Structural and Functional Characterization of Anti-SARS-CoV-2 Spike Monoclonal Antibodies Produced via Bicistronic Expression in CHO Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Synthesis and Vector Design
2.2. Transient Expression in ExpiCHOTM Cells
2.3. mAb Purification by Protein A Affinity Chromatography
2.4. SDS-PAGE and Western Blot Analysis
2.5. Evaluation of Secondary and Tertiary Structures
2.6. Quaternary Structure and Heterogeneity Assessment by SEC-HPLC
2.7. Transmission Electron Microscopy (TEM)
2.8. N-Glycosylation Profile Evaluation by HILIC-HPLC and LC-MS
2.8.1. High-Performance Hydrophilic Interaction Liquid Chromatography (HILIC-HPLC)
2.8.2. Hydrophilic Interaction Nano-Liquid Chromatography/Tandem Mass Spectrometry (LC-MS)
2.9. Evaluation of Affinity to the Target by Spot Blot and Localized Surface Plasmon Resonance (LSPR)
2.10. Statistical Analysis
3. Results and Discussion
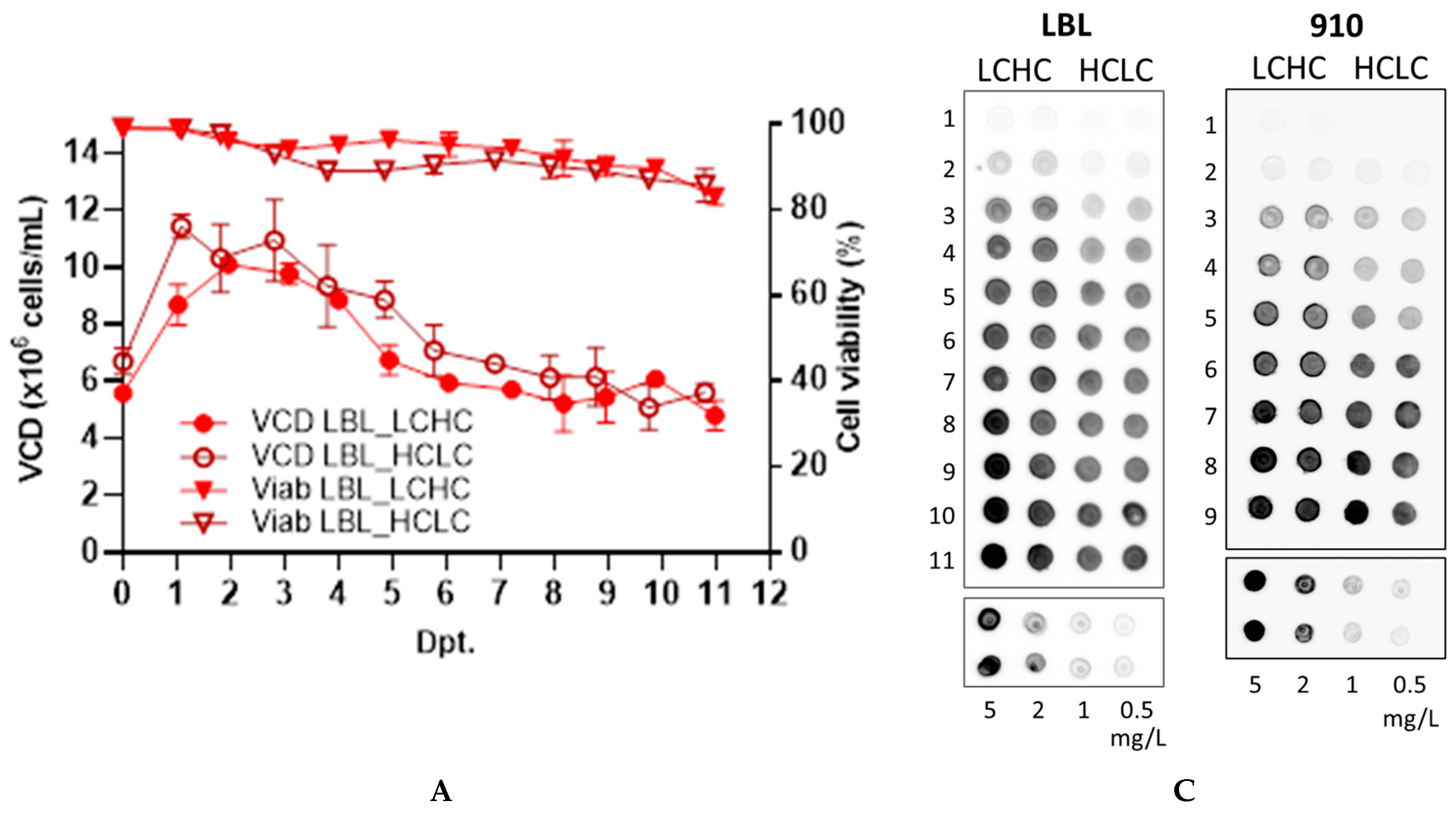
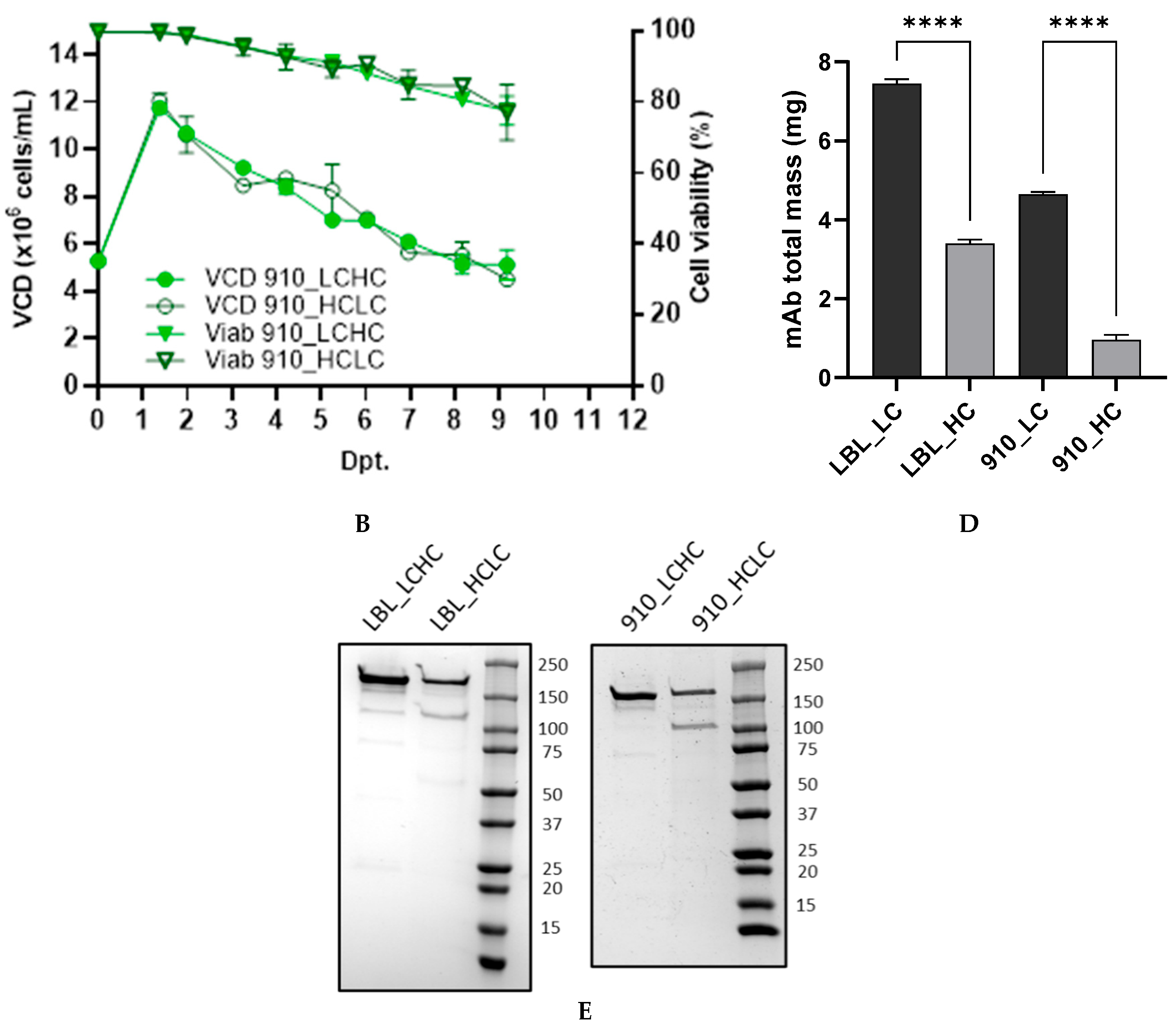
3.1. Transient mAb Production in ExpiCHO™ Cells
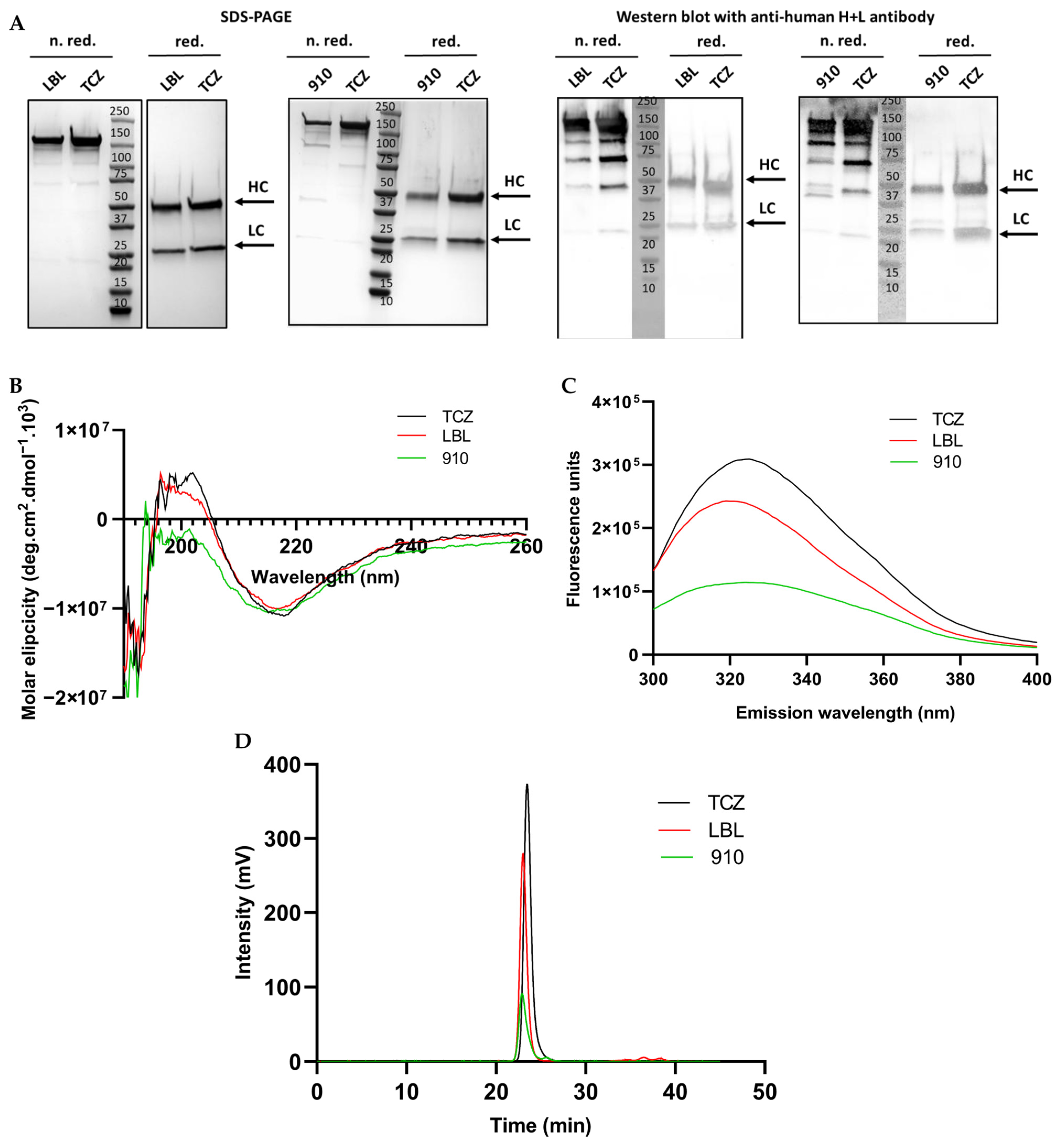
3.2. Structure Characterization
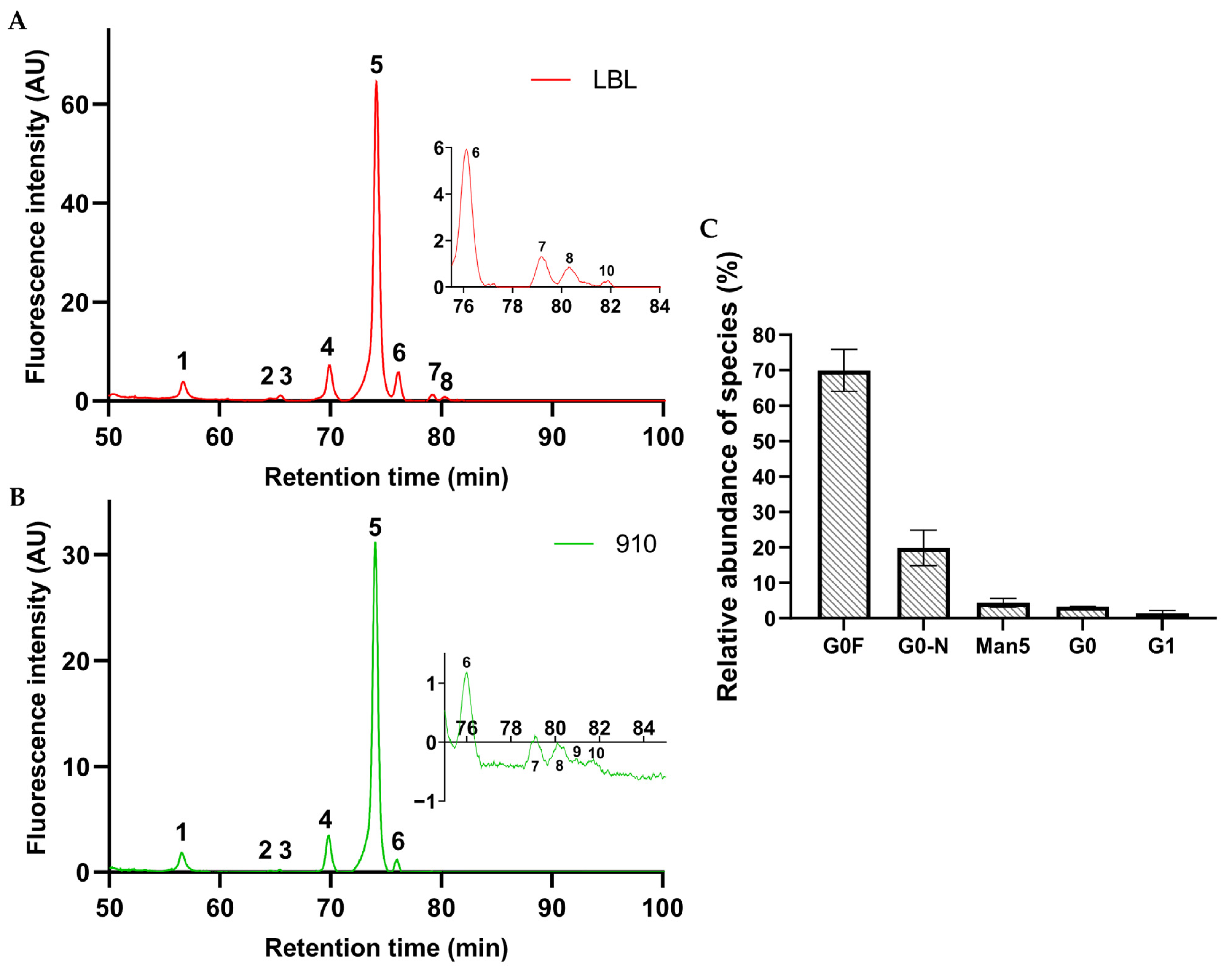
3.3. N-Glycosylation Profile Assessment
3.4. Functional Assays
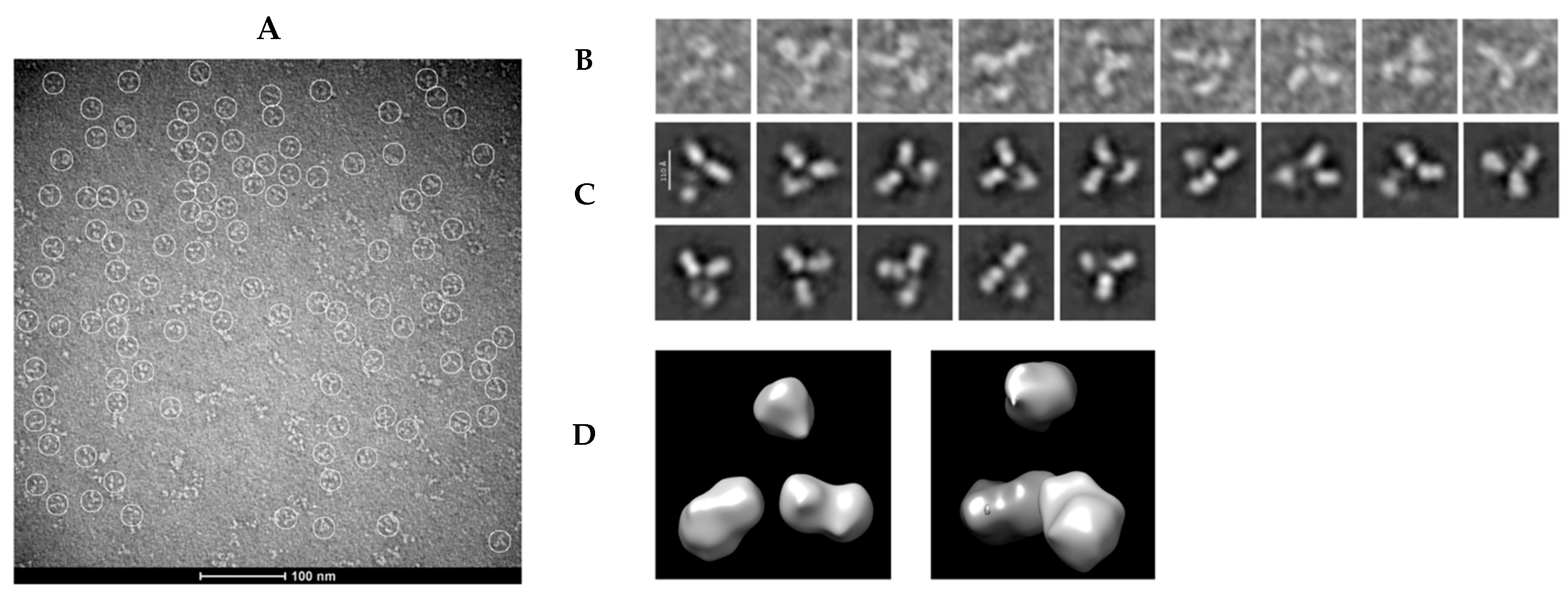
3.5. Negative-Staining Images and Reference-Free Class Averages of mAb LBL-01
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller-Pulido, A.; Jiménez-Pérez, M.I.; Gonzalez-Sanchez, F.A.; Rojo-Gutierrez, R.P.; Torres-Anguiano, E.; Aleman-Aguilar, J.P.; Garcia-Varela, R. Production of monoclonal antibodies for therapeutic purposes: A review. Int. Immunopharmacol. 2023, 120, 110376. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.C.; Ulitzka, M.; Grzeschik, J.; Kornmann, H.; Hock, B.; Kolmar, H. From cell line development to the formulated drug product: The art of manufacturing therapeutic monoclonal antibodies. Int. J. Pharm. 2021, 594, 120164. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.L.; Bardor, M.; Li, B.; Lee, J.J.; Song, Z.; Tong, Y.W. Comparison of internal ribosome entry site (IRES) and Furin-2A (F2A) for monoclonal antibody expression level and quality in CHO cells. PLoS ONE 2013, 8, e63247. [Google Scholar] [CrossRef]
- Ho, S.C.; Tong, Y.W.; Yang, Y. Generation of Monoclonal Antibody-Producing Mammalian Cell Lines. Pharm. Bioprocess. 2013, 1, 71–87. [Google Scholar] [CrossRef]
- Hunter, M.; Yuan, P.; Vavilala, D.; Fox, M. Optimization of Protein Expression in Mammalian Cells. Curr. Protoc. Protein Sci. 2018, 95, e77. [Google Scholar] [CrossRef]
- Gupta, K.; Parasnis, M.; Jain, R.; Dandekar, P. Vector-related stratagems for enhanced monoclonal antibody production in mammalian cells. Biotechnol. Adv. 2019, 37, 107415. [Google Scholar] [CrossRef]
- Li, J.; Menzel, C.; Meier, D.; Zhang, C.; Dübel, S.; Jostock, T. A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies. J. Immunol. Methods 2007, 318, 113–124. [Google Scholar] [CrossRef]
- Costa, A.R.; Rodrigues, M.E.; Henriques, M.; Azeredo, J.; Oliveira, R. Guidelines to Cell Engineering for Mono-clonal Antibody Production. Eur. J. Pharm. Biopharm. 2010, 74, 127–138. [Google Scholar] [CrossRef]
- Lai, T.; Yang, Y.; Ng, S.K. Advances in Mammalian Cell Line Development Technologies for Recombinant Protein Production. Pharmaceuticals 2013, 6, 579–603. [Google Scholar] [CrossRef]
- Li, Y.M.; Tian, Z.W.; Xu, D.H.; Wang, X.Y.; Wang, T.Y. Construction Strategies for Developing Expression Vectors for Recombinant Monoclonal Antibody Production in CHO Cells. Mol. Biol. Rep. 2018, 45, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Duke, G.M.; Hoffman, M.A.; Palmenberg, A.C. Sequence and structural elements that contribute to efficient encephalomyocarditis virus RNA translation. J. Virol. 1992, 66, 1602–1609. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Jostock, T.; Dübel, S. Analysis of IgG heavy chain to light chain ratio with mutant Encephalomyocarditis virus internal ribosome entry site. Protein Eng. Des. Sel. 2007, 20, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.L.; Bardor, M.; Feng, H.; Mariati; Tong, Y.W.; Song, Z.; Yap, M.G.S.; Yang, Y. IRES-mediated tricistronic vectors for enhancing generation of high monoclonal antibody expressing CHO cell lines. J. Biotechnol. 2012, 157, 130–139. [Google Scholar] [CrossRef]
- Ho, S.C.L.; Koh, E.Y.C.; van Beers, M.; Mueller, M.; Wan, C.; Teo, G.; Song, Z.; Tong, Y.W.; Bardor, M.; Yang, Y. Control of IgG LC:HC ratio in stably transfected CHO cells and study of the impact on expression, aggregation, glycosylation and conformational stability. J. Biotechnol. 2013, 165, 157–166. [Google Scholar] [CrossRef]
- Koh, E.Y.C.; Ho, S.C.L.; Mariati; Song, Z.; Bi, X.; Bardor, M.; Yang, Y. An internal ribosome entry site (IRES) mutant library for tuning expression level of multiple genes in mammalian cells. PLoS ONE 2013, 8, e82100. [Google Scholar] [CrossRef]
- Ho, S.C.L.; Wang, T.; Song, Z.; Yang, Y. IgG Aggregation Mechanism for CHO Cell Lines Expressing Excess Heavy Chains. Mol. Biotechnol. 2015, 57, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.M.; Ho, S.C.L.; Mariati, M.; Koh, E.; Tay, S.J.; Woen, S.; Zhang, P.; Yang, Y. Optimized Selection Marker and CHO Host Cell Combinations for Generating High Monoclonal Antibody Producing Cell Lines. Biotechnol. J. 2017, 12, 1700175. [Google Scholar] [CrossRef]
- Bayat, H.; Hoseinzadeh, S.; Pourmaleki, E.; Ahani, R.; Rahimpour, A. Evaluation of different vector design strategies for the expression of recombinant monoclonal antibody in CHO cells. Prep. Biochem. Biotechnol. 2018, 48, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Martin, T.; Barnes, A.; Wang, J.; Jimenez, R.B.; Rice, M.; Li, L.; Feng, H.; Zhang, S.; Chaerkady, R.; et al. A novel bicistronic gene design couples stable cell line selection with a fucose switch in a designer CHO host to produce native and afucosylated glycoform antibodies. mAbs 2018, 10, 416–430. [Google Scholar] [CrossRef]
- Cruz, T.A.; Pinho, M.B.; Castilho, L.R. Evaluation of different IRES-mediated tricistronic plasmid designs for expression of an anti-PCSK9 biosimilar monoclonal antibody in CHO cells. Biotechnol. Lett. 2020, 42, 2511–2522. [Google Scholar] [CrossRef]
- Toporova, V.A.; Argentova, V.V.; Aliev, T.K.; Panina, A.A.; Dolgikh, D.A.; Kirpichnikov, M.P. Optimization of recombinant antibody production based on the vector design and the level of metabolites for generation of Ig-producing stable cell lines. J. Genet. Eng. Biotechnol. 2023, 21, 23. [Google Scholar] [CrossRef]
- Bochkov, Y.A.; Palmenberg, A.C. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques 2006, 41, 283–292. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Mason, B.; Rossomando, T.; Li, N.; Liu, D.; Cheung, J.K.; Xu, W.; Raghava, S.; Katiyar, A.; et al. Structure, heterogeneity and developability assessment of therapeutic antibodies. mAbs 2018, 11, 239–264. [Google Scholar] [CrossRef]
- Cruz, T.A.; Larson, N.R.; Wei, Y.; Subelzu, N.; Wu, Y.; Schöneich, C.; Castilho, L.R.; Middaugh, C.R. Characterization of Critical Quality Attributes of an Anti-PCSK9 Monoclonal Antibody. Biologics 2024, 4, 294–313. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Albratty, M. Analytical Techniques for the Characterization and Quantification of Monoclonal Antibodies. Pharmaceuticals 2023, 16, 291. [Google Scholar] [CrossRef]
- Banach, B.B.; Cerutti, G.; Fahad, A.S.; Shen, C.H.; Oliveira De Souza, M.; Katsamba, P.S.; Tsybovsky, Y.; Wang, P.; Nair, M.S.; Huang, Y.; et al. Paired heavy- and light-chain signatures contribute to potent SARS-CoV-2 neutralization in public antibody responses. Cell Rep. 2021, 37, 109771. [Google Scholar] [CrossRef] [PubMed]
- Conde, L.; Oliveira, D.L.; Maciel, G.; Castro, F.; de Oliveira Albuquerque, A.; Rodrigues, D.; Meira, G.; Gabrielle, B.; Ferreira, S.S.; Cunha, M.S.; et al. Comprehensive analysis of the human SARS-CoV-2 memory B cell repertoire in low-resource settings. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Schlothauer, T.; Herter, S.; Koller, C.F.; Grau-Richards, S.; Steinhart, V.; Spick, C.; Kubbies, M.; Klein, C.; Umaña, P.; Mössner, E. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng. Des. Sel. 2016, 29, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef]
- Selman, M.H.J.; Hemayatkar, M.; Deelder, A.M.; Wuhrer, M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal. Chem. 2011, 83, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Alvin, K.; Latif, H.; Hsu, A.; Parikh, V.; Whitmer, T.; Tellers, M.; de la Cruz Edmonds, M.C.; Ly, J.; Salmon, P.; et al. Rapid protein production using CHO stable transfection pools. Biotechnol. Prog. 2010, 26, 1431–1437. [Google Scholar] [CrossRef]
- Jain, N.K.; Barkowski-Clark, S.; Altman, R.; Johnson, K.; Sun, F.; Zmuda, J.; Liu, C.Y.; Kita, A.; Schulz, R.; Neill, A.; et al. A high density CHO-S transient transfection system: Comparison of ExpiCHO and Expi293. Protein Expr. Purif. 2017, 134, 38–46. [Google Scholar] [CrossRef]
- Schlatter, S.; Stansfield, S.H.; Dinnis, D.M.; Racher, A.J.; Birch, J.R.; James, D.C. On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells. Biotechnol. Prog. 2005, 21, 122–133. [Google Scholar] [CrossRef]
- Bhoskar, P.; Belongia, B.; Smith, R.; Yoon, S.; Carter, T.; Xu, J. Free light chain content in culture media reflects recombinant monoclonal antibody productivity and quality. Biotechnol. Prog. 2013, 29, 1131–1139. [Google Scholar] [CrossRef]
- Mason, M.; Sweeney, B.; Cain, K.; Stephens, P.; Sharfstein, S.T. Identifying bottlenecks in transient and stable production of recombinant monoclonal-antibody sequence variants in Chinese hamster ovary cells. Biotechnol. Prog. 2012, 28, 846–855. [Google Scholar] [CrossRef]
- Mathias, S.; Wippermann, A.; Raab, N.; Zeh, N.; Handrick, R.; Gorr, I.; Schulz, P.; Fischer, S.; Gamer, M.; Otte, K. Unraveling what makes a monoclonal antibody difficult-to-express: From intracellular accumulation to incomplete folding and degradation via ERAD. Biotechnol. Bioeng. 2020, 117, 5–16. [Google Scholar] [CrossRef]
- Pybus, L.P.; James, D.C.; Dean, G.; Slidel, T.; Hardman, C.; Smith, A.; Daramola, O.; Field, R. Predicting the expression of recombinant monoclonal antibodies in Chinese hamster ovary cells based on sequence features of the CDR3 domain. Biotechnol. Prog. 2014, 30, 188–197. [Google Scholar] [CrossRef]
- Liu, H.; Gaza-Bulseco, G.; Chumsae, C.; Newby-Kew, A. Characterization of lower molecular weight artifact bands of recombinant monoclonal IgG1 antibodies on non-reducing SDS-PAGE. Biotechnol. Lett. 2007, 29, 1611–1622. [Google Scholar] [CrossRef]
- Zhu, Z.C.; Chen, Y.; Ackerman, M.S.; Wang, B.; Wu, W.; Li, B.; Obenauer-Kutner, L.; Zhao, R.; Tao, L.; Ihnat, P.M.; et al. Investigation of monoclonal antibody fragmentation artifacts in non-reducing SDS-PAGE. J. Pharm. Biomed. Anal. 2013, 83, 89–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y. Major cause of antibody artifact bands on non-reducing SDS-PAGE and methods for minimizing artifacts. Protein Expr. Purif. 2019, 164, 105459. [Google Scholar] [CrossRef]
- Wang, X.; An, Z.; Luo, W.; Xia, N.; Zhao, Q. Molecular and functional analysis of monoclonal antibodies in support of biologics development. Protein Cell 2018, 9, 74–85. [Google Scholar] [CrossRef]
- Harn, N.; Spitznagel, T.; Perkins, M.; Allan, C.; Shire, S.; Middaugh, C.R. Biophysical Signatures of Monoclonal Antibodies. In Current Trends in Monoclonal Antibody Development and Manufacturing; Shire, S., Gombotz, W., Bechtold-Peters, K., Andya, J., Eds.; Biotechnology: Pharmaceutical Aspects; Springer: New York, NY, USA, 2010; Volume XI, pp. 365–387. [Google Scholar] [CrossRef]
- Abbas, S.A.; Gaspar, G.; Sharma, V.K.; Patapoff, T.W.; Kalonia, D.S. Application of second-derivative fluorescence spectroscopy to monitor subtle changes in a monoclonal antibody structure. J. Pharm. Sci. 2013, 102, 52–61. [Google Scholar] [CrossRef]
- Ha, T.K.; Kim, D.; Kim, C.L.; Grav, L.M.; Lee, G.M. Factors affecting the quality of therapeutic proteins in recombinant Chinese hamster ovary cell culture. Biotechnol. Adv. 2022, 54, 107831. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Farkash, I.; Feferman, T.; Cohen-Saban, N.; Avraham, Y.; Morgenstern, D.; Mayuni, G.; Barth, N.; Lustig, Y.; Miller, L.; Shouval, D.S.; et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep. 2021, 37, 110114. [Google Scholar] [CrossRef]
- Pongracz, T.; Vidarsson, G.; Wuhrer, M. Antibody glycosylation in COVID-19. Glycoconj. J. 2022, 39, 335–344. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2023, 23, 381–399. [Google Scholar] [CrossRef]
- Winkler, E.S.; Gilchuk, P.; Yu, J.; Bailey, A.L.; Chen, R.E.; Chong, Z.; Zost, S.J.; Jang, H.; Huang, Y.; Allen, J.D.; et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell 2021, 184, 1804–1820.e16. [Google Scholar] [CrossRef]
- Reusch, D.; Haberger, M.; Maier, B.; Maier, M.; Kloseck, R.; Zimmermann, B.; Hook, M.; Szabo, Z.; Tep, S.; Wegstein, J.; et al. Comparison of methods for the analysis of therapeutic immunoglobulin G Fc-glycosylation profiles—Part 1: Separation-based methods. mAbs 2015, 7, 167–179. [Google Scholar] [CrossRef]
- Duivelshof, B.L.; Denorme, S.; Sandra, K.; Liu, X.; Beck, A.; Lauber, M.A.; Guillarme, D.; D’Atri, V. Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis. Pharmaceutics 2021, 13, 1744. [Google Scholar] [CrossRef]
- Campbell, M.P.; Royle, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M. GlycoBase and autoGU: Tools for HPLC-based glycan analysis. Bioinformatics 2008, 24, 1214–1216. [Google Scholar] [CrossRef]
- Campbell, M.P.; Zhao, S.; Abrahams, J.L.; Nguyen-Khuong, T.; Rudd, P.M. GlycoStore: A Platform for H/UPLC and Capillary Electrophoresis Glycan Data. In Glycosylation: Methods and Protocols; Davey, G.P., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2370, pp. 15–33. ISBN 978-1-0716-1684-0. [Google Scholar] [CrossRef]
- Kaur, H. Characterization of glycosylation in monoclonal antibodies and its importance in therapeutic antibody development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, B. Benchmark Glycan Profile of Therapeutic Monoclonal Antibodies Produced by Mammalian Cell Expression Systems. Pharm. Res. 2024, 41, 29–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Ju, B.; Ge, J.; Chan, J.F.W.; Cheng, L.; Wang, R.; Huang, W.; Fang, M.; Chen, P.; Zhou, B.; et al. Potent and protective IGHV3-53/3-66 public antibodies and their shared escape mutant on the spike of SARS-CoV-2. Nat. Commun. 2021, 12, 4210. [Google Scholar] [CrossRef]
- Sandin, S.; Ofverstedt, L.G.; Wikström, A.C.; Wrange, O.; Skoglund, U. Structure and flexibility of individual immunoglobulin G molecules in solution. Structure 2004, 12, 409–415. [Google Scholar] [CrossRef]


| LBL-01 | 910-30 | |||||
|---|---|---|---|---|---|---|
| Variant | Kon (M−1 s−1) | Koff (s−1) | KD (M) | Kon (M−1 s−1) | Koff (s−1) | KD (M) |
| D614G | 8.63 ± 0.00 × 104 | 7.21 ± 0.45 × 10−5 | 8.36 ± 0.05 × 10−10 | 9.06 ± 0.02 × 104 | 4.05 ± 0.78 × 10−5 | 4.47 ± 0.86 × 10−10 |
| Delta | 1.17 ± 0.00 × 105 | 2.30 ± 0.00 × 10−4 | 1.96 ± 0.03 × 10−9 | 1.43 ± 0.04 × 105 | 2.56 ± 0.01 × 10−3 | 1.79 ± 0.01 × 10−8 |
| Gamma | 8.38 ± 0.01 × 104 | 6.41 ± 0.56 × 10−5 | 7.66 ± 0.67 × 10−10 | ND | ND | ND |
| Omicron | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsili, F.F.; de Aquino, F.B.; Arruda, H.R.d.S.; Marques, M.A.; Cabral, K.M.d.S.; Almeida, M.d.S.; de Oliveira, G.A.P.; Maranhão, A.Q.; Carvalho, R.S.; Castilho, L.d.R. Structural and Functional Characterization of Anti-SARS-CoV-2 Spike Monoclonal Antibodies Produced via Bicistronic Expression in CHO Cells. Antibodies 2025, 14, 86. https://doi.org/10.3390/antib14040086
Marsili FF, de Aquino FB, Arruda HRdS, Marques MA, Cabral KMdS, Almeida MdS, de Oliveira GAP, Maranhão AQ, Carvalho RS, Castilho LdR. Structural and Functional Characterization of Anti-SARS-CoV-2 Spike Monoclonal Antibodies Produced via Bicistronic Expression in CHO Cells. Antibodies. 2025; 14(4):86. https://doi.org/10.3390/antib14040086
Chicago/Turabian StyleMarsili, Federico Francisco, Fernanda Bittencourt de Aquino, Hiam Rodrigo da Silva Arruda, Mayra Amorim Marques, Katia Maria dos Santos Cabral, Marcius da Silva Almeida, Guilherme Augusto Piedade de Oliveira, Andrea Queiroz Maranhão, Renato Sampaio Carvalho, and Leda dos Reis Castilho. 2025. "Structural and Functional Characterization of Anti-SARS-CoV-2 Spike Monoclonal Antibodies Produced via Bicistronic Expression in CHO Cells" Antibodies 14, no. 4: 86. https://doi.org/10.3390/antib14040086
APA StyleMarsili, F. F., de Aquino, F. B., Arruda, H. R. d. S., Marques, M. A., Cabral, K. M. d. S., Almeida, M. d. S., de Oliveira, G. A. P., Maranhão, A. Q., Carvalho, R. S., & Castilho, L. d. R. (2025). Structural and Functional Characterization of Anti-SARS-CoV-2 Spike Monoclonal Antibodies Produced via Bicistronic Expression in CHO Cells. Antibodies, 14(4), 86. https://doi.org/10.3390/antib14040086








