IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites
Abstract
1. Introduction
2. Materials and Methods
2.1. COVID-19 Vaccine Cohort
2.2. AGS Patient Cohort
2.3. Alpha-Gal IgE, Total IgE, and ABO Assays
2.4. IgG Assays
2.5. Statistical Analysis
3. Results
3.1. Demographics and Alpha-Gal Antibody Responses in the Vaccine and AGS Patient Cohorts
3.2. Alpha-Gal ImmunoCAP Assays Using Alpha-Gal HSA and BTG Yield Similar Results
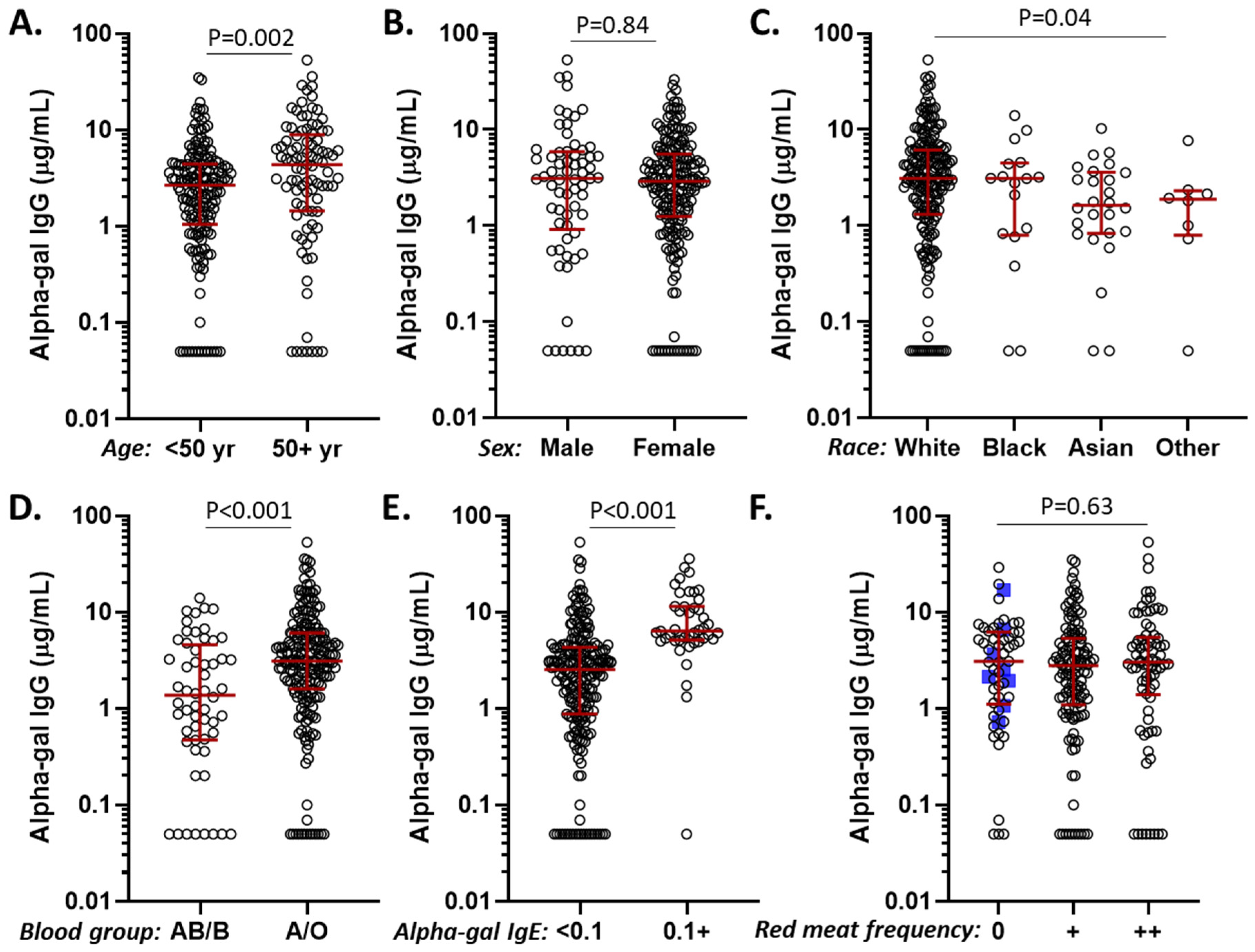
3.3. Alpha-Gal IgG Levels in Relation to Age, Sex, Race, Diet, ABO Blood Group, and Alpha-Gal IgE Sensitization
3.4. Alpha-Gal IgG in Relation to Mammalian Meat Allergy
3.5. Diagnostic Utility of Alpha-Gal IgE and IgG Antibody Levels
3.6. Tick Bites and Their Relation to Alpha-Gal IgE Sensitization and Alpha-Gal IgG Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landsteiner, K.; Miller, C.P. Serological Studies on the Blood of the Primates: III. Distribution of Serological Factors Related to Human Isoagglutinogens in the Blood of Lower Monkeys. J. Exp. Med. 1925, 42, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, R.M.; Galili, U.; Zhou, P.; Griffiss, J.M. Anti-alpha-galactosyl immunoglobulin A (IgA), IgG, and IgM in human secretions. Clin. Diagn. Lab. Immunol. 1995, 2, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Obukhova, P.; Rieben, R.; Bovin, N. Normal human serum contains high levels of anti-Gal alpha 1-4GlcNAc antibodies. Xenotransplantation 2007, 14, 627–635. [Google Scholar] [CrossRef]
- Mañez, R.; Blanco, F.J.; Díaz, I.; Centeno, A.; Lopez-Pelaez, E.; Hermida, M.; Davies, H.F.; Katopodis, A. Removal of bowel aerobic gram-negative bacteria is more effective than immunosuppression with cyclophosphamide and steroids to decrease natural alpha-galactosyl IgG antibodies. Xenotransplantation 2001, 8, 15–23. [Google Scholar] [CrossRef]
- Montassier, E.; Al-Ghalith, G.A.; Mathé, C.; Le Bastard, Q.; Douillard, V.; Garnier, A.; Guimon, R.; Raimondeau, B.; Touchefeu, Y.; Duchalais, E.; et al. Distribution of Bacterial α1,3-Galactosyltransferase Genes in the Human Gut Microbiome. Front. Immunol. 2019, 10, 3000. [Google Scholar] [CrossRef]
- Galili, U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol. Today 1993, 14, 480–482. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef]
- Konakci, K.Z.; Bohle, B.; Blumer, R.; Hoetzenecker, W.; Roth, G.; Moser, B.; Boltz-Nitulescu, G.; Gorlitzer, M.; Klepetko, W.; Wolner, E.; et al. Alpha-Gal on bioprostheses: Xenograft immune response in cardiac surgery. Eur. J. Clin. Investig. 2005, 35, 17–23. [Google Scholar] [CrossRef]
- Senage, T.; Paul, A.; Le Tourneau, T.; Fellah-Hebia, I.; Vadori, M.; Bashir, S.; Galinanes, M.; Bottio, T.; Gerosa, G.; Evangelista, A.; et al. The role of antibody responses against glycans in bioprosthetic heart valve calcification and deterioration. Nat. Med. 2022, 28, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kuravi, K.V.; Sorrells, L.T.; Nellis, J.R.; Rahman, F.; Walters, A.H.; Matheny, R.G.; Choudhary, S.K.; Ayares, D.L.; Commins, S.P.; Bianchi, J.R.; et al. Allergic response to medical products in patients with alpha-gal syndrome. J. Thorac. Cardiovasc. Surg. 2022, 164, e411–e424. [Google Scholar] [CrossRef] [PubMed]
- Perusko, M.; Grundstrom, J.; Eldh, M.; Hamsten, C.; Apostolovic, D.; van Hage, M. The alpha-Gal epitope—The cause of a global allergic disease. Front. Immunol. 2024, 15, 1335911. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Erickson, L.; Levin, M.; Ailsworth, S.M.; Commins, S.P.; Platts-Mills, T.A.E. Tick bites, IgE to galactose-alpha-1,3-galactose and urticarial or anaphylactic reactions to mammalian meat: The alpha-gal syndrome. Allergy 2024, 79, 1440–1454. [Google Scholar] [CrossRef]
- Commins, S.P. Diagnosis & management of alpha-gal syndrome: Lessons from 2500 patients. Expert. Rev. Clin. Immunol. 2020, 16, 667–677. [Google Scholar]
- Macdougall, J.D.; Thomas, K.O.; Iweala, O.I. The Meat of the Matter: Understanding and Managing Alpha-Gal Syndrome. Immunotargets Ther. 2022, 11, 37–54. [Google Scholar] [CrossRef]
- Wilson, J.M.; Nguyen, A.T.; Schuyler, A.J.; Commins, S.P.; Taylor, A.M.; Platts-Mills, T.A.E.; McNamara, C.A. IgE to the Mammalian Oligosaccharide Galactose-α-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1665–1669. [Google Scholar] [CrossRef]
- Vernon, S.T.; Kott, K.A.; Hansen, T.; Finemore, M.; Baumgart, K.W.; Bhindi, R.; Yang, J.; Hansen, P.S.; Nicholls, S.J.; Celermajer, D.S.; et al. Immunoglobulin E Sensitization to Mammalian Oligosaccharide Galactose-α-1,3 (α-Gal) Is Associated With Noncalcified Plaque, Obstructive Coronary Artery Disease, and ST-Segment–Elevated Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 352–361. [Google Scholar] [CrossRef]
- Commins, S.P.; James, H.R.; Kelly, L.A.; Pochan, S.L.; Workman, L.J.; Perzanowski, M.S.; Kocan, K.M.; Fahy, J.V.; Nganga, L.W.; Ronmark, E.; et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1286–1293.e6. [Google Scholar] [CrossRef]
- Ailsworth, S.M.; Susi, A.; Workman, L.J.; Ji, Y.S.; Patel, J.; Nelson, M.R.; Platts-Mills, T.A.E.; Nylund, C.M.; Wilson, J.M. Alpha-Gal IgE Prevalence Patterns in the United States: An Investigation of 3,000 Military Recruits. J. Allergy Clin. Immunol. Pract. 2024, 12, 175–184.e5. [Google Scholar] [CrossRef]
- Sharma, S.R.; Choudhary, S.K.; Vorobiov, J.; Commins, S.P.; Karim, S. Tick bite-induced alpha-gal syndrome and immunologic responses in an alpha-gal deficient murine model. Front. Immunol. 2024, 14, 1336883. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, J.L.; Cox, K.M.; Loo, W.M.; Qiao, H.; Tung, K.S.; Erickson, L.D. Cutaneous Exposure to Clinically Relevant Lone Star Ticks Promotes IgE Production and Hypersensitivity through CD4+ T Cell- and MyD88-Dependent Pathways in Mice. J. Immunol. 2019, 203, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Murangi, T.; Prakash, P.; Moreira, B.P.; Basera, W.; Botha, M.; Cunningham, S.; Facey-Thomas, H.; Halajian, A.; Joshi, L.; Ramjith, J.; et al. Ascaris lumbricoides and ticks associated with sensitization to galactose alpha1,3-galactose and elicitation of the alpha-gal syndrome. J. Allergy Clin. Immunol. 2022, 149, 698–707.e3. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Hodzic, A.; Roman-Carrasco, P.; Mateos-Hernandez, L.; Duscher, G.G.; Sinha, D.K.; Hemmer, W.; Swoboda, I.; Estrada-Pena, A.; de la Fuente, J. Environmental and Molecular Drivers of the alpha-Gal Syndrome. Front. Immunol. 2019, 10, 1210. [Google Scholar] [CrossRef]
- Saunders, E.F.; Sohail, H.; Myles, D.J.; Charnetzky, D.; Ayres, B.N.; Nicholson, W.L.; Commins, S.P.; Salzer, J.S. Alpha-Gal Syndrome after Ixodes scapularis Tick Bite and Statewide Surveillance, Maine, USA, 2014–2023. Emerg. Infect. Dis. 2025, 31, 809–813. [Google Scholar] [CrossRef]
- Chakrapani, N.; Swiontek, K.; Hübschen, J.M.; Fischer, J.; Ruiz-Castell, M.; Codreanu-Morel, F.; Hannachi, F.; Morisset, M.; Ollert, M.; Kuehn, A.; et al. Recurrent tick bites induce high IgG1 antibody responses to α-Gal in sensitized and non-sensitized forestry employees in Luxembourg. Clin. Transl. Allergy 2024, 14, e12396. [Google Scholar] [CrossRef]
- Ailsworth, S.M.; Keshavarz, B.; Richards, N.E.; Workman, L.J.; Murphy, D.D.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann. Allergy. Asthma. Immunol. 2023, 130, 67–73. [Google Scholar] [CrossRef]
- Richards, N.E.; Ailsworth, S.M.; Workman, L.J.; Bortz, P.S.; Patel, J.; MacCallum, M.; Canderan, G.; Murphy, D.; Muehling, L.M.; McGowan, E.C.; et al. Mammalian Meat Allergy and IgE to Alpha-Gal in Central Virginia: Findings From a COVID-19 Vaccine and Patient Cohort. J. Allergy Clin. Immunol. Pract. 2024, 12, 2817–2825.e2. [Google Scholar] [CrossRef]
- Seagroatt, V.; Anderson, S.G. The second international reference preparation for human serum immunoglobulin E and the first British standard for human serum immunoglobulin E. J. Biol. Stand. 1981, 9, 431–437. [Google Scholar] [CrossRef]
- Keshavarz, B.; Wiencek, J.R.; Workman, L.J.; Straesser, M.D.; Muehling, L.M.; Canderan, G.; Drago, F.; Bonham, C.A.; Sturek, J.M.; Ramani, C.; et al. Quantitative Measurement of IgG to Severe Acute Respiratory Syndrome Coronavirus-2 Proteins Using ImmunoCAP. Int. Arch. Allergy Immunol. 2021, 182, 417–424. [Google Scholar] [CrossRef]
- Makin, T.; Borish, L.; Nylund, C.M.; Wilson, J.M.; Lawrence, M.G. IgE deficiency is not associated with hypogammaglobulinemia in a large cohort of military recruits. Ann. Allergy Asthma Immunol. 2024, 133, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lupberger, E.; Hebsaker, J.; Blumenstock, G.; Aichinger, E.; Yazdi, A.S.; Reick, D.; Oehme, R.; Biedermann, T. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy 2017, 72, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, L.P.; Cristiano, L.M.; Dowling, A.P.G.; Wilson, J.M.; Platts-Mills, T.A.E.; Traister, R.S. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J. Allergy Clin. Immunol. Pract. 2019, 7, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Macher, B.A.; Buehler, J.; Shohet, S.B. Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1----3)-linked galactose residues. J. Exp. Med. 1985, 162, 573–582. [Google Scholar] [CrossRef]
- Yu, P.B.; Holzknecht, Z.E.; Bruno, D.; Parker, W.; Platt, J.L. Modulation of natural IgM binding and complement activation by natural IgG antibodies: A role for IgG anti-Gal alpha1-3Gal antibodies. J. Immunol. 1996, 157, 5163–5168. [Google Scholar] [CrossRef]
- Joral, A.; Azketa, N.; Sanchez, P.; Vélez-Del-Burgo, A.; Aranzabal-Soto, M.A.; Lizarza, S.; Martínez, J.; Postigo, I. The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy. Foods 2022, 11, 466. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, S.-Y.; Wu, L.-X.; Li, H.; Sun, Z.-J.; Li, J.-B.; Jiang, H.-X.; Chen, Z.-H.; Wang, Q.-B.; Chen, W.-W. Variable Food-Specific IgG Antibody Levels in Healthy and Symptomatic Chinese Adults. PLoS ONE 2013, 8, e53612. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the α-Gal Syndrome: Characteristics of 261 Children and Adults Reporting Red Meat Allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 2348–2358.e4. [Google Scholar] [CrossRef]
- Rispens, T.; Derksen, N.I.L.; Commins, S.P.; Platts-Mills, T.A.; Aalberse, R.C. IgE Production to α-Gal Is Accompanied by Elevated Levels of Specific IgG1 Antibodies and Low Amounts of IgE to Blood Group B. PLoS ONE 2013, 8, e55566. [Google Scholar] [CrossRef]
- McMorrow, I.M.; Comrack, C.A.; Nazarey, P.P.; Sachs, D.H.; DerSimonian, H. Relationship between ABO blood group and levels of Gal alpha,3Galactose-reactive human immunoglobulin G. Transplantation 1997, 64, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Bernth-Jensen, J.M.; Moller, B.K.; Jensenius, J.C.; Thiel, S. Biological variation of anti-alphaGal-antibodies studied by a novel Time-Resolved ImmunoFluorometric Assay. J. Immunol. Methods 2011, 373, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Apostolovic, D.; Rodrigues, R.; Thomas, P.; Starkhammar, M.; Hamsten, C.; van Hage, M. Immunoprofile of α-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018, 73, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Tesfazghi, M.T.; Zaydman, M.A.; Jackups, R., Jr.; Kim, B.S.; Scott, M.G.; Gronowski, A.M.; Grossman, B.J. The B antigen protects against the development of red meat allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 1790–1791.e3. [Google Scholar] [CrossRef]
- Taylor, M.L.; Kersh, G.J.; Salzer, J.S.; Jones, E.S.; Binder, A.M.; Armstrong, P.A.; Choudhary, S.K.; Commins, G.K.; Amelio, C.L.; Biggerstaff, B.J.; et al. Intrinsic risk factors for alpha-gal syndrome in a case-control study, 2019 to 2020. Ann. Allergy Asthma Immunol. 2024, 132, 759–764.e2. [Google Scholar] [CrossRef]
- Buonomano, R.; Tinguely, C.; Rieben, R.; Mohacsi, P.J.; Nydegger, U.E. Quantitation and characterization of anti-Galα1-3Gal antibodies in sera of 200 healthy persons. Xenotransplantation 1999, 6, 173–180. [Google Scholar] [CrossRef]
- Kollmann, D.; Nagl, B.; Ebner, C.; Emminger, W.; Wöhrl, S.; Kitzmüller, C.; Vrtala, S.; Mangold, A.; Ankersmit, H.-J.; Bohle, B. The quantity and quality of α-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy 2017, 72, 266–273. [Google Scholar] [CrossRef]
- Strobl, M.R.; Demir, H.; Sánchez Acosta, G.; Drescher, A.; Kitzmüller, C.; Möbs, C.; Pfützner, W.; Bohle, B. The role of IgG1 and IgG4 as dominant IgE-blocking antibodies shifts during allergen immunotherapy. J. Allergy Clin. Immunol. 2023, 151, 1371–1378.e5. [Google Scholar] [CrossRef]
- James, L.K.; Shamji, M.H.; Walker, S.M.; Wilson, D.R.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Kimber, I.; Till, S.J.; Durham, S.R. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J. Allergy Clin. Immunol. 2011, 127, e1–e5. [Google Scholar] [CrossRef]
- Zinkhan, S.; Thoms, F.; Augusto, G.; Vogel, M.; Bachmann, M.F. On the role of allergen-specific IgG subclasses for blocking human basophil activation. Front. Immunol. 2022, 13, 892631. [Google Scholar] [CrossRef]
- Apostolovic, D.; Mihailovic, J.; Commins, S.P.; Wijnveld, M.; Kazimirova, M.; Starkhammar, M.; Stockinger, H.; Platts-Mills, T.A.E.; Cirkovic Velickovic, T.; Hamsten, C.; et al. Allergenomics of the tick Ixodes ricinus reveals important α-Gal-carrying IgE-binding proteins in red meat allergy. Allergy 2020, 75, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Koschutnik, M.; Nitsche, C.; Laggner, M.; Polak, D.; Bohle, B.; Mangold, A.; Moser, B.; Mascherbauer, J.; Ankersmit, H.J. Inflammatory immune response in recipients of transcatheter aortic valves. JTCVS Open 2021, 6, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Workman, L.; Platts-Mills, T.; Maccallum, M.; Taylor, A.; McNamara, C.; Wilson, J.; Moverare, R. Isotype diversity of antibodies to alpha-gal Including IgG3 as well as IgE and the possible relevance to coronary artery disease. J. Allergy Clin. Immunol. 2025, 155, AB33. [Google Scholar] [CrossRef]
- McGill, S.K.; Commins, S.P.; Peery, A.F.; Galanko, J.; Keku, T.O.; Shaheen, N.J.; Anderson, C.; Sandler, R.S. Alpha-gal sensitization in a US screening population is not associated with a decreased meat intake or gastrointestinal symptoms. Am. J. Gastroenterol. 2023, 118, 1276–1281. [Google Scholar] [CrossRef]
- Mabelane, T.; Basera, W.; Botha, M.; Thomas, H.F.; Ramjith, J.; Levin, M.E. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr. Allergy Immunol. 2018, 29, 841–849. [Google Scholar] [CrossRef]
- Kersh, G.J.; Salzer, J.; Jones, E.S.; Binder, A.M.; Armstrong, P.A.; Choudhary, S.K.; Commins, G.K.; Amelio, C.L.; Kato, C.Y.; Singleton, J.; et al. Tick bite as a risk factor for alpha-gal-specific immunoglobulin E antibodies and development of alpha-gal syndrome. Ann. Allergy. Asthma. Immunol. 2023, 130, 472–478. [Google Scholar] [CrossRef]
- Makroo, R.N.; Kakkar, B.; Agrawal, S.; Chowdhry, M.; Prakash, B.; Karna, P. Retrospective analysis of forward and reverse ABO typing discrepancies among patients and blood donors in a tertiary care hospital. Transfus. Med. 2019, 29, 103–109. [Google Scholar] [CrossRef]
- Westman, M.; Asarnoj, A.; Ballardini, N.; Andersson, N.; Kiewiet, M.B.G.; Borres, M.P.; Apostolovic, D.; Kull, I.; Bergström, A.; Melén, E.; et al. Alpha-gal sensitization among young adults is associated with male sex and polysensitization. J. Allergy Clin. Immunol. Pract. 2022, 10, 333–335.e2. [Google Scholar] [CrossRef]
- Ching, S.J.; Susi, A.; Ailsworth, S.M.; Workman, L.J.; Platts-Mills, T.A.E.; Wilson, J.M.; Nylund, C.M. Incidence of Alpha-Gal IgE Sensitization in 3000 Military Personnel, Assessing Sex, Race, Installation, and Occupational Impacts. J. Clin. Med. 2024, 13, 7162. [Google Scholar] [CrossRef]
- Thompson, J.M.; Carpenter, A.; Kersh, G.J.; Wachs, T.; Commins, S.P.; Salzer, J.S. Geographic Distribution of Suspected Alpha-gal Syndrome Cases—United States, January 2017–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 815–820. [Google Scholar] [CrossRef]





| Characteristics | COVID Cohort (n = 267) | AGS Cohort (n = 38) | p |
|---|---|---|---|
| Age, y (median [interquartile range]) | 42 (31–55) | 62 (52–73) | <0.001 |
| Female sex, n (%) | 202 (75.7%) | 18 (47.4%) | <0.001 |
| Race and Ethnicity | 0.24 | ||
| Asian, n (%) | 26 (9.7%) | 1 (2.6%) | |
| White, n (%) | 216 (80.9%) | 29 (76.3%) | |
| Black, n (%) | 18 (6.7%) | 2 (5.3%) | |
| Hispanic, n (%) | 6 (2.3%) | 0 (0%) | |
| Other, n (%) | 1 (0.4%) | 1 (2.6%) | |
| Total IgE, IU/mL (geometric mean [95% CI]) | 22.5 [18.6–27.2] | 106.2 [71.82–157.1] | <0.001 |
| Mammalian Meat Allergy, n (%) | 8 (3.0%) | 38 (100%) | <0.001 |
| Variable | N (%) | Adjusted Linear Regression β (95% CI) | p-Value |
|---|---|---|---|
| Age 50+ | 93 (35%) | 0.05 (−0.13 to 0.22) | 0.60 |
| Male sex | 65 (24%) | −0.01 (−0.19 to 0.17) | 0.90 |
| White race | 216 (81%) | 0.08 (−0.12 to 0.27) | 0.46 |
| B or AB blood group | 56 (21%) | −0.30 (−0.49 to −0.12) | 0.002 |
| Alpha-gal IgE ≥ 0.1 IU/mL | 43 (16%) | 0.53 (0.32 to 0.75) | <0.001 |
| 1–2 Red meat servings/week | 129 (48%) | 0.004 (−0.19 to 0.20) | 0.97 |
| ≥3 Red meat servings/week | 77 (29%) | 0.03 (−0.19 to 0.24) | 0.80 |
| Variable | N (%) | Unadjusted Linear Regression β (95% CI) | p-Value | Adjusted Linear Regression β (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age 50+ | 48 (29%) | 0.03 (−0.19 to 0.26) | 0.78 | 0.009 (−0.21 to 0.23) | 0.93 |
| Male Sex | 40 (24%) | 0.17 (−0.07 to 0.40) | 0.16 | 0.19 (−0.04 to 0.42) | 0.10 |
| White Race | 127 (77%) | 0.04 (−0.19 to 0.29) | 0.69 | 0.12 (−0.30 to 0.18) | 0.64 |
| B or AB blood group | 37 (22%) | −0.42 (−0.65 to −0.18) | <0.001 | −0.41 (−0.65 to −0.18) | <0.001 |
| Tick/chigger bite | 97 (58%) | 0.25 (0.04 to 0.45) | 0.02 | 0.11 (0.04 to 0.46) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailsworth, S.M.; MacCallum, M.; Richards, N.E.; Workman, L.J.; Schoppee Bortz, P.; Makin, T.; Platts-Mills, T.A.E.; Wilson, J.M. IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites. Antibodies 2025, 14, 43. https://doi.org/10.3390/antib14020043
Ailsworth SM, MacCallum M, Richards NE, Workman LJ, Schoppee Bortz P, Makin T, Platts-Mills TAE, Wilson JM. IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites. Antibodies. 2025; 14(2):43. https://doi.org/10.3390/antib14020043
Chicago/Turabian StyleAilsworth, Samuel M., Matthew MacCallum, Nathan E. Richards, Lisa J. Workman, Pamela Schoppee Bortz, Thomas Makin, Thomas A. E. Platts-Mills, and Jeffrey M. Wilson. 2025. "IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites" Antibodies 14, no. 2: 43. https://doi.org/10.3390/antib14020043
APA StyleAilsworth, S. M., MacCallum, M., Richards, N. E., Workman, L. J., Schoppee Bortz, P., Makin, T., Platts-Mills, T. A. E., & Wilson, J. M. (2025). IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites. Antibodies, 14(2), 43. https://doi.org/10.3390/antib14020043






