Put, W.; Lenaerts, J.P.; van Damme, J.; Proost, P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: A naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 2008, 112, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Loos, T.; Opdenakker, G.; van Damme, J.; Proost, P. Citrullination of CXCL8 increases this chemokine’s ability to mobilize neutrophils into the blood circulation. Haematologica 2009, 94, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, F.G.; Wood, D.D.; Mei, J.; Raijmakers, R.; Tseveleki, V.; Dosch, H.M.; Probert, L.; Casaccia-Bonnefil, P.; Moscarello, M.A. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: A role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J. Neurosci. 2006, 26, 11387–11396. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Loos, T.; Mortier, A.; Schutyser, E.; Gouwy, M.; Noppen, S.; Dillen, C.; Ronsse, I.; Conings, R.; Struyf, S.; et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008, 205, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.S.; Slade, D.J.; Thompson, P.R.; Mowen, K.A. Activation of PAD4 in NET formation. Front. Immunol. 2012, 3, 360. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Ronnelid, J.; Lundberg, K.; Padyukov, L.; Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 2008, 26, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Payet, J.; Goulvestre, C.; Biale, L.; Avouac, J.; Wipff, J.; Job-Deslandre, C.; Batteux, F.; Dougados, M.; Kahan, A.; Allanore, Y. Anticyclic citrullinated peptide antibodies in rheumatoid and nonrheumatoid rheumatic disorders: Experience with 1162 patients. J. Rheumatol. 2014, 41, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Smeets, T.J.; Kraan, M.C.; Raats, J.M.; van Venrooij, W.J.; Tak, P.P. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 2004, 50, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Nogueira, L.; Clavel, C.; Sebbag, M.; Serre, G. Autoantibodies to citrullinated proteins: ACPA. Autoimmunity 2005, 38, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Peene, I.; Union, A.; Meheus, L.; Sebbag, M.; Serre, G.; Veys, E.M.; de Keyser, F. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: Relevance to antifilaggrin autoantibodies. Arthritis Rheum. 2001, 44, 2255–2262. [Google Scholar] [CrossRef]
- Snir, O.; Widhe, M.; Hermansson, M.; von, S.C.; Lindberg, J.; Hensen, S.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Toes, R.E.; et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010, 62, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Uysal, H.; Bockermann, R.; Nandakumar, K.S.; Sehnert, B.; Bajtner, E.; Engstrom, A.; Serre, G.; Burkhardt, H.; Thunnissen, M.M.; Holmdahl, R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 2009, 206, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, G.; Karimi, R.; Joshua, V.; Olsen, H.; Hensvold, A.H.; Harju, A.; Engstrom, M.; Grunewald, J.; Nyren, S.; Eklund, A.; et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Anzilotti, C.; Riente, L.; Pratesi, F.; Chimenti, D.; Delle, S.A.; Bombardieri, S.; Migliorini, P. IgG, IgA, IgM antibodies to a viral citrullinated peptide in patients affected by rheumatoid arthritis, chronic arthritides and connective tissue disorders. Rheumatology (Oxford) 2007, 46, 1579–1582. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; Willemze, A.; Robinson, D.B.; Peschken, C.A.; Markland, J.; van der Woude, D.; Elias, B.; Menard, H.A.; Newkirk, M.; Fritzler, M.J.; et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008, 58, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Suwannalai, P.; Willemze, A.; van, T.L.; Stoeken-Rijsbergen, G.; Levarht, N.; Drijfhout, J.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A. The fine specificity of IgM anti-citrullinated protein antibodies (ACPA) is different from that of IgG ACPA. Arthritis Res. Ther. 2011, 13, R195. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.H.; van Nies, J.A.; Chipping, J.; Marshall, T.; van der Helm-van Mil, A.H.; Symmons, D.P.; Verstappen, S.M. Rheumatoid factor and anti-citrullinated protein antibody positivity, but not level, are associated with increased mortality in patients with rheumatoid arthritis: Results from two large independent cohorts. Arthritis Res. Ther. 2014, 16, 483. [Google Scholar] [PubMed]
- Quinn, M.A.; Gough, A.K.; Green, M.J.; Devlin, J.; Hensor, E.M.; Greenstein, A.; Fraser, A.; Emery, P. Anti-CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology (Oxford) 2006, 45, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Ronnelid, J.; Wick, M.C.; Lampa, J.; Lindblad, S.; Nordmark, B.; Klareskog, L.; van Vollenhoven, R.F. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: Anti-CP status predicts worse disease activity and greater radiological progression. Ann. Rheum. Dis. 2005, 64, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Van Gaalen, F.A.; Linn-Rasker, S.P.; van Venrooij, W.J.; de Jong, B.A.; Breedveld, F.C.; Verweij, C.L.; Toes, R.E.; Huizinga, T.W. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: A prospective cohort study. Arthritis Rheum. 2004, 50, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.M.; van der Horst, A.R.; van, S.D.; van der Horst-Bruinsma, I.E.; van de Stadt, R.J.; Aarden, L.; Dijkmans, B.A.; Hamann, D. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann. Rheum. Dis. 2005, 64, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.; Huizinga, T.W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Soos, L.; Szabo, Z.; Fekete, A.; Kapitany, A.; Vegvari, A.; Sipka, S.; Szucs, G.; Szanto, S.; Lakos, G. Anti-citrullinated protein antibodies in rheumatoid arthritis: As good as it gets? Clin. Rev. Allergy Immunol. 2008, 34, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Nienhuis, R.L.; Mandema, E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann. Rheum. Dis. 1964, 23, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Young, B.J.; Mallya, R.K.; Leslie, R.D.; Clark, C.J.; Hamblin, T.J. Anti-keratin antibodies in rheumatoid arthritis. Br. Med. J. 1979, 2, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, M.; Simon, M.; Vincent, C.; Masson-Bessiere, C.; Girbal, E.; Durieux, J.J.; Serre, G. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1995, 95, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Girbal, E.; Sebbag, M.; Gomes-Daudrix, V.; Vincent, C.; Salama, G.; Serre, G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called “antikeratin antibodies”, autoantibodies specific for rheumatoid arthritis. J. Clin. Investig. 1993, 92, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Vincent, C.; Haftek, M.; Girbal, E.; Sebbag, M.; Gomes-Daudrix, V.; Serre, G. The rheumatoid arthritis-associated autoantibodies to filaggrin label the fibrous matrix of the cornified cells but not the profilaggrin-containing keratohyalin granules in human epidermis. Clin. Exp. Immunol. 1995, 100, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Despres, N.; Boire, G.; Lopez-Longo, F.J.; Menard, H.A. The Sa system: A novel antigen-antibody system specific for rheumatoid arthritis. J. Rheumatol. 1994, 21, 1027–1033. [Google Scholar] [PubMed]
- Hayem, G.; Chazerain, P.; Combe, B.; Elias, A.; Haim, T.; Nicaise, P.; Benali, K.; Eliaou, J.F.; Kahn, M.F.; Sany, J.; et al. Anti-Sa antibody is an accurate diagnostic and prognostic marker in adult rheumatoid arthritis. J. Rheumatol. 1999, 26, 7–13. [Google Scholar] [PubMed]
- Bang, H.; Egerer, K.; Gauliard, A.; Luthke, K.; Rudolph, P.E.; Fredenhagen, G.; Berg, W.; Feist, E.; Burmester, G.R. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, G.A.; Visser, H.; de Jong, B.A.; van den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; van Venrooij, W.J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef]
- Aggarwal, R.; Liao, K.; Nair, R.; Ringold, S.; Costenbader, K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Coenen, D.; Verschueren, P.; Westhovens, R.; Bossuyt, X. Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin. Chem. 2007, 53, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.L.; Carvalho, S.; Fortuna, J.; Pereira, M.H. Comparison of three anti-CCP antibody tests and rheumatoid factor in RA and control patients. Clin. Rev. Allergy Immunol. 2008, 34, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Baron, M.; Miyachi, K.; Fritzler, M.J.; Abu-Hakima, M.; Leclercq, S.; Bell, M.; Hudson, M.; Mathieu, J.P.; Taillefer, S.; et al. A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti-CCP assay (CCP3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin. Rheumatol. 2008, 27, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hoet, R.M.; Boerbooms, A.M.; Arends, M.; Ruiter, D.J.; van Venrooij, W.J. Antiperinuclear factor, a marker autoantibody for rheumatoid arthritis: Colocalisation of the perinuclear factor and profilaggrin. Ann. Rheum. Dis. 1991, 50, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Smolen, J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002, 4, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M.A.; Vossenaar, E.R.; van den Hoogen, F.H.; van Venrooij, W.J. Autoantibody systems in rheumatoid arthritis: Specificity, sensitivity and diagnostic value. Arthritis Res. 2002, 4, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Masson-Bessiere, C.; Sebbag, M.; Girbal-Neuhauser, E.; Nogueira, L.; Vincent, C.; Senshu, T.; Serre, G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J. Immunol. 2001, 166, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Iobagiu, C.; Magyar, A.; Nogueira, L.; Cornillet, M.; Sebbag, M.; Arnaud, J.; Hudecz, F.; Serre, G. The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J. Autoimmun. 2011, 37, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Sugiyama, D.; Kogata, Y.; Tsuji, G.; Nakazawa, T.; Kawano, S.; Saigo, K.; Morinobu, A.; Koshiba, M.; Kuntz, K.M.; et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann. Intern. Med. 2007, 146, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Szabo, Z.; Zeher, M.; Soos, L.; Danko, K.; Horvath, I.; Lakos, G. Superior performance of the CCP3.1 test compared to CCP2 and MCV in the rheumatoid factor-negative RA population. Immunol. Res. 2013, 56, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Tonutti, E.; Tozzoli, R.; Villalta, D. Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin. Chem. 2007, 53, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Longo, F.J.; Rodriguez-Mahou, M.; Sanchez-Ramon, S.; Estecha, A.; Balsera, M.; Plaza, R.; Fernandez-Cruz, E.; Perez, L.C. Anti-cyclic citrullinated peptide versus anti-Sa antibodies in diagnosis of rheumatoid arthritis in an outpatient clinic for connective tissue disease and spondyloarthritis. J. Rheumatol. 2006, 33, 1476–1481. [Google Scholar] [PubMed]
- Wagner, E.; Skoumal, M.; Bayer, P.M.; Klaushofer, K. Antibody against mutated citrullinated vimentin: A new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol. Int. 2009, 29, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Holm, B.E.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; Houen, G. Application of synthetic peptides for detection of anti-citrullinated peptide antibodies. Peptides 2016, 76, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Van de Stadt, L.A.; de Koning, M.H.; van de Stadt, R.J.; Wolbink, G.; Dijkmans, B.A.; Hamann, D.; van Schaardenburg, D. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011, 63, 3226–3233. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, W.J.; van Beers, J.J.; Pruijn, G.J. Anti-CCP Antibody, a Marker for the Early Detection of Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2008, 1143, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cerqueira, C.; Ossipova, E.; Gunasekera, S.; Hansson, M.; Mathsson, L.; Catrina, A.I.; Sommarin, Y.; Klareskog, L.; Lundberg, K.; Ronnelid, J.; et al. Targeting of anti-citrullinated protein/peptide antibodies in rheumatoid arthritis using peptides mimicking endogenously citrullinated fibrinogen antigens. Arthritis Res. Ther. 2015, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Bengtsson, C.; Kharlamova, N.; Reed, E.; Jiang, X.; Kallberg, H.; Pollak-Dorocic, I.; Israelsson, L.; Kessel, C.; Padyukov, L.; et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann. Rheum. Dis. 2013, 72, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Sokolove, J.; Lahey, L.J.; Bengtsson, C.; Saevarsdottir, S.; Alfredsson, L.; Delanoy, M.; Lindstrom, T.M.; Walker, R.P.; Bromberg, R.; et al. Identification of anticitrullinated protein antibody reactivities in a subset of anti-CCP-negative rheumatoid arthritis: Association with cigarette smoking and HLA-DRB1 ‘shared epitope’ alleles. Ann. Rheum. Dis. 2015, 74, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Kobayashi, A. Tyrosine kinases in rheumatoid arthritis. J. Inflamm. (London) 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006, 54, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Kulik, L.; Tomooka, B.; Braschler, K.J.; Arend, W.P.; Robinson, W.H.; Holers, V.M. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J. Clin. Investig. 2006, 116, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Nogueira, L.; Laurent, L.; Iobagiu, C.; Vincent, C.; Sebbag, M.; Serre, G. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008, 58, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Haisma, E.M.; Levarht, E.W.; van der Woude, D.; Ioan-Facsinay, A.; Daha, M.R.; Huizinga, T.W.; Toes, R.E. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009, 60, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, F.; Huang, C.; Huang, J.; Wang, Q.; Chen, Y.; Du, Y.; Zhao, L.; Gao, M.; Wang, F. Plasmapheresis Therapy in Combination with TNF-alpha Inhibitor and DMARDs: A Multi-Target Method for Treatment of Rheumatoid Arthritis. Mod. Rheumatol. 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; de Vita, S.; Blasone, N.; Visentini, D.; Pezzarini, E.; Pontarini, E.; Fabro, C.; Quartuccio, L.; Mazzolini, S.; Curcio, F.; et al. Serum levels of anti-CCP antibodies, anti-MCV antibodies and RF IgA in the follow-up of patients with rheumatoid arthritis treated with rituximab. Autoimmun. Highlights 2010, 1, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Van Steendam, K.; Tilleman, K.; de Ceuleneer, M.; de Keyser, F.; Elewaut, D.; Deforce, D. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010, 12, R132. [Google Scholar] [CrossRef] [PubMed]
- James, E.A.; Rieck, M.; Pieper, J.; Gebe, J.A.; Yue, B.B.; Tatum, M.; Peda, M.; Sandin, C.; Klareskog, L.; Malmstrom, V.; et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014, 66, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, P.L.; de Hair, M.J.; Doorensplet, M.E.; van Schaik, B.D.; Esveldt, R.E.; van de Sande, M.G.; Cantaert, T.; Gerlag, D.M.; Bae Baeten, D.; van Kampen, A.H.; et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 2012, 71, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Cantaert, T.; Brouard, S.; Thurlings, R.M.; Pallier, A.; Salinas, G.F.; Braud, C.; Klarenbeek, P.L.; de Vries, N.; Zhang, Y.; Soulillou, J.P.; et al. Alterations of the synovial T cell repertoire in anticitrullinated protein antibody-positive rheumatoid arthritis. Ahtritis Rheum. 2009, 60, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Southwood, S.; Sette, A.; Jevnikar, A.M.; Bell, D.A.; Cairns, E. Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 2003, 171, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Wang, D.; Jevnikar, A.M.; Cairns, E.; Bell, D.A. The relationship between predicted peptide-MHC class II affinity and T-cell activation in a HLA-DRbeta1*0401 transgenic mouse model. Arthritis Res. Ther. 2003, 5, R40–R48. [Google Scholar] [CrossRef] [PubMed]
- James, E.A.; Moustakas, A.K.; Bui, J.; Papadopoulos, G.K.; Bondinas, G.; Buckner, J.H.; Kwok, W.W. HLA-DR1001 presents “altered-self” peptides derived from joint-associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. 2010, 62, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Law, S.C.; Street, S.; Yu, C.H.; Capini, C.; Ramnoruth, S.; Nel, H.J.; van Gorp, E.; Hyde, C.; Lau, K.; Pahau, H.; et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012, 14, R118. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Rieck, M.; Gebe, J.A.; Yue, B.B.; Rawlings, C.A.; Nepom, G.; Malmstrom, V.; Buckner, J.H. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 2011, 63, 2873–2883. [Google Scholar] [CrossRef] [PubMed]
- Chemin, K.; Pollastro, S.; James, E.; Ge, C.; Albrecht, I.; Herrath, J.; Gerstner, C.; Tandre, K.; Sampaio Rizzi, T.; Rönnblom, L.; et al. A novel HLA-DRB1*10:01 restricted T cell epitope from cirullinated type II collagen relevant for rheumatoid ahtritis. Arthritis Rheum. 2016, 68, 1124–1125. [Google Scholar]
- Feitsma, A.L.; van der Voort, E.I.; Franken, K.L.; el Bannoudi, H.; Elferink, B.G.; Drijfhout, J.W.; Huizinga, T.W.; de Vries, R.R.; Toes, R.E.; Ioan-Facsinay, A. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 117–125. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Snieder, H.; Rigby, A.S.; Koskenvuo, M.; Kaprio, J.; Aho, K.; Silman, A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000, 43, 30–37. [Google Scholar] [CrossRef]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Stastny, P. Mixed lymphocyte cultures in rheumatoid arthritis. J. Clin. Investig. 1976, 57, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Holoshitz, J. The rheumatoid arthritis HLA-DRB1 shared epitope. Curr. Opin. Rheumatol. 2010, 22, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bhayani, H.R.; Hedrick, S.M. The role of polymorphic amino acids of the MHC molecule in the selection of the T cell repertoire. J. Immunol. 1991, 146, 1093–1098. [Google Scholar] [PubMed]
- Wucherpfennig, K.W.; Strominger, J.L. Selective binding of self peptides to disease-associated major histocompatibility complex (MHC) molecules: A mechanism for MHC-linked susceptibility to human autoimmune diseases. J. Exp. Med. 1995, 181, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Kapitany, A.; Szabo, Z.; Lakos, G.; Aleksza, M.; Vegvari, A.; Soos, L.; Karanyi, Z.; Sipka, S.; Szegedi, G.; Szekanecz, Z. Associations between serum anti-CCP antibody, rheumatoid factor levels and HLA-DR4 expression in Hungarian patients with rheumatoid arthritis. Isr. Med. Assoc. J. 2008, 10, 32–36. [Google Scholar] [PubMed]
- Snir, O.; Widhe, M.; von, S.C.; Lindberg, J.; Padyukov, L.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Lundeberg, J.; Holmdahl, R.; et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2009, 68, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Begovich, A.B.; Carlton, V.E.; Honigberg, L.A.; Schrodi, S.J.; Chokkalingam, A.P.; Alexander, H.C.; Ardlie, K.G.; Huang, Q.; Smith, A.M.; Spoerke, J.M.; et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004, 75, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Padyukov, L.; Remmers, E.F.; Purcell, S.; Lee, A.T.; Karlson, E.W.; Wolfe, F.; Kastner, D.L.; Alfredsson, L.; Altshuler, D.; et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: Association of susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 2005, 77, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Kallberg, H.; Bengtsson, C.; Grunewald, J.; Ronnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapaa-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; le, C.S.; Huizinga, T.W.; de Vries, R.R.; Toes, R.E. The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum. 2007, 56, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kallberg, H.; Padyukov, L.; Plenge, R.M.; Ronnelid, J.; Gregersen, P.K.; van der Helm-van Mil, A.H.; Toes, R.E.; Huizinga, T.W.; Klareskog, L.; Alfredsson, L. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am. J. Hum. Genet. 2007, 80, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, J.; van der Helm-van Mil, A.H.; Toes, R.E.; Chokkalingam, A.P.; Carlton, V.E.; Begovich, A.B.; Huizinga, T.W. Association of the PTPN22 C1858T single-nucleotide polymorphism with rheumatoid arthritis phenotypes in an inception cohort. Arthritis Rheum. 2005, 52, 2948–2950. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Seielstad, M.; Padyukov, L.; Lee, A.T.; Remmers, E.F.; Ding, B.; Liew, A.; Khalili, H.; Chandrasekaran, A.; Davies, L.R.; et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—A genomewide study. N. Engl. J. Med. 2007, 357, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, J.C.; Flornes, L.; Eklow, C.; Backdahl, L.; Ribbhammar, U.; Guo, J.P.; Smolnikova, M.; Dissen, E.; Seddighzadeh, M.; Brookes, A.J.; et al. Association of arthritis with a gene complex encoding C-type lectin-like receptors. Arthritis Rheum. 2007, 56, 2620–2632. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.; Padyukov, L.; Kurreeman, F.A.; Liljedahl, U.; Wiman, A.C.; Alfredsson, L.; Toes, R.; Ronnelid, J.; Klareskog, L.; Huizinga, T.W.; et al. Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef] [PubMed]
- Anzilotti, C.; Merlini, G.; Pratesi, F.; Tommasi, C.; Chimenti, D.; Migliorini, P. Antibodies to viral citrullinated peptide in rheumatoid arthritis. J. Rheumatol. 2006, 33, 647–651. [Google Scholar] [PubMed]
- Burkhardt, H.; Sehnert, B.; Bockermann, R.; Engstrom, A.; Kalden, J.R.; Holmdahl, R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur. J. Immunol. 2005, 35, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.; Tatzer, V.; Wait, R.; Peston, D.; Lundberg, K.; Donatien, P.; Moyes, D.; Taylor, P.C.; Venables, P.J. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R1421–R1429. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Tommasi, C.; Anzilotti, C.; Chimenti, D.; Migliorini, P. Deiminated Epstein-Barr virus nuclear antigen 1 is a target of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, M.; Moinard, N.; Auger, I.; Clavel, C.; Arnaud, J.; Nogueira, L.; Roudier, J.; Serre, G. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur. J. Immunol. 2006, 36, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Suzuki, A.; Sawada, T.; Ohsaka, M.; Inoue, T.; Yamada, R.; Yamamoto, K. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann. Rheum. Dis. 2006, 65, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, D.; Rantapaa-Dahlqvist, S.; Ioan-Facsinay, A.; Onnekink, C.; Schwarte, C.M.; Verpoort, K.N.; Drijfhout, J.W.; Huizinga, T.W.; Toes, R.E.; Pruijn, G.J. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann. Rheum. Dis. 2010, 69, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Dam, C.E.; Houen, G.; Trier, N.H. The dependency on neighboring amino acids for reactivity of anti-citrullinated protein antibodies to citrullinated proteins. Scand. J. Clin. Lab. Investig. 2016, 76, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Leth, M.L.; Hansen, P.R.; Houen, G. Cross-reactivity of a human IgG(1) anticitrullinated fibrinogen monoclonal antibody to a citrullinated profilaggrin peptide. Protein Sci. 2012, 21, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Union, A.; Meheus, L.; Humbel, R.L.; Conrad, K.; Steiner, G.; Moereels, H.; Pottel, H.; Serre, G.; de Keyser, F. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002, 46, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Dam, C.E.; Olsen, D.T.; Hansen, P.R.; Houen, G. Contribution of Peptide Backbone to Anti-Citrullinated Peptide Antibody Reactivity. PLoS ONE 2015, 10, e0144707. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Holm, B.E.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Houen, G. Physical characteristics of a citrullinated pro-filaggrin epitope recognized by anti-citrullinated protein antibodies in rheumatoid arthritis sera. PLoS ONE 2016, 11, e0168542. [Google Scholar] [CrossRef] [PubMed]
- Babos, F.; Szarka, E.; Nagy, G.; Majer, Z.; Sarmay, G.; Magyar, A.; Hudecz, F. Role of N- or C-terminal biotinylation in autoantibody recognition of citrullin containing filaggrin epitope peptides in rheumatoid arthritis. Bioconjug. Chem. 2013, 24, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Tommasi, C.; Anzilotti, C.; Puxeddu, I.; Sardano, E.; Di, C.G.; Migliorini, P. Antibodies to a new viral citrullinated peptide, VCP2: Fine specificity and correlation with anti-cyclic citrullinated peptide (CCP) and anti-VCP1 antibodies. Clin. Exp. Immunol. 2011, 164, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.D.; Ackerley, C.A.; Brand, B.; Zhang, L.; Raijmakers, R.; Mastronardi, F.G.; Moscarello, M.A. Myelin localization of peptidylarginine deiminases 2 and 4: Comparison of PAD2 and PAD4 activities. Lab. Investig. 2008, 88, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kobylyansky, A.G.; Nekrasov, A.N.; Kozlova, V.I.; Sandin, M.Y.; Alikhanov, B.A.; Demkin, V.V. Detection of new epitopes of antibodies to filaggrin in filaggrin protein molecule. Bull. Exp. Biol. Med. 2011, 151, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Dorow, D.S.; Shi, P.T.; Carbone, F.R.; Minasian, E.; Todd, P.E.; Leach, S.J. Two large immunogenic and antigenic myoglobin peptides and the effects of cyclisation. Mol. Immunol. 1985, 22, 1255–1264. [Google Scholar] [CrossRef]
- Nair, D.T.; Singh, K.; Siddiqui, Z.; Nayak, B.P.; Rao, K.V.; Salunke, D.M. Epitope recognition by diverse antibodies suggests conformational convergence in an antibody response. J. Immunol. 2002, 168, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; el-Bannoudi, H.; Scherer, H.U.; van der Woude, D.; Menard, H.A.; Lora, M.; Trouw, L.A.; Huizinga, T.W.; Toes, R.E. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann. Rheum. Dis. 2011, 70, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Willemze, A.; Bohringer, S.; Knevel, R.; Levarht, E.W.; Stoeken-Rijsbergen, G.; Houwing-Duistermaat, J.J.; van der Helm-van Mil, A.H.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A. The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Ann. Rheum. Dis. 2012, 71, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.; Perez-Pampin, E.; Calaza, M.; Gomez-Reino, J.J.; Gonzalez, A. Association of anti-citrullinated vimentin and anti-citrullinated alpha-enolase antibodies with subsets of rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Goldbach-Mansky, R.; Lee, J.; McCoy, A.; Hoxworth, J.; Yarboro, C.; Smolen, J.S.; Steiner, G.; Rosen, A.; Zhang, C.; Menard, H.A.; et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000, 2, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Goules, J.D.; Goules, A.V.; Tzioufas, A.G. Fine specificity of anti-citrullinated peptide antibodies discloses a heterogeneous antibody population in rheumatoid arthritis. Clin. Exp. Immunol. 2013, 174, 10–17. [Google Scholar] [CrossRef] [PubMed]
| Physiological Functions of Citrullination | References |
|---|---|
| Skin keratinization | [7] |
| Brain development | [7,21] |
| Fertility | [20] |
| Hair formation | [22] |
| NET formation | [37] |
| Gene regulation | [24,25,26,27,28,29,30] |
| Immune functions | [7,33,34,35,36] |
| Antibody | Specificity | Sensitivity | Reference |
|---|---|---|---|
| Anti-perinuclear factor | 90 | 50–70 | [68] |
| Anti-keratin antibodies | 94 | 45 | [69] |
| Anti-citrullinated filaggrin | >90 | 60 | [70] |
| Anti-citrullinated fibrinogen | >90 | 55 | [71] |
| Anti-fibrin | 90 | 75 | [72] |
| Anti-CCP1 | 96 | 53 | [63] |
| Anti-CCP2 | 94–95 | 68–74 | [73,74] |
| Anti-CCP3 | 86–96 | 68–79 | [67,73,74,75] |
| Anti-CCP3.1 | 98 | 83 | [74] |
| Anti-Sa antibodies | 99 | 43 | [76] |
| Anti-mutated citrullinated vimentin | 97 | 72 | [77] |
| Anti-citrullinated EBNA-1 | 85 | 67 | [78,79] |
| Protein | Epitope | No of Cit Residues | Most Important Residue | Reference |
|---|---|---|---|---|
| Enolase | 5 KIHA-Cit-EFDS-Cit-GNPTVE 21 | 2 | Cit 15 | [121] |
| Fibrin | α36 GP-Cit-VVE-Cit-HQSACKDS 50 | 2 | Cit 42 | [73] |
| Fibrin | β60 Cit-PAPPISGGGY-Cit-A-Cit 74 | 3 | Cit 74 | [73] |
| Collagen II | 358 GA-Cit-GLTG-Cit-PGDAGPPGPP 375 | 2 | Cit 360 | [44] |
| Filaggrin | 306 SHQEST-Cit-G-Cit-SRGRSGRSG 324 | 2 | Cit 312 | [3] |
| EBNA-1 | GGRRGRGRERA-Cit-GGSRERAR | 1 | - | [79] |
| EBNA-1 | ARGGSRERARGRGRG-Cit-GEKR | 1 | - | [79] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trier, N.H.; Houen, G. Epitope Specificity of Anti-Citrullinated Protein Antibodies. Antibodies 2017, 6, 5. https://doi.org/10.3390/antib6010005
Trier NH, Houen G. Epitope Specificity of Anti-Citrullinated Protein Antibodies. Antibodies. 2017; 6(1):5. https://doi.org/10.3390/antib6010005
Chicago/Turabian StyleTrier, Nicole H., and Gunnar Houen. 2017. "Epitope Specificity of Anti-Citrullinated Protein Antibodies" Antibodies 6, no. 1: 5. https://doi.org/10.3390/antib6010005
APA StyleTrier, N. H., & Houen, G. (2017). Epitope Specificity of Anti-Citrullinated Protein Antibodies. Antibodies, 6(1), 5. https://doi.org/10.3390/antib6010005





