Evaluating the Role of Basiliximab Induction in Simultaneous Liver–Kidney Transplantation: A Multicenter Propensity-Score-Matched Analysis
Abstract
1. Introduction
Aims
2. Materials and Methods
2.1. Data Source and Collection
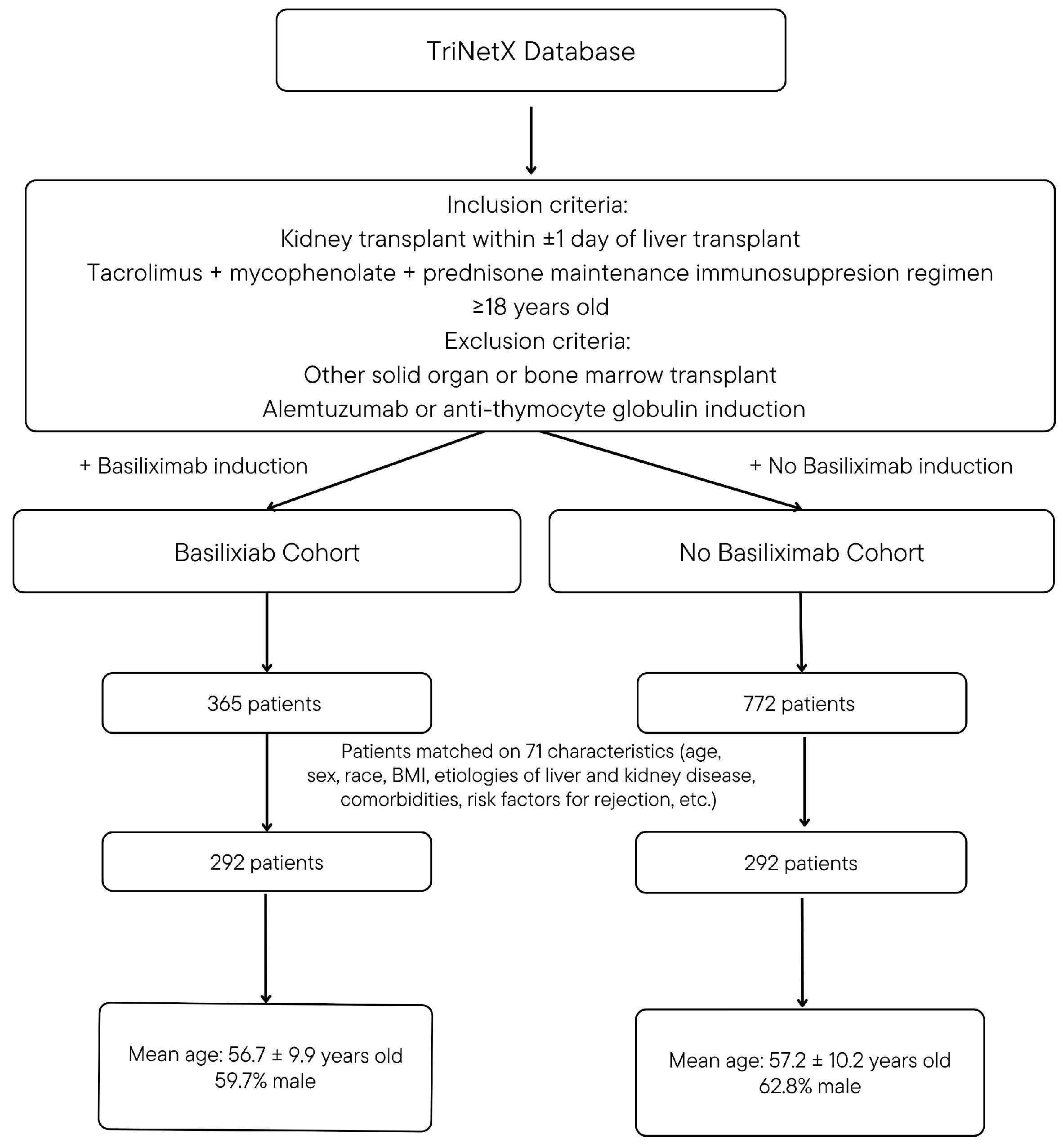
2.2. Study Design and Population
2.3. Propensity Score Matching Variables
2.4. Primary Analysis: Graft and Infectious Outcomes
2.5. Secondary Analysis: Descriptive Outcomes
2.6. Statistical Analysis
3. Results
3.1. Propensity Score Matching Results
3.2. Delayed Kidney Graft Function/Liver Primary Non-Function
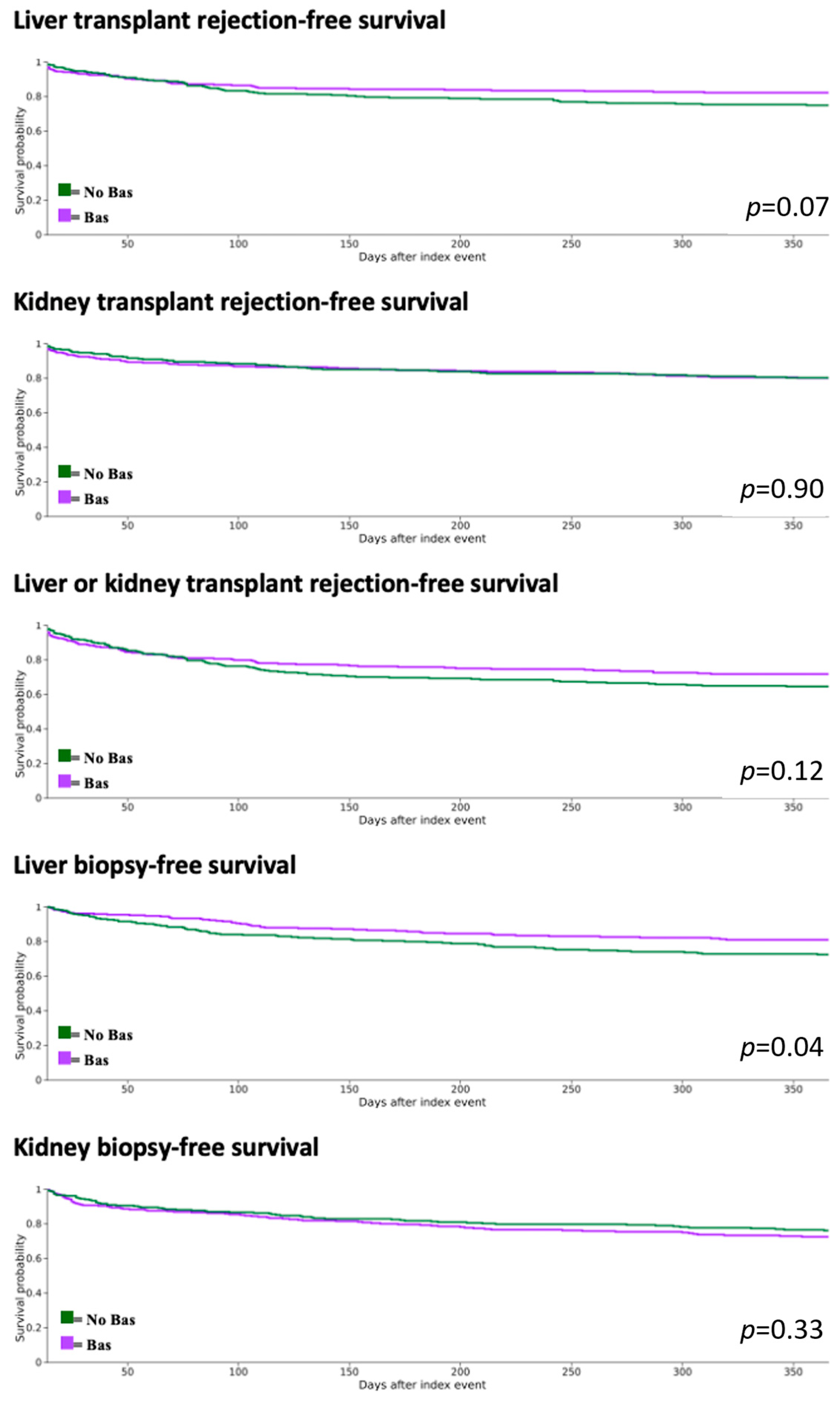
3.3. Graft and Recipient Outcomes
3.4. Infectious Outcomes
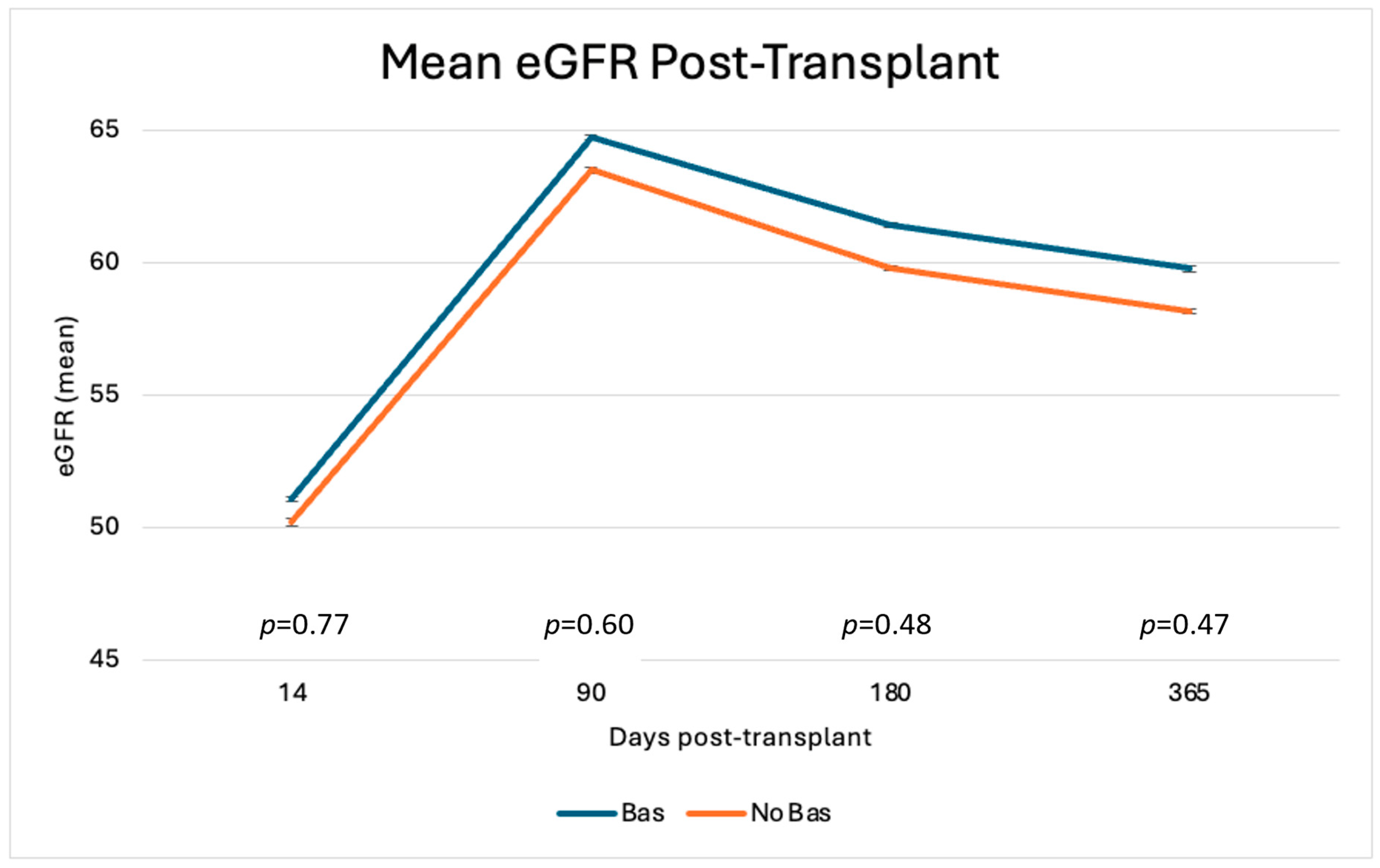
3.5. Descriptive Outcomes
4. Discussion
Limitations/Generalizability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liver-Scientific Registry of Transplant Recipients. Available online: https://srtr.transplant.hrsa.gov/ADR/Chapter?name=Liver&year=2022 (accessed on 20 August 2025).
- Parajuli, S.; Hidalgo, L.G.; Foley, D. Immunology of simultaneous liver and kidney transplants with identification and prevention of rejection. Front. Transpl. 2022, 1, 991546. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Bin Kim, H.; Kim, J.-M.; Kwon, H.E.; Kim, Y.H.; Ko, Y.; Sung, F.S.; Jung, J.H.; Baek, C.H.; Kim, H.; et al. Immunoprotective Effect of Liver Allograft on Patients with Combined Liver and Kidney Transplantation. Ann. Transpl. 2024, 29, e942763-1. [Google Scholar] [CrossRef] [PubMed]
- Kamal, L.; Yu, J.W.; Reichman, T.W.; Kang, L.; Bandyopadhyay, D.; Kumar, D.; King, A.; Gautam, U.; Bhati, C.; Yakubu, I.; et al. Impact of Induction Immunosuppression Strategies in Simultaneous Liver/Kidney Transplantation. Transplantation 2020, 104, 395–403. [Google Scholar] [CrossRef]
- Boyd, A.; Brown, A.; Patel, J.; Nightingale, P.; Perera, M.T.P.; Ferguson, J.; Neuberger, J.; Rajoriya, N. Basiliximab with Delayed Tacrolimus Improves Short-Term Renal Outcomes Post-Liver Transplantation—A Real-World Experience. Transplant. Proc. 2021, 53, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Mohammed, M.; Fülöp, T.; Malik, S. Outcomes of thymoglobulin versus basiliximab induction therapies in living donor kidney transplant recipients with mild to moderate immunological risk–a retrospective analysis of UNOS database. Ann. Med. 2023, 55, 2215536. [Google Scholar] [CrossRef]
- Yao, X.; Weng, G.; Wei, J.; Gao, W. Basiliximab induction in kidney transplantation with donation after cardiac death donors. Exp. Ther. Med. 2016, 11, 2541–2546. [Google Scholar] [CrossRef]
- Basiliximab Induction Therapy for Live Donor Kidney Transplantation: A Long-Term Follow-Up of Prospective Randomized Controlled Study-PubMed. Available online: https://pubmed-ncbi-nlm-nih-gov.libux.utmb.edu/18327678/ (accessed on 21 August 2025).
- Ruiz, I.; Sparkes, T.; Masters, B.; Barth, R.; Maluf, D.; Freedman, S. Impact of Steroid Only Induction on Rejection in Simultaneous Liver-Kidney Transplantation. Prog. Transplant. 2022, 32, 363–369. [Google Scholar] [CrossRef]
- AbdulRahim, N.; Anderson, L.; Kotla, S.; Liu, H.; Ariyamuthu, V.K.; Ghanta, M.; MacConmara, M.; Tujios, S.R.; Mufti, A.; Mohan, S.; et al. Lack of Benefit and Potential Harm of Induction Therapy in Simultaneous Liver-Kidney Transplants. Liver Transplant. 2019, 25, 411–424. [Google Scholar] [CrossRef]
- TriNetX Admin TriNetX Real-World Evidence Platform Validates Outcomes of Randomized Clinical Trials. TriNetX, 5 February 2019. Available online: https://trinetx.com/press-releases/real-world-evidence-platform-validates-outcomes-of-randomized-clinical-trials/ (accessed on 20 August 2025).
- Stapff, M.P. Using real world data to assess cardiovascular outcomes of two antidiabetic treatment classes. World J. Diabetes 2018, 9, 252–257. [Google Scholar] [CrossRef]
- Palchuk, M.B.; London, J.W.; Perez-Rey, D.; Drebert, Z.J.; Winer-Jones, J.P.; Thompson, C.N.; Esposito, J.; Claerhout, B. A global federated real-world data and analytics platform for research. JAMIA Open 2023, 6, ooad035. [Google Scholar] [CrossRef]
- Mankowski, M.A.; Bae, S.; Strauss, A.T.; Lonze, B.E.; Orandi, B.J.; Stewart, D.; Massie, A.B.; McAdams-DeMarco, M.A.; Oermann, E.K.; Habal, M.; et al. Generalizability of kidney transplant data in electronic health records-The Epic Cosmos database vs the Scientific Registry of Transplant Recipients. Am. J. Transplant. 2025, 25, 744–755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yen, F.-S.; Hsu, C.-C.; Yeh, Y.-K.; Cheng, W.-Y.; Liao, P.-L.; Hwu, C.-M.; Wei, J.C.-C. The impact of sodium-glucose cotransporter-2 inhibitors on dialysis risk and mortality in kidney transplant patients with diabetes. Am. J. Transplant. 2025, 25, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.; Hung, Y.; Huang, J.; Hsu, C.; Cheng, W.; Hwu, C.; Wei, J.C. Effects of SGLT2 inhibitors on transplant survival and key clinical outcomes in heart transplant recipients with diabetes. J. Intern. Med. 2025, 297, 532–542. [Google Scholar] [CrossRef]
- Lai, G.-S.; Li, J.-R.; Chen, C.-S.; Wang, S.-S.; Lin, C.-Y.; Yang, C.-J.; Ho, H.-C.; Hung, S.-C.; Chiu, K.-Y.; Yang, C.-K. Temporal trends in malignancy incidence and outcomes among kidney transplantation recipients: A multi-center real-world evidence study using the TriNetX network (2000–2010 vs. 2010–2021). Front Immunol. 2025, 16, 1626135. [Google Scholar] [CrossRef]
- Johnson, J.C.; Malik, M.; Engebretsen, T.L.; Mujtaba, M.; Lea, A.S.; Stevenson, H.L.; Kueht, M.L. Assessing Long-Term Adverse Outcomes in Older Kidney Transplant Recipients: A Propensity Score-Matched Comparison of Early Steroid Withdrawal Versus Continuous Steroid Immunosuppression Using a Large Real-World Database. Drugs Aging 2024, 41, 915–927. [Google Scholar] [CrossRef]
- Publication Guidelines. TriNetX. Available online: https://trinetx.com/real-world-resources/case-studies-publications/trinetx-publication-guidelines/ (accessed on 19 August 2025).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Murthy, B.; Pallister, Z.; Kueht, M.; Cotton, R.; Galvan, N.T.N.; Etheridge, W.; Liu, H.; Goss, J.; O’mahony, C. Profiling risk for acute rejection in kidney transplantation: Recipient age is a robust risk factor. J. Nephrol. 2017, 30, 859–868. [Google Scholar] [CrossRef]
- Cippà, P.E.; Schiesser, M.; Ekberg, H.; van Gelder, T.; Mueller, N.J.; Cao, C.A.; Fehr, T.; Bernasconi, C. Risk stratification for rejection and infection after kidney transplantation. CJASN 2015, 10, 2213–2220. [Google Scholar] [CrossRef]
- Lebranchu, Y.; Baan, C.; Biancone, L.; Legendre, C.; Morales, J.M.; Naesens, M.; Thomusch, O.; Friend, P. Pretransplant identification of acute rejection risk following kidney transplantation. Transpl. Int. 2014, 27, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Abbes, S.; Metjian, A.; Gray, A.; Martinu, T.; Snyder, L.; Chen, D.; Ellis, M.; Arepally, G.M.; Onwuemene, O. HLA sensitization in solid organ transplantation: A primer on terminology, testing, and clinical significance for the apheresis practitioner. Ther. Apher. Dial. 2017, 21, 441–450. [Google Scholar] [CrossRef] [PubMed]
- OPTN/UNOS. Affected Policy Language. Organ Procurement and Transplantation Network. 31 January 2019. Available online: https://optn.transplant.hrsa.gov/media/2795/20190131_nlrb_policy.pdf (accessed on 20 August 2025).
- Ponticelli, C.; Reggiani, F.; Moroni, G. Delayed Graft Function in Kidney Transplant: Risk Factors, Consequences and Prevention Strategies. J. Pers. Med. 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Cederborg, A.; Norén, Å.; Barten, T.; Lindkvist, B.; Bennet, W.; Herlenius, G.; Castedal, M.; Marschall, H.-U.; Åberg, F. Renal function after liver transplantation: Real-world experience with basiliximab induction and delayed reduced-dose tacrolimus. Dig. Liver Dis. 2022, 54, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Aoki, N.; Nagano, K.; Hakamata, M.; Bamba, Y.; Shibata, S.; Koizumi, T.; Ohshima, Y.; Watanabe, S.; Moro, H.; et al. Factors associated with cytomegalovirus antigenemia in patients with rheumatic disease: A retrospective study. J. Infect. Chemother. 2022, 28, 1471–1477. [Google Scholar] [CrossRef] [PubMed]

| Cohort Characteristics, After Propensity-Score Matching (Mean ± SD; n (% Cohort)) | |||
|---|---|---|---|
| Bas | No Bas | p Value | |
| Demographics | |||
| Age | 56.7 ± 9.9 | 57.2 ± 10.2 | 0.62 |
| Male | 174 (59.7%) | 183 (62.8%) | 0.44 |
| Black or African American | 52 (17.7%) | 43 (14.6%) | 0.31 |
| Hispanic or Latino | 17 (5.9%) | 29 (10.1%) | 0.07 |
| White | 193 (66.3%) | 200 (68.4%) | 0.60 |
| Diagnoses | |||
| BMI | 28.4 ± 6.3 | 28.0 ± 5.9 | 0.54 |
| Overweight and obesity | 105 (35.8%) | 109 (37.2%) | 0.73 |
| Viral hepatitis | 91 (31.3%) | 88 (30.2%) | 0.79 |
| Unspecified viral hepatitis C | 70 (24.0%) | 68 (23.3%) | 0.84 |
| Unspecified viral hepatitis B | 14 (4.9%) | 10 (3.5%) | 0.40 |
| Alcoholic liver disease | 148 (50.7%) | 146 (50.0%) | 0.87 |
| Fatty liver | 67 (22.9%) | 75 (25.7%) | 0.44 |
| Nonalcoholic steatohepatitis | 103 (35.4%) | 89 (30.6%) | 0.22 |
| Liver cell carcinoma | 39 (13.2%) | 37 (12.8%) | 0.90 |
| Primary biliary cirrhosis | 15 (5.2%) | 13 (4.5%) | 0.70 |
| Primary hypertension | 187 (63.9%) | 187 (64.2%) | 0.93 |
| Diabetes mellitus | 148 (50.7%) | 149 (51.0%) | 0.93 |
| Hepatorenal syndrome | 148 (50.7%) | 146 (50.0%) | 0.87 |
| Markers of Illness Severity | |||
| Portal hypertension | 225 (77.1%) | 217 (74.3%) | 0.44 |
| Abdominal paracentesis | 137 (46.9%) | 143 (49.0%) | 0.62 |
| Hepatic encephalopathy | 78 (26.7%) | 73 (25.0%) | 0.63 |
| Respiratory failure | 97 (33.3%) | 97 (33.7%) | 0.93 |
| Shock | 64 (21.9%) | 70 (24.0%) | 0.55 |
| Hemodialysis | 110 (37.8%) | 119 (40.6%) | 0.50 |
| Peritoneal dialysis, CRRT, hemofiltration | 63 (21.5%) | 53 (18.1%) | 0.30 |
| Critical Care services | 110 (37.8%) | 114 (38.9%) | 0.80 |
| Model for end-stage liver disease score (n, % with value) | 37.9 ± 8.2 (17, 5.9%) | 36.4 ± 8.0 (10, 3.5%) | 0.65 |
| Medications | |||
| Midodrine | 146 (50.0%) | 149 (51.0%) | 0.80 |
| Vasopressin | 48 (16.3%) | 54 (18.4%) | 0.51 |
| Norepinephrine | 61 (20.8%) | 65 (22.2%) | 0.69 |
| Phenylephrine | 77 (26.4%) | 73 (25.0%) | 0.70 |
| Albumin | 198 (67.7%) | 208 (71.2%) | 0.37 |
| Vitamin K | 96 (33.0%) | 97 (31.3%) | 0.66 |
| Octreotide | 116 (39.9%) | 118 (40.3%) | 0.93 |
| Labs | |||
| Sodium [moles/volume] | 135.5 ± 5.0 | 135.3 ± 4.8 | 0.59 |
| Bilirubin, total [mass/volume] | 5.0 ± 8.2 | 6.1 ± 10.1 | 0.20 |
| Platelets [#/volume] | 90.4 ± 66.4 | 92.3 ± 66.5 | 0.74 |
| INR in Plasma or Blood | 1.6 ± 0.6 | 1.6 ± 0.7 | 0.09 |
| Albumin [mass/volume] | 3.1 ± 0.7 | 3.1 ± 0.7 | 0.85 |
| Creatinine [mass/volume] | 4.0 ± 2.9 | 3.7 ± 2.6 | 0.25 |
| Cytomegalovirus IgG Ab [units/volume] in Serum or Plasma (n, % cohort) | 5.4 ± 12.3 (21, 7.3%) | 26.5 ± 79.1 (27, 9.4%) | 0.23 |
| Varicella zoster virus IgG Ab [Presence] in Serum | 10 (3.5%) | 21 (7.3%) | 0.50 |
| Epstein–Barr virus capsid IgG Ab [Presence] in Serum | 10 (3.5%) | 14 (4.9%) | 0.40 |
| HLA Ab in Serum by Flow cytometry (FC) (n, % with value) | 0.4 ± 0.5 (17, 5.9%) | 0.3 ± 0.5 (10, 3.5%) | 0.26 |
| Procedures | |||
| Backbench preparation of living donor renal allograft | 11 (3.91%) | 11 (3.91%) | 1 |
| Backbench preparation of deceased donor renal allograft | 217 (74.22%) | 217 (80.01%) | 0.11 |
| Backbench preparation of deceased whole liver graft | 217 (74.44%) | 243 (83.20%) | 0.06 |
| Backbench preparation of deceased or living renal allograft | 38 (12.89%) | 56 (19.14%) | 0.05 |
| Transfusion of Red Blood Cells | 58 (19.8%) | 43 (14.6%) | 0.20 |
| Transfusion, blood or components | 44 (14.9%) | 44 (14.9%) | 1 |
| Previous liver or kidney transplant | |||
| Liver transplant | 11 (3.8%) | 11 (3.8%) | 1 |
| Kidney transplant rejection diagnosis | 14 (4.9%) | 12 (4.1%) | 0.68 |
| Kidney transplant failure diagnosis | 16 (5.6%) | 15 (5.3%) | 0.85 |
| Liver transplant rejection diagnosis | 13 (4.5%) | 13 (4.5%) | 1 |
| Liver transplant failure diagnosis | 14 (4.9%) | 14 (4.5%) | 0.84 |
| Cumulative Incidence (%) | p | Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|---|---|
| Delayed graft function (need for any dialysis) | Bas | 27.45% | 0.64 | 1.081 (0.771, 1.515) |
| No Bas | 25.54% | |||
| Delayed graft function (need for hemodialysis) | Bas | 12.94% | 0.08 | 0.677 (0.433, 1.059) |
| No Bas | 18.08% | |||
| Primary liver non-function | Bas | 2.75% | 0.04 | 7.038 (0.866, 57.207) |
| No Bas | 0.39% |
| Outcome | Cohort | 3-Month CI (%)/Mean # | p | 6-Month CI (%)/Mean # | p | 1 Year CI (%)/Mean # | p |
|---|---|---|---|---|---|---|---|
| Kidney transplant rejection (Diagnosis only) | Bas | 10.41% | 0.88 | 15.35% | 0.93 | 19.97% | 0.90 |
| No bas | 10.19% | 15.30% | 19.79% | ||||
| Liver transplant rejection (Diagnosis only) | Bas | 12.60% | 0.97 | 16.14% | 0.15 | 17.80% | 0.07 |
| No bas | 12.81% | 21.82% | 25.08% | ||||
| Kidney or liver transplant rejection (Diagnosis or treated) | Bas | 17.25% | 0.48 | 24.16% | 0.11 | 28.29% | 0.12 |
| No bas | 20.08% | 31.42% | 35.47% | ||||
| Liver biopsy | Bas | 7.61% | 0.004 | 14.55% | 0.001 | 18.16% | 0.04 |
| No bas | 15.39% | 25.30% | 25.47% | ||||
| Kidney biopsy | Bas | 12.90% | 0.95 | 20.74% | 0.68 | 27.55% | 0.33 |
| No bas | 12.73% | 21.87% | 23.93% | ||||
| Hemodialysis | Bas | 15.72% | 0.82 | 17.20% | 0.90 | 18.01% | 0.93 |
| No bas | 16.44% | 17.53% | 17.54% | ||||
| Kidney graft failure (eGFR < 15) | Bas | 12.91% | 0.30 | 14.03% | 0.15 | 15.28% | 0.05 |
| No bas | 16.08% | 18.71% | 22.36% | ||||
| Liver re-transplant | Bas | 0.38% | 1.00 | 0.38% | 0.78 | 0.38% | 0.78 |
| No bas | 0.38% | 0.38% | 0.38% | ||||
| Hospitalizations (mean #) | Bas | 19.83 | 0.15 | 25.55 | 0.17 | 29.65 | 0.25 |
| No bas | 17.06 | 21.73 | 25.56 | ||||
| Mortality | Bas | 2.46% | 0.61 | 3.93% | 0.24 | 7.83% | 0.98 |
| No bas | 3.17% | 6.17% | 7.64% |
| Outcome | Cohort | 3-Month CI (%) | p | 6-Month CI (%) | p | 1 Year CI (%) | p |
|---|---|---|---|---|---|---|---|
| CMV | Bas | 5.25% | 0.003 | 14.57% | 0.02 | 22.97% | 0.03 |
| No bas | 12.38% | 21.93% | 30.47% | ||||
| EBV | Bas | 0.35% | 0.17 | 0.35% | 0.06 | 0.36% | 0.03 |
| No bas | 1.42% | 2.18% | 2.60% | ||||
| BK Virus | Bas | 1.79% | 0.54 | 5.12% | 0.4 | 6.35% | 0.81 |
| No bas | 2.51% | 6.63% | 6.63% | ||||
| JC Virus | Bas | 0.00% | 1 | 0.00% | 1 | 0.00% | 0.31 |
| No bas | 0.00% | 0.38% | 0.38% | ||||
| VZV | Bas | 0.00% | 0.31 | 1.12% | 0.70 | 1.98% | 0.50 |
| No bas | 0.35% | 1.47% | 2.72% | ||||
| Composite Viremia (CMV, EBV, BK, JC, VZV) | Bas | 7.04% | 0.002 | 18.60% | 0.006 | 27.00% | 0.01 |
| No bas | 15.22% | 27.83% | 35.49% | ||||
| Composite pneumonia | Bas | 10.58% | 0.57 | 16.58% | 0.61 | 20.60% | 0.09 |
| No bas | 9.17% | 11.54% | 15.15% | ||||
| Pyelonephritis | Bas | 5.01% | 0.47 | 7.94% | 0.64 | 12.04% | 0.17 |
| No bas | 6.52% | 6.96% | 8.20% | ||||
| Sepsis | Bas | 15.37% | 0.99 | 22.15% | 0.85 | 28.51% | 0.81 |
| No bas | 15.47% | 22.93% | 27.27% |
| Outcome | Cohort | 14 Days (Mean ± SD) | p | 3 Months (Mean ± SD) | p | 6 Months (Mean ± SD) | p | 12 Months (Mean ± SD) | p |
|---|---|---|---|---|---|---|---|---|---|
| AST | Bas | 30.99 ± 45.84 | 0.24 | 30.65 ± 67.93 | 0.66 | 60.60 ± 459.87 | 0.56 | 61.43 ± 429.12 | 0.41 |
| No bas | 52.31 ± 271.39 | 34.36 ± 103.50 | 90.95 ± 679.26 | 37.51 ± 101.38 | |||||
| ALT | Bas | 51.77 ± 66.3 | 0.22 | 31.47 ± 46.91 | 0.44 | 44.7 ± 199.66 | 0.63 | 42.88 ± 184.96 | 0.51 |
| No bas | 64.14 ± 136.65 | 35.57 ± 63 | 54.56 ± 224.97 | 34.54 ± 51.34 | |||||
| Total bilirubin | Bas | 1.71 ± 3.17 | 0.42 | 0.82 ± 2.79 | 0.58 | 0.95 ± 2.96 | 0.13 | 0.93 ± 2.78 | 0.19 |
| No bas | 1.97 ± 3.52 | 0.95 ± 1.87 | 1.5 ± 4.24 | 1.35 ± 4.07 | |||||
| INR | Bas | 1.16 ± 0.30 | 0.31 | 1.17 ± 0.35 | 0.36 | 1.24 ± 0.61 | 0.40 | 1.17 ± 0.48 | 0.86 |
| No bas | 1.19 ± 0.36 | 1.21 ± 0.46 | 1.19 ± 0.49 | 1.18 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koi, A.; Engebretsen, T.; Lea, A.S.; Arango, D.; Stevenson, H.L.; Kueht, M.L. Evaluating the Role of Basiliximab Induction in Simultaneous Liver–Kidney Transplantation: A Multicenter Propensity-Score-Matched Analysis. Antibodies 2025, 14, 91. https://doi.org/10.3390/antib14040091
Koi A, Engebretsen T, Lea AS, Arango D, Stevenson HL, Kueht ML. Evaluating the Role of Basiliximab Induction in Simultaneous Liver–Kidney Transplantation: A Multicenter Propensity-Score-Matched Analysis. Antibodies. 2025; 14(4):91. https://doi.org/10.3390/antib14040091
Chicago/Turabian StyleKoi, Avery, Trine Engebretsen, Alfred S. Lea, Daniel Arango, Heather L. Stevenson, and Michael L. Kueht. 2025. "Evaluating the Role of Basiliximab Induction in Simultaneous Liver–Kidney Transplantation: A Multicenter Propensity-Score-Matched Analysis" Antibodies 14, no. 4: 91. https://doi.org/10.3390/antib14040091
APA StyleKoi, A., Engebretsen, T., Lea, A. S., Arango, D., Stevenson, H. L., & Kueht, M. L. (2025). Evaluating the Role of Basiliximab Induction in Simultaneous Liver–Kidney Transplantation: A Multicenter Propensity-Score-Matched Analysis. Antibodies, 14(4), 91. https://doi.org/10.3390/antib14040091





.png)

