1. Introduction
Dupilumab, a fully human monoclonal antibody targeting the interleukin-4 receptor α subunit (IL-4Rα), is the first biologic therapy approved for the treatment of moderate-to-severe atopic dermatitis (AD). By blocking the shared receptor for IL-4 and IL-13, dupilumab inhibits key type 2 inflammatory pathways, resulting in significant reductions in eczema severity, pruritus, and sleep disturbance, with a favorable safety profile established across pivotal randomized clinical trials and subsequent real-world studies [
1,
2,
3].
Despite its demonstrated efficacy, important uncertainties remain regarding its safety in specific subpopulations. A systematic review by Braddock et al. [
4] highlighted the conflicting and incomplete evidence concerning the role of IL-4 and IL-13 signaling in carcinogenesis, raising questions about the use of dupilumab in patients with current or prior malignancies. These concerns are particularly relevant because pivotal trials excluded individuals with active cancer, hematologic malignancies, or severe comorbidities, thereby limiting the generalizability of trial findings to these high-risk groups.
In clinical practice, however, dermatologists often treat AD patients with concomitant hematologic disorders. These include premalignant plasma cell dyscrasias such as monoclonal gammopathy of undetermined significance (MGUS), hematologic malignancies like Hodgkin and non-Hodgkin lymphomas, acute and chronic leukemias, and myelodysplastic syndromes. Non-malignant conditions are also frequently encountered, ranging from immune cytopenias and hypereosinophilic syndromes (HESs) to inherited hemoglobinopathies and myeloproliferative neoplasms. Managing AD in these patients poses therapeutic challenges, as traditional systemic immunosuppressants may exacerbate underlying hematologic disease or increase infection risk.
While randomized clinical trials provide high internal validity, their restrictive eligibility criteria reduce external validity and leave an evidence gap for complex populations with relevant comorbidities. Real-world studies can bridge this gap by evaluating broader and more heterogeneous cohorts that mirror routine clinical practice. However, such studies also carry intrinsic limitations, including retrospective design, non-standardized follow-up, and potential unmeasured confounding.
The present multicenter retrospective study aimed to provide the largest real-world evaluation to date of the safety and effectiveness of dupilumab in patients with moderate-to-severe AD and concomitant hematologic disorders, addressing a clinically important population often excluded from clinical trials but frequently encountered in daily dermatologic practice.
2. Materials and Methods
This retrospective study included adolescents and adults (≥15 years) with a confirmed diagnosis of AD and at least one concomitant hematologic comorbidity who were treated with dupilumab (Sanofi Genzyme, Paris, France; Regeneron Pharmaceuticals, Tarrytown, NY, USA) between November 2018 and May 2025 across participating Italian dermatology centers. Treatment eligibility followed national and international standards, specifically the Italian Medicines Agency (AIFA) reimbursement criteria and the EuroGuiDerm guideline recommendations, which require the presence of severe AD, defined as an Eczema Area and Severity Index (EASI) score ≥ 24, together with prior failure, intolerance, or contraindication to cyclosporine A. In addition, patients could have received previous systemic therapies, including other biologic agents or small-molecule inhibitors, which were discontinued due to inefficacy or safety concerns. During the observation period, concomitant topical anti-inflammatory therapies, including corticosteroids and calcineurin inhibitors, were permitted and used intermittently for the management of localized flares, reflecting routine clinical practice. Importantly, no additional systemic immunosuppressive or immunomodulatory therapies were administered concurrently with dupilumab, ensuring that treatment outcomes could be attributed with greater confidence to dupilumab itself. Patient data were retrospectively retrieved from medical records. Eligible patients were required to have completed at least one post-baseline assessment and to have a minimum follow-up duration of four months in order to ensure the adequate documentation of clinical response and safety outcomes. Follow-up evaluations were generally scheduled in alignment with routine clinical practice, typically at approximately weeks 16, 36, 52, 104, and 156.
Disease severity was assessed at each visit using the EASI. This composite score evaluates four clinical signs of AD (erythema, edema/papulation, excoriations, and lichenification) across four body regions (head/neck, trunk, upper limbs, and lower limbs). Each sign is graded on a scale from 0 to 3, and the regional scores are weighted by the corresponding body surface area involved. The total EASI score ranges from 0 (clear skin) to 72 (most severe disease). Assessments were performed by trained dermatologists following standard guidance. Symptoms were captured using patient-reported Numerical Rating Scales (NRSs) ranging from 0 (none) to 10 (worst imaginable). We recorded the mean pruritus NRS and mean sleep-loss NRS as the average severity over the preceding 7 days, which reflects common clinical practice for adults with AD and other dermatologic conditions [
5,
6]. Laboratory assessments included total serum IgE (kU/L), measured using each center’s routine immunoassay platform, and absolute eosinophil counts, obtained from automated hematology analyzers and expressed as ×10
3/µL. Given the multicenter, real-world setting, assay platforms varied among sites; all values were converted to uniform units prior to analysis and were evaluated in accordance with good clinical practice. Hematologic outcomes at the last available follow-up were categorized as follows: resolution, the complete disappearance of hematologic disease signs/symptoms with the normalization of relevant laboratory/imaging findings, as reported by the hematology specialist; stability, no clinically meaningful change from the baseline; and progression, a documented worsening of hematologic disease based on specialist assessment, laboratory data, or imaging.
Descriptive statistics, including medians, ranges, means with standard deviations, and proportions, were used to summarize the data, providing an accurate and transparent representation of the observed trends. The normality of the distributions was assessed using the Kolmogorov–Smirnov test. For paired comparisons between consecutive timepoints, the Wilcoxon signed-rank test was applied, as the variables were not normally distributed. A p-value < 0.05 was considered statistically significant for all analyses.
Ethical approval was not required due to the retrospective design of the study and the use of anonymized data. All patients had previously provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and all applicable data protection regulations.
3. Results
A total of 139 adult and adolescent patients with moderate-to-severe AD and at least one hematologic comorbidity were enrolled from 21 dermatology centers (mean age: 60 years [SD 21.2]; 58.3% male). All patients were receiving treatment with dupilumab. Population characteristics are summarized in
Table 1 and
Table 2.
The most common clinical phenotype was classic AD (62.6%), followed by prurigo-like (27.3%), nummular eczema (4.3%), erythroderma (3.6%), portrait eczema (1.4%; involving the face, neck, and upper chest), and palmo-plantar involvement (0.7%).
Atopic comorbidities were frequent, including allergic rhinitis in 40.3% of patients, asthma in 32.4%, conjunctivitis in 20.9%, and both nasal polyposis and eosinophilic esophagitis in 2.9% each. The most common non-atopic comorbidities were hypertension (28.8%), cardiovascular disease (21.6%), and diabetes (10.1%), with smaller proportions affected by chronic renal failure (2.2%) or Chronic Obstructive Pulmonary Disease (COPD) (2.2%).
Regarding prior treatments, systemic corticosteroids had been used in 89.2% of patients, cyclosporine in 34.5%, phototherapy in 33.1%, methotrexate in 5.8%, acitretin in 2.2%, and tralokinumab in 2.9%.
The most frequently reported hematologic conditions were plasma cell dyscrasias, including MGUS and multiple myeloma (n = 45). Leukemias were identified in 13 cases, comprising chronic lymphocytic leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia. Non-Hodgkin lymphomas were reported in 12 cases, while 10 patients were diagnosed with Hodgkin lymphoma. Myeloproliferative neoplasms, including essential thrombocythemia, polycythemia vera, and idiopathic myelofibrosis, were observed in 13 patients, and 7 patients had a diagnosis of myelodysplastic syndrome. Other comorbidities included 7 cases of autoimmune thrombocytopenia, 10 cases of thalassemic trait, and 4 cases of iron metabolism disorders such as hereditary hemochromatosis. Immunodeficiencies, including common variable immunodeficiency and IgA deficiency, were observed in four patients. Thrombophilic mutations, such as factor II and factor V Leiden variants, were also present in four cases each. Additional diagnoses included primary immunologic syndromes such as hyper-IgE syndrome in two cases, eosinophilic syndromes including HES in two cases, and Kaposi sarcoma in one case. Among the 139 cases, only 12 patients received a new hematologic diagnosis after initiating dupilumab, with a mean time to diagnosis of 1.6 years (SD, 0.82). These included six cases of MGUS and one case each of hereditary hemochromatosis, Hodgkin lymphoma, non-Hodgkin lymphoma, chronic myeloid leukemia, polycythemia vera, and myelodysplastic syndrome. These diagnoses were identified incidentally during routine care or prompted by new symptoms, as no systematic hematologic screening was performed. The median follow-up duration was 52 weeks (range, 4–156).
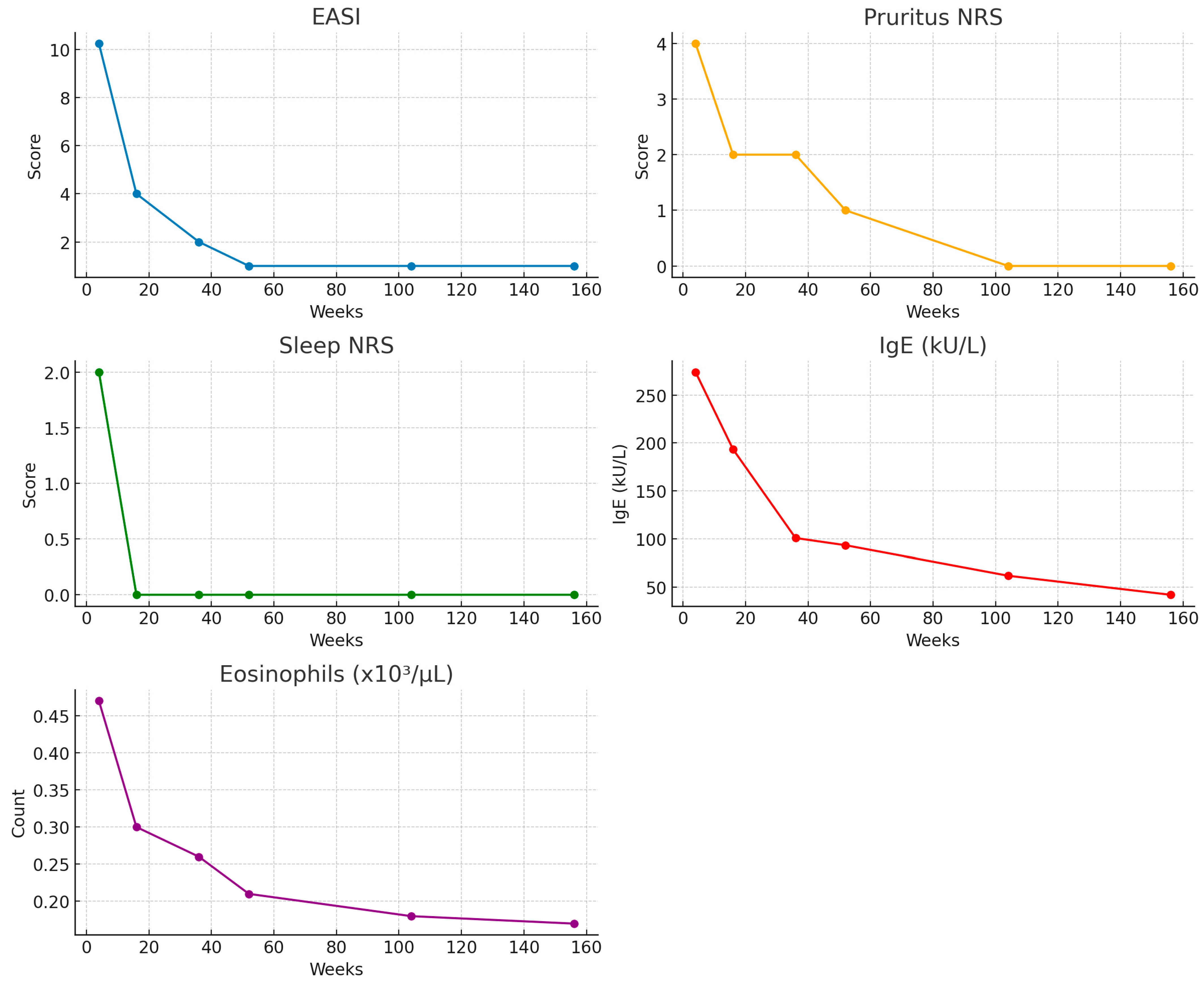
A marked clinical improvement was observed over time across all efficacy measures (
Figure 1,
Table 3).
Median EASI scores progressively declined from 26.0 at the baseline to 10.25 at week 4, 4.0 at week 16, 2.0 at week 36, and 1.0 at weeks 52, 104, and 156. NRS pruritus decreased from a median of 9.0 at the baseline to 4.0 at week 4, 2.0 at weeks 16 and 36, 1.0 at week 52, and 0.0 thereafter. Similarly, sleep disturbance scores dropped from 8.0 at the baseline to 2.0 at week 4, and they reached 0.0 from week 16 onwards, showing a sustained response. Serum IgE levels showed a similar trend, with median concentrations decreasing from 286.0 kU/L at the baseline to 274.0 at week 4, 193.5 at week 16, 101.0 at week 36, and 93.5 at week 52 and stabilizing at 61.95 and 42.1 kU/L at weeks 104 and 156, respectively. Finally, median eosinophil counts were 0.46 × 103/µL at the baseline, remained stable at 0.47 at week 4, and subsequently declined to 0.30 at week 16, 0.26 at week 36, 0.21 at week 52, 0.18 at week 104, and 0.17 at week 156.
Significant improvements were observed across all evaluated variables (
Table 4).
EASI scores decreased significantly from the baseline to week 4 (p < 0.001), week 4 to week 16 (p < 0.001), week 16 to week 36 (p < 0.001), and week 36 to week 52 (p = 0.049), and no further change was observed from week 52 to week 104 (p = 0.66 and 0.60, respectively). Pruritus NRS showed significant reductions from the baseline to week 4 (p < 0.001), week 4 to week 16 (p < 0.001), and week 36 to week 52 (p = 0.009), whereas other interval changes were not significant. Sleep NRS scores significantly improved from the baseline to week 4 (p < 0.001), week 4 to week 16 (p < 0.001), and week 36 to week 52 (p = 0.015), with borderline significance between week 16 and week 36 (p = 0.050). Serum IgE levels decreased significantly across all consecutive timepoints (all p < 0.001). Eosinophil counts showed no change from the baseline to week 4 (p = 0.964) but significantly decreased from week 4 to week 16 (p < 0.001), week 16 to week 36 (p = 0.020), and week 36 to week 52 (p < 0.001), with no further change thereafter.
By the end of the observation period, hematologic conditions remained stable in 115 patients, had resolved in 23, and showed progression in a single case.
4. Discussion
To our knowledge, this multicenter retrospective study represents the largest real-world analysis conducted to date evaluating the safety and effectiveness of dupilumab in patients with moderate-to-severe AD and concomitant hematologic conditions, a population typically excluded from pivotal clinical trials. This is particularly relevant, given the increasing clinical need to manage complex patients with multimorbidity in daily practice. Dupilumab, a fully human monoclonal antibody directed against the interleukin-4 receptor α (IL-4Rα), is currently approved for the treatment of moderate-to-severe AD across a broad age spectrum, including adults, adolescents, and children as young as 6 months, as well as for asthma with an eosinophilic phenotype or oral corticosteroid dependence [
7], and chronic rhinosinusitis with nasal polyposis [
8]. More recently, its therapeutic indications have expanded to include prurigo nodularis in adults [
9], eosinophilic esophagitis [
10], and bullous pemphigoid [
11]. Beyond its approved indications, dupilumab has been increasingly employed in an off-label setting for the management of a variety of type 2 inflammatory and rare dermatologic disorders, reflecting its broad immunomodulatory activity. These include alopecia areata [
12], hypereosinophilic syndrome [
13], and eosinophilic annular erythema [
14], among others. The growing spectrum of diseases treated with dupilumab underscores the need for robust safety data in special populations, such as patients with underlying hematologic malignancies or immune dysregulation, for whom immunomodulatory therapies raise specific concerns regarding efficacy, tolerability, and long-term outcomes.
In this multicenter, real-world cohort, we observed rapid and sustained clinical improvement in patients treated with dupilumab, as reflected by significant reductions in EASI scores, pruritus NRS, and sleep NRS within the first 4 to 36 weeks of therapy. The absence of significant change in EASI, pruritus NRS, and sleep-loss NRS beyond week 52 suggests that most patients reached a clinical plateau and maintained an effective response up to week 156. A biomarker analysis revealed a consistent decline in serum IgE levels across all timepoints, supporting the mechanistic role of IL-4/IL-13 blockade in downregulating IgE production [
3]. Eosinophil counts, as expected from previous reports, did not decrease in the early treatment phase but showed a delayed yet significant reduction after week 4, with further decreases through week 52 [
3]. This pattern is consistent with the known transient eosinophilia or stability observed at treatment initiation, reflecting a redistribution, rather than an immediate suppression of circulating eosinophils.
Patients with active malignancy or significant comorbidities were systematically excluded from pivotal dupilumab clinical trials, creating an important evidence gap for clinicians who manage complex real-world populations. Our findings suggest that dupilumab remains both effective and well tolerated in patients with concomitant hematologic disorders, thereby extending the external validity of previous trial data. Notably, 8.6% of patients developed a new hematologic diagnosis during treatment; however, no causal link can be inferred. This limitation is primarily due to the retrospective, observational nature of the study, the absence of systematic hematologic screening protocols, and the possibility of pre-existing but previously undiagnosed conditions. Consequently, these observations should be interpreted as descriptive associations, rather than evidence of a treatment-related effect. It is also worth highlighting that, in current clinical practice, the initiation of dupilumab does not require specific hematologic investigations beyond routine baseline assessments. This may contribute to the underrecognition of asymptomatic or subclinical hematologic conditions at treatment onset, which could later manifest during follow-up. Importantly, the broad spectrum of hematologic disorders included in our cohort, ranging from premalignant plasma cell dyscrasias to hematologic malignancies and immune-mediated cytopenias, did not appear to compromise treatment efficacy.
As highlighted by Braddock et al. [
4], preclinical studies have reported conflicting effects, with some suggesting that these cytokines promote tumor development and metastasis, while others indicate potential protective roles. The therapeutic blockade of IL-4/IL-13 could, therefore, yield opposing outcomes, depending on tumor type and stage. For example, IL-13 signaling via the IL-13Rα2 receptor has been implicated in tumor progression in some models [
15,
16]. Additionally, evidence from Ingram et al. suggests that IL-4Ra inhibition may exert either beneficial or deleterious effects based on the timing within the cancer course [
17].
IL-4 and IL-13 are type 2 cytokines involved not only in the inflammatory pathways of atopic dermatitis but also in regulating the tumor microenvironment. Both signal through the shared IL-4Rα subunit—the pharmacologic target of dupilumab—via type I (IL-4Rα/γc) and type II (IL-4Rα/IL-13Rα1) receptor complexes, activating downstream JAK/STAT6, PI3K/AKT, and ERK pathways [
18,
19]. These cascades can promote cell proliferation, survival, migration, and resistance to apoptosis—processes relevant to oncogenesis and tumor progression [
19,
20]. In the tumor microenvironment, IL-4 and IL-13 also polarize macrophages toward an M2 phenotype, which supports tumor growth and suppresses anti-tumor immunity. In patients with hematologic disease, immune surveillance is frequently impaired due to the underlying disorder or its treatments. Theoretically, IL-4Rα inhibition may reduce type-2 cytokine–mediated tumor-promoting immune suppression, but it could also alter protective immune surveillance, potentially impacting infection risk or anti-tumor immunity in immunocompromised hosts [
20]. Conversely, in certain tumor contexts, IL-4 and IL-13 contribute to the activation and survival of cytotoxic T lymphocytes and natural killer (NK) cells, supporting immune surveillance against malignant cells [
11]. In addition, they can influence the maturation and antigen-presenting capacity of dendritic cells, supporting the priming of anti-tumor immunity [
21].
More recently, a systematic review by Guo et al. [
22] synthesized evidence from both clinical trials and real-world studies and found no consistent signal of an increased cancer incidence associated with dupilumab use. Although isolated reports have described new or worsening malignancies, most frequently cutaneous T-cell lymphomas [
23], as well as myelodysplastic syndromes and leukemias [
24], these cases were often characterized by preexisting or previously undiagnosed hematologic disease, making attribution to dupilumab treatment uncertain. Importantly, the majority of patients treated with dupilumab across diverse settings have not demonstrated an increased risk of neoplastic progression, and the overall evidence to date supports a favorable oncologic safety profile. Nevertheless, the interpretation of these findings requires caution. Most of the available data derive from post-marketing surveillance, retrospective analyses, and individual case reports, all of which are inherently limited by reporting bias, heterogeneity in baseline patient characteristics, and a lack of standardized long-term follow-up. Overall, current evidence suggests that dupilumab does not confer an increased cancer risk and may be safely considered in patients with stable or historical hematologic comorbidities. However, the absence of prospective, systematically monitored trials in immunocompromised and oncologic populations highlights an ongoing need for vigilance, structured pharmacovigilance registries, and collaborative studies aimed at clarifying long-term safety in this vulnerable subgroup.
The study’s limitations include the retrospective design, the lack of a comparator arm, and the absence of mandatory baseline hematologic screening, which may have limited the ability to distinguish preexisting from incident hematologic conditions. Nevertheless, the large sample size, extended follow-up, and multicenter setting enhance the reliability and generalizability of the findings. Moreover, the inclusion of patients previously treated with tralokinumab could raise concerns regarding potential carryover effects. All such patients had discontinued tralokinumab due to inadequate clinical response, but, in line with good clinical practice, treatment with dupilumab was initiated without waiting for the full tralokinumab elimination period; in some cases, the interval between the two biologics was shorter than approximately 3 months required for >90% drug clearance. Therefore, a pharmacologic carryover effect during the first months of dupilumab therapy in these patients cannot be entirely excluded. Finally, circulating cytokines IL-4 and IL-13, which are the therapeutic targets of dupilumab, were not measured, as such testing is not routinely performed in AD care across Italian centers; therefore, pathophysiologic correlations between cytokine levels and clinical outcomes could not be explored.
5. Conclusions
In conclusion, dupilumab demonstrates a favorable safety and efficacy profile for the treatment of moderate-to-severe AD in patients with diverse hematologic comorbidities, including including those with a history of neoplastic disease. Clinical benefits were rapid and sustained, and hematologic conditions remained stable or improved in most cases. Importantly, no consistent evidence of disease progression attributable to IL-4Rα blockade was observed. Long-term prospective studies and dedicated registries are warranted to better define the impact of dupilumab on immune surveillance, clarify potential risks in specific hematologic subgroups, and provide guidance for clinicians managing these complex patients.
Author Contributions
Conceptualization, L.B., M.R. and S.B.; methodology, L.B., S.B. and A.V.M.; software, L.B.; validation, L.B., S.B., A.V.M. and M.R.; formal analysis, L.B.; investigation, L.B., S.B., S.M.F., A.V.M., F.B., A.N., M.B. (Matteo Bianco), A.C., N.Z., M.O., S.R., E.P., M.N., M.M., G.G., Z.F., E.E., C.F., G.D.B., I.T., A.B., D.D., N.G., F.V., G.P., F.R., M.B.G., L.C., R.B., M.B. (Manfredo Bruni) and M.R.; resources, all authors; data curation, L.B., F.B., A.C., E.P. and S.R.; writing—original draft preparation, L.B.; writing—review and editing, S.B., A.V.M., M.R. and A.C.; visualization, L.B.; supervision, M.R. and A.V.M.; project administration, M.R.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required due to the retrospective design of the study and the use of anonymized data. The study was conducted in accordance with the Declaration of Helsinki and all applicable data protection regulations.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
Conflicts of Interest
Mariateresa Rossi has received personal fees for advisory board meetings from Sanofi, AbbVie, Novartis, and Cantabria. Alessandra Narcisi has served on advisory boards and received honoraria and research grants from multiple companies, including Almirall, AbbVie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi Genzyme, Amgen, and Boehringer Ingelheim. Silvia Mariel Ferrucci has been the principal investigator in clinical trials for multiple companies, including AbbVie, Almirall, Galderma, Leo Pharma, Sanofi, Amgen, Novartis, and Bayer, and has received honoraria for lectures from Novartis and Menarini. Simone Ribero has received honoraria and served as a consultant or advisory board member for companies including AbbVie, Almirall, Leo Pharma, Eli Lilly, Novartis, Pfizer, and Sanofi Genzyme. Michela Ortoncelli has served as a consultant or advisory board member for AbbVie, Leo Pharma, and Sanofi Genzyme. Maddalena Napolitano has acted as speaker, consultant, and/or advisory board member for AbbVie, Eli Lilly, Leo Pharma, Novartis, and Sanofi. Anna Balato has received honoraria for participation in advisory boards or as a speaker for several pharmaceutical companies, including AbbVie, Celgene, Janssen Cilag, Eli Lilly, Novartis, Pfizer, Sanofi Genzyme, and UCB Pharma. Andrea Carugno has been a consultant, speaker, or participant in advisory boards for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen-Cilag, Leo Pharma, Novartis, Sanofi, and UCB Pharma. Mario Bruno Guanti has served as an advisory board member and/or consultant and received fees and honoraria or participated in clinical studies for AbbVie, Leo Pharma, Sanofi Genzyme, Novartis, Cantabria, and Eli Lilly. Elena Pezzolo has been a consultant and speaker for Sanofi Genzyme, AbbVie, Leo Pharma, Novartis, Janssen, Almirall, Pfizer, Galderma, and Boehringer Ingelheim. All other authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Atopic dermatitis |
| MGUS | Monoclonal gammopathy of undetermined significance |
| HES | Hypereosinophilic syndrome |
| EASI | Eczema Area and Severity Index |
| NRS | Numerical Rating Scale |
| IgE | Immunoglobulin E |
| IL-4 | Interleukin 4 |
| IL-13 | Interleukin 13 |
| IL-4Rα | Interleukin-4 receptor alpha |
| IL-13Rα2 | Interleukin-13 receptor alpha 2 |
| IL-4Rα/γc | Interleukin-4 receptor alpha/common gamma chain |
References
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Simpson, E.L.; Silverberg, J.I.; Worm, M.; Honari, G.; Masuda, K.; Syguła, E.; Schuttelaar, M.L.A.; Mortensen, E.; Laws, E.; Akinlade, B.; et al. Dupilumab treatment improves signs, symptoms, quality of life, and work productivity in patients with atopic hand and foot dermatitis: Results from a phase 3, randomized, double-blind, placebo-controlled trial. J. Am. Acad. Dermatol. 2024, 90, 1190–1199. [Google Scholar] [CrossRef]
- Rossi, M.; Bettolini, L.; Artelli, G.L.; Fraghì, A.; Tomasi, C.; Calzavara-Pinton, P. Dupilumab treatment efficacy and impact on clinical scores, serum biomarkers, and itch in adult patients with atopic dermatitis: A retrospective analysis. J. Asthma Allergy. 2023, 16, 1233–1240. [Google Scholar] [CrossRef]
- Braddock, M.; Hanania, N.A.; Sharafkhaneh, A.; Colice, G.; Carlsson, M. Potential risks related to modulating interleukin-13 and Interleukin-4 signalling: A systematic review. Drug Saf. 2018, 41, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Bettolini, L.; Maione, V.; Arisi, M.; Rovaris, S.; Romanò, C.; Tomasi, C.; Calzavara-Pinton, P.; Zerbinati, N.; Bighetti, S. The influence of genital lichen sclerosus on sexual health and well-being: A tripartite comparative analysis. J. Low. Genit. Tract. Dis. 2025, 29, 81–87. [Google Scholar] [CrossRef]
- Maione, V.; Bettolini, L.; Cozzi, C.; Bighetti, S.; Tomasi, C.; Calzavara-Pinton, P. Sexual quality of life in patients with pemphigus: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 761–768. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Giacco, S.D.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines-recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Mollanazar, N.; Ständer, S.; Kwatra, S.G.; Kim, B.S.; Laws, E.; Mannent, L.P.; Amin, N.; Akinlade, B.; Staudinger, H.W.; et al. Dupilumab in patients with prurigo nodularis: Two randomized, double-blind, placebo-controlled phase 3 trials. Nat. Med. 2023, 29, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Murrell, D.F.; Joly, P.; Werth, V.P.; Ujiie, H.; Worm, M.; Mangold, A.R.; Avetisova, E.; Maloney, J.; Laws, E.; Mortensen, E.; et al. Study design of a phase 2/3 randomized controlled trial of dupilumab in adults with bullous pemphigoid: LIBERTY-BP ADEPT. Adv. Ther. 2024, 41, 2991–3002. [Google Scholar] [CrossRef]
- Mateos-Haro, M.; Novoa-Candia, M.; Vanegas, G.S.; Correa-Pérez, A.; Gil, A.G.; Fernández-García, S.; Ortega-Quijano, D.; Rodriguez, M.G.U.; Saceda-Corralo, D. Bennouna-Dalero, T.; et al. Treatments for alopecia areata: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 10, CD013719. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, Y.; Chang, J.; Sun, X.; Zhang, Y.; Zhang, M.; Maurer, M.; Li, Y.; Zhao, Z.; Tong, X. Dupilumab as a novel steroid-sparing treatment for hypereosinophilic syndrome. JAAD Case Rep. 2022, 29, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Bettolini, L.; Maione, V.; Bighetti, S.; Venturini, M.; Incardona, P.; Calzavara-Pinton, P.; Brunetti, A.P.; Stabile, G.; Rongioletti, F. Eosinophilic annular erythema: Clinicopathologic analysis and therapeutic outcomes from a multicenter cohort. Dermatol. Pract. Concept. 2025, 15, 5143. [Google Scholar] [CrossRef] [PubMed]
- Papageorgis, P.; Ozturk, S.; Lambert, A.W.; Neophytou, C.M.; Tzatsos, A.; Wong, C.K.; Thiagalingam, S.; Constantinou, A.I. Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis. Breast Cancer Res. 2015, 17, 98. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer. 2012, 131, 344–356. [Google Scholar] [CrossRef]
- Ingram, N.; Northwood, E.L.; Perry, S.L.; Marston, G.; Snowden, H.; Taylor, J.C.; Scott, N.; Bishop, D.T.; Coletta, P.L.; Hull, M.A. Reduced type II interleukin-4 receptor signalling drives initiation, but not progression, of colorectal carcinogenesis: Evidence from transgenic mouse models and human case-control epidemiological observations. Carcinogenesis 2013, 34, 2341–2349. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and their receptors in pancreatic cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef]
- Melo-Cardenas, J.; Bezavada, L.; Crawford, J.C.; Gurbuxani, S.; Cotton, A.; Kang, G.; Gossett, J.; Marinaccio, C.; Weinberg, R.; Hoffman, R.; et al. IL-13/IL-4 signaling contributes to fibrotic progression of the myeloproliferative neoplasms. Blood 2022, 140, 2805–2817. [Google Scholar] [CrossRef]
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, L.; Bu, D.; Liu, F. Tumors in the setting of dupilumab use: A review of the literature. World Allergy Organ. J. 2024, 18, 101006. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Inano, S.; Kitano, T. T-cell lymphoma associated with dupilumab. Ann. Hematol. 2023, 102, 1601–1602. [Google Scholar] [CrossRef]
- Owji, S.; Dubin, D.P.; Yassky, D.; Han, J.; Tan, K.; Jagannath, S.; Parekh, S.; Gulati, N. Dupilumab in multiple myeloma: A case series. Clin. Lymphoma Myeloma Leuk. 2022, 22, 928–932. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).