Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Dye
2.2. Cloning, Expression and Purification of the Anti-CD44 and Anti-EpCAM Antibodies
2.3. Conjugation of the Photosensitizer Dye WB692-CB2
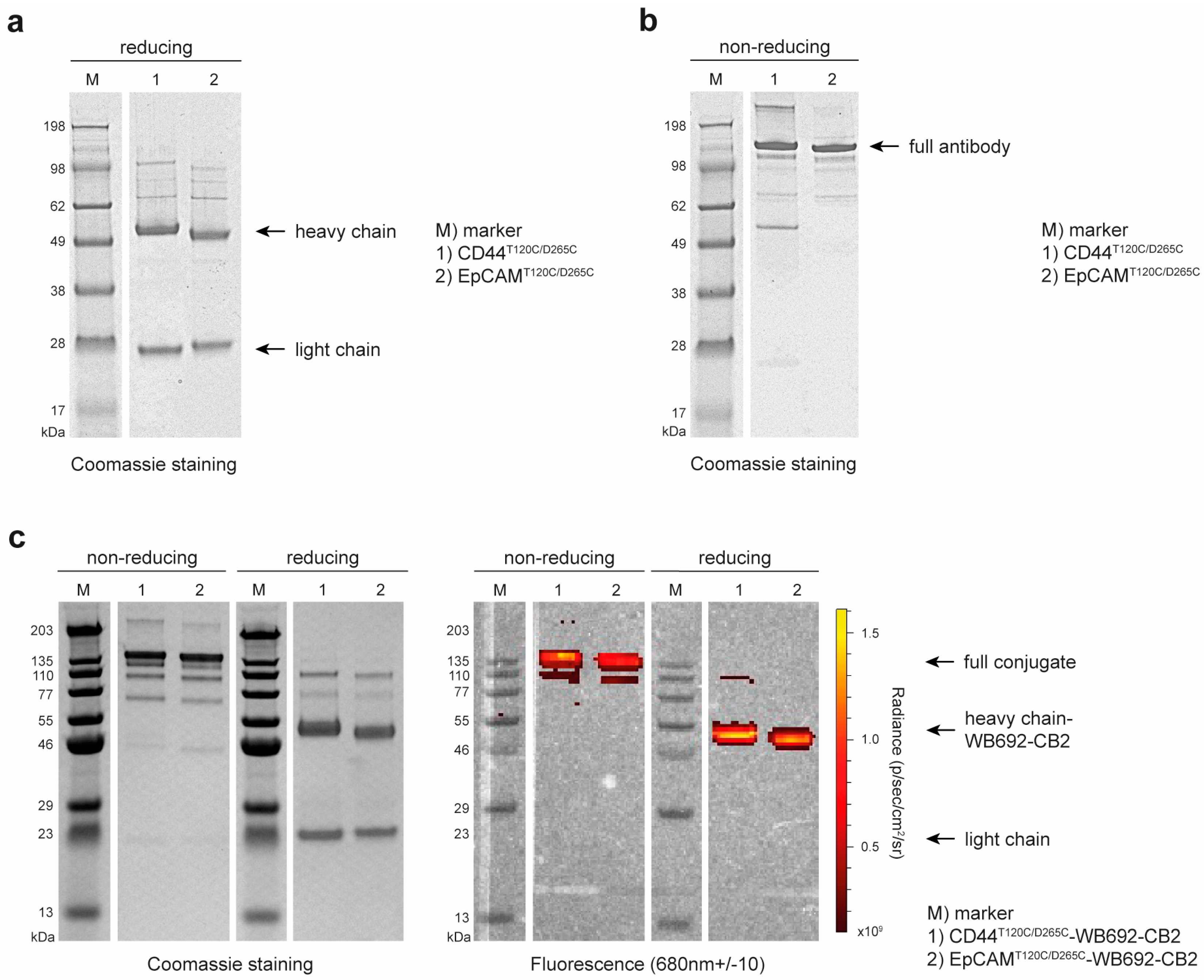
2.4. SDS-PAGE and Western Blots
2.5. Flow Cytometry
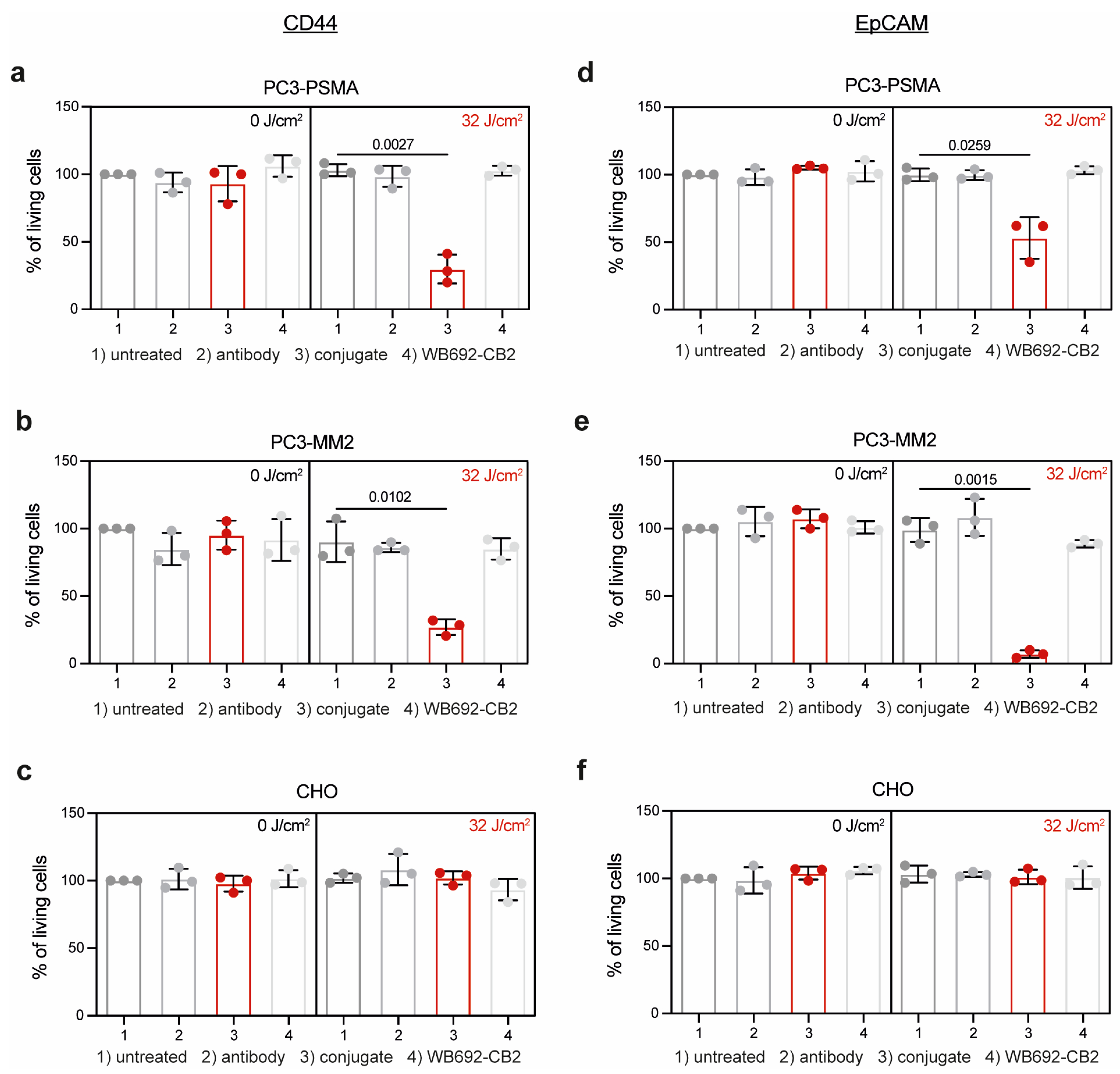
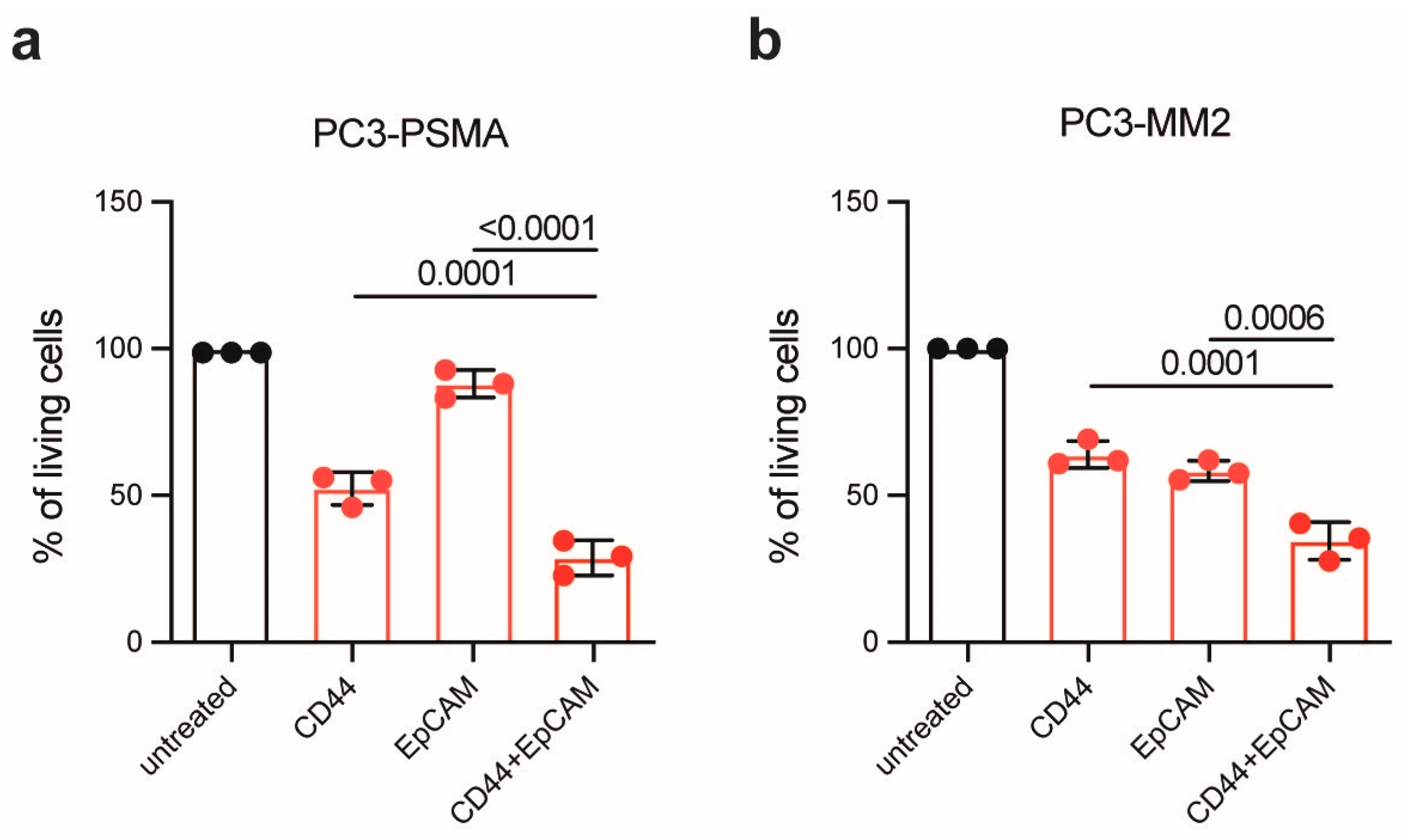
2.6. Photoimmunotherapy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The lancet commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F.; Moul, J.W. Rising prostate-specific antigen after primary prostate cancer therapy. Nat. Clin. Pract. Urol. 2005, 2, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Cheng, L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: Preclinical models, emerging technologies, and future applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef]
- Wolf, I.; Gratzke, C.; Wolf, P. Prostate cancer stem cells: Clinical aspects and targeted therapies. Front. Oncol. 2022, 12, 935715. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.; Zhang, C.; Sheng, W.; Al-Rawe, M.; Zeppernick, F.; Meinhold-Heerlein, I.; Hussain, A.F. Near infrared photoimmunotherapy: A review of recent progress and their target molecules for cancer therapy. Int. J. Mol. Sci. 2023, 24, 2655. [Google Scholar] [CrossRef]
- Rowehl, R.A.; Crawford, H.; Dufour, A.; Ju, J.; Botchkina, G.I. Genomic analysis of prostate cancer stem cells isolated from a highly metastatic cell line. Cancer Genom. Proteom. 2008, 5, 301–310. [Google Scholar]
- Lee, Y.C.; Jin, J.K.; Cheng, C.J.; Huang, C.F.; Song, J.H.; Huang, M.; Brown, W.S.; Zhang, S.; Yu-Lee, L.Y.; Yeh, E.T.; et al. Targeting constitutively activated β1 integrins inhibits prostate cancer metastasis. Mol. Cancer Res. MCR 2013, 11, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Botchkina, G.I.; Zuniga, E.S.; Rowehl, R.H.; Park, R.; Bhalla, R.; Bialkowska, A.B.; Johnson, F.; Golub, L.M.; Zhang, Y.; Ojima, I.; et al. Prostate cancer stem cell-targeted efficacy of a new-generation taxoid, sbt-1214 and novel polyenolic zinc-binding curcuminoid, cmc2.24. PLoS ONE 2013, 8, e69884. [Google Scholar] [CrossRef]
- Wolf, I.; Storz, J.; Schultze-Seemann, S.; Esser, P.R.; Martin, S.F.; Lauw, S.; Fischer, P.; Peschers, M.; Melchinger, W.; Zeiser, R.; et al. A new silicon phthalocyanine dye induces pyroptosis in prostate cancer cells during photoimmunotherapy. Bioact. Mater. 2024, 41, 537–552. [Google Scholar] [CrossRef]
- Birzele, F.; Cannarile, M.; Feuerhake, F.; Fischer, T.; Heil, F.; Honold, K.; Nopora, A.; Schmitt-Graeff, A.; Voss, E.; Weigand, S. Markers for Responsiveness to Anti-cd44 Antibodies. Patent WO2014198843A1, 12 June 2014. [Google Scholar]
- Cizeau, J.; Macdonald, G.; Premsukh, A. Optimized Nucleic Acid Sequences for the Expression of vb4-845. Patent WO2009039630A1, 26 September 2008. [Google Scholar]
- Steinwand, M.; Droste, P.; Frenzel, A.; Hust, M.; Dübel, S.; Schirrmann, T. The influence of antibody fragment format on phage display based affinity maturation of igg. mAbs 2014, 6, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.A.; Okamura, S.M.; De Magalhaes Filho, C.D.; Bergeron, D.M.; Rodriguez, A.; West, M.; Yadav, D.; Heim, R.; Fong, J.J.; Garcia-Guzman, M. Cancer-targeted photoimmunotherapy induces antitumor immunity and can be augmented by anti-pd-1 therapy for durable anticancer responses in an immunologically active murine tumor model. Cancer Immunol. Immunother. 2023, 72, 151–168. [Google Scholar] [CrossRef]
- Hou, J.; Hsu, J.M.; Hung, M.C. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol. Cell 2021, 81, 4579–4590. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Okuyama, S.; Fukushima, H.; Choyke, P.L.; Kobayashi, H. Simultaneously combined cancer cell- and ctla4-targeted nir-pit causes a synergistic treatment effect in syngeneic mouse models. Mol. Cancer Ther. 2021, 20, 2262–2273. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Thurber, G.M.; Weissleder, R. Quantitating antibody uptake in vivo: Conditional dependence on antigen expression levels. Mol. Imaging Biol. 2011, 13, 623–632. [Google Scholar] [CrossRef] [PubMed][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, I.; Schultze-Seemann, S.; Gratzke, C.; Wolf, P. Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer. Antibodies 2025, 14, 5. https://doi.org/10.3390/antib14010005
Wolf I, Schultze-Seemann S, Gratzke C, Wolf P. Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer. Antibodies. 2025; 14(1):5. https://doi.org/10.3390/antib14010005
Chicago/Turabian StyleWolf, Isis, Susanne Schultze-Seemann, Christian Gratzke, and Philipp Wolf. 2025. "Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer" Antibodies 14, no. 1: 5. https://doi.org/10.3390/antib14010005
APA StyleWolf, I., Schultze-Seemann, S., Gratzke, C., & Wolf, P. (2025). Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer. Antibodies, 14(1), 5. https://doi.org/10.3390/antib14010005






