SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Acquisition
2.2. Assessment of Humoral Immunity
2.3. Statistics
3. Results
3.1. Patient Characteristics
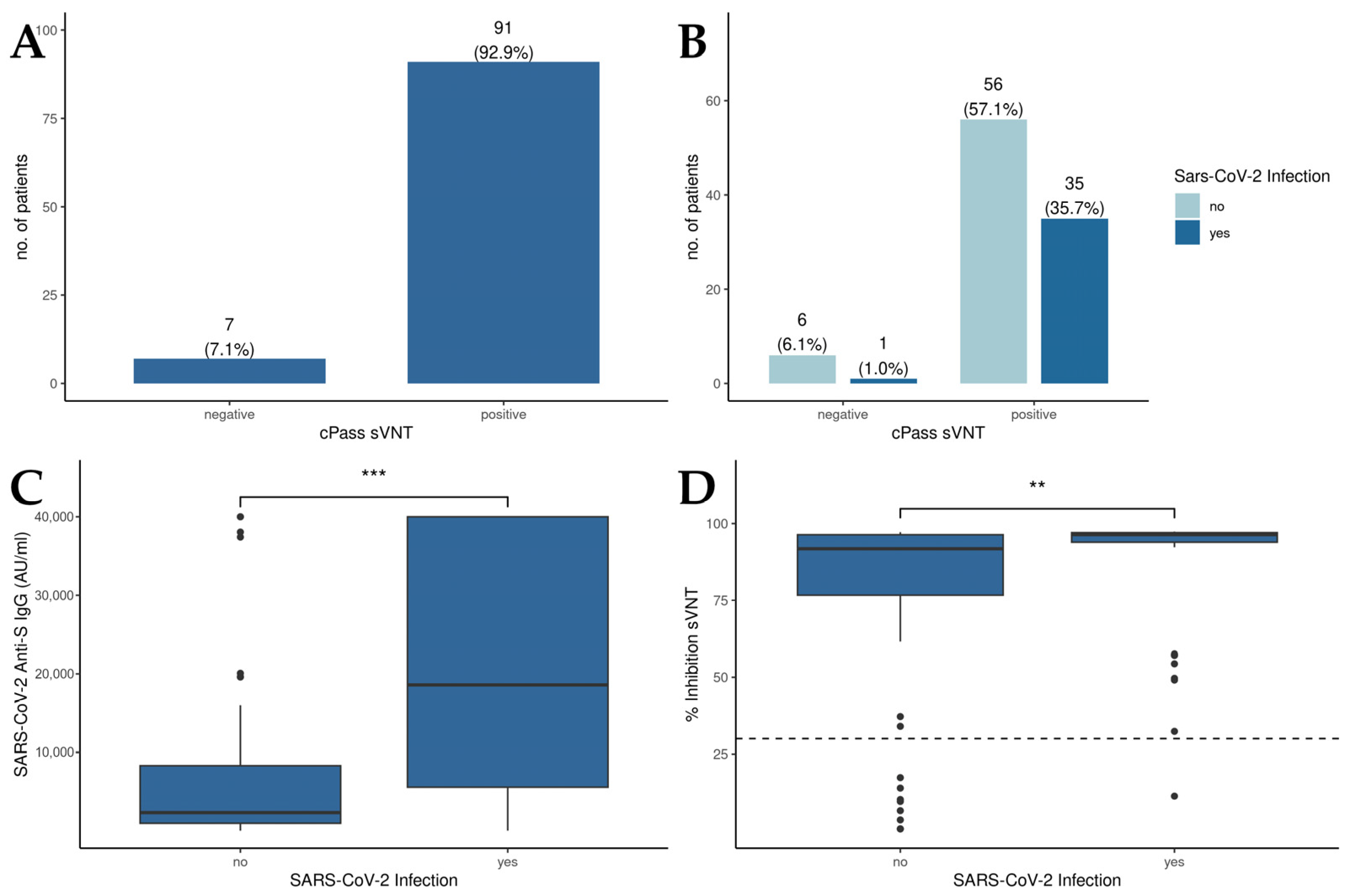
3.2. SARS-CoV-2 Infection Augments the Humoral Immune Response in Vaccinated Liver Transplant Recipients
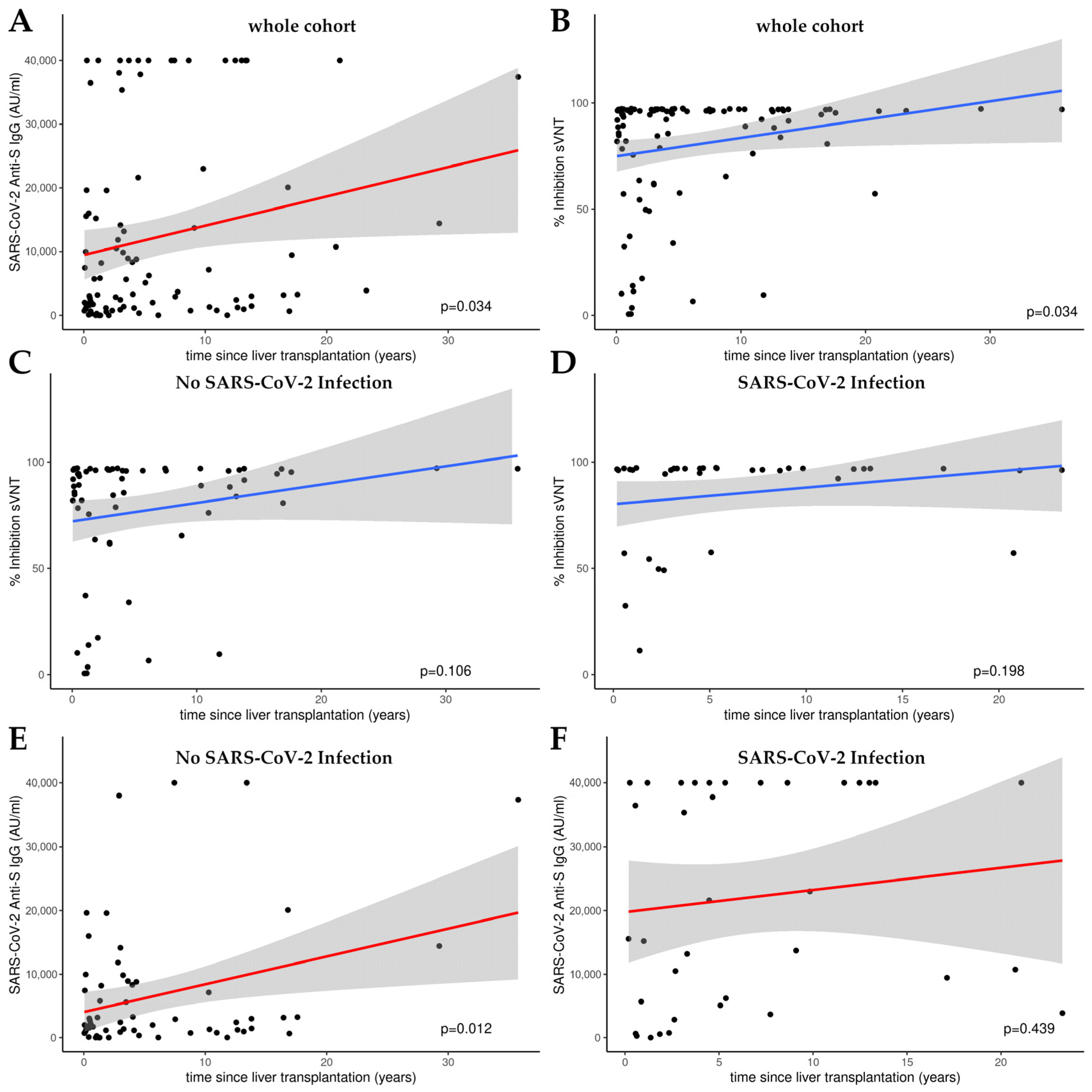
3.3. Time-Dependent Amplification of Humoral Immune Response in Liver Transplant Recipients: A Linear Model Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| Abbreviation | Definition |
| AU | arbitrary units |
| BAU | binding antibody units |
| CMIA | chemiluminescence microparticle assay |
| ELISA | enzyme-linked immunosorbent assay |
| hACE2 | human angiotensin-converting enzyme 2 |
| RBD | receptor-binding domain |
| sVNT | surrogate virus neutralization test |
References
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Imam, A.; Abukhalaf, S.A.; Merhav, H.; Abu-Gazala, S.; Cohen-Arazi, O.; Pikarsky, A.J.; Safadi, R.; Khalaileh, A. Prognosis and Treatment of Liver Transplant Recipients in the COVID-19 Era: A Literature Review. Ann. Transplant. 2020, 25, e926196. [Google Scholar] [CrossRef]
- Thuluvath, P.J.; Robarts, P.; Chauhan, M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J. Hepatol. 2021, 75, 1434–1439. [Google Scholar] [CrossRef]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162–172.e9. [Google Scholar] [CrossRef]
- Harberts, A.; Schaub, G.M.; Ruether, D.F.; Duengelhoef, P.M.; Brehm, T.T.; Karsten, H.; Fathi, A.; Jahnke-Triankowski, J.; Fischer, L.; Addo, M.M.; et al. Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2022, 20, 2558–2566.e5. [Google Scholar] [CrossRef]
- Herrera, S.; Colmenero, J.; Pascal, M.; Escobedo, M.; Castel, M.A.; Sole-González, E.; Palou, E.; Egri, N.; Ruiz, P.; Mosquera, M.; et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021, 21, 3971–3979. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.-R.; Kühn, J.E.; Hennies, M.; Strauss, M.; Rennebaum, F.; Schomacher, T.; Boeckel, G.; Lorentzen, E.; Bokemeyer, A.; et al. Humoral Immune Response in IBD Patients Three and Six Months after Vaccination with the SARS-CoV-2 mRNA Vaccines mRNA-1273 and BNT162b2. Biomedicines 2022, 10, 171. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.-R.; Lorentzen, E.; Nowacki, T.M. Impaired Humoral Immunity with Concomitant Preserved T Cell Reactivity in IBD Patients on Treatment with Infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA Vaccine BNT162b2: A Pilot Study. J. Pers. Med. 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Schoefbaenker, M.; Neddermeyer, R.; Guenther, T.; Mueller, M.M.; Romberg, M.-L.; Classen, N.; Hennies, M.T.; Hrincius, E.R.; Ludwig, S.; Kuehn, J.E.; et al. Surrogate Virus Neutralisation Test Based on Nanoluciferase-Tagged Antigens to Quantify Inhibitory Antibodies against SARS-CoV-2 and Characterise Omicron-Specific Reactivity in a Vaccination Cohort. Vaccines 2023, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Ayala-Borges, B.; Escobedo, M.; Egri, N.; Herrera, S.; Crespo, M.; Mirabet, S.; Arias-Cabrales, C.; Vilella, A.; Palou, E.; Mosquera, M.M.; et al. Impact of SARS-CoV-2 Infection on Humoral and Cellular Immunity in a Cohort of Vaccinated Solid Organ Transplant Recipients. Vaccines 2023, 11, 1845. [Google Scholar] [CrossRef]
- Kirchner, T.; Heinrich, S.; Bonifacius, A.; Engel, B.; Ruhl, L.; Pink, I.; Thomas, N.; Martens, J.; Hoeper, M.M.; Blasczyk, R.; et al. Reduced humoral but stable cellular SARS-CoV-2-specific immunity in liver transplant recipients in the first year after COVID-19. PLoS ONE 2022, 17, e0276929. [Google Scholar] [CrossRef]
- Caballero-Marcos, A.; Salcedo, M.; Alonso-Fernández, R.; Rodríguez-Perálvarez, M.; Olmedo, M.; Graus Morales, J.; Cuervas-Mons, V.; Cachero, A.; Loinaz-Segurola, C.; Iñarrairaegui, M.; et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am. J. Transplant. 2021, 21, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- van Thiel, D.H.; el-Ashmawy, L.; Love, K.; Gavaler, J.S.; Starzl, T.E. Response to hepatitis B vaccination by liver transplant candidates. Dig. Dis. Sci. 1992, 37, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Loinaz, C.; de Juanes, J.R.; Gonzalez, E.M.; López, A.; Lumbreras, C.; Gómez, R.; Gonzalez-Pinto, I.; Jiménez, C.; Garcia, I.; Fuertes, A. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology 1997, 44, 235–238. [Google Scholar] [PubMed]
| No SARS-CoV-2 Infection (n = 62) | SARS-CoV-2 Infection (n = 36) | p-Value | ||
|---|---|---|---|---|
| Patient characteristics | Age, years median (IQR) | 62.5 (17.8) | 60.5 (15.5) | 0.563 |
| Sex, male, total | 35 (35.7%) | 20 (20.4%) | 0.931 | |
| Months after transplantation, median (IQR) | 36.7 (111) | 54.6 (92) | 0.218 | |
| Retransplantation | 7 (7.1%) | 6 (6.1%) | 0.449 | |
| Pre-existing conditions | Diabetes mellitus | 14 (14.2%) | 8 (8.2%) | 0.140 |
| Kidney insufficiency | 24 (24.5%) | 10 (10.2%) | 0.273 | |
| Inflammatory disease | 16 (16.3%) | 12 (12.2%) | 0.427 | |
| Medication | Tacrolimus | 50 (51%) | 30 (30.6%) | 0.740 |
| Everolimus | 19 (19.4%) | 12 (12.2%) | 0.783 | |
| Mycophenolatmofetil | 36 (36.7%) | 18 (18.4%) | 0.439 | |
| Ciclosporin | 3 (3.1%) | 2 (2%) | 0.876 | |
| Prednisolon | 17 (17.3%) | 9 (9.2%) | 0.794 | |
| Sirolimus | 2 (2%) | 2 (2%) | 0.574 | |
| Immunosuppressive therapy | 0.957 1 | |||
| Immunosuppressive monotherapy | 11 (11.2%) | 6 (6.1%) | 0.892 | |
| Immunosuppressive dual therapy | 36 (36.7%) | 22 (22.4%) | 0.767 | |
| Immunosuppressive triple therapy | 15 (15.3%) | 8 (8.2%) | 0.824 | |
| Indication for liver transplantation | 0.993 1 | |||
| Hepatocellular carcinoma | 7 (7.1%) | 10 (10.2%) | 0.676 | |
| Primary sclerosing cholangitis | 6 (6.1%) | 6 (6.1%) | 1 | |
| Secondary sclerosing cholangitis | 1 (1%) | 2 (2%) | 0.901 | |
| Autoimmune hepatitis | 2 (2%) | 4 (4.1%) | 0.858 | |
| Ethanol related | 4 (4.1%) | 9 (9.2%) | 0.632 | |
| Drug induced liver injury | 2 (2%) | 3 (3.1%) | 0.876 | |
| Viral hepatitis | 3 (3.1%) | 4 (4.1%) | 0.727 | |
| Budd Chiari | 1 (1%) | 1 (1%) | 1 | |
| Non-alcoholic steatohepatitis | 1 (1%) | 4 (4.1%) | 0.426 | |
| Cryptogene | 6 (6.1%) | 10 (10.2%) | 0.945 | |
| Other | 3 (3.1%) | 9 (9.2%) | 0.544 | |
| No SARS-CoV-2 Infection (n = 62) | SARS-CoV-2 Infection (n = 36) | p-Value | ||
|---|---|---|---|---|
| Manufacturer of first vaccine | 0.727 | |||
| mRNA (BNT162b2 and mRNA-1273) | 58 (93.5%) | 33 (91.7%) | n.d. | |
| vector-based (ChAdOx1-S and Ad26.COV2.S) | 4 (6.5%) | 3 (8.3%) | n.d. | |
| no vaccination | 0 | 0 | n.d. | |
| Manufacturer of second vaccine | 0.070 | |||
| mRNA (BNT162b2 and mRNA-1273) | 62 (100%) | 33 (91.7%) | 0.021 | |
| vector-based (ChAdOx1-S and Ad26.COV2.S) | 0 (0%) | 2 (5.6%) | 0.061 | |
| no vaccination | 0 (0%) | 1 (2.8%) | 0.187 | |
| Manufacturer of third vaccine | 0.049 | |||
| mRNA (BNT162b2 and mRNA-1273) | 58 (93.5%) | 29 (80.6%) | n.d. | |
| vector-based (ChAdOx1-S and Ad26.COV2.S) | 0 | 0 | n.d. | |
| no vaccination | 4 (6.5%) | 7 (19.4%) | n.d. | |
| Manufacturer of fourth vaccine | 0.040 | |||
| mRNA (BNT162b2 and mRNA-1273) | 34 (54.8%) | 12 (33.3%) | n.d. | |
| vector-based (ChAdOx1-S and Ad26.COV2.S) | 0 | 0 | n.d. | |
| no vaccination | 28 (45.2%) | 24 (66.7%) | n.d. | |
| Manufacturer of fifth vaccine | 0.901 | |||
| mRNA (BNT162b2 and mRNA-1273) | 2 (3.2%) | 1 (2.8%) | n.d. | |
| vector-based (ChAdOx1-S and Ad26.COV2.S) | 0 | 0 | n.d. | |
| no vaccination | 60 (96.8%) | 35 (97.2%) | n.d. | |
| Vaccination History | vaccinations | 0.101 | ||
| 1 vaccination | 0 (0%) | 1 (2.8%) | 0.187 | |
| 2 vaccinations | 3 (4.8%) | 6 (16.7%) | 0.051 | |
| 3 vaccinations | 25 (40.3%) | 17 (47.2%) | 0.506 | |
| 4 vaccinations | 32 (51.6%) | 11 (30.6%) | 0.043 | |
| 5 vaccinations | 2 (3.2%) | 1 (2.8%) | 0.901 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiprasito, J.B.; Nowacki, T.; Vollenberg, R.; Meier, J.A.; Rennebaum, F.; Schomacher, T.; Trebicka, J.; Fischer, J.; Lorentzen, E.U.; Tepasse, P.-R. SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients. Antibodies 2024, 13, 78. https://doi.org/10.3390/antib13030078
Adiprasito JB, Nowacki T, Vollenberg R, Meier JA, Rennebaum F, Schomacher T, Trebicka J, Fischer J, Lorentzen EU, Tepasse P-R. SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients. Antibodies. 2024; 13(3):78. https://doi.org/10.3390/antib13030078
Chicago/Turabian StyleAdiprasito, Jan Basri, Tobias Nowacki, Richard Vollenberg, Jörn Arne Meier, Florian Rennebaum, Tina Schomacher, Jonel Trebicka, Julia Fischer, Eva U. Lorentzen, and Phil-Robin Tepasse. 2024. "SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients" Antibodies 13, no. 3: 78. https://doi.org/10.3390/antib13030078
APA StyleAdiprasito, J. B., Nowacki, T., Vollenberg, R., Meier, J. A., Rennebaum, F., Schomacher, T., Trebicka, J., Fischer, J., Lorentzen, E. U., & Tepasse, P.-R. (2024). SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients. Antibodies, 13(3), 78. https://doi.org/10.3390/antib13030078






