B Cell Help by CD1d-Rectricted NKT Cells
Abstract
:1. Introduction
2. T Dependent Antibody Responses
3. T Independent Antibody Responses
4. CD1d-Restricted Natural Killer T Cells
5. Strategic iNKT Cell Localization for B Cell Help
6. Cognate iNKT Cell Help for B Cells
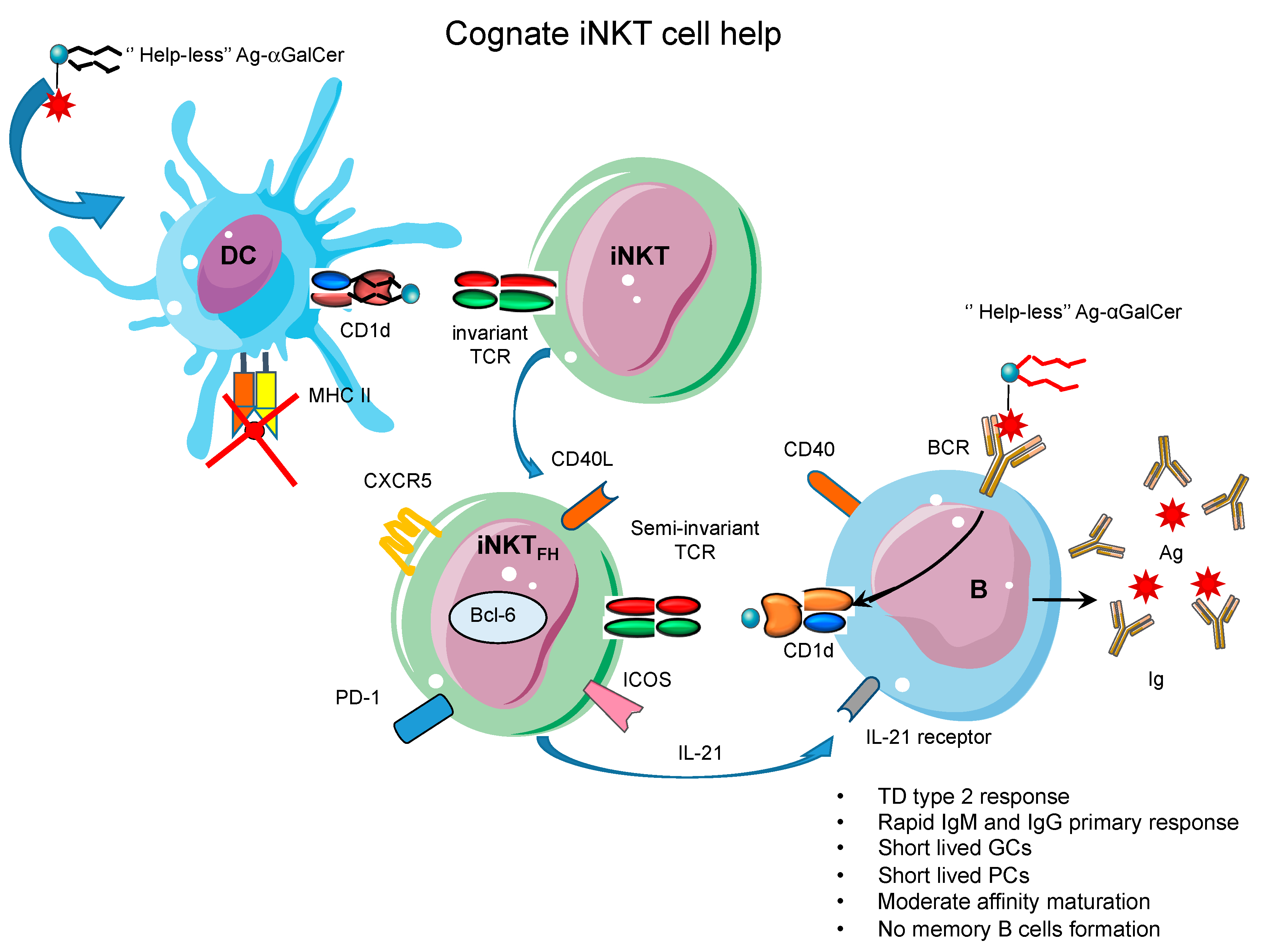
7. Distinct Dynamics of B Cell Response Helped by iNKTFH Cells
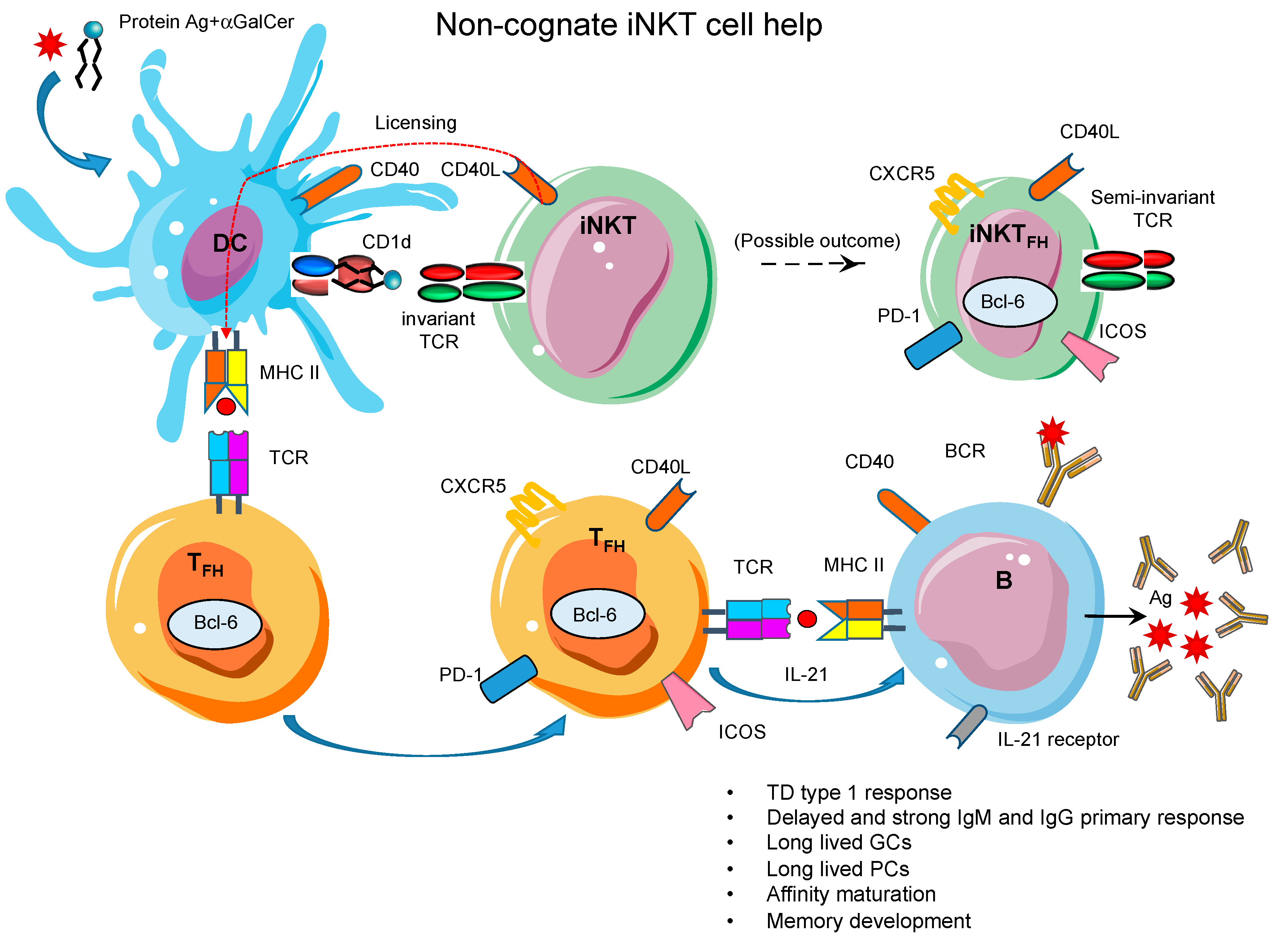
8. Non-Cognate iNKT Cell Help for B Cells
9. Spontaneous iNKT Cell Help to B Cells
10. Type 2 NKT Cell Help to B Cells
11. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TD | T dependent |
| TI | T Independent |
| GC | Germinal Centers |
| Ag | antigen |
| NKT | Natural Killer T cells |
| TCR | T cell Receptor |
| αGalCer | αgalactosyl ceramide |
| MZ B | marginal zone B cells |
| DCs | dendritic cells |
| PC | plasma cells |
| NP hapten | 4-Hydroxy-3-nitrophenyl acetyl hapten |
| BCR | B Cell Receptor |
References
- Mitchell, G.F.; Miller, J.F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J. Exp. Med. 1968, 128, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Dellabona, P.; Abrignani, S.; Casorati, G. iNKT-cell help to B cells: A cooperative job between innate and adaptive immune responses. Eur. J. Immunol. 2014, 44, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Ann. Rev. Immunol. 2005, 23, 487–513. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, I.C. Germinal centers. Annu. Rev. Immunol. 1994, 12, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Ann. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Cyster, J.G. How T cells earn the follicular rite of passage. Immunity 2011, 35, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Tangye, S.G.; Moser, B.; Mackay, C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 2005, 5, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Vinuesa, C.G. The elusive identity of T follicular helper cells. Trends Immunol. 2010, 31, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Rigby, R.J.; Wong, R.; Silva, D.; Withers, D.; Anderson, G.; Verma, N.K.; Brink, R.; Hutloff, A.; Goodnow, C.C.; et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity 2009, 30, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Bauquet, A.T.; Jin, H.; Paterson, A.M.; Mitsdoerffer, M.; Ho, I.C.; Sharpe, A.H.; Kuchroo, V.K. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009, 10, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Liu, D.; Li, J.; Zhang, X.; Chen, X.; Hou, S.; Peng, L.; Xu, C.; Liu, W.; et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Cook, M.C.; Angelucci, C.; Athanasopoulos, V.; Rui, L.; Hill, K.M.; Yu, D.; Domaschenz, H.; Whittle, B.; Lambe, T.; et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005, 435, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Good-Jacobson, K.L.; Szumilas, C.G.; Chen, L.; Sharpe, A.H.; Tomayko, M.M.; Shlomchik, M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010, 11, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hams, E.; McCarron, M.J.; Amu, S.; Yagita, H.; Azuma, M.; Chen, L.; Fallon, P.G. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J. Immunol. 2011, 186, 5648–5655. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Kersh, E.N.; Cannons, J.; Schwartzberg, P.L.; Ahmed, R. SAP is required for generating long-term humoral immunity. Nature 2003, 421, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, R.; Cannons, J.L.; Zhao, F.; Yusuf, I.; Lao, C.; Locci, M.; Schwartzberg, P.L.; Crotty, S. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity 2012, 36, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Liston, A.; Vinuesa, C.G. T-follicular helper cell differentiation and the co-option of this pathway by non-helper cells. Immunol. Rev. 2012, 247, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Tato, A.; Leon, B.; Graf, B.A.; Moquin, A.; Adams, P.S.; Lund, F.E.; Randall, T.D. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012, 36, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Choi, Y.S.; Diamond, J.A.; Yang, J.A.; Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012, 209, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Rigby, R.J.; Wong, R.K.; Yu, D.; Brink, R.; Cannons, J.L.; Schwartzberg, P.L.; Cook, M.C.; Walters, G.D.; Vinuesa, C.G. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009, 206, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Morita, R.; Bourdery, L.; Bentebibel, S.E.; Zurawski, S.M.; Banchereau, J.; Ueno, H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009, 31, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Batten, M.; Ramamoorthi, N.; Kljavin, N.M.; Ma, C.S.; Cox, J.H.; Dengler, H.S.; Danilenko, D.M.; Caplazi, P.; Wong, M.; Fulcher, D.A.; et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 2010, 207, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef] [PubMed]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Chang, P.P. Innate B cell helpers reveal novel types of antibody responses. Nat. Immunol. 2013, 14, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Magri, G.; Miyajima, M.; Bascones, S.; Mortha, A.; Puga, I.; Cassis, L.; Barra, C.M.; Comerma, L.; Chudnovskiy, A.; Gentile, M.; et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 2014, 15, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Salio, M.; Silk, J.D.; Jones, E.Y.; Cerundolo, V. Biology of CD1- and MR1-Restricted T Cells. Ann. Rev. Immunol. 2014, 32, 323–366. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What's in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Bonneville, M.; Kearney, J.F. Autoreactivity by design: Innate B and T lymphocytes. Nat. Rev. Immunol. 2001, 1, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Dellabona, P.; Padovan, E.; Casorati, G.; Brockhaus, M.; Lanzavecchia, A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J. Exp. Med. 1994, 180, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Kain, L.; Webb, B.; Anderson, B.L.; Deng, S.; Holt, M.; Costanzo, A.; Zhao, M.; Self, K.; Teyton, A.; Everett, C.; et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity 2014, 41, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; Cox, D.G.; Lockridge, J.L.; Wang, X.; Chen, X.; Scharf, L.; Trott, D.L.; Ndonye, R.M.; Veerapen, N.; Besra, G.S.; et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009, 7, e1000228. [Google Scholar] [CrossRef] [PubMed]
- Exley, M.A.; Hou, R.; Shaulov, A.; Tonti, E.; Dellabona, P.; Casorati, G.; Akbari, O.; Akman, H.O.; Greenfield, E.A.; Gumperz, J.E.; et al. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur. J. Immunol. 2008, 38, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Benlagha, K.; Weiss, A.; Beavis, A.; Teyton, L.; Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 2000, 191, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Naidenko, O.V.; Gapin, L.; Nakayama, T.; Taniguchi, M.; Wang, C.R.; Koezuka, Y.; Kronenberg, M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000, 192, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Weiss, A.; Benlagha, K.; Kyin, T.; Teyton, L.; Bendelac, A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 2001, 193, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Macho-Fernandez, E.; Brigl, M. The Extended Family of CD1d-Restricted NKT Cells: Sifting through a Mixed Bag of TCRs, Antigens, and Functions. Front. Immunol. 2015, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- homas, S.Y.; Scanlon, S.T.; Griewank, K.G.; Constantinides, M.G.; Savage, A.K.; Barr, K.A.; Meng, F.; Luster, A.D.; Bendelac, A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 2011, 208, 1179–1188. [Google Scholar]
- Barral, P.; Sanchez-Nino, M.D.; van Rooijen, N.; Cerundolo, V.; Batista, F.D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 2012, 31, 2378–2390. [Google Scholar] [CrossRef] [PubMed]
- King, I.L.; Amiel, E.; Tighe, M.; Mohrs, K.; Veerapen, N.; Besra, G.; Mohrs, M.; Leadbetter, E.A. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J. Immunol. 2013, 191, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Tonti, E.; Galli, G.; Malzone, C.; Abrignani, S.; Casorati, G.; Dellabona, P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood 2009, 113, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.P.; Barral, P.; Fitch, J.; Pratama, A.; Ma, C.S.; Kallies, A.; Hogan, J.J.; Cerundolo, V.; Tangye, S.G.; Bittman, R.; et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 2012, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Barral, P.; Polzella, P.; Bruckbauer, A.; van Rooijen, N.; Besra, G.S.; Cerundolo, V.; Batista, F.D. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat. Immunol. 2010, 11, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kastenmuller, W.; Torabi-Parizi, P.; Subramanian, N.; Lammermann, T.; Germain, R.N. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012, 150, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Nuti, S.; Tavarini, S.; Galli-Stampino, L.; De Lalla, C.; Casorati, G.; Dellabona, P.; Abrignani, S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 2003, 197, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.A.; Devera, T.S.; Lang, M.L. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood 2008, 111, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Brigl, M.; Illarionov, P.; Cohen, N.; Luteran, M.C.; Pillai, S.; Besra, G.S.; Brenner, M.B. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8339–8344. [Google Scholar] [CrossRef] [PubMed]
- Barral, P.; Eckl-Dorna, J.; Harwood, N.E.; De Santo, C.; Salio, M.; Illarionov, P.; Besra, G.S.; Cerundolo, V.; Batista, F.D. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8345–8350. [Google Scholar] [CrossRef] [PubMed]
- Tonti, E.; Fedeli, M.; Napolitano, A.; Iannacone, M.; von Andrian, U.H.; Guidotti, L.G.; Abrignani, S.; Casorati, G.; Dellabona, P. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J. Immunol. 2012, 188, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- King, I.L.; Fortier, A.; Tighe, M.; Dibble, J.; Watts, G.F.; Veerapen, N.; Haberman, A.M.; Besra, G.S.; Mohrs, M.; Brenner, M.B.; et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 2011, 13, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Detre, C.; Keszei, M.; Garrido-Mesa, N.; Kis-Toth, K.; Castro, W.; Agyemang, A.F.; Veerapen, N.; Besra, G.S.; Carroll, M.C.; Tsokos, G.C.; et al. SAP expression in invariant NKT cells is required for cognate help to support B-cell responses. Blood 2012, 120, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rampuria, P.; Lang, M.L. CD1d-dependent expansion of NKT follicular helper cells in vivo and in vitro is a product of cellular proliferation and differentiation. Int. Immunol. 2015, 27, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.B.; Joshi, S.K.; Rampuria, P.; Devera, T.S.; Lang, G.A.; Stohl, W.; Lang, M.L. BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand. J. Immunol. 2013, 191, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Deng, S.; Reboulet, R.; Mathew, R.; Teyton, L.; Savage, P.B.; Bendelac, A. Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, 16097–16102. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, M.; Stallforth, P.; Kalinichenko, A.; Rathwell, D.C.; Gronewold, T.M.; Adibekian, A.; Mori, L.; Landmann, R.; Seeberger, P.H.; De Libero, G. A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat. Chem. Biol. 2014, 10, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.P.; Vinuesa, C.G. Detection of mouse natural killer T follicular helper (NKT(FH)) cells by flow cytometry. Methods Mol. Biol. 2015, 1291, 135–141. [Google Scholar] [PubMed]
- Deng, S.; Bai, L.; Reboulet, R.; Matthew, R.; Engler, D.A.; Teyton, L.; Bendelac, A.; Savage, P.B. A peptide-free, liposome-based oligosaccharide vaccine, adjuvanted with a natural killer T cell antigen, generates robust antibody responses. Chem. Sci. 2014, 5, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Pittoni, P.; Tonti, E.; Malzone, C.; Uematsu, Y.; Tortoli, M.; Maione, D.; Volpini, G.; Finco, O.; Nuti, S.; et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA 2007, 104, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.T.; Thomas, S.Y.; Ferreira, C.M.; Bai, L.; Krausz, T.; Savage, P.B.; Bendelac, A. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J. Exp. Med. 2011, 208, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.A.; Exley, M.A.; Lang, M.L. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology 2006, 119, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Wolter, E.; Fillatreau, S.; Meisel, H.; Kaufmann, S.H.; Schonrich, G. NKT cells determine titer and subtype profile of virus-specific IgG antibodies during herpes simplex virus Infection. J. Immunol. 2014, 192, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Mattner, J.; Savage, P.B.; Leung, P.; Oertelt, S.S.; Wang, V.; Trivedi, O.; Scanlon, S.T.; Pendem, K.; Teyton, L.; Hart, J.; et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 2008, 3, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.B.; Devera, T.S.; Rampuria, P.; Lang, G.A.; Lang, M.L. Type II NKT cells facilitate Alum-sensing and humoral immunity. J. Leuk. Biol. 2012, 92, 883–893. [Google Scholar] [CrossRef] [PubMed]
- de Fost, M.; Out, T.A.; de Wilde, F.A.; Tjin, E.P.; Pals, S.T.; van Oers, M.H.; Boot, R.G.; Aerts, J.F.; Maas, M.; Vom Dahl, S.; et al. Immunoglobulin and free light chain abnormalities in Gaucher disease type I: Data from an adult cohort of 63 patients and review of the literature. Ann. Hematol. 2008, 87, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Boddupalli, C.S.; Verma, R.; Liu, J.; Yang, R.; Pastores, G.M.; Mistry, P.K.; Dhodapkar, M.V. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood 2015, 125, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.K.; Liu, J.; Yang, M.; Nottoli, T.; McGrath, J.; Jain, D.; Zhang, K.; Keutzer, J.; Chuang, W.L.; Mehal, W.Z.; et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. USA 2010, 107, 19473–19478. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yang, J.Q.; Kim, P.J.; Singh, R.R. Homeostatic regulation of marginal zone B cells by invariant natural killer T cells. PLoS One 2011, 6, e26536. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Neparidze, N.; Zhang, L.; Nair, S.; Monesmith, T.; Sundaram, R.; Miesowicz, F.; Dhodapkar, K.M.; Dhodapkar, M.V. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 2013, 121, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Tefit, J.N.; Crabe, S.; Orlandini, B.; Nell, H.; Bendelac, A.; Deng, S.; Savage, P.B.; Teyton, L.; Serra, V. Efficacy of ABX196, a new NKT agonist, in prophylactic human vaccination. Vaccine 2014, 32, 6138–6145. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clerici, L.; Casorati, G.; Dellabona, P. B Cell Help by CD1d-Rectricted NKT Cells. Antibodies 2015, 4, 279-294. https://doi.org/10.3390/antib4040279
Clerici L, Casorati G, Dellabona P. B Cell Help by CD1d-Rectricted NKT Cells. Antibodies. 2015; 4(4):279-294. https://doi.org/10.3390/antib4040279
Chicago/Turabian StyleClerici, Livia, Giulia Casorati, and Paolo Dellabona. 2015. "B Cell Help by CD1d-Rectricted NKT Cells" Antibodies 4, no. 4: 279-294. https://doi.org/10.3390/antib4040279
APA StyleClerici, L., Casorati, G., & Dellabona, P. (2015). B Cell Help by CD1d-Rectricted NKT Cells. Antibodies, 4(4), 279-294. https://doi.org/10.3390/antib4040279




