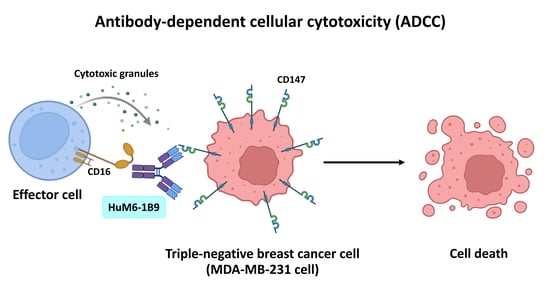
Potentiating Antibody-Dependent Cellular Cytotoxicity in Triple-Negative Breast Cancer via the Humanized Anti-CD147 Antibody
Abstract
1. Introduction
2. Methods
2.1. Cell Lines
2.2. Isolation of PBMCs
2.3. Production and Purification of HuM6-1B9
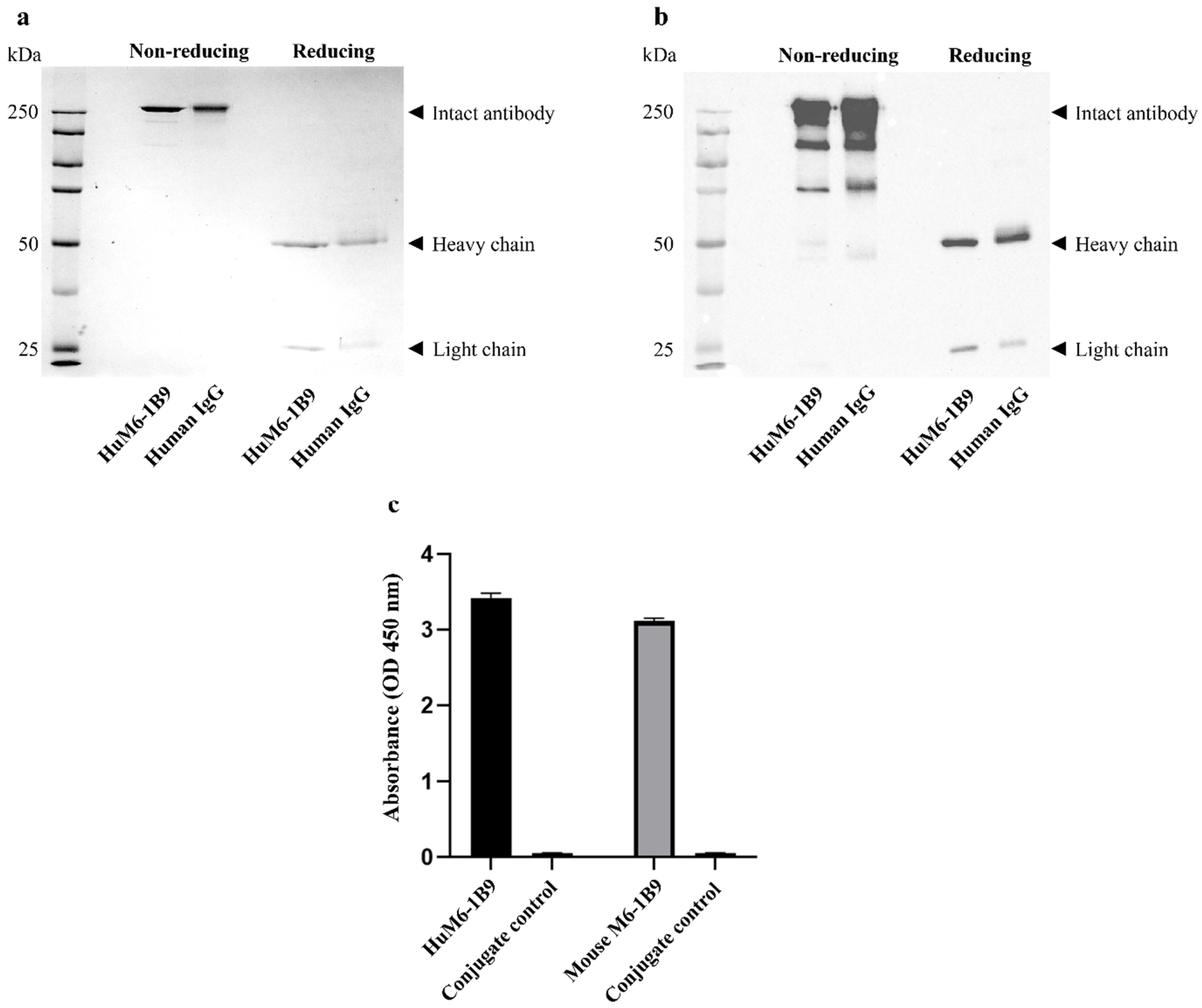
2.4. Determination of Purity of HuM6-1B9
2.5. Examination of Binding Activity of HuM6-1B9
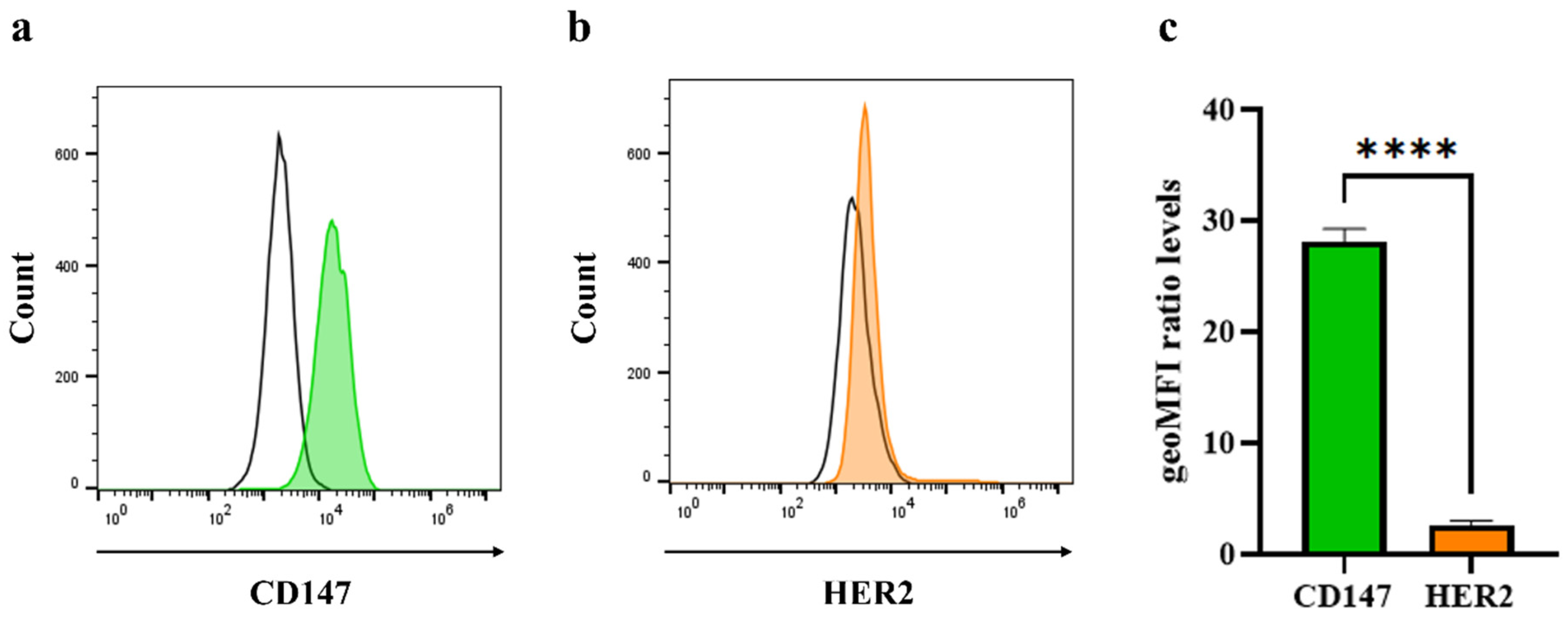
2.6. Analysis of Cell Surface Molecule on Triple-Negative Breast Cancer Cells
2.7. Assessment of HuM6-1B9 Mediated ADCC
2.8. Statistical Analysis
3. Results
3.1. Assessment of Purity and Binding Activity of HuM6-1B9
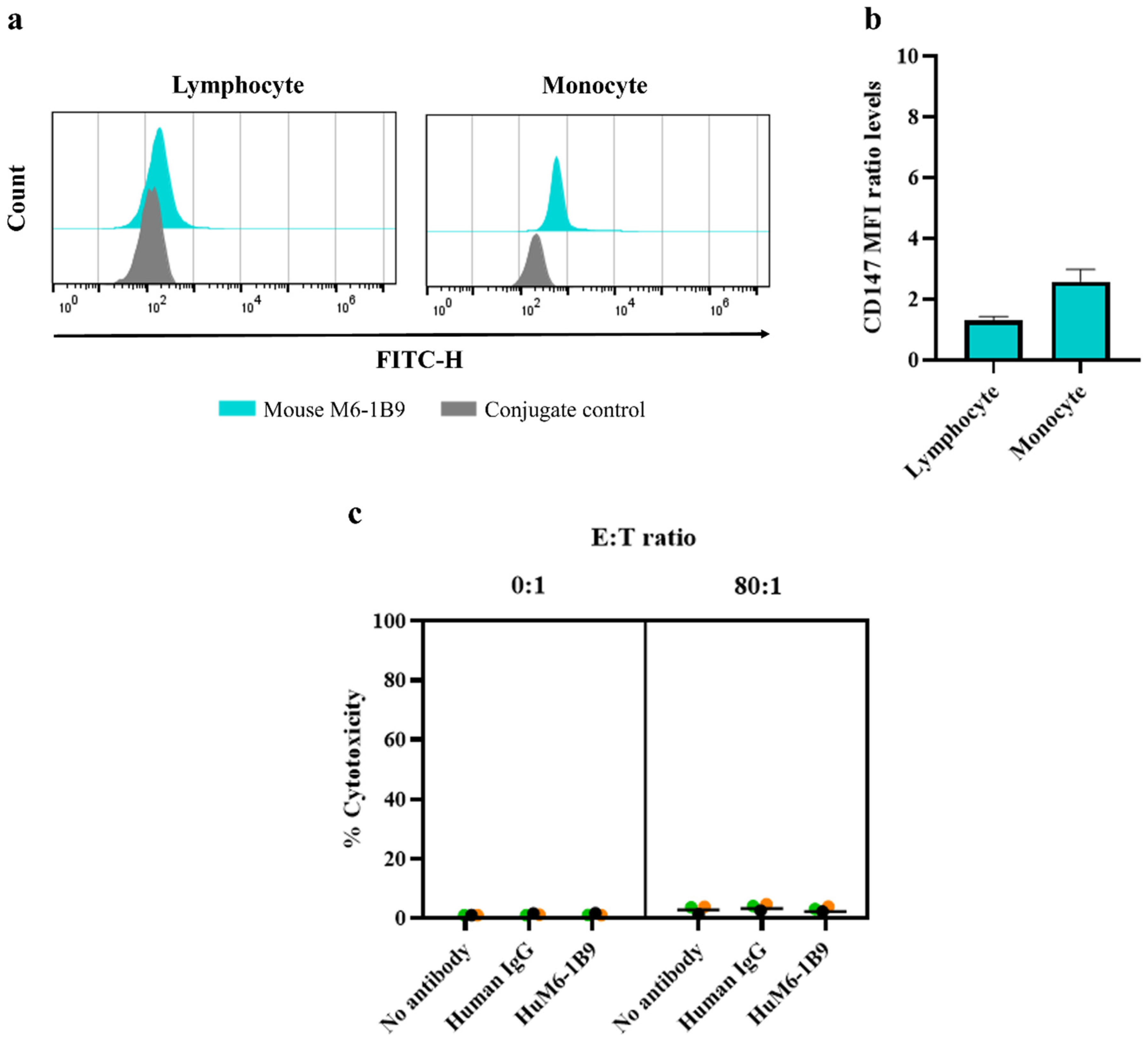
3.2. Detection of CD147 and HER2 Molecules on Triple-Negative Breast Cancer Cells
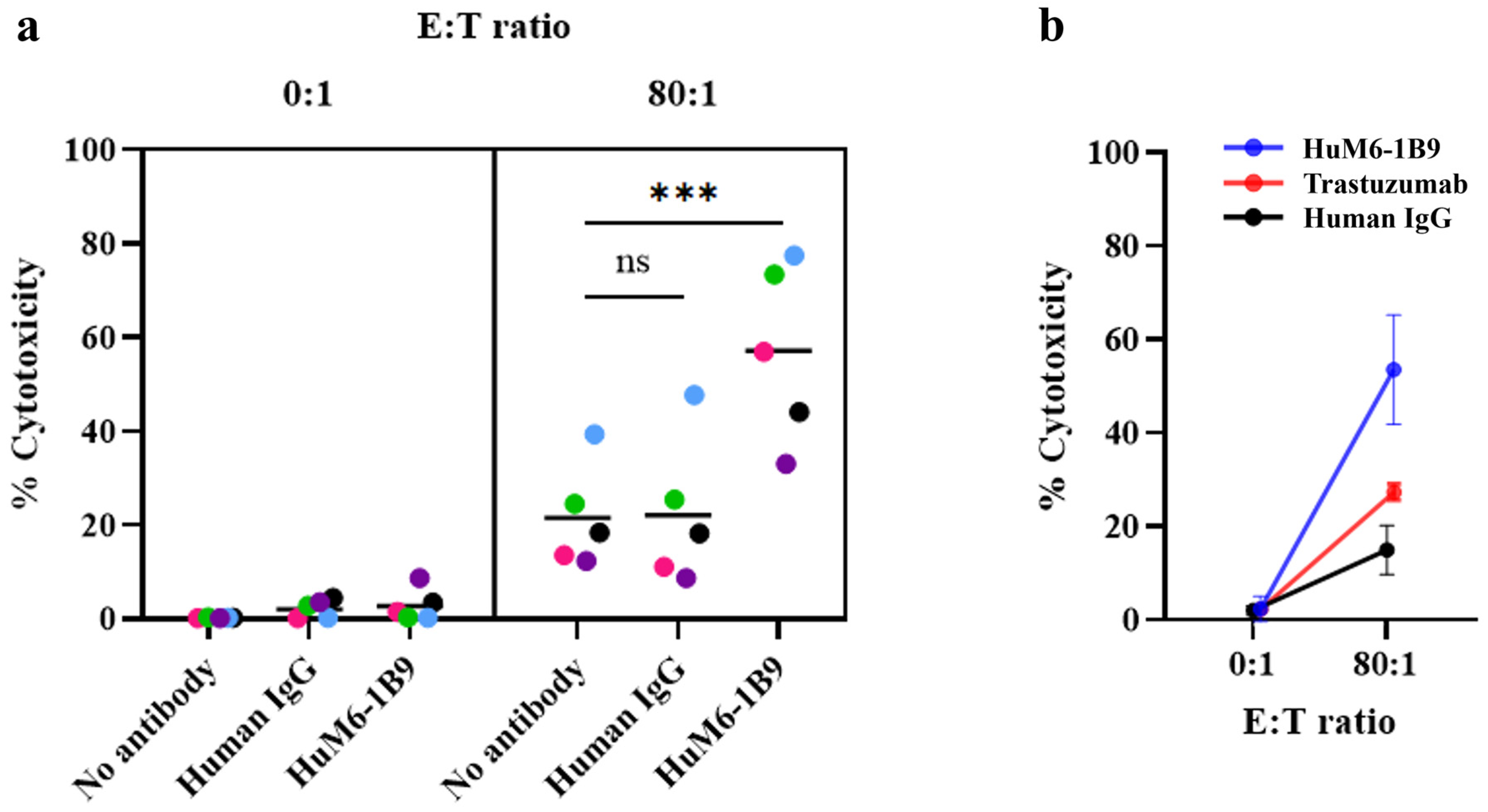
3.3. Evaluation of HuM6-1B9-Mediated ADCC in MDA-MB-231 Cells
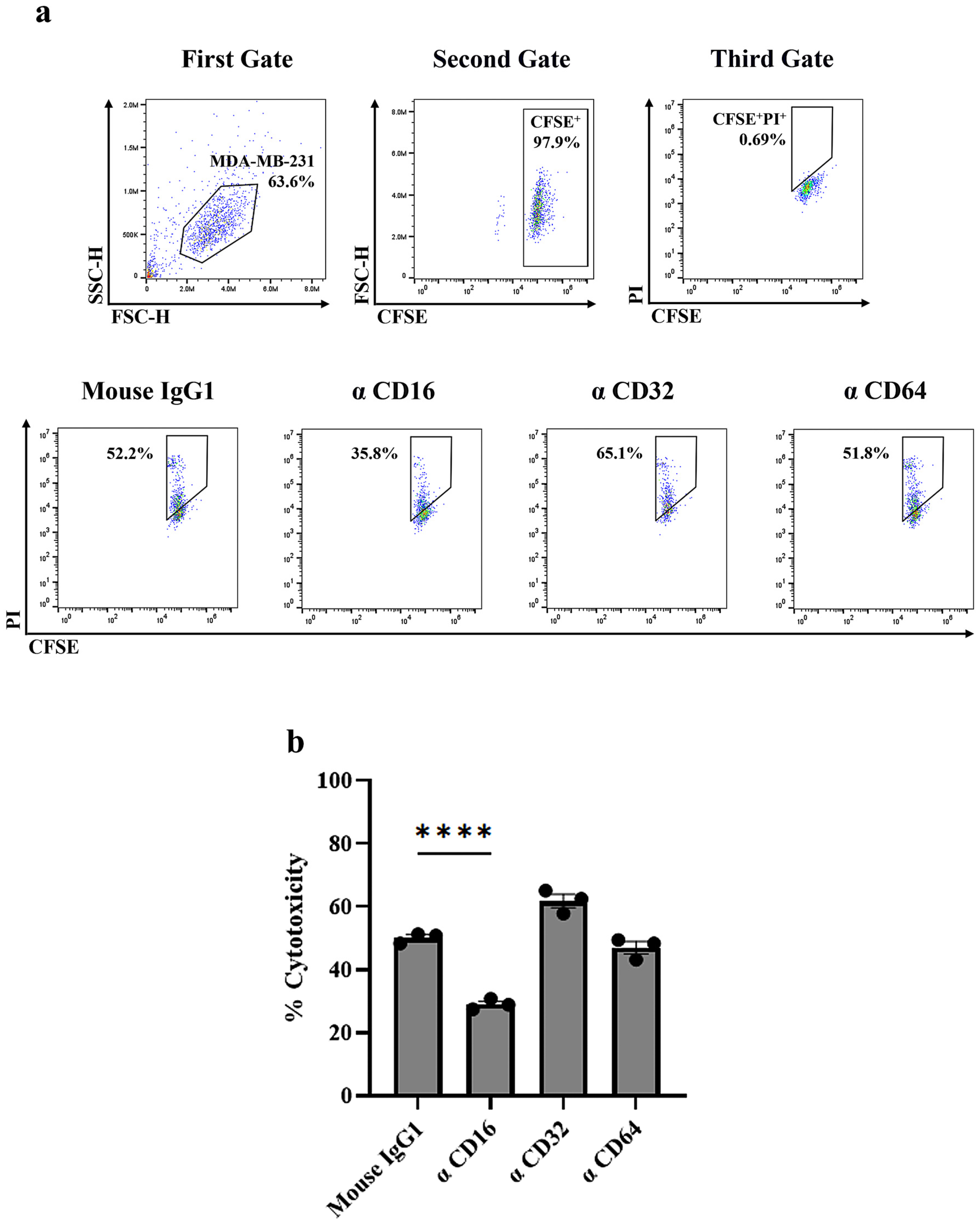
3.4. Identification of FcγR Involved in HuM6-1B9-Mediated ADCC
3.5. Investigation of HuM6-1B9 ADCC Activity Against PBMCs from Healthy Donors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, A.; Fumagalli, D.; Sotiriou, C.; Piccart, M. Is the differentiation into molecular subtypes of breast cancer important for staging, local and systemic therapy, and follow up? Cancer Treat. Rev. 2014, 40, 1089–1095. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Shi, S.; Ma, H.Y.; Sang, Y.Z.; Ju, Y.B.; Wang, X.G.; Zhang, Z.G. CD147 expression as a clinicopathological and prognostic indicator in breast cancer: A meta-analysis and bioinformatics analysis. BMC Cancer 2024, 24, 1429. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Plasilova, M.L.; Hayse, B.; Killelea, B.K.; Horowitz, N.R.; Chagpar, A.B.; Lannin, D.R. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine 2016, 95, e4614. [Google Scholar] [CrossRef]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: Approved treatment options and their mechanisms of action. J. Cancer Res. Clin. Oncol. 2023, 149, 3701–3719. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Chen, L.; Zhong, W.D.; Zhang, Z.; Mi, L.; Zhang, Y.; Liao, C.G.; Bian, H.J.; Jiang, J.L.; et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology 2009, 54, 677–687. [Google Scholar] [CrossRef]
- Xiong, L.; Edwards, C.K.; Zhou, L. The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature. Int. J. Mol. Sci. 2014, 15, 17411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ma, W.; Zhang, M.; Tang, D.; Shi, Q.; Xu, S.; Zhang, X.; Liu, Y.; Song, Y.; Liu, L.; et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med. Oncol. 2013, 30, 335. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nakada, M.T.; Kesavan, P.; McCabe, F.; Millar, H.; Rafferty, P.; Bugelski, P.; Yan, L. Extracellular Matrix Metalloproteinase Inducer Stimulates Tumor Angiogenesis by Elevating Vascular Endothelial Cell Growth Factor and Matrix Metalloproteinases. Cancer Res. 2005, 65, 3193–3199. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Yan, Y.-q.; Bai, C.-z.; Guo, J.-q. CD147 promotes breast cancer migration and invasion by inducing epithelial-mesenchymal transition via the MAPK/ERK signaling pathway. BMC Cancer 2023, 23, 1214. [Google Scholar] [CrossRef] [PubMed]
- Knutti, N.; Huber, O.; Friedrich, K. CD147 (EMMPRIN) controls malignant properties of breast cancer cells by interdependent signaling of Wnt and JAK/STAT pathways. Mol. Cell Biochem. 2019, 451, 197–209. [Google Scholar] [CrossRef]
- Zhou, S.; Liao, L.; Chen, C.; Zeng, W.; Liu, S.; Su, J.; Zhao, S.; Chen, M.; Kuang, Y.; Chen, X.; et al. CD147 mediates chemoresistance in breast cancer via ABCG2 by affecting its cellular localization and dimerization. Cancer Lett. 2013, 337, 285–292. [Google Scholar] [CrossRef]
- Jia, L.; Xu, H.; Zhao, Y.; Jiang, L.; Yu, J.; Zhang, J. Expression of CD147 mediates tumor cells invasion and multidrug resistance in hepatocellular carcinoma. Cancer Investig. 2008, 26, 977–983. [Google Scholar] [CrossRef]
- Xiong, L.; Ding, L.; Ning, H.; Wu, C.; Fu, K.; Wang, Y.; Zhang, Y.; Liu, Y.; Zhou, L. CD147 knockdown improves the antitumor efficacy of trastuzumab in HER2-positive breast cancer cells. Oncotarget 2016, 7, 57737–57751. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Landras, A.; Reger de Moura, C.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef]
- Lian, C.; Guo, Y.; Zhang, J.; Chen, X.; Peng, C. Targeting CD147 is a Novel Strategy for Antitumor Therapy. Curr. Pharm. Des. 2017, 23, 4410–4421. [Google Scholar] [CrossRef]
- Fan, X.Y.; He, D.; Sheng, C.B.; Wang, B.; Wang, L.J.; Wu, X.Q.; Xu, L.; Jiang, J.L.; Li, L.; Chen, Z.N. Therapeutic anti-CD147 antibody sensitizes cells to chemoradiotherapy via targeting pancreatic cancer stem cells. Am. J. Transl. Res. 2019, 11, 3543–3554. [Google Scholar]
- Wang, M.; Zhang, S.; Sun, Q.; Yang, X.; Wang, Y.; Shang, R.; Zhu, Y.; Yao, H.; Li, Y. Dual effects of an anti-CD147 antibody for Esophageal cancer therapy. Cancer Biol. Ther. 2019, 20, 1443–1452. [Google Scholar] [CrossRef]
- Chen, Z.-N.; Mi, L.; Xu, J.; Song, F.; Zhang, Q.; Zhang, Z.; Xing, J.-L.; Bian, H.-J.; Jiang, J.-L.; Wang, X.-H.; et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical Phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 435–444. [Google Scholar] [CrossRef]
- Pamonsupornwichit, T.; Sornsuwan, K.; Juntit, O.A.; Yasamut, U.; Takheaw, N.; Kasinrerk, W.; Wanachantararak, P.; Kodchakorn, K.; Nimmanpipug, P.; Intasai, N.; et al. Engineered CD147-Deficient THP-1 Impairs Monocytic Myeloid-Derived Suppressor Cell Differentiation but Maintains Antibody-Dependent Cellular Phagocytosis Function for Jurkat T-ALL Cells with Humanized Anti-CD147 Antibody. Int. J. Mol. Sci. 2024, 25, 6626. [Google Scholar] [CrossRef]
- Abhinandan, K.R.; Martin, A.C.R. Analyzing the “Degree of Humanness” of Antibody Sequences. J. Mol. Biol. 2007, 369, 852–862. [Google Scholar] [CrossRef]
- Yuita, H.; Nakamura, R.; Tsukada, J.; Yukinaga, H.; Hung, H.T.; Fukuchi, K. Abstract B127: Novel anti-CD147 antibody DS-1471a exerts antitumor effect in hepatocellular carcinoma Patient Derived Xenograft models and its efficacy correlates with the expression of CD147. Mol. Cancer Ther. 2023, 22, B127. [Google Scholar] [CrossRef]
- Fukuchi, K.; Nanai, K.; Yuita, H.; Maru, C.; Tsukada, J.; Ishigami, M.; Nagai, Y.; Nakano, Y.; Yoshimura, C.; Yoneda, K.; et al. Novel Antibody Exerts Antitumor Effect Through Downregulation of CD147 and Activation of Multiple Stress Signals. J. Oncol. 2022, 2022, 3552793. [Google Scholar] [CrossRef]
- Intasai, N.; Rangnoi, K.; Yamabhai, M.; Pamonsupornwichit, T.; Thongkum, W.; Yasamut, U.; Chupradit, K.; Takheaw, N.; Nimmanpipug, P.; Tayapiwatana, C. Immunoreactivity of humanized single-chain variable fragment against its functional epitope on domain 1 of CD147. Sci. Rep. 2022, 12, 6719. [Google Scholar] [CrossRef]
- Salinas-Jazmín, N.; Hisaki-Itaya, E.; Velasco-Velázquez, M.A. A flow cytometry-based assay for the evaluation of antibody-dependent cell-mediated cytotoxicity (ADCC) in cancer cells. Methods Mol. Biol. 2014, 1165, 241–252. [Google Scholar]
- Qiao, Z.; Li, X.; Kang, N.; Yang, Y.; Chen, C.; Wu, T.; Zhao, M.; Liu, Y.; Ji, X. A Novel Specific Anti-CD73 Antibody Inhibits Triple-Negative Breast Cancer Cell Motility by Regulating Autophagy. Int. J. Mol. Sci. 2019, 20, 1057. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, L.; Xing, Y.; Meng, T.; Ma, L.; Pei, J.; Cong, Y.; Zhang, X.; Ren, Z.; Wang, X.; et al. Dual Mechanisms of Novel CD73-Targeted Antibody and Antibody-Drug Conjugate in Inhibiting Lung Tumor Growth and Promoting Antitumor Immune-Effector Function. Mol. Cancer Ther. 2020, 19, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Joshkon, A.; Ladjimi, A.; Traboulsi, W.; Bachelier, R.; Robert, S.; Foucault-Bertaud, A.; Leroyer, A.S.; Bardin, N.; Somasundaram, I.; et al. Soluble CD146 as a Potential Target for Preventing Triple Negative Breast Cancer MDA-MB-231 Cell Growth and Dissemination. Int. J. Mol. Sci. 2022, 23, 974. [Google Scholar] [CrossRef]
- Zabouo, G.; Imbert, A.-M.; Jacquemier, J.; Finetti, P.; Moreau, T.; Esterni, B.; Birnbaum, D.; Bertucci, F.; Chabannon, C. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 2009, 11, R1. [Google Scholar] [CrossRef]
- Liu, M.; Tsang, J.Y.S.; Lee, M.; Ni, Y.B.; Chan, S.K.; Cheung, S.Y.; Hu, J.; Hu, H.; Tse, G.M.K. CD147 expression is associated with poor overall survival in chemotherapy treated triple-negative breast cancer. J. Clin. Pathol. 2018, 71, 1007–1014. [Google Scholar] [CrossRef]
- Dai, L.; Guinea, M.C.; Slomiany, M.G.; Bratoeva, M.; Grass, G.D.; Tolliver, L.B.; Maria, B.L.; Toole, B.P. CD147-dependent heterogeneity in malignant and chemoresistant properties of cancer cells. Am. J. Pathol. 2013, 182, 577–585. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Zhou, P.; Qiao, Y.; Li, Y.; Xu, Y.; Shi, X. Generation of a High-Affinity Nanobody Against CD147 for Tumor Targeting and Therapeutic Efficacy Through Conjugating Doxorubicin. Front. Immunol. 2022, 13, 852700. [Google Scholar] [CrossRef]
- Jin, B.K.; Odongo, S.; Radwanska, M.; Magez, S. NANOBODIES®: A Review of Diagnostic and Therapeutic Applications. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef]
- Giles, A.; Hao, S.; Padget, M.; Song, H.; Wei, Z.; Lynes, J.; Sanchez, V.; Liu, Y.; Jung, J.; Cao, X.; et al. Efficient ADCC killing of meningioma by avelumab and a high-Affinity natural killer cell line, haNK. JCI Insight 2019, 4, e130688. [Google Scholar] [CrossRef]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.Y.; Quek, J.K.S.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mahaweni, N.M.; Olieslagers, T.I.; Rivas, I.O.; Molenbroeck, S.J.J.; Groeneweg, M.; Bos, G.M.J.; Tilanus, M.G.J.; Voorter, C.E.M.; Wieten, L. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 2018, 8, 15983. [Google Scholar] [CrossRef] [PubMed]
- Mata-Molanes, J.J.; Rebollo-Liceaga, J.; Martínez-Navarro, E.M.; Manzano, R.G.; Brugarolas, A.; Juan, M.; Sureda, M. Relevance of Fc Gamma Receptor Polymorphisms in Cancer Therapy with Monoclonal Antibodies. Front. Oncol. 2022, 12, 926289. [Google Scholar] [CrossRef]
- Roßkopf, S.; Eichholz, K.M.; Winterberg, D.; Diemer, K.J.; Lutz, S.; Münnich, I.A.; Klausz, K.; Rösner, T.; Valerius, T.; Schewe, D.M.; et al. Enhancing CDC and ADCC of CD19 Antibodies by Combining Fc Protein-Engineering with Fc Glyco-Engineering. Antibodies 2020, 9, 63. [Google Scholar] [CrossRef]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef]
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother 2017, 44, 327–336. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Bachelot, T.; Wright, G.S.; Saura, C.; et al. Margetuximab Versus Trastuzumab in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results from a Randomized Phase 3 Trial. J. Clin. Oncol. 2023, 41, 198–205. [Google Scholar] [CrossRef]
- Izadi, A.; Naimi, A.; Amjadi, E.; Beheshtiparvar, D.; Soltan, M. The Prevalence of PD-L1 Expression in Triple-Negative Breast Cancer Patients and Its Correlation with Survival Rates and Other Prognostic Factors: A Survival Analysis. Adv. Biomed. Res. 2024, 13, 86. [Google Scholar] [CrossRef]
- Ignatov, T.; Gorbunow, F.; Eggemann, H.; Ortmann, O.; Ignatov, A. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res. Treat. 2019, 175, 401–408. [Google Scholar] [CrossRef]
- Dawood, S.; Resetkova, E.; Gonzalez-Angulo, A.M. Trastuzumab Administration Associated with Change in HER2 Status. Clin. Breast Cancer 2008, 8, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S.; et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Sinberger, L.A.; Salmon-Divon, M.; Ben-Dror, J.; Shachar, S.S.; Sonnenblick, A. Impact of the OncotypeDX score and HER2 RNA PCR levels on HER2-low IHC levels in primary and metastasized tumors. BMC Cancer 2023, 23, 1031. [Google Scholar] [CrossRef]
- Schreiber, A.R.; O’Bryant, C.L.; Kabos, P.; Diamond, J.R. The emergence of targeted therapy for HER2-low triple-negative breast cancer: A review of fam-trastuzumab deruxtecan. Expert. Rev. Anticancer. Ther. 2023, 23, 1061–1069. [Google Scholar] [CrossRef]
- Horn, D.M.; Alpert, A.E.; Duggan, M.G.; Garcia, N.A.; Jacobson, M. Biosimilar Competition and Payments in Medicare: The Case of Trastuzumab. JCO Oncol. Pract. 2023, 19, e476–e483. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health (CADTH). Trastuzumab Deruxtecan (Enhertu). Available online: https://www.ncbi.nlm.nih.gov/books/NBK595132/ (accessed on 8 February 2025).
- Li, y.; Wang, M.; Fan, T.; Wang, Y.; Chen, L.; Zhu, C.; Li, Z.; Mou, L.; Zhang, Z.; Chen, L.; et al. CD147 promotes cisplatin resistance in ovarian cancer by inhibiting FOXM1 degradation via PI3k/Akt-GSK3β pathway. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Dong, S.; Li, S.; Wang, X.; Liang, S.; Zhang, W.; Li, L.; Xu, Q.; Shi, B.; Cheng, Z.; Zhang, X.; et al. CD147 Mediates 5-Fluorouracil Resistance in Colorectal Cancer by Reprogramming Glycolipid Metabolism. Front. Oncol. 2022, 12, 813852. [Google Scholar] [CrossRef]
- Huang, D.-F.; Zhang, W.-J.; Chen, J.; Jiao, Z.-G.; Wang, X.-L.; Rao, D.-Y.; Li, W.-S.; Hu, D.; Xie, F.-F.; Wang, X.-X.; et al. Hepatocellular carcinoma cell-derived small extracellular vesicle-associated CD147 serves as a diagnostic marker and promotes endothelial cell angiogenesis via the PI3K/Akt pathway. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 532–547. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Zhang, Y.; Wang, C.; Wang, W.; Zheng, A. OIP5-AS1/CD147/TRPM7 axis promotes gastric cancer metastasis by regulating apoptosis related PI3K-Akt signaling. Front. Oncol. 2023, 13, 1221445. [Google Scholar] [CrossRef]
- Costa, R.L.B.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef]
- Zha, D.; Li, Y.; Luo, Y.; Liu, Y.; Lin, Z.; Lin, C.; Chen, S.; Wu, J.; Yu, L.; Chen, S.; et al. Synthesis and in vitro anticancer evaluation of novel flavonoid-based amide derivatives as regulators of the PI3K/AKT signal pathway for TNBC treatment. RSC Med. Chem. 2022, 13, 1082–1099. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongheang, K.; Pamonsupornwichit, T.; Sornsuwan, K.; Juntit, O.-a.; Chokepaichitkool, T.; Thongkum, W.; Yasamut, U.; Tayapiwatana, C. Potentiating Antibody-Dependent Cellular Cytotoxicity in Triple-Negative Breast Cancer via the Humanized Anti-CD147 Antibody. Antibodies 2025, 14, 36. https://doi.org/10.3390/antib14020036
Thongheang K, Pamonsupornwichit T, Sornsuwan K, Juntit O-a, Chokepaichitkool T, Thongkum W, Yasamut U, Tayapiwatana C. Potentiating Antibody-Dependent Cellular Cytotoxicity in Triple-Negative Breast Cancer via the Humanized Anti-CD147 Antibody. Antibodies. 2025; 14(2):36. https://doi.org/10.3390/antib14020036
Chicago/Turabian StyleThongheang, Kanyarat, Thanathat Pamonsupornwichit, Kanokporn Sornsuwan, On-anong Juntit, Tawan Chokepaichitkool, Weeraya Thongkum, Umpa Yasamut, and Chatchai Tayapiwatana. 2025. "Potentiating Antibody-Dependent Cellular Cytotoxicity in Triple-Negative Breast Cancer via the Humanized Anti-CD147 Antibody" Antibodies 14, no. 2: 36. https://doi.org/10.3390/antib14020036
APA StyleThongheang, K., Pamonsupornwichit, T., Sornsuwan, K., Juntit, O.-a., Chokepaichitkool, T., Thongkum, W., Yasamut, U., & Tayapiwatana, C. (2025). Potentiating Antibody-Dependent Cellular Cytotoxicity in Triple-Negative Breast Cancer via the Humanized Anti-CD147 Antibody. Antibodies, 14(2), 36. https://doi.org/10.3390/antib14020036