The Role of CD2 Family Members in NK-Cell Regulation of B-Cell Antibody Production
Abstract
:1. Introduction
2. Evidence for NK-Cell Regulation of B-Cell Responses
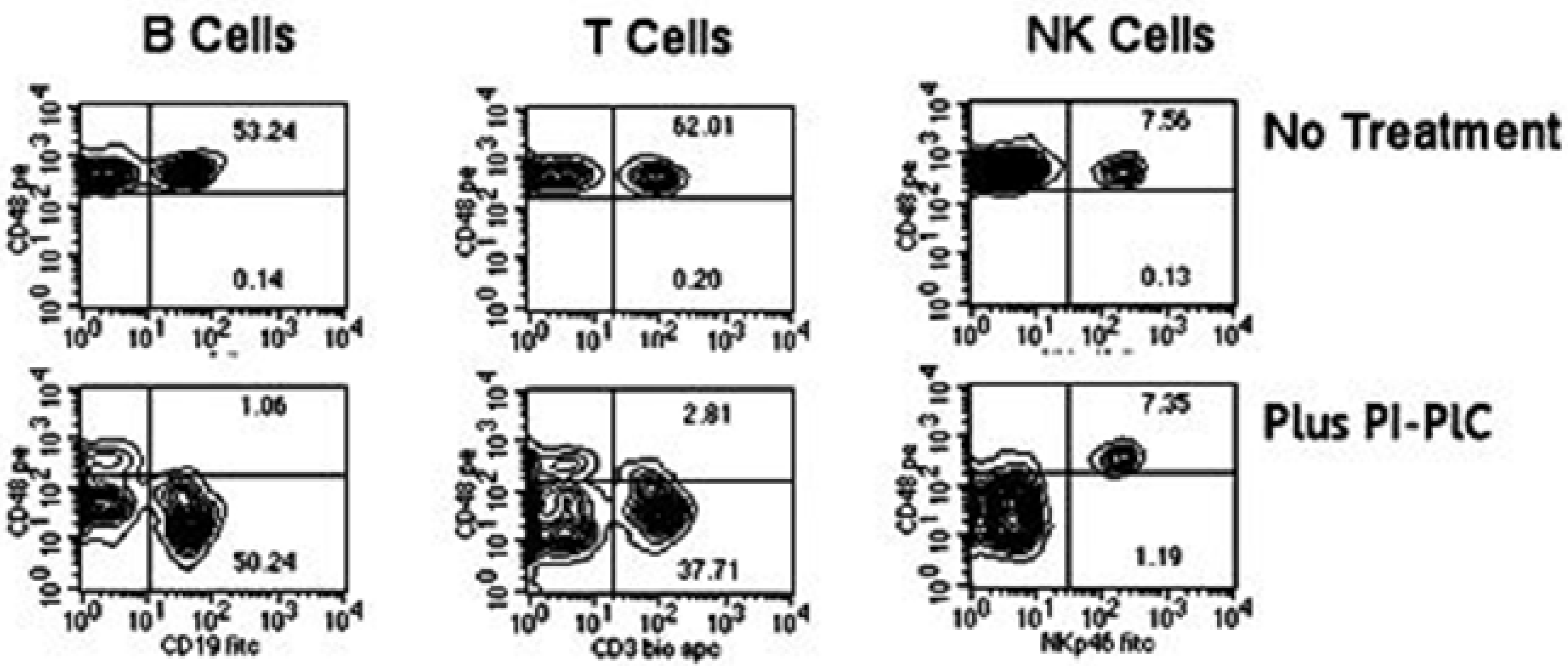

3. The Role of NK Cells in the Production of Anti-Nuclear-Antibodies
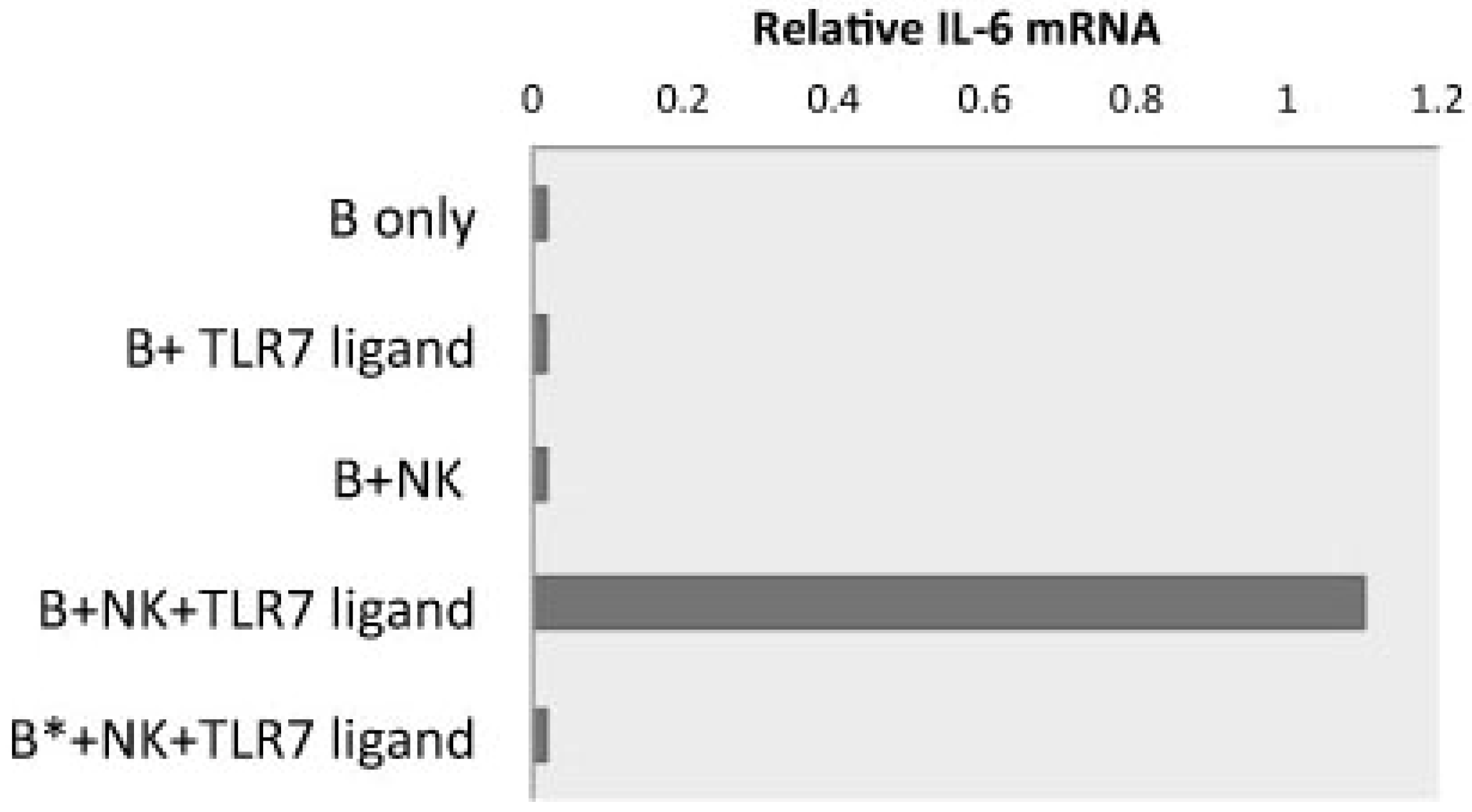
4. Functional Relevance of Preferential IgG Subclass Switch
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Abruzzo, L.V.; Rowley, D.A. Homeostasis of the antibody responses, Immunoregulation by NK cells. Science 1983, 222, 581–585. [Google Scholar]
- Wilder, J.A.; Koh, C.Y.; Yuan, D. The role of NK cells during in vivo antigen-specific antibody responses. J. Immunol. 1996, 156, 146–152. [Google Scholar]
- Koh, C.Y.; Yuan, D. The effect of NK cell activation by tumor cells on antigen-specific antibody responses. J. Immunol. 1997, 159, 4745–4752. [Google Scholar]
- Satoskar, A.R.; Stamm, L.M.; Zhang, X.; Okano, M.; David, J.R.; Terhorst, C.; Wang, B. NK cell-deficient mice develop a Th1-like response but fail to mount an efficient antigen-specific IgG2a antibody response. J. Immunol. 1999, 163, 5298–5302. [Google Scholar]
- Szomolanyi-Tsuda, E.; Brien, J.D.; Dorgan, J.E.; Garcea, R.L.; Woodland, R.T.; Welsh, R.M. Antiviral T-cell-independent type 2 antibody responses induced in vivo in the absence of T and NK cells. Virology 2001, 280, 160–168. [Google Scholar] [CrossRef]
- Markine-Goriaynoff, D.; Hulhoven, X.; Cambiaso, C.L.; Monteyne, P.; Briet, T.; Gonzalez, M.-D.; Coulie, P.; Coutelier, J.-P. Natural killer cell activation after infection with lactate dehydrogenase-elevating virus. J. Gen. Virol. 2002, 83, 2709–2716. [Google Scholar]
- Yuan, D.; Bibi, R.; Dang, T. The role of adjuvant on the regulatory effects of NK cells on B cell responses as revealed by a new model of NK cell deficiency. Int. Immunol. 2004, 16, 707–716. [Google Scholar] [CrossRef]
- Hawn, T.R.; Ozinsky, A.; Underhill, D.M.; Buckner, F.S.; Akira, S.; Aderem, A. Leishmania major activates IL-1 alpha expression in macrophages through a MyD88-dependent pathway. Microbe. Infect. 2002, 4, 763–771. [Google Scholar] [CrossRef]
- Scanga, C.A.; Aliberti, J.; Jankovic, D.; Tilloy, F.; Bennouna, S.; Denkers, E.Y.; Medzhitov, R.; Sher, A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002, 168, 5997–6001. [Google Scholar]
- Becker, I.; Salaiza, N.; Aguirre, M.; Delgado, J.; Carrillo-Carrasco, N.; Kobeh, L.G.; Ruiz, A.; Cervantes, R.; Torres, A.P.; Cabrera, N.; et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 2003, 130, 65–74. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; e Sousa, C.R. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Huang, L.Y.; Ishii, K.J.; Akira, S.; Aliberti, J.; Golding, B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J. Immunol. 2005, 175, 3964–3970. [Google Scholar]
- Szomolanyi-Tsuda, E.; Liang, X.; Welsh, R.M.; Kurt-Jones, E.A.; Finberg, R.W. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 2006, 80, 4286–4291. [Google Scholar] [CrossRef]
- Barr, T.A.; Brown, S.; Ryan, G.; Zhao, J.; Gray, D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 2007, 37, 3040–3053. [Google Scholar] [CrossRef]
- Zhu, J.; Martinez, J.; Huang, X.; Yang, Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 2007, 109, 619–625. [Google Scholar] [CrossRef]
- Miyake, T.; Kumagai, Y.; Kato, H.; Guo, Z.; Matsushita, K.; Satoh, T.; Kawagoe, T.; Kumar, H.; Jang, M.H.; Kawai, T.; et al. Poly I:C-induced activation of NK cells by CD8alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J. Immunol. 2009, 183, 2522–2528. [Google Scholar] [CrossRef]
- Makela, S.M.; Osterlund, P.; Julkunen, I. TLR ligands induce synergistic interferon-beta and interferon-lambda1 gene expression in human monocyte-derived dendritic cells. Mol. Immunol. 2011, 48, 505–515. [Google Scholar] [CrossRef]
- Martinez, J.; Huang, X.; Yang, Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010, 6, e1000811. [Google Scholar] [CrossRef]
- Gao, N.; Jennings, P.; Guo, Y.; Yuan, D. Regulatory role of natural killer (NK) cells on antibody responses to Brucella abortus. Innate Immun. 2011, 17, 152–163. [Google Scholar] [CrossRef]
- Nabel, G.; Allard, W.J.; Cantor, H. A cloned cell line mediating natural killer cell function inhibits immunoglobulin secretion. J. Exp. Med. 1982, 156, 658–663. [Google Scholar] [CrossRef]
- Becker, J.C.; Kolanus, W.; Lonnemann, C.; Schmidt, R.E. Human natural killer clones enhance in vitro antibody production by tumour necrosis factor alpha and gamma interferon. Scand. J. Immunol. 1990, 32, 153–162. [Google Scholar] [CrossRef]
- Snapper, C.M.; Yamaguchi, H.; Moorman, M.A.; Sneed, R.; Smoot, D.; Mond, J.J. Natural killer cells induce activated murine B cells to secrete Ig. J. Immunol. 1993, 151, 5251–5260. [Google Scholar]
- Gray, J.; Horwitz, D. Activated human NK cells can stimulate resting B cells to secrete immunoglobulin. J. Immunol. 1995, 154, 5656–5664. [Google Scholar]
- Vos, Q.; Snapper, C.M.; Mond, J.J. Heterogeneity in the ability of cytotoxic murine NK cell clones to enhance Ig secretion in vitro. Int. Immunol. 1999, 11, 159–168. [Google Scholar] [CrossRef]
- Gao, N.; Dang, T.; Dunnick, W.A.; Collins, J.T.; Blazar, B.R.; Yuan, D. Receptors and Counterreceptors Involved in NK-B Cell Interactions. J. Immunol. 2005, 174, 4113–4119. [Google Scholar]
- Jennings, P.; Yuan, D. NK cell enhancement of antigen presentation by B lymphocytes. J. Immunol. 2009, 182, 2879–2887. [Google Scholar] [CrossRef]
- Gao, N.; Dang, T.; Yuan, D. IFN-gamma-dependent and -independent initiation of switch recombination by NK cells. J. Immunol. 2001, 167, 2011–2018. [Google Scholar]
- Sinha, S.K.; Gao, N.; Guo, Y.; Yuan, D. Mechanism of induction of NK activation by 2B4 (CD244) via its cognate ligand. J. Immunol. 2010, 185, 5205–5210. [Google Scholar] [CrossRef]
- Clarkson, N.G.; Simmonds, S.J.; Puklavec, M.J.; Brown, M.H. Direct and indirect interactions of the cytoplasmic region of CD244 (2B4) in mice and humans with FYN kinase. J. Biol. Chem. 2007, 282, 25385–25394. [Google Scholar] [CrossRef]
- Thet, S.; Yuan, D. University of Texas Medical Center: Dallas, TX, USA, Unpublished work. 2013.
- Yuan, D.; Guo, Y.; Thet, S. Enhancement of Antigen-Specific Immunoglobulin G Responses by Anti-CD48. J. Innate Immun. 2013, 5, 174–184. [Google Scholar] [CrossRef]
- Gonzalez-Cabrero, J.; Wise, C.J.; Latchman, Y.; Freeman, G.J.; Sharpe, A.H.; Reiser, H. CD48-deficient mice have a pronounced defect in CD4(+) T cell activation. Proc. Natl. Acad. Sci. USA 1999, 96, 1019–1023. [Google Scholar] [CrossRef]
- Bortnick, A.; Allman, D. What is and what should always have been: Long-lived plasma cells induced by T cell-independent antigens. J. Immunol. 2013, 190, 5913–5918. [Google Scholar] [CrossRef]
- Bajenoff, M.; Breart, B.; Huang, A.Y.C.; Qi, H.; Cazareth, J.; Braud, V.M.; Germain, R.N. Nicolas Glaichenhaus, Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 2006, 203, 619–631. [Google Scholar] [CrossRef]
- Salazar-Mather, T.P.; Ishikawa, R.; Biron, C.A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 1996, 157, 3054–3064. [Google Scholar]
- Li, S.; Yan, Y.; Lin, Y.; Bullens, D.M.; Rutgeerts, O.; Goebels, J.; Segers, C.; Boon, L.; Kasran, A.; De Vos, R.; et al. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood 2007, 110, 3926–3935. [Google Scholar] [CrossRef]
- Fogel, L.A.; Sun, M.M.; Geurs, T.L.; Carayannopoulos, L.N.; French, A.R. Markers of nonselective and specific NK cell activation. J. Immunol. 2013, 190, 6269–6276. [Google Scholar] [CrossRef]
- Gerosa, F.; Baldani-Guerra, B.; Nisii, C.; Marchesini, V.; Carra, G.; Trinchieri, G. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 2002, 195, 327–333. [Google Scholar] [CrossRef]
- Piccioli, D.; Sbrana, S.; Melandri, E.; Valiante, N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 2002, 195, 335–341. [Google Scholar] [CrossRef]
- Koka, R.; Burkett, P.; Chien, M.; Chai, S.; Boone, D.L.; Ma, A. Cutting edge: Murine dendritic cells require IL-15R alpha to prime NK cells. J. Immunol. 2004, 173, 3594–3598. [Google Scholar]
- Lucas, M.; Schachterle, W.; Oberle, K.; Aichele, P.; Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007, 26, 503–517. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Son, Y.-I.; Redlinger, R.; Coates, P.T.; Giermasz, A.; Morel, P.A.; Storkus, W.J.; Kalinski, P. Dendritic cells mediate NK cell help for Th1 and CTL responses: Two-signal requirement for the induction of NK cell helper function. J. Immunol. 2003, 171, 2366–2373. [Google Scholar]
- Yoshida, O.; Akbar, F.; Miyake, T.; Abe, M.; Matsuura, B.; Hiasa, Y.; Onji, M. Impaired dendritic cell functions because of depletion of natural killer cells disrupt antigen-specific immune responses in mice: Restoration of adaptive immunity in natural killer-depleted mice by antigen-pulsed dendritic cell. Clin. Exp. Immunol. 2008, 152, 174–181. [Google Scholar] [CrossRef]
- Reid-Yu, S.A.; Small, C.L.; Coombes, B.K. CD3 NK1.1 cells aid in the early induction of a Th1 response to an attaching and effacing enteric pathogen. Eur. J. Immunol. 2013, 43, 2638–2649. [Google Scholar] [CrossRef]
- Kelly, M.N.; Zheng, M.; Ruan, S.; Kolls, J.; D’Souza, A.; Shellito, J.E. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. 2013, 190, 285–295. [Google Scholar] [CrossRef]
- Gasteiger, G.; Hemmers, S.; Firth, M.A.; Floc’h, A.L.; Huse, M.; Sun, J.C.; Rudensky, A.Y. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J. Exp. Med. 2013, 210, 1167–1178. [Google Scholar] [CrossRef]
- Gasteiger, G.; Hemmers, S.; Bos, P.D.; Sun, J.C.; Rudensky, A.Y. IL-2-dependent adaptive control of NK cell homeostasis. J. Exp. Med. 2013, 210, 1179–1187. [Google Scholar] [CrossRef]
- Kerdiles, Y.; Ugolini, S.; Vivier, E. T cell regulation of natural killer cells. J. Exp. Med. 2013, 210, 1065–1068. [Google Scholar] [CrossRef]
- Wandstrat, A.E.; Nguyen, C.; Limaye, N.; Chan, A.Y.; Subramanian, S.; Tian, X.-H.; Yim, Y.-S.; Pertsemlidis, A.; Garner, H.R., Jr.; Morel, L.; et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity 2004, 21, 769–780. [Google Scholar] [CrossRef]
- Morel, L.; Yu, Y.; Blenman, K.R.; Caldwell, R.A.; Wakeland, E.K. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mamm. Genome. 1996, 7, 335–339. [Google Scholar] [CrossRef]
- Morel, L.; Mohan, C.; Yu, Y.; Croker, B.P.; Tian, N.; Deng, A.; Wakeland, E.K. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J. Immunol. 1997, 158, 6019–6028. [Google Scholar]
- Sobel, E.S.; Mohan, C.; Morel, L.; Schiffenbauer, J.; Wakeland, E.K. Genetic dissection of SLE pathogenesis: Adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J. Immunol. 1999, 162, 2415–2421. [Google Scholar]
- Garni-Wagner, B.A.; Purohit, A.; Mathew, P.A.; Bennett, M.; Kumar, V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J. Immunol. 1993, 151, 60–70. [Google Scholar]
- Nakajima, H.; Cella, M.; Langen, H.; Friedlein, A.; Colonna, M. Activating interactions in human NK cell recognition: The role of 2B4-CD48. Eur. J. Immunol. 1999, 29, 1676–1683. [Google Scholar] [CrossRef]
- Tangye, S.G.; Lazetic, S.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. Cutting edge: Human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 1999, 162, 6981–6985. [Google Scholar]
- Schatzle, J.D.; Sheu, S.; Stepp, S.E.; Mathew, P.A.; Bennett, M.; Kumar, V. Characterization of inhibitory and stimulatory forms of the murine natural killer cell receptor 2B4. Proc. Natl. Acad. Sci. USA 1999, 96, 3870–3875. [Google Scholar] [CrossRef]
- Stepp, S.E.; Schatzle, J.D.; Bennett, M.; Kumar, V.; Mathew, P.A. Gene structure of the murine NK cell receptor 2B4: Presence of two alternatively spliced isoforms with distinct cytoplasmic domains. Eur. J. Immunol. 1999, 29, 2392–2399. [Google Scholar] [CrossRef]
- Eissmann, P.; Beauchamp, L.; Wooters, J.; Tilton, J.C.; Long, E.O.; Watzl, C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244). Blood 2005, 105, 4722–4729. [Google Scholar] [CrossRef]
- Lee, K.M.; Bhawan, S.; Majima, T.; Wei, H.; Nishimura, M.I.; Yagita, H.; Kumar, V. Cutting edge: The NK cell receptor 2B4 augments antigen-specific T cell cytotoxicity through CD48 ligation on neighboring T cells. J. Immunol. 2003, 170, 4881–4885. [Google Scholar]
- Velikovsky, C.A.; Deng, L.; Chlewicki, L.K.; Fernández, M.M.; Kumar, V.; Mariuzza, R.A. Structure of natural killer receptor 2B4 bound to CD48 reveals basis for heterophilic recognition in signaling lymphocyte activation molecule family. Immunity 2007, 27, 572–584. [Google Scholar] [CrossRef]
- Gao, N.; Schwartzberg, P.; Wilder, J.A.; Blazar, B.R.; Yuan, D. B cell induction of IL-13 expression in NK cells: Role of CD244 and SLAM-associated protein. J. Immunol. 2006, 176, 2758–2764. [Google Scholar]
- Taniguchi, R.T.; Guzior, D.; Kumar, V. 2B4 inhibits NK-cell fratricide. Blood 2007, 110, 2020–2023. [Google Scholar] [CrossRef]
- Waggoner, S.N.; Taniguchi, R.T.; Mathew, P.A.; Kumar, V.; Welsh, R.M. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J. Clin. Invest. 2010, 120, 1925–1938. [Google Scholar] [CrossRef]
- Jennings, P.; Taniguch, R.T.; Mathew, P.A.; Kumar, V.; Welsh, R.M. Antigen-specific responses and ANA production in B6.Sle1b mice: A role for SAP. J. Autoimmun. 2008, 31, 345–353. [Google Scholar] [CrossRef]
- Yuan, D.; Chan, A.; Schwartzberg, P.; Wakeland, E.K.; Yuan, D. The role of NK cells in the development of autoantibodies. Autoimmunity 2011, 31, 345–353. [Google Scholar]
- Czar, M.J.; Kersh, E.N.; Mijares, L.A.; Lanier, G.; Lewis, J.; Yap, G.; Chen, A.; Sher, A.; Duckett, C.S.; Ahmed, R.; et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl. Acad. Sci. USA 2001, 98, 7449–7454. [Google Scholar] [CrossRef]
- Cannons, J.L.; Yu, L.J.; Hill, B.; Mijares, L.A.; Dombroski, D.; Nichols, K.E.; Antonellis, A.; Koretzky, G.A.; Gardner, K.; Schwartzberg, P.L. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity 2004, 21, 693–706. [Google Scholar] [CrossRef]
- Nichols, K.E.; Hom, J.; Gong, S.-Y.; Ganguly, A.; Ma, C.S.; Cannons, J.L.; Tangye, S.G.; Schwartzberg, P.L.; Koretzky, G.A.; Stein, P.L. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 2005, 11, 340–345. [Google Scholar] [CrossRef]
- Kumar, K.R.; Li, L.; Yan, M.; Bhaskarabhatla, M.; Mobley, A.B.; Nguyen, C.; Mooney, J.M.; Schatzle, J.D.; Wakeland, E.K.; Mohan, C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science 2006, 312, 1665–1669. [Google Scholar] [CrossRef]
- Cannons, J.L.; Qi, H.; Lu, K.T.; Dutta, M.; Gomez-Rodriguez, J.; Cheng, J.; Wakeland, E.K.; Germain, R.N.; Schwartzberg, P.L. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 2010, 32, 253–265. [Google Scholar] [CrossRef]
- Dutta, M.; Kraus, Z.J.; Gomez-Rodriguez, J.; Hwang, S.; Cannons, J.L.; Cheng, J.; Lee, S.-Y.; Wiest, D.L.; Wakeland, E.K.; Schwartzberg, P.L. A role for Ly108 in the induction of promyelocytic zinc finger transcription factor in developing thymocytes. J. Immunol. 2013, 190, 2121–2128. [Google Scholar] [CrossRef]
- Fossati, L.; Sobel, E.S.; Iwamoto, M.; Cohen, P.L.; Eisenberg, R.A.; Izui, S. The Yaa gene-mediated acceleration of murine lupus: Yaa- T cells from non-autoimmune mice collaborate with Yaa+ B cells to produce lupus autoantibodies in vivo. Eur. J. Immunol. 1995, 25, 3412–3417. [Google Scholar] [CrossRef]
- Subramanian, S.; Tus, K.; Li, Q.-Z.; Wang, A.; Tian, X.-H.; Zhou, J.; Liang, C.; Bartov, G.; McDaniel, L.D.; Zhou, X.J.; et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA 2006, 103, 9970–9975. [Google Scholar] [CrossRef]
- Avalos, A.M.; Uccellini, M.B.; Lenert, P.; Viglianti, G.A.; Marshak-Rothstein, A. FcgammaRIIB regulation of BCR/TLR-dependent autoreactive B-cell responses. Eur. J. Immunol. 2010, 40, 2692–2698. [Google Scholar] [CrossRef]
- Lau, C.M.; Broughton, C.; Tabor, A.S.; Akira, S.; Flavell, R.A.; Mamula, M.J.; Christensen, S.R.; Shlomchik, M.J.; Viglianti, G.A.; Rifkin, I.R.; et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005, 202, 1171–1177. [Google Scholar] [CrossRef]
- Berland, R.; Fernandez, L.; Kari, E.; Han, J.-H.; Lomakin, I.; Akira, S.; Wortis, H.H.; Kearney, J.F.; Ucci, A.A.; Imanishi-Kari, T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 2006, 25, 429–440. [Google Scholar] [CrossRef]
- Santiago-Raber, M.L.; Dunand-Sauthier, I.; Wu, T.; Li, Q.-Z.; Uematsu, S.; Akira, S.; Reith, W.; Mohan, C.; Kotzin, B.L.; Izui, S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J. Autoimmun. 2010, 34, 339–348. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, H.; Yamamoto, M.; Jones, L.A.; Dayalan, J.; Hopkins, R.; Zhou, X.J.; Yarovinsky, F.; Connolly, J.E.; Curotto de Lafaille, M.A. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J. Immunol. 2012, 189, 5786–5796. [Google Scholar] [CrossRef]
- Sinha, S.; Guo, Y.; Thet, S.; Yuan, D. IFN type I and type II independent enhancement of B cell TLR7 expression by natural killer cells. J. Leukoc. Biol. 2012, 92, 713–722. [Google Scholar] [CrossRef]
- Ank, N.; Iversen, M.B.; Bartholdy, C.; Staeheli, P.; Hartmann, R.; Jensen, U.B.; Dagnaes-Hansen, F.; Thomsen, A.R.; Chen, Z.; Haugen, H. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 2008, 180, 2474–2485. [Google Scholar]
- Zhou, Z.; Hamming, O.J.; Ank, N.; Paludan, S.R.; Nielsen, A.L.; Hartmann, R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007, 81, 7749–7758. [Google Scholar] [CrossRef]
- Green, N.M.; Laws, A.; Kiefer, K.; Busconi, L.; Kim, Y.-M.; Brinkmann, M.M.; Trail, E.H.; Yasuda, K.; Christensen, S.R.; Shlomchik, M.J.; et al. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J. Immunol. 2009, 183, 1569–1576. [Google Scholar] [CrossRef]
- Bessa, J.; Jegerlehner, A.; Hinton, H.J.; Pumpens, P.; Saudan, P.; Schneider, P.; Bachmann, M.F. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J. Immunol. 2009, 183, 3788–3799. [Google Scholar] [CrossRef]
- Thibault, D.L.; Graham, K.L.; Lee, L.Y.; Balboni, I.; Hertzog, P.J.; Utz, P.J. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Res. Ther. 2009, 11, R112. [Google Scholar] [CrossRef]
- Bao, Y.; Han, Y.; Chen, Z.; Xu, S.; Cao, X. IFN-alpha-producing PDCA-1+ Siglec-H- B cells mediate innate immune defense by activating NK cells. Eur. J. Immunol. 2011, 41, 657–668. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef]
- Maeda, K.; Malykhin, A.; Teague-Weber, B.N.; Sun, X.-H.; Darise Farris, A.; Mark Coggeshall, K. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood 2009, 113, 4534–4540. [Google Scholar] [CrossRef]
- Tipping, P.G.; Kitching, A.R. Glomerulonephritis, Th1 and Th2: What's new? Clin. Exp. Immunol. 2005, 142, 207–215. [Google Scholar]
- Kipps, T.J.; Parham, P.; Punt, J.; Herzenberg, L.A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J. Exp. Med. 1985, 161, 1–17. [Google Scholar] [CrossRef]
- Steplewski, Z.; Lubeck, M.D.; Scholz, D.; Loibner, H.; McDonald, S.J.; Koprowski, H. Tumor cell lysis and tumor growth inhibition by the isotype variants of MAb BR55-2 directed against Y oligosaccharide. In Vivo 1991, 5, 79–83. [Google Scholar]
- Koh, C.Y.; Yuan, D. The functional relevance of NK-cell-mediated upregulation of antigen-specific IgG2a responses. Cell. Immunol. 2000, 204, 135–142. [Google Scholar] [CrossRef]
- Gupta, N.; Arthos, J.; Khazanie, P.; Steenbeke, T.D.; Censoplano, N.M.; Chung, E.A.; Cruz, C.C.; Chaikin, M.A.; Daucher, M.; Kottilil, S.; et al. Targeted lysis of HIV-infected cells by natural killer cells armed and triggered by a recombinant immunoglobulin fusion protein: Implications for immunotherapy. Virology 2005, 332, 491–497. [Google Scholar] [CrossRef]
- Mochizuki, Y.; De Ming, T.; Hayashi, T.; Itoh, M.; Hotta, H.; Homma, M. Protection of mice against Sendai virus pneumonia by non-neutralizing anti-F monoclonal antibodies. Microbiol. Immunol. 1990, 34, 171–183. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Immune memory redefined: Characterizing the longevity of natural killer cells. Immunol. Rev. 2010, 236, 83–94. [Google Scholar] [CrossRef]
- Vivier, E.; Beilke, J.N.; Lanier, L.L. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Soudja, S.M.; Ruiz, A.L.; Marie, J.C.; Lauvau, G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 2012, 37, 549–562. [Google Scholar] [CrossRef]
- Karre, K. NK cells, MHC class I molecules and the missing self. Scand. J. Immunol. 2002, 55, 221–228. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Keyel, P.A.; Yang, L.; Pingel, J.T.; Cheng, T.P. Achim Schneeberger, Wayne M. Yokoyama, Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 2008, 205, 1829–1841. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. Cutting edge: Viral infection breaks NK cell tolerance to "missing self". J. Immunol. 2008, 181, 7453–7457. [Google Scholar]
- Harada, M.; Lin, T.; Kurosawa, S.; Maeda, T.; Umesue, M.; Itoh, O.; Matsuzaki, G.; Nomoto, K. Natural killer cells inhibit the development of autoantibody production in (C57BL/6 x DBA/2) F1 hybrid mice injected with DBA/2 spleen cells. Cell. Immunol. 1995, 161, 42–49. [Google Scholar] [CrossRef]
- Nilsson, N.; Carlsten, H. Enhanced natural but diminished antibody-mediated cytotoxicity in the lungs of MRLlpr/lpr mice. Clin. Exp. Immunol. 1996, 105, 480–485. [Google Scholar]
- Liang, Z.; Xie, C.; Chen, C.; Kreska, D.; Hsu, K.; Li, L.; Zhou, X.J.; Mohan, C. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J. Exp. Med. 2004, 199, 381–398. [Google Scholar] [CrossRef]
- Santiago-Raber, M.L.; Laporte, C.; Reininger, L.; Izui, S. Genetic basis of murine lupus. Autoimmun. Rev. 2004, 3, 33–39. [Google Scholar] [CrossRef]
- Wang, A.; Batteux, F.; Wakeland, E.K. The role of SLAM/CD2 polymorphisms in systemic autoimmunity. Curr. Opin. Immunol. 2010, 22, 706–714. [Google Scholar] [CrossRef]
- Orange, J.S. Unraveling human natural killer cell deficiency. J. Clin. Invest. 2012, 122, 798–801. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yuan, D. The Role of CD2 Family Members in NK-Cell Regulation of B-Cell Antibody Production. Antibodies 2014, 3, 1-15. https://doi.org/10.3390/antib3010001
Yuan D. The Role of CD2 Family Members in NK-Cell Regulation of B-Cell Antibody Production. Antibodies. 2014; 3(1):1-15. https://doi.org/10.3390/antib3010001
Chicago/Turabian StyleYuan, Dorothy. 2014. "The Role of CD2 Family Members in NK-Cell Regulation of B-Cell Antibody Production" Antibodies 3, no. 1: 1-15. https://doi.org/10.3390/antib3010001
APA StyleYuan, D. (2014). The Role of CD2 Family Members in NK-Cell Regulation of B-Cell Antibody Production. Antibodies, 3(1), 1-15. https://doi.org/10.3390/antib3010001



