Physiologically Based Pharmacokinetic Model for Prediction of Immunoglobulins Exposure in Pregnant Women
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Nonpregnancy PBPK Model Development
2.3. Pregnancy PBPK Model for Predicting Exposure of Immunoglobulins
2.4. Simulation of IVIG PK in Nonpregnant and Pregnant Women
2.5. Software and Statistical Evaluations
3. Results
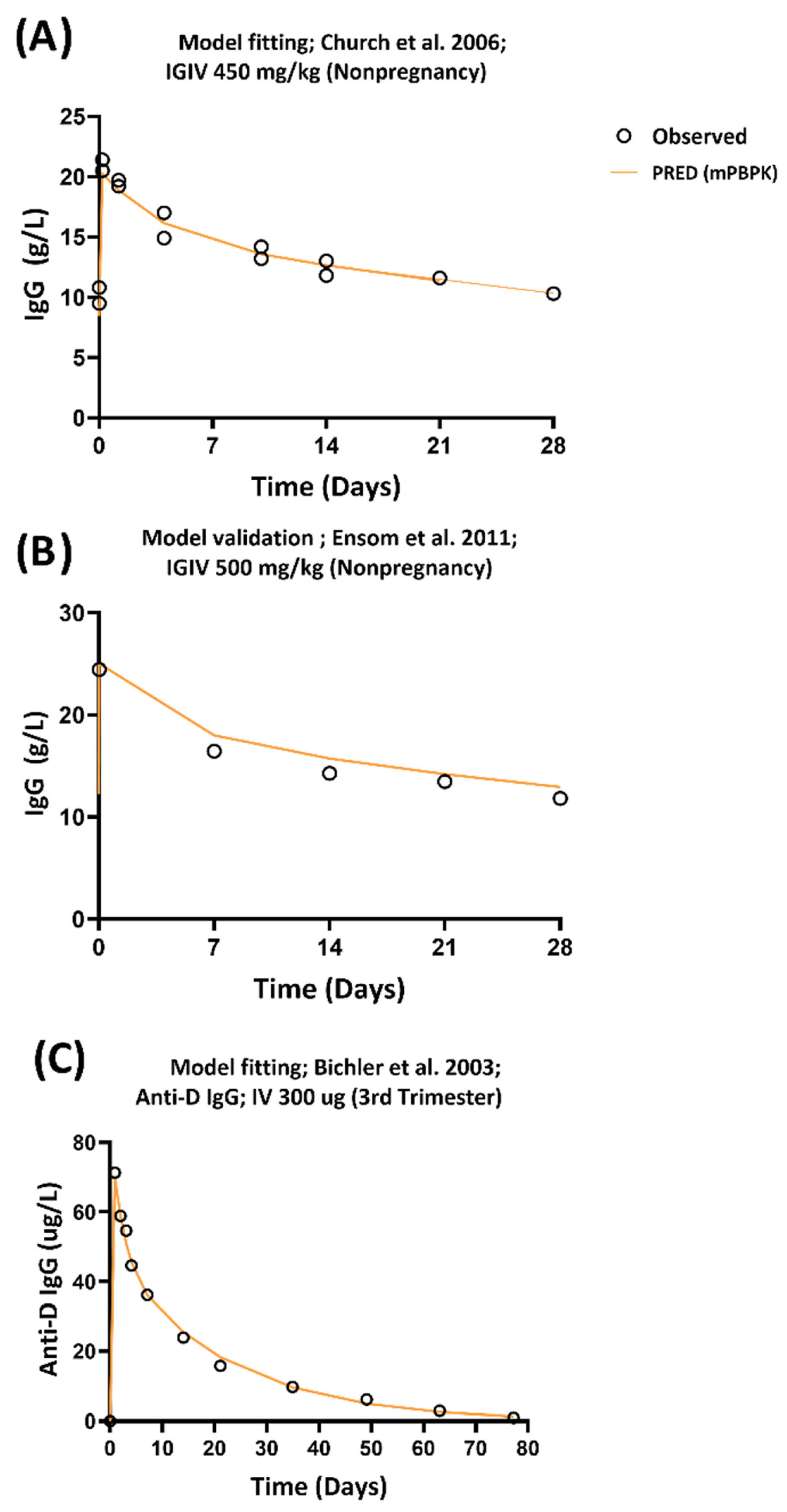
3.1. Development of PBPK Model
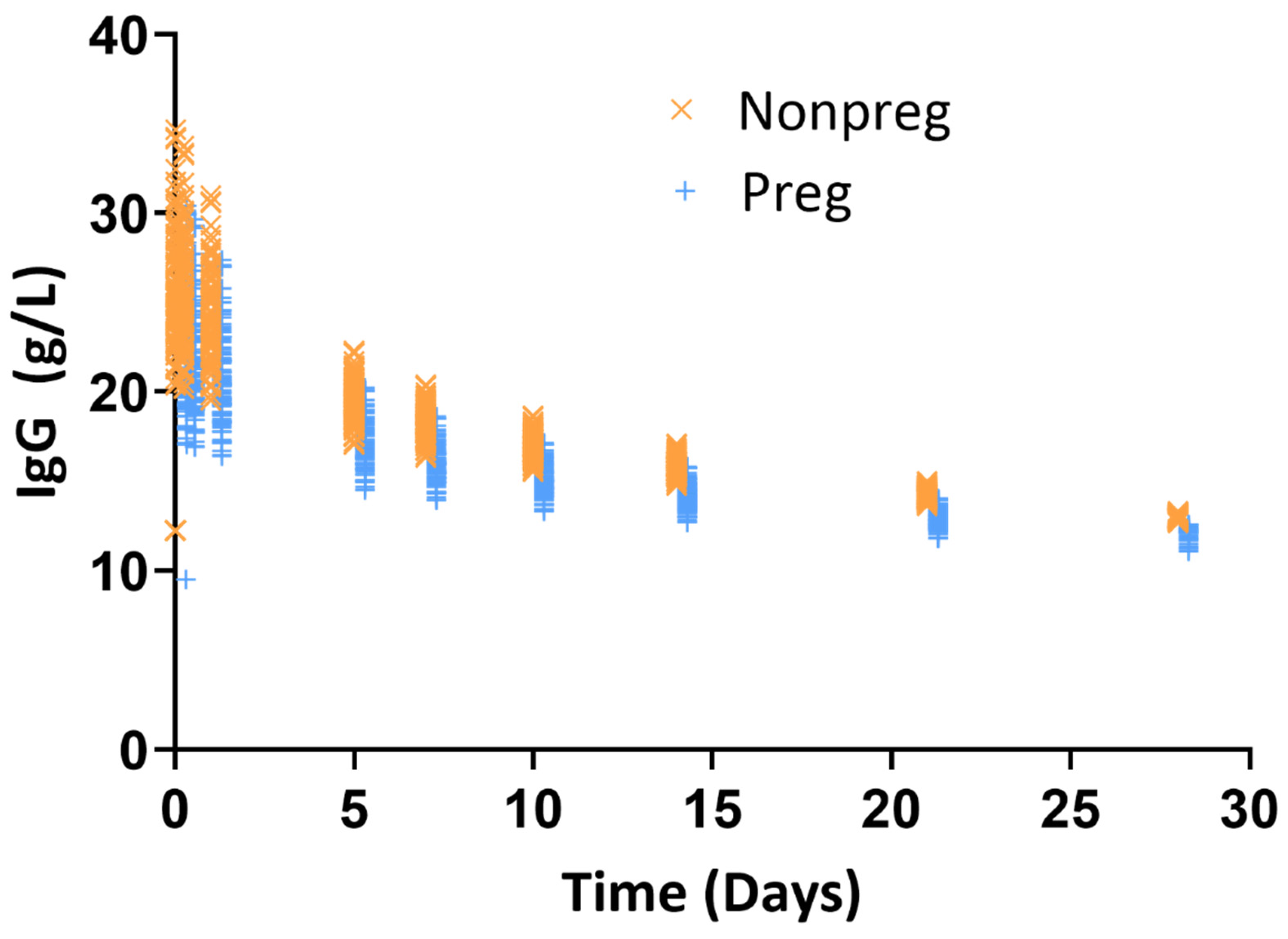
3.2. Evaluation of mPBPK Models in Pregnant Women
3.3. Quantification of Exposure Difference in Nonpregnant Versus Pregnant Women Following IVIG Administration
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Hartung, H.P.; Mouthon, L.; Ahmed, R.; Jordan, S.; Laupland, K.B.; Jolles, S. Clinical applications of intravenous immunoglobulins (IVIg)—beyond immunodeficiencies and neurology. Clin. Exp. Immunol. 2009, 158 (Suppl. S1), 23–33. [Google Scholar] [CrossRef]
- Jolles, S.; Orange, J.S.; Gardulf, A.; Stein, M.R.; Shapiro, R.; Borte, M.; Berger, M. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin. Exp. Immunol. 2015, 179, 146–160. [Google Scholar] [CrossRef]
- Mahmood, I.; Tegenge, M.A.; Golding, B. Considerations for Optimizing Dosing of Immunoglobulins Based on Pharmacokinetic Evidence. Antibodies 2020, 9, 24. [Google Scholar] [CrossRef]
- D’Mello, R.J.; Hsu, C.-D.; Chaiworapongsa, P.; Chaiworapongsa, T. Update on the Use of Intravenous Immunoglobulin in Pregnancy. NeoReviews 2021, 22, e7–e24. [Google Scholar] [CrossRef]
- FDA Package Insert: WinRho® SDF [Rho(D) Immune Globulin Intravenous (Human)]. Available online: https://www.fda.gov/media/77860/download (accessed on 21 March 2022).
- FDA Package Insert: Rh0 (D) Immune Globulin Intravenous (Human) (Rhophylac). Available online: https://www.fda.gov/media/75013/download (accessed on 21 March 2022).
- Tegenge, M.A.; Mahmood, I.; Forshee, R. Clinical Pharmacology Review of Plasma-derived and Recombinant Protein Products: CBER Experience and Perspectives on Model-Informed Drug Development. Haemophilia 2019, 25, e240–e246. [Google Scholar] [CrossRef]
- Koleba, T.; Ensom, M.H. Pharmacokinetics of intravenous immunoglobulin: A systematic review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006, 26, 813–827. [Google Scholar] [CrossRef]
- Kerr, J.; Quinti, I.; Eibl, M.; Chapel, H.; Spath, P.J.; Sewell, W.A.; Salama, A.; van Schaik, I.N.; Kuijpers, T.W.; Peter, H.H. Is dosing of therapeutic immunoglobulins optimal? A review of a three-decade long debate in europe. Front. Immunol. 2014, 5, 629. [Google Scholar] [CrossRef]
- Malek, A.; Sager, R.; Kuhn, P.; Nicolaides, K.H.; Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 1996, 36, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, D.; Hermann Jr, U.; Morell, A.; von Muralt, G.; Barandun, S. Transplacental passage of intravenous immunoglobulin in the last trimester of pregnancy. J. Pediatr. 1986, 109, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Israel, E.; Simister, N.; Freiberg, E.; Caplan, A.; Walker, W. Immunoglobulin G binding sites on the human foetal intestine: A possible mechanism for the passive transfer of immunity from mother to infant. Immunology 1993, 79, 77. [Google Scholar] [PubMed]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Pitcher-Wilmott, R.; Hindocha, P.; Wood, C. The placental transfer of IgG subclasses in human pregnancy. Clin. Exp. Immunol. 1980, 41, 303. [Google Scholar] [PubMed]
- Bi, Y.; Liu, J.; Li, F.; Yu, J.; Bhattaram, A.; Bewernitz, M.; Li, R.J.; Ahn, J.; Earp, J.; Ma, L.; et al. Model-Informed Drug Development in Pediatric Dose Selection. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S60–S69. [Google Scholar] [CrossRef]
- Green, F.G.; Park, K.; Burckart, G.J. Methods Used for Pediatric Dose Selection in Drug Development Programs Submitted to the US FDA 2012-2020. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S28–S35. [Google Scholar] [CrossRef]
- Manolis, E.; Musuamba, F.T.; Karlsson, K.E. The European Medicines Agency Experience With Pediatric Dose Selection. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S22–S27. [Google Scholar] [CrossRef]
- De Sousa Mendes, M.; Hirt, D.; Urien, S.; Valade, E.; Bouazza, N.; Foissac, F.; Blanche, S.; Treluyer, J.M.; Benaboud, S. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br. J. Clin. Pharmacol. 2015, 80, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Lumen, A.; Nguyen, C.; Wesley, B.; Wang, J.; Beitz, J.; Crentsil, V. Application of physiologically based pharmacokinetic modeling for sertraline dosing recommendations in pregnancy. npj Syst. Biol. Appl. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ke, A.; Nallani, S.; Zhao, P.; Rostami-Hodjegan, A.; Unadkat, J. A PBPK model to predict disposition of CYP3A-metabolized drugs in pregnant women: Verification and discerning the site of CYP3A induction. CPT Pharmacomet. Syst. Pharmacol. 2012, 1, 1–10. [Google Scholar] [CrossRef]
- Xia, B.; Heimbach, T.; Gollen, R.; Nanavati, C.; He, H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013, 15, 1012–1024. [Google Scholar] [CrossRef]
- Dallmann, A.; Pfister, M.; van den Anker, J.; Eissing, T. Physiologically based pharmacokinetic modeling in pregnancy: A systematic review of published models. Clin. Pharmacol. Ther. 2018, 104, 1110–1124. [Google Scholar] [CrossRef]
- Ke, A.B.; Nallani, S.C.; Zhao, P.; Rostami-Hodjegan, A.; Unadkat, J.D. Expansion of a PBPK model to predict disposition in pregnant women of drugs cleared via multiple CYP enzymes, including CYP2B6, CYP2C9 and CYP2C19. Br. J. Clin. Pharmacol. 2014, 77, 554–570. [Google Scholar] [CrossRef]
- Church, J.A.; Leibl, H.; Stein, M.R.; Melamed, I.R.; Rubinstein, A.; Schneider, L.C.; Wasserman, R.L.; Pavlova, B.G.; Birthistle, K.; Mancini, M.; et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J. Clin. Immunol. 2006, 26, 388–395. [Google Scholar] [CrossRef]
- Bichler, J.; Schöndorfer, G.; Pabst, G.; Andresen, I. Pharmacokinetics of anti-D IgG in pregnant RhD-negative women. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 39–45. [Google Scholar]
- Ensom, M.H.; Stephenson, M.D. A two-center study on the pharmacokinetics of intravenous immunoglobulin before and during pregnancy in healthy women with poor obstetrical histories. Hum. Reprod. 2011, 26, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Tiblad, E.; Wikman, A.; Rane, A.; Jansson, Y.; Westgren, M. Pharmacokinetics of 250 μg anti-D IgG in the third trimester of pregnancy: An observational study. Acta Obstet. Gynecol. Scand. 2012, 91, 587–592. [Google Scholar] [CrossRef]
- MacKenzie, I.; Roseman, F.; Findlay, J.; Thompson, K.; Jackson, E.; Scott, J.; Reed, M. General obstetrics: The kinetics of routine antenatal prophylactic intramuscular injections of polyclonal anti-D immunoglobulin. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 97–101. [Google Scholar] [CrossRef]
- Cao, Y.; Balthasar, J.P.; Jusko, W.J. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J. Pharmacokinet. Pharmacodyn. 2013, 40, 597–607. [Google Scholar] [CrossRef]
- Ward, R.M.; Varner, M.W. Principles of pharmacokinetics in the pregnant woman and fetus. Clin. Perinatol. 2019, 46, 383–398. [Google Scholar] [CrossRef]
- Aksu, G.; Genel, F.; Koturoglu, G.; Kurugol, Z.; Kutukculer, N. Serum immunoglobulin (IgG, IgM, IgA) and IgG subclass concentrations in healthy children: A study using nephelometric technique. Turk. J. Pediatr. 2006, 48, 19. [Google Scholar] [PubMed]
- Smith, G.; Griffiths, B.; Mollison, D.; Mollison, P. Uptake of IgG after intramuscular and subcutaneous injection. Lancet 1972, 299, 1208–1212. [Google Scholar] [CrossRef]
- NHANES 2011-2014 Adult Data. Available online: https://www.openlab.psu.edu/nhanes/ (accessed on 10 November 2021).
- Abduljalil, K.; Furness, P.; Johnson, T.N.; Rostami-Hodjegan, A.; Soltani, H. Anatomical, Physiological and Metabolic Changes with Gestational Age during Normal Pregnancy. Clin. Pharmacokinet. 2012, 51, 365–396. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Pharmacokinetics in Pregnancy—Study Design, Data Analysis, and Impact on Dosing and Labeling. Available online: https://www.fda.gov/media/71353/download (accessed on 22 March 2022).
- Cao, Y.; Jusko, W.J. Applications of minimal physiologically-based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2012, 39, 711–723. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, Y.; Jusko, W.J. Across-Species Scaling of Monoclonal Antibody Pharmacokinetics Using a Minimal PBPK Model. Pharm. Res. 2015, 32, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Tegenge, M.A. Spreadsheet-Based Minimal Physiological Models for the Prediction of Clearance of Therapeutic Proteins in Pediatric Patients. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S108–S116. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Guideline General Principles for Model-Informed Drug Development M15; Draft Version. Available online: https://database.ich.org/sites/default/files/ICH_M15_EWG_Step2_DraftGuideline_2024_1031.pdf (accessed on 11 February 2025).
- Bjorkman, S. Reduction and lumping of physiologically based pharmacokinetic models: Prediction of the disposition of fentanyl and pethidine in humans by successively simplified models. J. Pharmacokinet. Pharmacodyn. 2003, 30, 285–307. [Google Scholar] [CrossRef]
- Hardiansyah, D.; Ng, C.M. Effects of the FcRn developmental pharmacology on the pharmacokinetics of therapeutic monoclonal IgG antibody in pediatric subjects using minimal physiologically-based pharmacokinetic modelling. MAbs 2018, 10, 1144–1156. [Google Scholar] [CrossRef]
- Levy, G.; Mager, D.E.; Cheung, W.K.; Jusko, W.J. Comparative pharmacokinetics of coumarin anticoagulants L: Physiologic modeling of S-warfarin in rats and pharmacologic target-mediated warfarin disposition in man. J. Pharm. Sci. 2003, 92, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Themans, P.; Marquet, P.; Winkin, J.J.; Musuamba, F.T. Towards a Generic Tool for Prediction of Meropenem Systemic and Infection-Site Exposure: A Physiologically Based Pharmacokinetic Model for Adult Patients with Pneumonia. Drugs R D 2019, 19, 177–189. [Google Scholar] [CrossRef]

| Parameters for Typical 70 kg Adult [Reference] | Parameters for Pregnant Women | |
|---|---|---|
| Plasma volume (L) | 2.6 [29] | 2.78–3.67 ** |
| Leaky tissue volume (L) | 4.37 [29] | 4.37 * (BW/70) |
| Tight tissue volume (L) | 8.11 [29] | 8.11 *(BW/70) |
| Lymph volume (L) | 5.2 [29] | 5.2 * (BW/70) |
| Total lymph flow (L/day) | 2.9 [29] | 2.9 * (BW/70) |
| Lymphatic capillary reflection coefficient | 0.2 [29] | 0.2 |
| Vascular reflection coefficient | σ1 * = 0.97 (0.94–0.99) σ2 * = 0.94 (0.93–0.95) | σ1 = 0.97 σ2 = 0.94 |
| Clearance (L/day) | 0.045 (0.034–0.055) | 0.045 * (BW/70)0.75 |
| Nonpregnant | Pregnant (Third Trimester) | % Mean Change | |
|---|---|---|---|
| Dose (g/kg) | 0.5 | 0.5 | 0 |
| Total Dose (g) | 37 ± 9 | 46 ± 11 | 24 |
| Cmax (g/L) | 26 ± 3 | 22 ± 3 | −15 |
| Cmax_adjusted (g/L) * | 25 | 16 | −32 |
| Ctrough (g/L) | 13 ± 0.1 | 12 ± 0.3 | −8 |
| Ctrough_adjusted (g/L) * | 12 | 9 | −26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tegenge, M.A. Physiologically Based Pharmacokinetic Model for Prediction of Immunoglobulins Exposure in Pregnant Women. Antibodies 2025, 14, 99. https://doi.org/10.3390/antib14040099
Tegenge MA. Physiologically Based Pharmacokinetic Model for Prediction of Immunoglobulins Exposure in Pregnant Women. Antibodies. 2025; 14(4):99. https://doi.org/10.3390/antib14040099
Chicago/Turabian StyleTegenge, Million A. 2025. "Physiologically Based Pharmacokinetic Model for Prediction of Immunoglobulins Exposure in Pregnant Women" Antibodies 14, no. 4: 99. https://doi.org/10.3390/antib14040099
APA StyleTegenge, M. A. (2025). Physiologically Based Pharmacokinetic Model for Prediction of Immunoglobulins Exposure in Pregnant Women. Antibodies, 14(4), 99. https://doi.org/10.3390/antib14040099






