Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Blood Samples
2.2. Anisakis spp. Antigens and Determination of Specific Antibodies
2.3. Sample Classification
2.4. Climate Classification
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| % IgG Positive | % IgE Positive | |
|---|---|---|
| Gran Canaria (GC) | 11.7 | 13.8 |
| Tenerife (TF) | 12 | 4.1 |
| Fuerteventura (FV) | 18.7 | 1.9 |
| Lanzarote (LZ) | 11.8 | 6.5 |
| La Palma (LP) | 35.3 | 4.9 |
| La Gomera (G) | 6.3 | 3.1 |
| El Hierro (H) | 8.8 | 16.3 |
| Temperate, mild (Tm) | 28 | 7 |
| Temperate, cold (Tc) | 21.3 | 6.5 |
| Dry desert (Dd) | 15.8 | 4.8 |
| Dry steppe (Ds) | 10 | 3.3 |
References
- Morozińska-Gogol, J. Anisakis spp. as etiological agent of zoonotic disease and allergy in European region—An overview. Ann. Parasitol. 2019, 65, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Cattaneo, P.; Castelletti, M.; Bernardi, C. Prevalence and Mean Intensity of Anisakidae Parasite in Seafood Caught in the Mediterranean Sea Focusing on Fish Species at Risk of Being Raw-consumed. A Meta Analysis and Systematic Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Dinas, S.; Diakou, A.; Vasiliadis, K.; Chaintoutis, S.C.; Massa, E.; Konstantinou, G.N.; Totsi, A.; Xakis, A.; Papavasiliou, C. First Case of Human Anisakiosis in Greece: Acute Invasive Infection Mimicking Peritoneal Malignancy. Pathogens 2024, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Pravettoni, V.; Primavesi, L.; Piantanida, M. Anisakis simplex: Current knowledge. Eur. Ann. Allergy Clin. Immunol. 2012, 44, 150–156. [Google Scholar] [PubMed]
- Bao, M.; Pierce, G.J.; Strachan, N.J.C.; Pascual, S.; González-Muñoz, M.; Levsen, A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends Food Sci. Technol. 2019, 86, 298–310. [Google Scholar] [CrossRef]
- Herrador, Z.; Daschner, Á.; Perteguer, M.J.; Benito, A. Epidemiological Scenario of Anisakidosis in Spain Based on Associated Hospitalizations: The Tip of the Iceberg. Clin. Infect. Dis. 2019, 69, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish—From infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Wootten, R. Anisakis and anisakiasis. Adv. Parasitol. 1978, 16, 93–163. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Nascetti, G. Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845, An update. Parasite 2006, 13, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Nascetti, G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv. Parasitol. 2008, 66, 47–148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C. Aspects of substrate utilization and energy requirement during molluscan phagocytosis. J. Invertebr. Pathol. 1976, 27, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W. Anisakis simplex (Rudolphi, 1809, det. Krabbe, 1878) (Nematoda: Ascaridoidea): Morphology and morphometry of larvae from euphausiids and fish, and a review of the life-history and ecology. J. Helminthol. 1983, 57, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Petithory, J.C.; Marty, B. L’Anisakiase en France. Lettre l’Infectiol. 1988, 3, 96–99. [Google Scholar]
- Cong, W.; Elsheikha, H.M. Biology, Epidemiology, Clinical Features, Diagnosis, and Treatment of Selected Fish-borne Parasitic Zoonoses. Yale J. Biol. Med. 2021, 94, 297–309. [Google Scholar] [PubMed]
- Tejada Yabar, M.; López Ramón, J. Evaluación de la Presencia de Nematodos del Género Anisakis en Los Pescados de Acuicultura Marina Españoles; Informe Final; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain; European Commission: Brussels, Belgium, 2012. Available online: https://www.mapa.gob.es/dam/mapa/contenido/pesca/temas--nuevo/fondos-europeos/fondo-europeo-de-la-pesca/acciones-colectivas/apromar---informe-anisakis-2012.pdf (accessed on 25 May 2025).
- Fioravanti, M.L.; Gustinelli, A.; Rigos, G.; Buchmann, K.; Caffara, M.; Pascual, S.; Pardo, M.Á. Negligible risk of zoonotic anisakid nematodes in farmed fish from European mariculture, 2016 to 2018. Eurosurveillance 2021, 26, 1900717. [Google Scholar] [CrossRef] [PubMed]
- Martin-Carrillo, N.; García-Livia, K.; Baz-González, E.; Abreu-Acosta, N.; Dorta-Guerra, R.; Valladares, B.; Foronda, P. Morphological and Molecular Identification of Anisakis spp. (Nematoda: Anisakidae) in Commercial Fish from the Canary Islands Coast (Spain): Epidemiological Data. Animals 2022, 12, 2634. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.D.; Carretón, E.; Morchón, R.; Falcón-Cordón, Y.; Falcón-Cordón, S.; Simón, F.; Montoya-Alonso, J.A. The Canary Islands as a model of risk of pulmonary dirofilariasis in a hyperendemic area. Parasitol. Res. 2018, 117, 933–936. [Google Scholar] [CrossRef] [PubMed]
- ISTAC (Instituto Canario de Estadística). Población Según Sexos. Provincias por Comunidades Autónomas y Años; Instituto Canario de Estadística: Las Palmas de Gran Canaria, Spain, 2015. Available online: https://www3.gobiernodecanarias.org/istac/statistical-visualizer/visualizer/data.html?resourceType=dataset&agencyId=ISTAC&resourceId=E30245A_000001&version=~latest#visualization/table (accessed on 8 May 2022).
- García-Palacios, L.; González, M.L.; Esteban, M.I.; Mirabent, E.; Perteguer, M.J.; Cuéllar, C. Enzyme-linked immunosorbent assay, immunoblot analysis and RAST fluoroimmunoassay analysis of serum responses against crude larval antigens of Anisakis simplex in a Spanish random population. J. Helminthol. 1996, 70, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Daschner, A.; Cuéllar, C.; Sánchez-Pastor, S.; Pascual, C.Y.; Martín-Esteban, M. Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol. 2002, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Cuéllar, C. Immunoglobulins anti-Anisakis simplex in patients with gastrointestinal diseases. J. Helminthol. 2002, 76, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ponce, E.; Molina, J.M.; Hernández, S. Seroprevalence of goat toxoplasmosis on Grand Canary Island (Spain). Prev. Vet. Med. 1995, 24, 229–234. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Alimentación y Medio Ambiente. Atlas Climático de los Archipiélagos de Canarias, Madeira y Azores; AEMET Agencia Estatal de Meteorología: Madrid, Spain, 2012. [CrossRef]
- ISTAC (Instituto Canario de Estadística). Encuesta sobre el Gasto Turístico; Instituto Canario de Estadística: Las Palmas de Gran Canaria, Spain, 2023. Available online: https://investigacion.turismodeislascanarias.com/en/report/tourist-profile-canary-islands-2022 (accessed on 14 July 2023).
- Rodríguez-Ponce, E.; González, J.F.; Conde de Felipe, M.; Hernández, J.N.; Raduan Jaber, J. Epidemiological survey of zoonotic helminths in feral cats in Gran Canaria island (Macaronesian archipelago-Spain). Acta Parasitol. 2016, 61, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J.C. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef] [PubMed]
- Mladineo, I.; Poljak, V.; Martínez-Sernández, V.; Ubeira, F.M. Anti-Anisakis IgE seroprevalence in the healthy Croatian coastal population and associated risk factors. PLoS Negl. Trop. Dis. 2014, 8, e2673. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Madrid, E.; Cuesta, C.; Gimeno, C.; Baquedano-Rodríguez, M.; Soriano-Sánchez, I.; Bolívar, A.M.; Sáez-Durán, S.; Trelis, M.; Debenedetti, Á.L. Anisakid Nematodes and Potential Risk of Human Anisakiasis through the Consumption of Hake, Merluccius spp., Sold Fresh in Spanish Supermarkets. Pathogens 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Costales, E.; González-Quevedo, A.L.; Lorenzo-Bernardo, L.; de la Hoz-Martín, M.P.; Rodero, M.; Puente, P.; Moreno-Torres, I.; Cuéllar, C.; González-Fernández, J. Prevalence of Anisakiasis in Madrid (Spain) after 20 Years of Preventive Legislation. Pathogens 2024, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gómez, A.; Moreno-Ancillo, A.; López-Serrano, M.C.; Suarez-de-Parga, J.M.; Daschner, A.; Caballero, M.T.; Barranco, P.; Cabanas, R. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol. Res. 2004, 93, 378–384. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- Valiñas, B.; Lorenzo, S.; Eiras, A.; Figueiras, A.; Sanmartín, M.L.; Ubeira, F.M. Prevalence of and risk factors for IgE sensitization to Anisakis simplex in a Spanish population. Allergy 2001, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura Pesca y Alimentación. Informe del Consumo de Alimentación en ESPAÑA 2020. 2021. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/ultimos-datos/default.aspx (accessed on 22 April 2022).
- González, J.A. El Mercado Pesquero de Canarias. Guía del Consumidor de Pescado; INTERREG V-A MAC 2014-2020. MARISCOMAC (MAC/2.3d/097); Universidad de Las Palmas de Gran Canaria: Las Palmas de Gran Canaria, Spain, 2021; 180p, ISBN 978-84-09-35681-2. [Google Scholar]
- Castellanos Garzón, J.A.; Ubeira, F.; Pustovrh, M.; Salazar, L.; Daschner, Á.; Cuéllar, C. First Report of IgE Sensitization to Anisakis Simplex in a Healthy Population in Colombia. Res. Sq. 2021, 1–9. [Google Scholar] [CrossRef]
- Figueiredo Junior, I.; Vericimo, M.A.; Cardoso, L.R.; São Clemente, S.C.; do Nascimento, E.R.; Teixeira, G.A. Cross-sectional study of serum reactivity to Anisakis simplex in healthy adults in Niterói, Brazil. Acta Parasitol. 2013, 58, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, J.O.; Choi, S.H.; Cho, M.K.; Yu, H.S.; Cha, H.J.; Ock, M. Seroprevalence of antibodies against Anisakis simplex larvae among health-examined residents in three hospitals of southern parts of Korea. Korean J. Parasitol. 2011, 49, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mladineo, I.; Popović, M.; Drmić-Hofman, I.; Poljak, V. A case report of Anisakis pegreffii (Nematoda, Anisakidae) identified from archival paraffin sections of a Croatian patient. BMC Infect. Dis. 2016, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.; Florvaag, E.; Van Do, T.; Johansson, S.G.; Levsen, A.; Vaali, K. IgE sensitization to the fish parasite Anisakis simplex in a Norwegian population: A pilot study. Scand. J. Immunol. 2012, 75, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.; Nepstad, I.; Florvaag, E.; Egaas, E.; Van Do, T. An extended study of seroprevalence of anti-Anisakis simplex IgE antibodies in Norwegian blood donors. Scand. J. Immunol. 2014, 79, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Puente, P.; Anadón, A.M.; Rodero, M.; Romarís, F.; Ubeira, F.M.; Cuéllar, C. Anisakis simplex: The high prevalence in Madrid (Spain) and its relation with fish consumption. Exp. Parasitol. 2008, 118, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Del Rey Moreno, A.; Valero, A.; Mayorga, C.; Gómez, B.; Torres, M.; Hernández, J.; Ortiz, M.; Maldonado, J.L. Sensitization to Anisakis simplex s.l. in a healthy population. Acta Trop. 2006, 97, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Abattouy, N.; Valero, A.; Martín-Sánchez, J.; Peñalver, M.C.; Lozano, J. Sensitization to Anisakis simplex species in the population of northern Morocco. J. Investig. Allergol. Clin. Immunol. 2012, 22, 514–519. [Google Scholar] [PubMed]
- Rubio, C.; Gutiérrez, A.; Burgos, A.; Hardisson, A. Total dietary intake of mercury in the Canary Islands, Spain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Armani, A.; Nucera, D.; Costanzo, F.; Mattiucci, S.; Bruschi, F. Human anisakiasis in Italy: A retrospective epidemiological study over two decades. Parasite 2018, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Yera, H.; Fréalle, É.; Dutoit, E.; Dupouy-Camet, J. A national retrospective survey of anisakidosis in France (2010–2014): Decreasing incidence, female predominance, and emerging allergic potential. Parasite 2018, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol Res. 2020, 119, 3585–3594, Correction in Parasitol. Res. 2021, 120, 1925–1926. https://doi.org/10.1007/s00436-021-07096-w. [Google Scholar] [CrossRef] [PubMed]
- Anshary, H.; Sriwulan; Freeman, M.A.; Ogawa, K. Occurrence and molecular identification of Anisakis Dujardin, 1845 from marine fish in southern Makassar Strait, Indonesia. Korean J. Parasitol. 2014, 52, 9–19. [Google Scholar] [CrossRef] [PubMed]


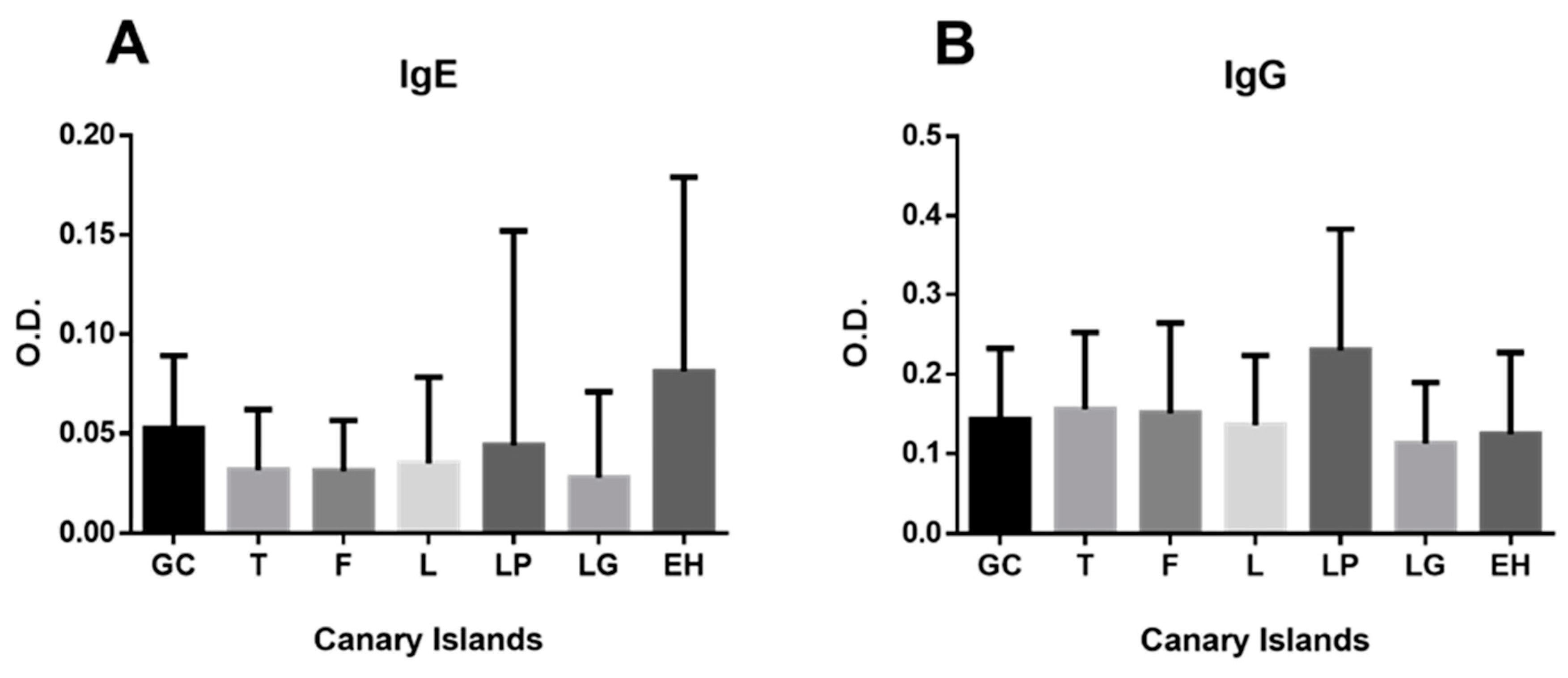
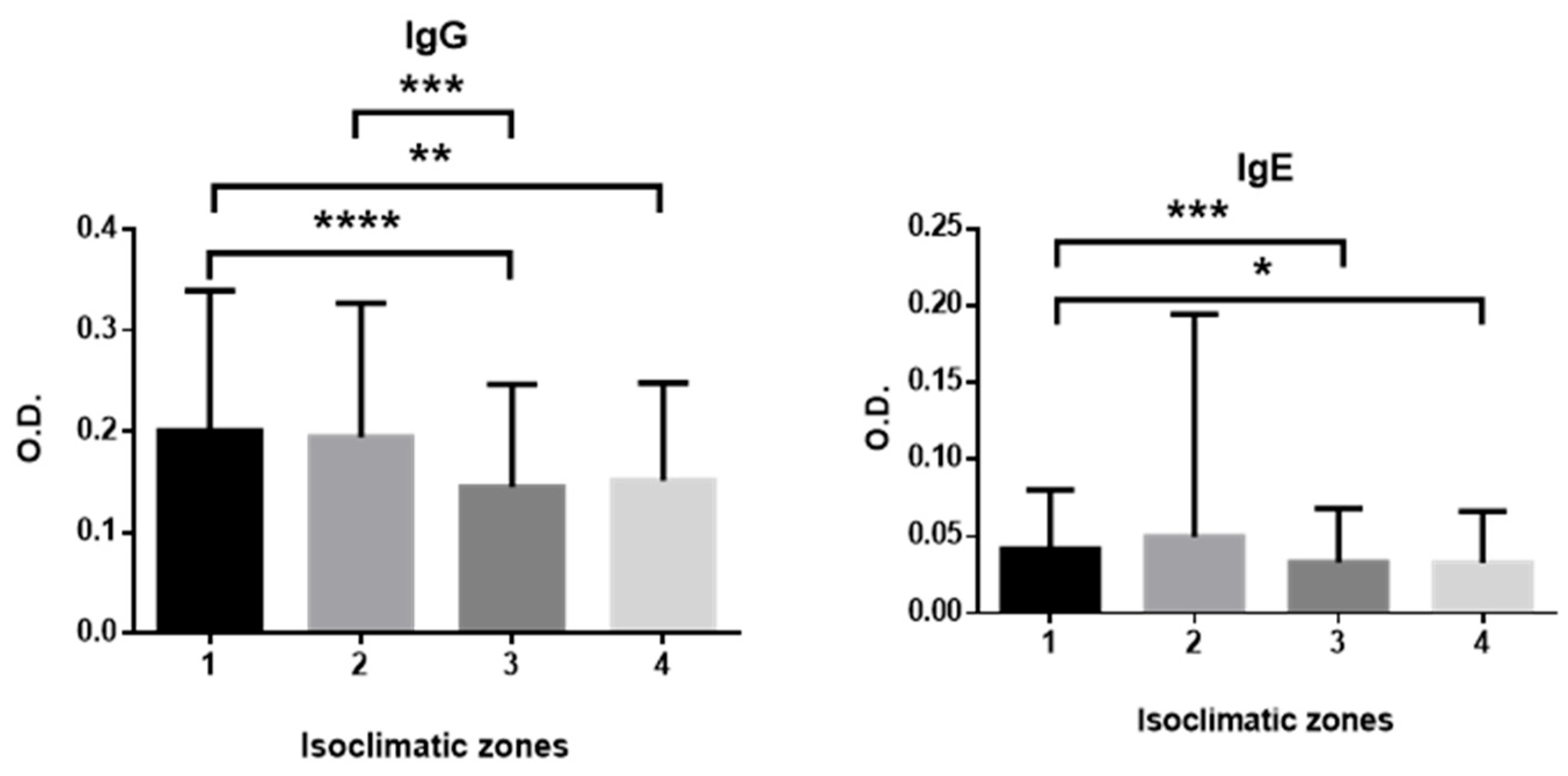
| Island | N (IgG) | N (IgE) | Mean Age ± SE (Years) | Min Age | Max Age |
|---|---|---|---|---|---|
| Gran Canaria (GC) | 94 | 94 | 40 ± 2 | 4 | 75 |
| Tenerife (TF) | 217 | 217 | 49 ± 1 | 2 | 90 |
| Fuerteventura (FV) | 214 | 214 | 42 ± 1 | 2 | 73 |
| Lanzarote (LZ) | 187 | 185 | 49 ± 1 | 13 | 81 |
| La Palma (LP) | 187 | 182 | 47 ± 1 | 8 | 80 |
| La Gomera (G) | 64 | 64 | 43 ± 2 | 10 | 83 |
| El Hierro (H) | 80 | 80 | 44 ± 2 | 2 | 82 |
| Total | 1043 | 1036 | 45.5 ± 0.5 | 2 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, E.; Rodero, M.; Montoya-Alonso, J.A.; Santana-Hernández, K.M.; Ventura, M.R.; Cuéllar, C.; Rodríguez-Ponce, E. Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands. Antibodies 2025, 14, 60. https://doi.org/10.3390/antib14030060
González-Rodríguez E, Rodero M, Montoya-Alonso JA, Santana-Hernández KM, Ventura MR, Cuéllar C, Rodríguez-Ponce E. Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands. Antibodies. 2025; 14(3):60. https://doi.org/10.3390/antib14030060
Chicago/Turabian StyleGonzález-Rodríguez, Eligia, Marta Rodero, J. Alberto Montoya-Alonso, Kevin M. Santana-Hernández, Myriam R. Ventura, Carmen Cuéllar, and Eligia Rodríguez-Ponce. 2025. "Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands" Antibodies 14, no. 3: 60. https://doi.org/10.3390/antib14030060
APA StyleGonzález-Rodríguez, E., Rodero, M., Montoya-Alonso, J. A., Santana-Hernández, K. M., Ventura, M. R., Cuéllar, C., & Rodríguez-Ponce, E. (2025). Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands. Antibodies, 14(3), 60. https://doi.org/10.3390/antib14030060









