Three-Dimensional Modeling of Camelus dromedarius T Cell Receptor Gamma (TRG)_Delta (TRD)/CD1D Complex Reveals Different Binding Interactions Depending on the TRD CDR3 Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystal Structure Sampling Through Fold Recognition, Multiple Sequence Alignments (MSA), and 3D Modeling of C. dromedarius TRG and TRD in Complex with C. dromedarius CD1D Antigen and B2M
2.2. FoldX Energy Calculations
3. Results
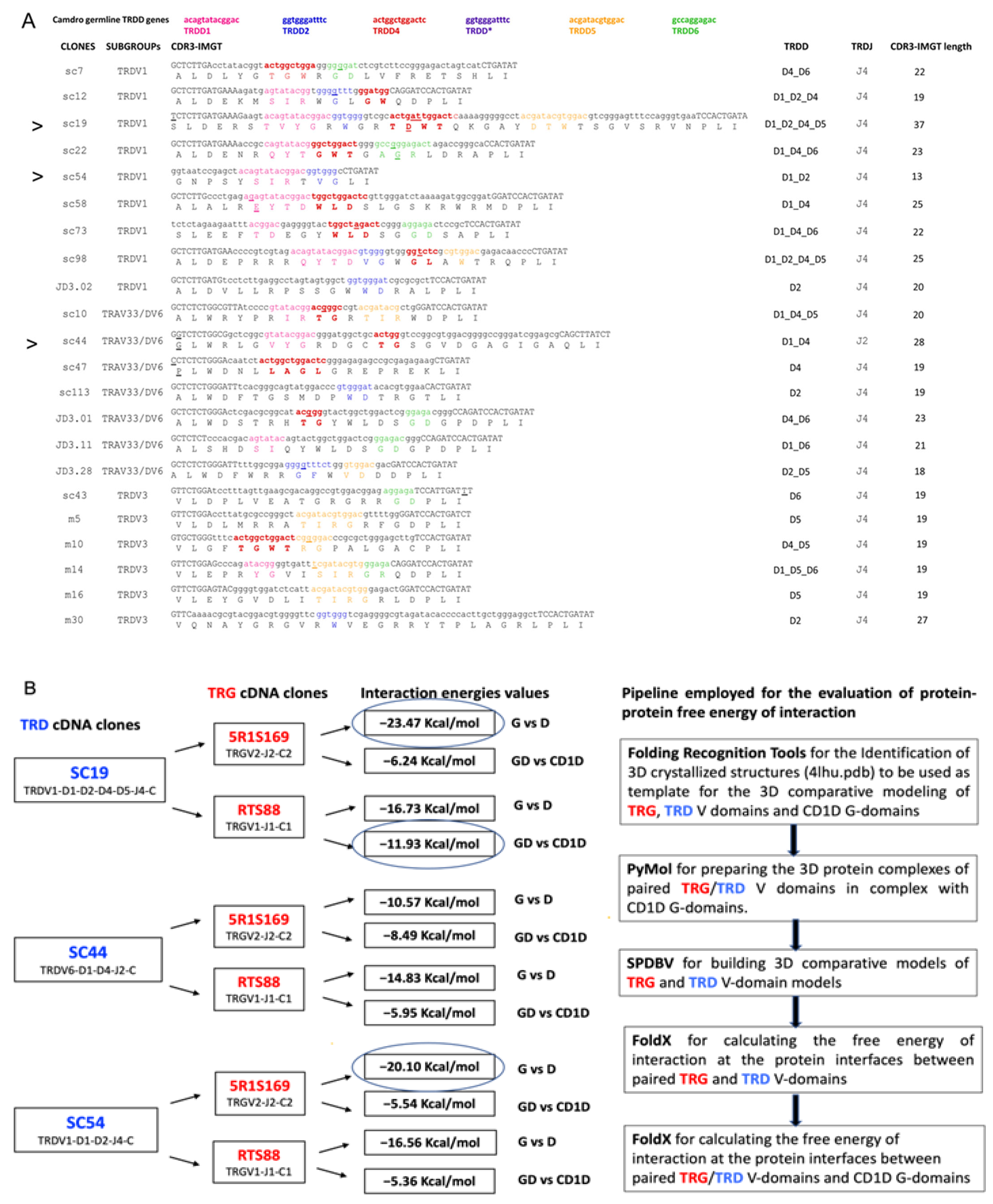
3.1. Identification and Selection of Dromedary TRD cDNA Clones Having a Long CDR3
3.2. Combination of C. dromedarius TRG and TRD cDNA Clones and 3D Modeling of the Paired V-Gamma and V-Delta Domains in Complex with the CD1D G-Domains and B2M
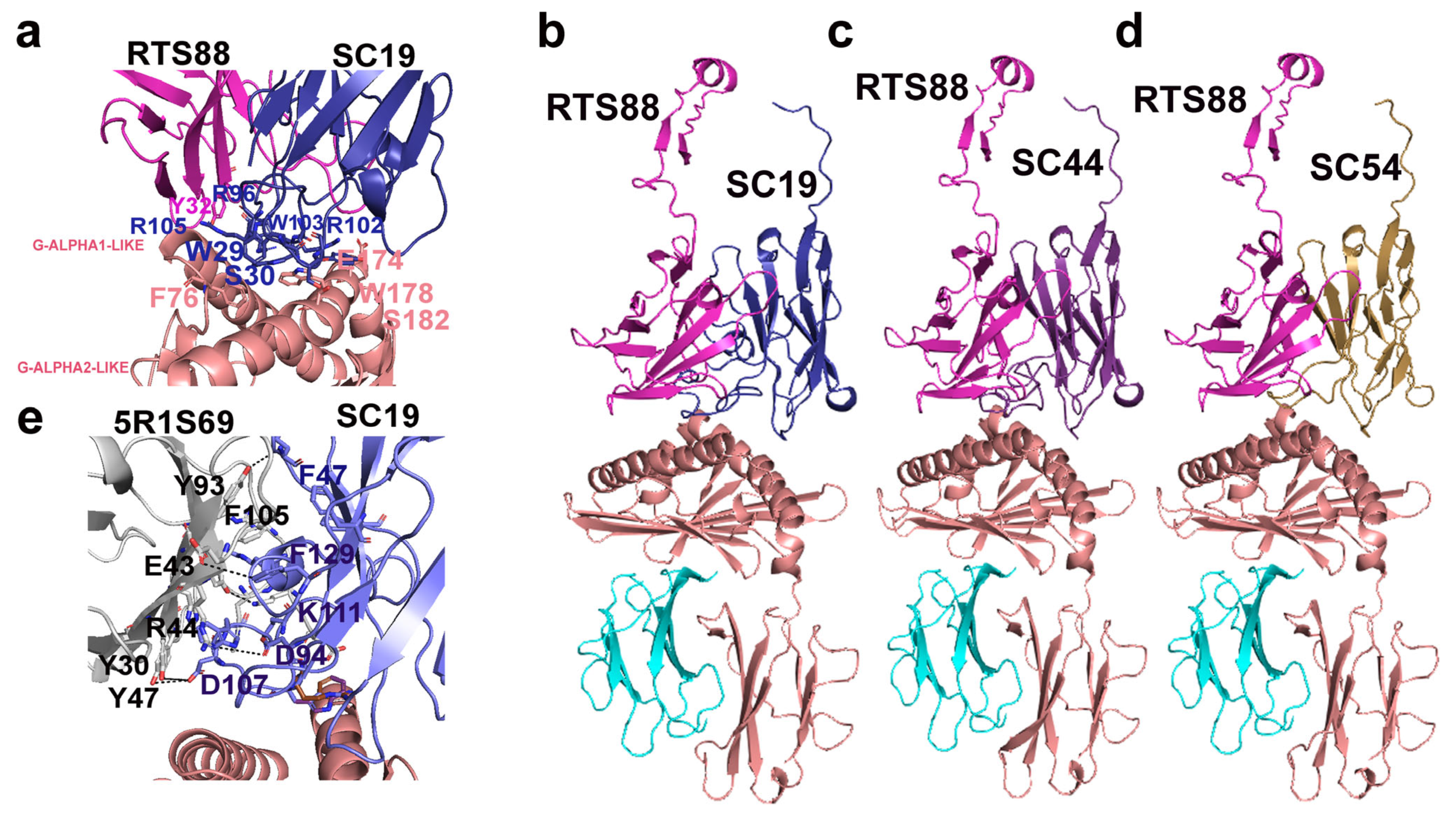
3.3. Involvement of the Long TRD CDR3 in the CD1D-Restriction of γδ T Cell in Dromedaries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefranc, M.-P. Immunoglobulin and T Cell Receptor Genes: IMGT® and the Birth and Rise of Immunoinformatics. Front. Immunol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Lefranc, G. The Immunoglobulin Factsbook; Academic Press: Cambridge, MA, USA, 2001; ISBN 012441351X. [Google Scholar]
- Lefranc, M.-P.; Lefranc, G. Immunoglobulins or Antibodies: IMGT® Bridging Genes, Structures and Functions. Biomedicines 2020, 8, 319. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Lefranc, G. The T Cell Receptor FactsBook; Academic Press: London, UK, 2001; ISBN 0124413528. [Google Scholar]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human Gamma Delta T Cells: Evolution and Ligand Recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef]
- Van Rhijn, I.; Le Nours, J. CD1 and MR1 Recognition by Human γδ T Cells. Mol. Immunol. 2021, 133, 95–100. [Google Scholar] [CrossRef]
- Lefranc, M.-P. Nomenclature of the Human Immunoglobulin Genes. Curr. Protoc. Immunol. 2000, 40, A.1P.1–A.1P.37. [Google Scholar] [CrossRef]
- Lefranc, M.-P. Nomenclature of the Human T Cell Receptor Genes. Curr. Protoc. Immunol. 2000, 40, A.1O.1–A.1O.23. [Google Scholar] [CrossRef]
- Lefranc, M.-P. WHO-IUIS Nomenclature Subcommittee for Immunoglobulins and T Cell Receptors Report. Immunogenetics 2007, 59, 899–902. [Google Scholar] [CrossRef]
- Lefranc, M.-P. WHO-IUIS Nomenclature Subcommittee for Immunoglobulins and T Cell Receptors Report August 2007, 13th International Congress of Immunology, Rio de Janeiro, Brazil. Dev. Comp. Immunol. 2008, 32, 461–463. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Bruford, E.; Campo, E.; Lefranc, M.-P. Immunogenetics in Hematopathology and Hematology: Why a Common Language Is Important. Leukemia 2024, 38, 1474–1476. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Pommié, C.; Ruiz, M.; Giudicelli, V.; Foulquier, E.; Truong, L.; Thouvenin-Contet, V.; Lefranc, G. IMGT Unique Numbering for Immunoglobulin and T Cell Receptor Variable Domains and Ig Superfamily V-like Domains. Dev. Comp. Immunol. 2003, 27, 55–77. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Pommié, C.; Kaas, Q.; Duprat, E.; Bosc, N.; Guiraudou, D.; Jean, C.; Ruiz, M.; Da Piédade, I.; Rouard, M.; et al. IMGT Unique Numbering for Immunoglobulin and T Cell Receptor Constant Domains and Ig Superfamily C-like Domains. Dev. Comp. Immunol. 2005, 29, 185–203. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Duprat, E.; Kaas, Q.; Tranne, M.; Thiriot, A.; Lefranc, G. IMGT Unique Numbering for MHC Groove G-DOMAIN and MHC Superfamily (MhcSF) G-LIKE-DOMAIN. Dev. Comp. Immunol. 2005, 29, 917–938. [Google Scholar] [CrossRef]
- Wu, D.; Yin, R.; Chen, G.; Ribeiro-Filho, H.V.; Cheung, M.; Robbins, P.F.; Mariuzza, R.A.; Pierce, B.G. Structural Characterization and AlphaFold Modeling of Human T Cell Receptor Recognition of NRAS Cancer Neoantigens. bioRxiv 2024. [Google Scholar] [CrossRef]
- Saotome, K.; Dudgeon, D.; Colotti, K.; Moore, M.J.; Jones, J.; Zhou, Y.; Rafique, A.; Yancopoulos, G.D.; Murphy, A.J.; Lin, J.C.; et al. Structural Analysis of Cancer-Relevant TCR-CD3 and Peptide-MHC Complexes by CryoEM. Nat. Commun. 2023, 14, 2401. [Google Scholar] [CrossRef]
- Dong, D.; Zheng, L.; Lin, J.; Zhang, B.; Zhu, Y.; Li, N.; Xie, S.; Wang, Y.; Gao, N.; Huang, Z. Structural Basis of Assembly of the Human T Cell Receptor–CD3 Complex. Nature 2019, 573, 546–552. [Google Scholar] [CrossRef]
- Rödström, K.E.J.; Regenthal, P.; Bahl, C.; Ford, A.; Baker, D.; Lindkvist-Petersson, K. Two Common Structural Motifs for TCR Recognition by Staphylococcal Enterotoxins. Sci. Rep. 2016, 6, 25796. [Google Scholar] [CrossRef]
- Chan, K.F.; Gully, B.S.; Gras, S.; Beringer, D.X.; Kjer-Nielsen, L.; Cebon, J.; McCluskey, J.; Chen, W.; Rossjohn, J. Divergent T-Cell Receptor Recognition Modes of a HLA-I Restricted Extended Tumour-Associated Peptide. Nat. Commun. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Le Nours, J.; Praveena, T.; Pellicci, D.G.; Gherardin, N.A.; Ross, F.J.; Lim, R.T.; Besra, G.S.; Keshipeddy, S.; Richardson, S.K.; Howell, A.R.; et al. Atypical Natural Killer T-Cell Receptor Recognition of CD1d-Lipid Antigens. Nat. Commun. 2016, 7, 10570. [Google Scholar] [CrossRef]
- Birkinshaw, R.W.; Pellicci, D.G.; Cheng, T.Y.; Keller, A.N.; Sandoval-Romero, M.; Gras, S.; De Jong, A.; Uldrich, A.P.; Moody, D.B.; Godfrey, D.I.; et al. αβ T Cell Antigen Receptor Recognition of CD1a Presenting Self Lipid Ligands. Nat. Immunol. 2015, 16, 258–266. [Google Scholar] [CrossRef]
- Rödström, K.E.J.; Regenthal, P.; Lindkvist-Petersson, K. Structure of Staphylococcal Enterotoxin E in Complex with TCR Defines the Role of TCR Loop Positioning in Superantigen Recognition. PLoS ONE 2015, 10, e0131988. [Google Scholar] [CrossRef]
- Roy, S.; Ly, D.; Li, N.S.; Altman, J.D.; Piccirilli, J.A.; Moody, D.B.; Adams, E.J. Molecular Basis of Mycobacterial Lipid Antigen Presentation by CD1c and Its Recognition by αβ T Cells. Proc. Natl. Acad. Sci. USA 2014, 111, E4648–E4657. [Google Scholar] [CrossRef]
- Van Rhijn, I.; Kasmar, A.; De Jong, A.; Gras, S.; Bhati, M.; Doorenspleet, M.E.; De Vries, N.; Godfrey, D.I.; Altman, J.D.; De Jager, W.; et al. A Conserved Human T Cell Population Targets Mycobacterial Antigens Presented by CD1b. Nat. Immunol. 2013, 14, 706–713. [Google Scholar] [CrossRef]
- Broughton, S.E.; Petersen, J.; Theodossis, A.; Scally, S.W.; Loh, K.L.; Thompson, A.; van Bergen, J.; Kooy-Winkelaar, Y.; Henderson, K.N.; Beddoe, T.; et al. Biased T Cell Receptor Usage Directed against Human Leukocyte Antigen DQ8-Restricted Gliadin Peptides Is Associated with Celiac Disease. Immunity 2012, 37, 611–621. [Google Scholar] [CrossRef]
- Deng, L.; Langley, R.J.; Brown, P.H.; Xu, G.; Teng, L.; Wang, Q.; Gonzales, M.I.; Callender, G.G.; Nishimura, M.I.; Topalian, S.L.; et al. Structural Basis for the Recognition of Mutant Self by a Tumor-Specific, MHC Class II-Restricted T Cell Receptor. Nat. Immunol. 2007, 8, 398–408. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Borg, N.A.; Pellicci, D.G.; Beddoe, T.; Kostenko, L.; Clements, C.S.; Williamson, N.A.; Smyth, M.J.; Besra, G.S.; Reid, H.H.; et al. A Structural Basis for Selection and Cross-Species Reactivity of the Semi-Invariant NKT Cell Receptor in CD1d/Glycolipid Recognition. J. Exp. Med. 2006, 203, 661–673. [Google Scholar] [CrossRef]
- Gadola, S.D.; Koch, M.; Marles-Wright, J.; Lissin, N.M.; Shepherd, D.; Matulis, G.; Harlos, K.; Villiger, P.M.; Stuart, D.I.; Jakobsen, B.K.; et al. Structure and Binding Kinetics of Three Different Human CD1d-α-Galactosylceramide-Specific T Cell Receptors. J. Exp. Med. 2006, 203, 699–710. [Google Scholar] [CrossRef]
- Chen, J.L.; Stewart-Jones, G.; Bossi, G.; Lissin, N.M.; Wooldridge, L.; Choi, E.M.L.; Held, G.; Dunbar, P.R.; Esnouf, R.M.; Sami, M.; et al. Structural and Kinetic Basis for Heightened Immunogenicity of T Cell Vaccines. J. Exp. Med. 2005, 201, 1243–1255. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Clements, C.S.; Brooks, A.G.; Purcell, A.W.; McCluskey, J.; Rossjohn, J. The 1.5 Å Crystal Structure of a Highly Selected Antiviral T Cell Receptor Provides Evidence for a Structural Basis of Immunodominance. Structure 2002, 10, 1521–1532. [Google Scholar] [CrossRef]
- Hoque, M.; Grigg, J.B.; Ramlall, T.; Jones, J.; McGoldrick, L.L.; Lin, J.C.; Olson, W.C.; Smith, E.; Franklin, M.C.; Zhang, T.; et al. Structural Characterization of Two γδ TCR/CD3 Complexes. Nat. Commun. 2025, 16, 318. [Google Scholar] [CrossRef]
- Wegrecki, M.; Ocampo, T.A.; Gunasinghe, S.D.; von Borstel, A.; Tin, S.Y.; Reijneveld, J.F.; Cao, T.P.; Gully, B.S.; Le Nours, J.; Moody, D.B.; et al. Atypical Sideways Recognition of CD1a by Autoreactive γδ T Cell Receptors. Nat. Commun. 2022, 13, 3872. [Google Scholar] [CrossRef]
- Allison, T.J.; Winter, C.C.; Fournié, J.J.; Bonneville, M.; Garboczi, D.N. Structure of a Human γδ T-Cell Antigen Receptor. Nature 2001, 411, 820–824. [Google Scholar] [CrossRef]
- Xin, W.; Huang, B.; Chi, X.; Liu, Y.; Xu, M.; Zhang, Y.; Li, X.; Su, Q.; Zhou, Q. Structures of Human γδ T Cell Receptor–CD3 Complex. Nature 2024, 630, 222–229. [Google Scholar] [CrossRef]
- Kaas, Q.; Ruiz, M.; Lefranc, M.-P. IMGT/3Dstructure-DB and IMGT/StructuralQuery, a Database and a Tool for Immunoglobulin, T Cell Receptor and MHC Structural Data. Nucleic Acids Res. 2004, 32, D208–D210. [Google Scholar] [CrossRef]
- Ehrenmann, F.; Lefranc, M.-P. Imgt/3Dstructure-DB: Querying the IMGT Database for 3D Structures in Immunology and Immunoinformatics (IG or Antibodies, TR, MH, RPI, and FPIA). Cold Spring Harb. Protoc. 2011, 2011, 750–761. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Lefranc, G. IMGT/3Dstructure-DB: T-Cell Receptor TR Paratope and Peptide/Major Histocompatibility pMH Contact Sites and Epitope. Methods Mol. Biol. 2022, 2453, 533–570. [Google Scholar] [CrossRef]
- Ehrenmann, F.; Kaas, Q.; Lefranc, M.-P. IMGT/3Dstructure-DB and IMGT/DomainGapAlign: A Database and a Tool for Immunoglobulins or Antibodies, T Cell Receptors, MHC, IgSF and MHcSF. Nucleic Acids Res. 2009, 38, D301–D307. [Google Scholar] [CrossRef]
- Ehrenmann, F.; Lefranc, M.-P. IMGT/DomainGapAlign: IMGT Standardized Analysis of Amino Acid Sequences of Variable, Constant, and Groove Domains (IG, TR, MH, IgSF, MhSF). Cold Spring Harb. Protoc. 2011, 2011, 737–749. [Google Scholar] [CrossRef]
- Pommié, C.; Levadoux, S.; Sabatier, R.; Lefranc, G.; Lefranc, M.-P. IMGT Standardized Criteria for Statistical Analysis of Immunoglobulin V-Region Amino Acid Properties. J. Mol. Recognit. 2004, 17, 17–32. [Google Scholar] [CrossRef]
- Ehrenmann, F.; Giudicelli, V.; Duroux, P.; Lefranc, M.-P. IMGT/Collier de Perles: IMGT Standardized Representation of Domains (IG, TR, and IgSF Variable and Constant Domains, MH and MhSF Groove Domains). Cold Spring Harb. Protoc. 2011, 2011, 726–736. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Lefranc, G. Using IMGT Unique Numbering for IG Allotypes and Fc-Engineered Variants of Effector Properties and Half-Life of Therapeutic Antibodies. Immunol. Rev. 2024, 328, 473–506. [Google Scholar] [CrossRef]
- Antonacci, R.; Massari, S.; Linguiti, G.; Jambrenghi, A.C.; Giannico, F.; Lefranc, M.-P.; Ciccarese, S. Evolution of the T-Cell Receptor (TR) Loci in the Adaptive Immune Response: The Tale of the TRG Locus in Mammals. Genes 2020, 11, 624. [Google Scholar] [CrossRef]
- Antonacci, R.; Mineccia, M.; Lefranc, M.-P.; Ashmaoui, H.M.E.; Lanave, C.; Piccinni, B.; Pesole, G.; Hassanane, M.S.; Massari, S.; Ciccarese, S. Expression and Genomic Analyses of Camelus dromedarius T Cell Receptor Delta (TRD) Genes Reveal a Variable Domain Repertoire Enlargement Due to CDR3 Diversification and Somatic Mutation. Mol. Immunol. 2011, 48, 1384–1396. [Google Scholar] [CrossRef]
- Vaccarelli, G.; Antonacci, R.; Tasco, G.; Yang, F.; Giordano, L.; El Ashmaoui, H.M.; Hassanane, M.S.; Massari, S.; Casadio, R.; Ciccarese, S. Generation of Diversity by Somatic Mutation in the Camelus dromedarius T-Cell Receptor Gamma Variable Domains. Eur. J. Immunol. 2012, 42, 3416–3428. [Google Scholar] [CrossRef]
- Ciccarese, S.; Vaccarelli, G.; Lefranc, M.-P.; Tasco, G.; Consiglio, A.; Casadio, R.; Linguiti, G.; Antonacci, R. Characteristics of the Somatic Hypermutation in the Camelus dromedarius T Cell Receptor Gamma (TRG) and Delta (TRD) Variable Domains. Dev. Comp. Immunol. 2014, 46, 300–313. [Google Scholar] [CrossRef]
- Ciccarese, S.; Burger, P.A.; Ciani, E.; Castelli, V.; Linguiti, G.; Plasil, M.; Massari, S.; Horin, P.; Antonacci, R. The Camel Adaptive Immune Receptors Repertoire as a Singular Example of Structural and Functional Genomics. Front. Genet. 2019, 10, 997. [Google Scholar] [CrossRef]
- Massari, S.; Linguiti, G.; Giannico, F.; D’addabbo, P.; Ciccarese, S.; Antonacci, R. The Genomic Organisation of the Tra/Trd Locus Validates the Peculiar Characteristics of Dromedary δ-Chain Expression. Genes 2021, 12, 544. [Google Scholar] [CrossRef]
- Hussein, B.A.; Hallner, A.; Wennström, L.; Brune, M.; Martner, A.; Hellstrand, K.; Bernson, E.; Thorén, F.B. Impact of NK Cell Activating Receptor Gene Variants on Receptor Expression and Outcome of Immunotherapy in Acute Myeloid Leukemia. Front. Immunol. 2021, 12, 796072. [Google Scholar] [CrossRef]
- Linguiti, G.; Tragni, V.; Pierri, C.L.; Massari, S.; Lefranc, M.-P.; Antonacci, R.; Ciccarese, S. 3D Structures Inferred from CDNA Clones Identify the CD1D-Restricted γδ T Cell Receptor in Dromedaries. Front. Immunol. 2022, 13, 928860. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; Mcpherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-Lipid Antigen Recognition by the γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef]
- Lobley, A.; Sadowski, M.I.; Jones, D.T. PGenTHREADER and PDomTHREADER: New Methods for Improved Protein Fold Recognition and Superfamily Discrimination. Bioinformatics 2009, 25, 1761–1767. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER Server for Protein 3D Structure Prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef]
- Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. J. Clin. Med. 2019, 8, 2117. [Google Scholar] [CrossRef]
- Pierri, C.L.; Parisi, G.; Porcelli, V. Computational Approaches for Protein Function Prediction: A Combined Strategy from Multiple Sequence Alignment to Molecular Docking-Based Virtual Screening. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2010, 1804, 1695–1712. [Google Scholar] [CrossRef]
- Mercurio, I.; Tragni, V.; Busto, F.; De Grassi, A.; Pierri, C.L. Protein Structure Analysis of the Interactions between SARS-CoV-2 Spike Protein and the Human ACE2 Receptor: From Conformational Changes to Novel Neutralizing Antibodies. Cell. Mol. Life Sci. 2020, 78, 1501–1522. [Google Scholar] [CrossRef]
- Pierri, C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular Modeling of Antibodies for the Treatment of TNFα-Related Immunological Diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving Physical Realism, Stereochemistry, and Side-Chain Accuracy in Homology Modeling: Four Approaches That Performed Well in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 114–122. [Google Scholar] [CrossRef]
- Patskovsky, Y.; Natarajan, A.; Patskovska, L.; Nyovanie, S.; Joshi, B.; Morin, B.; Brittsan, C.; Huber, O.; Gordon, S.; Michelet, X.; et al. Author Correction: Molecular Mechanism of Phosphopeptide Neoantigen Immunogenicity. Nat. Commun. 2023, 14, 4522. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, J.; Guan, H.; Chen, T.-T.; Teng, Y.; Wei, Z.; Li, Z.; Ouyang, S.; Chen, X. Structural and Functional Characterization of a Fish Type I Subgroup d IFN Reveals Its Binding to Receptors. J. Immunol. 2024, 212, 1207–1220. [Google Scholar] [CrossRef]
- Slater, B.T.; Han, X.; Chen, L.; Xiong, Y. Structural Insight into T Cell Coinhibition by PD-1H (VISTA). Proc. Natl. Acad. Sci. USA 2020, 117, 1648–1657. [Google Scholar] [CrossRef]
- Bovay, A.; Zoete, V.; Rizkallah, P.J.; Beck, K.; Delbreil, P.; Speiser, D.E.; Cole, D.K.; Fuertes Marraco, S.A. Identification of a Superagonist Variant of the Immunodominant Yellow Fever Virus Epitope NS4b 214-222 by Combinatorial Peptide Library Screening. Mol. Immunol. 2020, 125, 43–50. [Google Scholar] [CrossRef]
- Sibener, L.V.; Fernandes, R.A.; Kolawole, E.M.; Carbone, C.B.; Liu, F.; McAffee, D.; Birnbaum, M.E.; Yang, X.; Su, L.F.; Yu, W.; et al. Isolation of a Structural Mechanism for Uncoupling T Cell Receptor Signaling from Peptide-MHC Binding. Cell 2018, 174, 672–687.e27. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Alignment of Alzheimer’s Disease Amyloid-β Peptide and Herpes Simplex Virus-1 PUL15 C-Terminal Nuclease Domain. J. Alzheimer’s Dis. Rep. 2020, 4, 373–377. [Google Scholar] [CrossRef]
- Tragni, V.; Mercurio, I.; Paoletti, D.P.; Onofrio, A.; Laera, L.; Cafferati Beltrame, L.; Sgobba, M.N.; Guerra, L.; Volpicella, M.; De Grassi, A.; et al. Deconstructing SARS-CoV-2 Neutralization: A Modular Molecular Framework for Computational Design and Comparison of Antibodies and Nanobodies Targeting the Spike RBD. J. Med. Virol. 2023, 95, e28875. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Gonzalez, J.; Arboleda-Bustos, C.E.; Mendoza, N.; Martinez, C.; Pinzon, A. Computational Modeling of the Effect of Five Mutations on the Structure of the ACE2 Receptor and Their Correlation with Infectivity and Virulence of Some Emerged Variants of SARS-CoV-2 Suggests Mechanisms of Binding Affinity Dysregulation. Chem. Biol. Interact. 2022, 368, 110244. [Google Scholar] [CrossRef]
- Goswami, A.; Kumar, M.; Ullah, S.; Gore, M.M. De Novo Design of Anti-Variant COVID-19 Vaccine. Biol. Methods Protoc. 2023, 8, bpad021. [Google Scholar] [CrossRef]
- Sung, K.H.; Josewski, J.; Dübel, S.; Blankenfeldt, W.; Rau, U. Structural Insights into Antigen Recognition of an Anti-β-(1,6)-β-(1,3)-D-Glucan Antibody. Sci. Rep. 2018, 8, 13652. [Google Scholar] [CrossRef]
- Ilmiawan, L.; Tjandrawinata, R.R.; Prasasty, V.D. In Silico Study Binding Affinity of Regdanvimab-RBD Spike Protein SARS CoV-2 Omicron Variant Indonesia. J. Appl. Pharm. Sci. 2024, 14, 261–272. [Google Scholar] [CrossRef]
- Simister, P.C.; Border, E.C.; Vieira, J.F.; Pumphrey, N.J. Structural Insights into Engineering a T-Cell Receptor Targeting MAGE-A10 with Higher Affinity and Specificity for Cancer Immunotherapy. J. Immunother. Cancer 2022, 10, e004600. [Google Scholar] [CrossRef]
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A Graphical Interface for the FoldX Forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX Web Server: An Online Force Field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef]
- Lanzarotti, E.; Marcatili, P.; Nielsen, M. Identification of the Cognate Peptide-MHC Target of T Cell Receptors Using Molecular Modeling and Force Field Scoring. Mol. Immunol. 2018, 94, 91–97. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Kula, T.; Elledge, S.J. T-Switch: A Specificity-Based Engineering Platform for Developing Safe and Effective T Cell Therapeutics. Immunity 2024, 57, 2945–2958.e5. [Google Scholar] [CrossRef]
- Schaap-Johansen, A.L.; Vujović, M.; Borch, A.; Hadrup, S.R.; Marcatili, P. T Cell Epitope Prediction and Its Application to Immunotherapy. Front. Immunol. 2021, 12, 712488. [Google Scholar] [CrossRef]
- van der Kant, R.; Karow-Zwick, A.R.; Van Durme, J.; Blech, M.; Gallardo, R.; Seeliger, D.; Aßfalg, K.; Baatsen, P.; Compernolle, G.; Gils, A.; et al. Prediction and Reduction of the Aggregation of Monoclonal Antibodies. J. Mol. Biol. 2017, 429, 1244–1261. [Google Scholar] [CrossRef]
- Buß, O.; Rudat, J.; Ochsenreither, K. FoldX as Protein Engineering Tool: Better Than Random Based Approaches? Comput. Struct. Biotechnol. J. 2018, 16, 25–33. [Google Scholar] [CrossRef]
- Rosace, A.; Bennett, A.; Oeller, M.; Mortensen, M.M.; Sakhnini, L.; Lorenzen, N.; Poulsen, C.; Sormanni, P. Automated Optimisation of Solubility and Conformational Stability of Antibodies and Proteins. Nat. Commun. 2023, 14, 1937. [Google Scholar] [CrossRef]
- Sanchez, R.; Sali, A.; Sánchez, R.; Sali, A. Comparative Protein Structure Modeling. Introduction and Practical Examples with Modeller. Methods Mol. Biol. 2000, 143, 97–129. [Google Scholar]
- Martí-Renom, M.A.; Stuart, A.C.; Fiser, A.; Sánchez, R.; Melo, F.; Šali, A. Comparative Protein Structure Modeling of Genes and Genomes. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 291–325. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Linguiti, G.; Kossida, S.; Pierri, C.L.; Jabado-Michaloud, J.; Folch, G.; Massari, S.; Lefranc, M.-P.; Ciccarese, S.; Antonacci, R. The t Cell Receptor (Trb) Locus in Tursiops Truncatus: From Sequence to Structure of the Alpha/Beta Heterodimer in the Human/Dolphin Comparison. Genes 2021, 12, 571. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. Protein Structure and Function Prediction Using I-TASSER. Curr. Protoc. Bioinform. 2015, 52, 5.8.1–5.8.15. [Google Scholar] [CrossRef]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo—Distance Constraints Applied on Model Quality Estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef]
- Kryshtafovych, A.; Schwede, T.; Topf, M.; Fidelis, K.; Moult, J. Critical Assessment of Methods of Protein Structure Prediction (CASP)—Round XV. Proteins Struct. Funct. Bioinform. 2023, 91, 1539–1549. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Croll, T.I.; Sammito, M.D.; Kryshtafovych, A.; Read, R.J. Evaluation of Template-Based Modeling in CASP13. Proteins Struct. Funct. Bioinform. 2019, 87, 1113–1127. [Google Scholar] [CrossRef]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of Protein Models with Three-Dimensional Profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Praveena, T.; Govindarajan, S.; Almeida, C.F.; Pellicci, D.G.; Arkins, W.C.; Van Rhijn, I.; Venken, K.; Elewaut, D.; Godfrey, D.I.; et al. Lipidomic Scanning of Self-Lipids Identifies Headless Antigens for Natural Killer T Cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2321686121. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tsujimura, T.; Drexler, H.G. Proposal for the Designation of the Natural Killer Antigens-Positive γδ T-Cell Subset as γδ NKT-Cells: Nomenclature Based on Immunoprofile. Hum. Cell 2021, 34, 1278–1279. [Google Scholar] [CrossRef]
- Mallevaey, T.; Clarke, A.J.; Scott-Browne, J.P.; Young, M.H.; Roisman, L.C.; Pellicci, D.G.; Patel, O.; Vivian, J.P.; Matsuda, J.L.; McCluskey, J.; et al. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity 2011, 34, 315–326. [Google Scholar] [CrossRef]
- Macho-Fernandez, E.; Brigl, M. The Extended Family of CD1d-Restricted NKT Cells: Sifting through a Mixed Bag of TCRs, Antigens, and Functions. Front. Immunol. 2015, 6, 362. [Google Scholar] [CrossRef]
- Wun, K.S.; Ross, F.; Patel, O.; Besra, G.S.; Porcelli, S.A.; Richardson, S.K.; Keshipeddy, S.; Howell, A.R.; Godfrey, D.I.; Rossjohn, J. Human and Mouse Type I Natural Killer T Cell Antigen Receptors Exhibit Different Fine Specificities for CD1d-Antigen Complex. J. Biol. Chem. 2012, 287, 39139–39148. [Google Scholar] [CrossRef]
- Pellicci, D.G.; Uldrich, A.P.; Le Nours, J.; Ross, F.; Chabrol, E.; Eckle, S.B.G.; de Boer, R.; Lim, R.T.; McPherson, K.; Besra, G.; et al. The Molecular Bases of δ/αβ T Cell-Mediated Antigen Recognition. J. Exp. Med. 2014, 211, 2599–2615. [Google Scholar] [CrossRef]
- Marrack, P.; Scott-Browne, J.P.; Dai, S.; Gapin, L.; Kappler, J.W. Evolutionarily Conserved Amino Acids That Control TCR-MHC Interaction. Annu. Rev. Immunol. 2008, 26, 171–203. [Google Scholar] [CrossRef]
- Ekeruche-Makinde, J.; Miles, J.J.; Van Den Berg, H.A.; Skowera, A.; Cole, D.K.; Dolton, G.; Schauenburg, A.J.A.; Tan, M.P.; Pentier, J.M.; Llewellyn-Lacey, S.; et al. Peptide Length Determines the Outcome of TCR/Peptide-MHCI Engagement. Blood 2013, 121, 1112–1123. [Google Scholar] [CrossRef]
- Cao, T.P.; Shahine, A.; Cox, L.R.; Besra, G.S.; Moody, D.B.; Rossjohn, J. A Structural Perspective of How T Cell Receptors Recognize the CD1 Family of Lipid Antigen–Presenting Molecules. J. Biol. Chem. 2024, 300, 107511. [Google Scholar] [CrossRef]
- Ling Sok, C.; Rossjohn, J.; Gully, B.S. The Evolving Portrait of Gd TCR Recognition Determinants. J. Immunol. 2024, 213, 543–552. [Google Scholar]
- Taylor, W.R. Exploring Protein Fold Space. Biomolecules 2020, 10, 193. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Selvaraj, S. Inter-Residue Interactions in Protein Folding and Stability. Prog. Biophys. Mol. Biol. 2004, 86, 235–277. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-Restricted Antigens by Natural Killer T Cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef]
- Modi, T.; Campitelli, P.; Kazan, I.C.; Ozkan, S.B. Protein Folding Stability and Binding Interactions through the Lens of Evolution: A Dynamical Perspective. Curr. Opin. Struct. Biol. 2021, 66, 207–215. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of Protein Fold Stability on Immunogenicity and Its Implications for Vaccine Design. Expert. Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Deseke, M.; Prinz, I. Ligand Recognition by the γδ TCR and Discrimination between Homeostasis and Stress Conditions. Cell. Mol. Immunol. 2020, 17, 914–924. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.; Veerapen, N.; Besra, G.; Cresswell, P. Calreticulin Controls the Rate of Assembly of CD1d Molecules in the Endoplasmic Reticulum. J. Biol. Chem. 2010, 285, 38283–38292. [Google Scholar] [CrossRef]
- Girardi, E.; Zajonc, D.M. Molecular Basis of Lipid Antigen Presentation by CD1d and Recognition by Natural Killer T Cells. Immunol. Rev. 2012, 250, 167–179. [Google Scholar] [CrossRef]
- DeWitt, W.S.; Yu, K.K.Q.; Wilburn, D.B.; Sherwood, A.; Vignali, M.; Day, C.L.; Scriba, T.J.; Robins, H.S.; Swanson, W.J.; Emerson, R.O.; et al. A Diverse Lipid Antigen–Specific TCR Repertoire Is Clonally Expanded during Active Tuberculosis. J. Immunol. 2018, 201, 888–896. [Google Scholar] [CrossRef]
- De Libero, G.; Mori, L. How T Lymphocytes Recognize Lipid Antigens. FEBS Lett. 2006, 580, 5580–5587. [Google Scholar] [CrossRef]
- Pierce, B.G.; Vreven, T.; Weng, Z. Modeling T Cell Receptor Recognition of CD1-Lipid and MR1-Metabolite Complexes. BMC Bioinform. 2014, 15, 319. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 5081634. [Google Scholar] [CrossRef]
- Xu, B.; Pizarro, J.C.; Holmes, M.A.; McBeth, C.; Groh, V.; Spies, T.; Strong, R.K. Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl. Acad. Sci. 2011, 108, 2414–2419. [Google Scholar] [CrossRef]
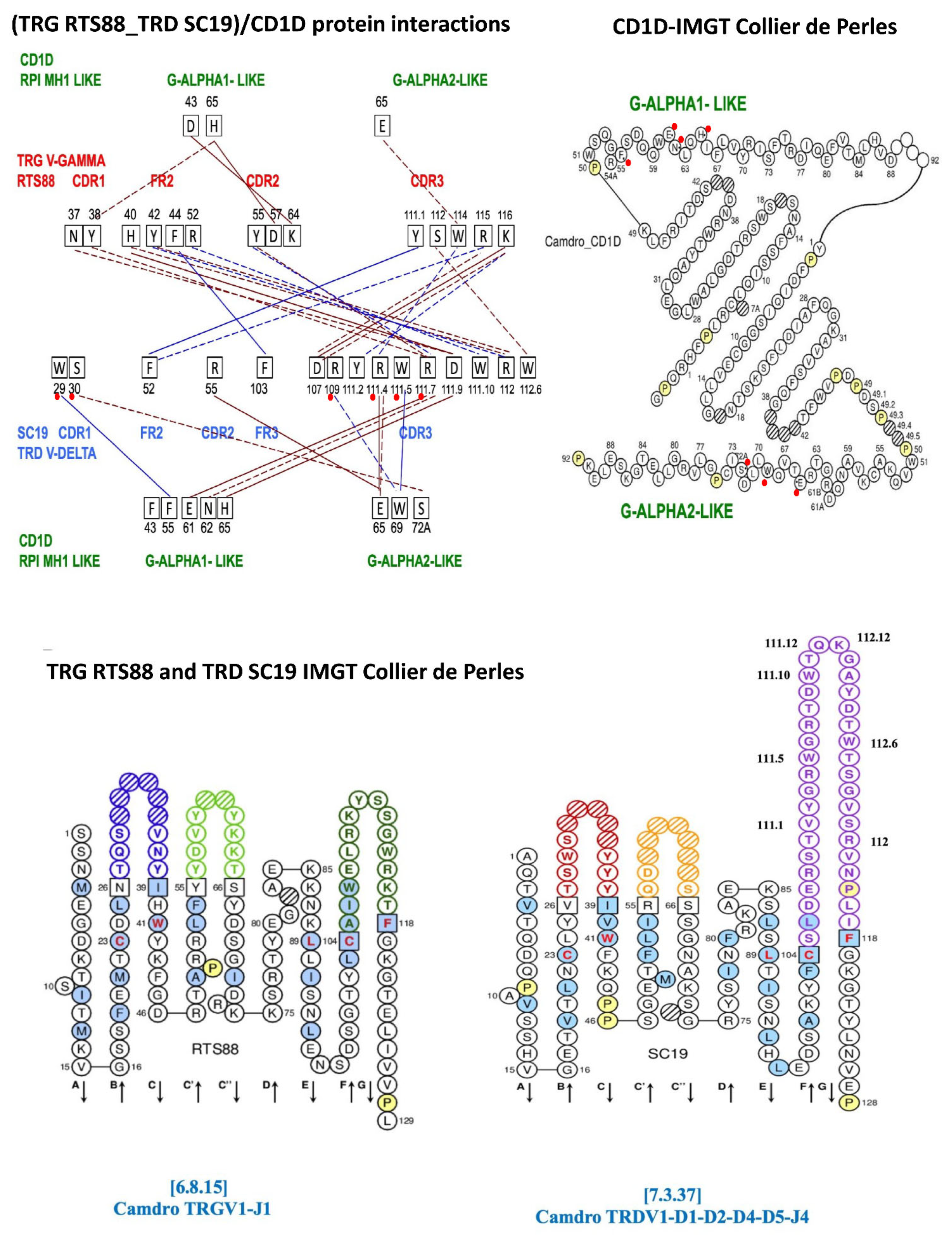
| A. Protein–protein interactions between the Camdro TRG V-GAMMA (clone RTS88) and TRD V-DELTA (clone SC19) domains. | |||||||||
| Camdro TRG | Camdro TRD | ||||||||
| RTS88 V-GAMMA TRGV1-TRGJ1-1 [6.8.15] | Pr-Pr | SC19 V-DELTA TRDV1-D1-D2-D4-D5-TRDJ4 [7.3.37] | |||||||
| CDR1-IMGT | ASN (N) | 37 | - - - - - | ASP (D) | 111.9 | CDR3-IMGT | |||
| CDR1-IMGT | TYR (Y) | 38 | - - - - - | ARG (R) | 111.7 | CDR3-IMGT | |||
| FR2-IMGT | HIS (H) | 40 |  | ASP (D) | 111.9 | CDR3-IMGT | |||
| HIS (H) | 40 | - - - - - | ARG (R) | 112 | CDR3-IMGT | ||||
| FR2-IMGT | TYR (Y) | 42 | - - - - - | ARG (R) | 112 | ||||
| TYR (Y) | 42 | - - - - - | ARG (R) | 112 | |||||
| FR2-IMGT | PHE (F) | 44 |  | PHE (F) | 103 | FR3-IMGT | |||
| FR2-IMGT | ARG (R) | 52 | - - - - - | TRP (W) | 111.10 | CDR3-IMGT | |||
| FR2-IMGT | TYR (Y) | 55 | - - - - - | ARG (R) | 111.7 | CDR3-IMGT | |||
| TYR (Y) | 55 | - - - - - | ARG (R) | 111.7 | |||||
| CDR3-IMGT | TYR (Y) | 111.1 |  | PHE (F) | 52 | FR2-IMGT | |||
| CDR3-IMGT | SER (S) | 112 | - - - - - | TRP (W) | 112.6 | CDR3-IMGT | |||
| CDR3-IMGT | TRP (W) | 114 | - - - - - | ARG (R) | 111.4 | CDR3-IMGT | |||
| CDR3-IMGT | ARG (R) | 115 | - - - - - | PHE (F) | 52 | FR2-IMGT | |||
| ARG (R) | 115 | - - - - - | ASP (D) | 107 | CDR3-IMT | ||||
| CDR3-IMGT | LYS (K) | 116 |  | ASP (D) | 107 | CDR3 | |||
| LYS (K) | 116 | - - - - - | ASP (D) | 107 | CDR3 | ||||
| LYS (K) | 116 | - - - - - | TYR (Y) | 111.2 | CDR3-IMGT | ||||
| B. Protein–protein interactions between the Camdro V-gamma_V-delta domains in complex with the Camdro CD1D G-ALPHA-1-LIKE and G-ALPHA-2-LIKE domains. | |||||||||
| Camdro TRG V-GAMMA | Camdro CD1D | Camdro TRD V-DELTA | |||||||
| RTS88 TRGV1-TRGJ1-1 [6.8.15] | SC19 TRDV1-TRDD1-D2-D4-D5-TRDJ4 [7.3.37] | ||||||||
| V-REGION | AA | IMGT | Pr-Pr | G-ALPHA1-LIKE | Pr-Pr | AA | IMGT | V-REGION | |
| PHE (F) | 55 |  | TRP (W) | 29 | CDR1-IMGT | ||||
| GLU (E) | 61 |  | ARG (R) | 111.7 | CDR3-IMGT | ||||
| ASN (N) | 62 | - - - - - | ARG (R) | 111.7 | CDR3-IMGT | ||||
| HIS (H) | 65 |  | ASP (D) | 111.9 | CDR3-IMGT | ||||
| CDR1-IMGT | TYR (Y) | 38 | - - - - - | HIS (H) | 65 | ||||
| CDR2-IMGT | ASP (D) | 57 |  | HIS (H) | 65 | ||||
| CDR2-IMGT | LYS (K) | 64 |  | ASP (D) | 43 | ||||
| G-ALPHA2-LIKE | |||||||||
| SER (S) | 72A | - - - - - | SER (S) | 30 | CDR1-IMGT | ||||
| TRP (W) | 69 | - - - - - | ARG (R) | 109 | CDR3-IMGT | ||||
| TRP (W) | 69 |  | TRP (W) | 111.5 | CDR3-IMGT | ||||
| GLU (E) | 65 |  | ARG (R) | 55 | FR2-IMGT | ||||
| GLU (E) | 65 |  | ARG (R) | 111.4 | CDR3-IMGT | ||||
| GLU (E) | 65 | - - - - - | ARG (R) | 111.4 | CDR3-IMGT | ||||
| CDR3-IMGT | TRP (W) | 114 | - - - - - | GLU (E) | 65 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccarese, S.; Lefranc, M.-P.; Perrone, G.C.M.; D’Addabbo, P.; Pierri, C.L. Three-Dimensional Modeling of Camelus dromedarius T Cell Receptor Gamma (TRG)_Delta (TRD)/CD1D Complex Reveals Different Binding Interactions Depending on the TRD CDR3 Length. Antibodies 2025, 14, 46. https://doi.org/10.3390/antib14020046
Ciccarese S, Lefranc M-P, Perrone GCM, D’Addabbo P, Pierri CL. Three-Dimensional Modeling of Camelus dromedarius T Cell Receptor Gamma (TRG)_Delta (TRD)/CD1D Complex Reveals Different Binding Interactions Depending on the TRD CDR3 Length. Antibodies. 2025; 14(2):46. https://doi.org/10.3390/antib14020046
Chicago/Turabian StyleCiccarese, Salvatrice, Marie-Paule Lefranc, Giulia C. M. Perrone, Pietro D’Addabbo, and Ciro Leonardo Pierri. 2025. "Three-Dimensional Modeling of Camelus dromedarius T Cell Receptor Gamma (TRG)_Delta (TRD)/CD1D Complex Reveals Different Binding Interactions Depending on the TRD CDR3 Length" Antibodies 14, no. 2: 46. https://doi.org/10.3390/antib14020046
APA StyleCiccarese, S., Lefranc, M.-P., Perrone, G. C. M., D’Addabbo, P., & Pierri, C. L. (2025). Three-Dimensional Modeling of Camelus dromedarius T Cell Receptor Gamma (TRG)_Delta (TRD)/CD1D Complex Reveals Different Binding Interactions Depending on the TRD CDR3 Length. Antibodies, 14(2), 46. https://doi.org/10.3390/antib14020046










