Platform-Specific Fc N-Glycan Profiles of an Antisperm Antibody
Abstract
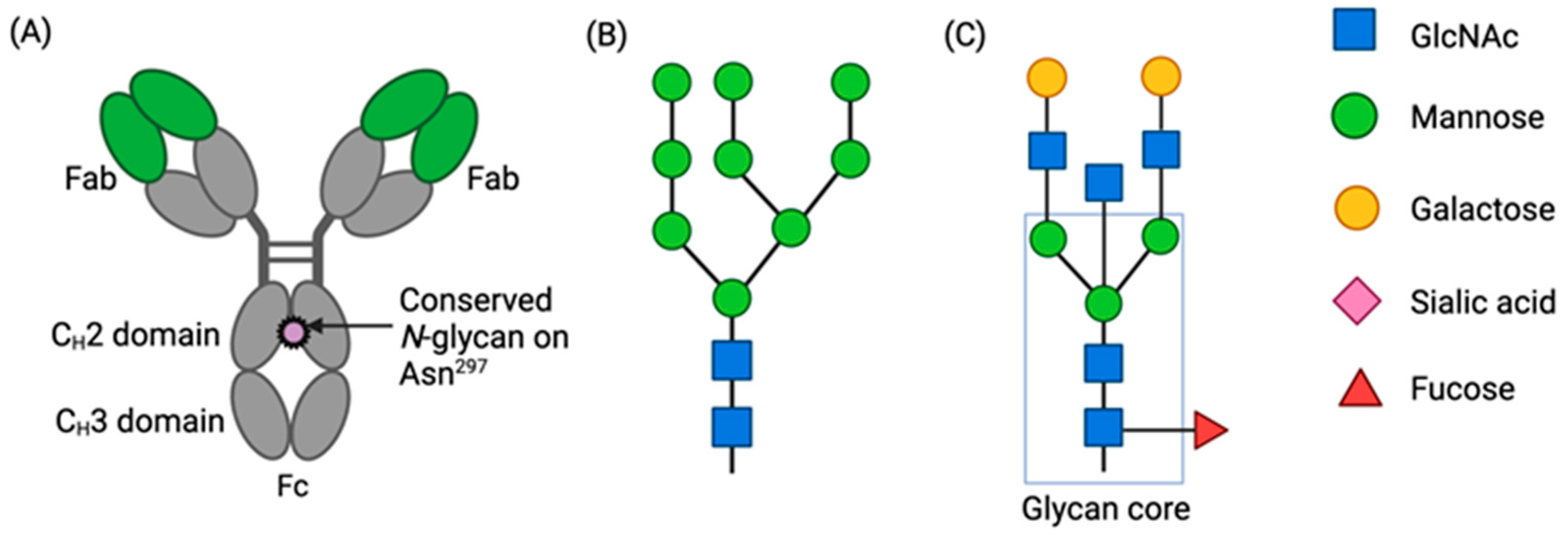
1. Introduction
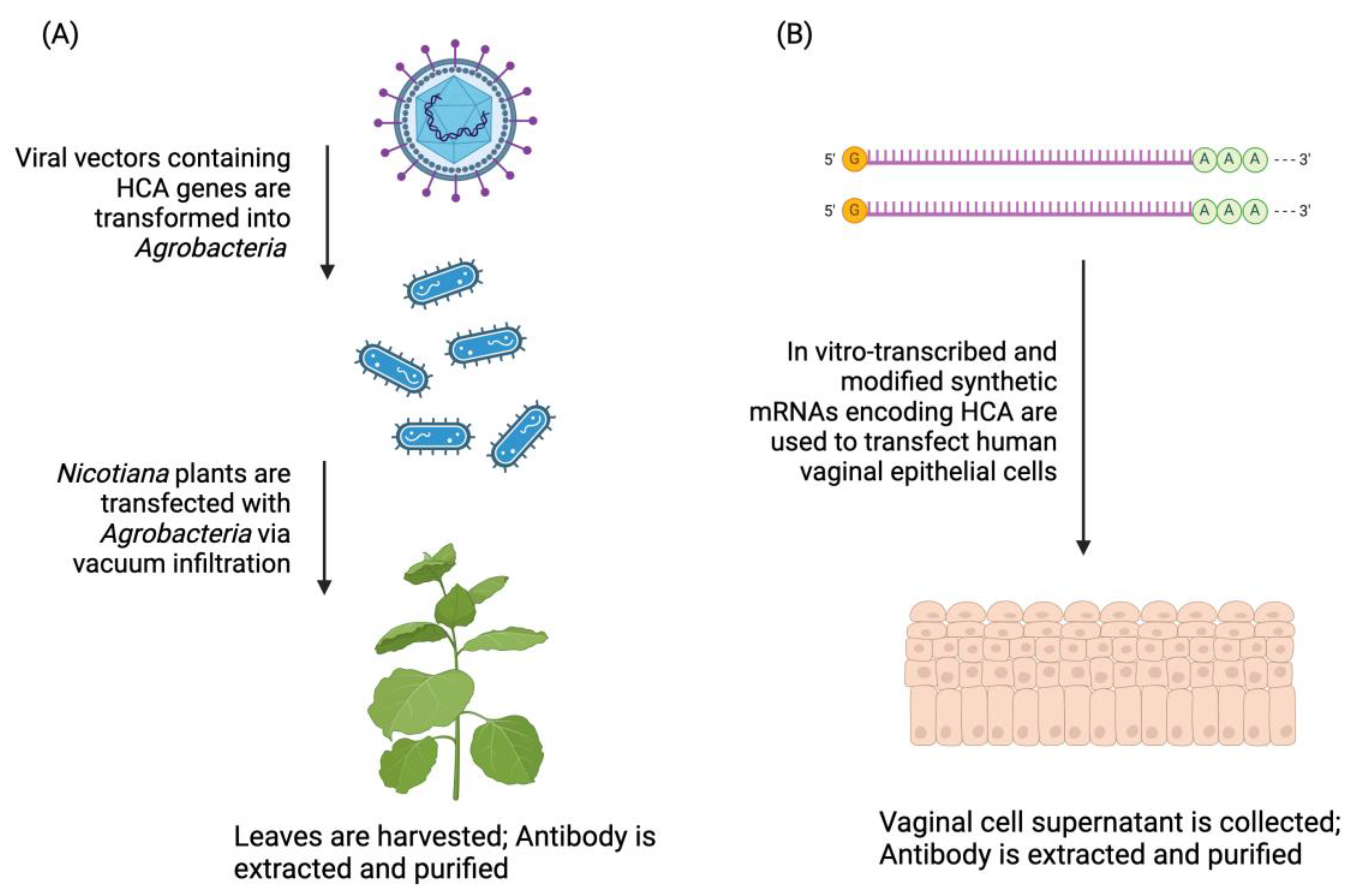
2. Materials and Methods
2.1. mRNA HCA Production (HCAmRNA_VK2)
2.2. Nicotiana HCA Production (HCA-N)
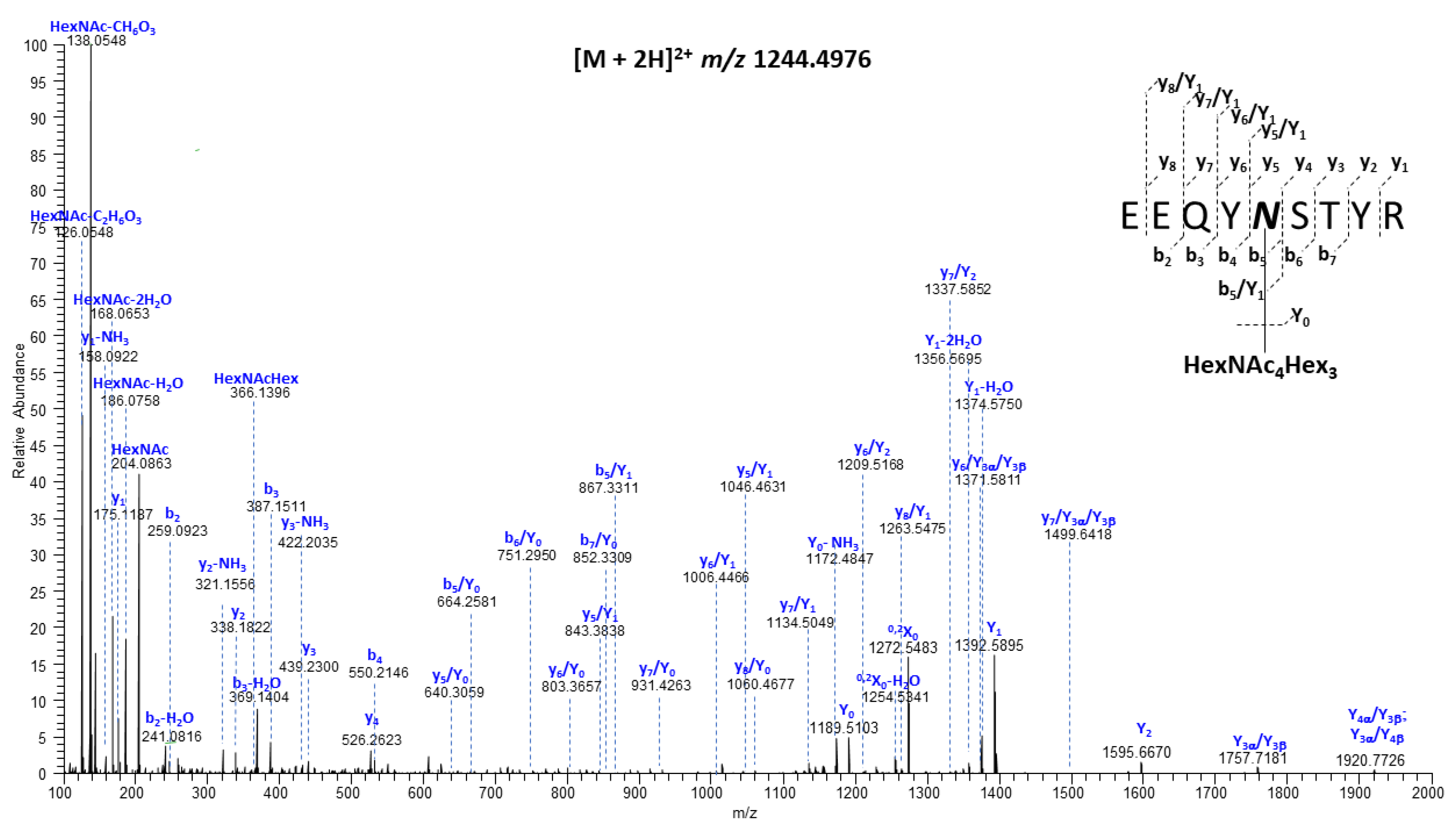
2.3. Mass Spectrometry Analysis
2.4. Antibody-Dependent Sperm Phagocytosis
2.5. Statistical Analysis
3. Results
3.1. Sequence Coverage Was Sufficient for Both HCA-N and HCAmRNA
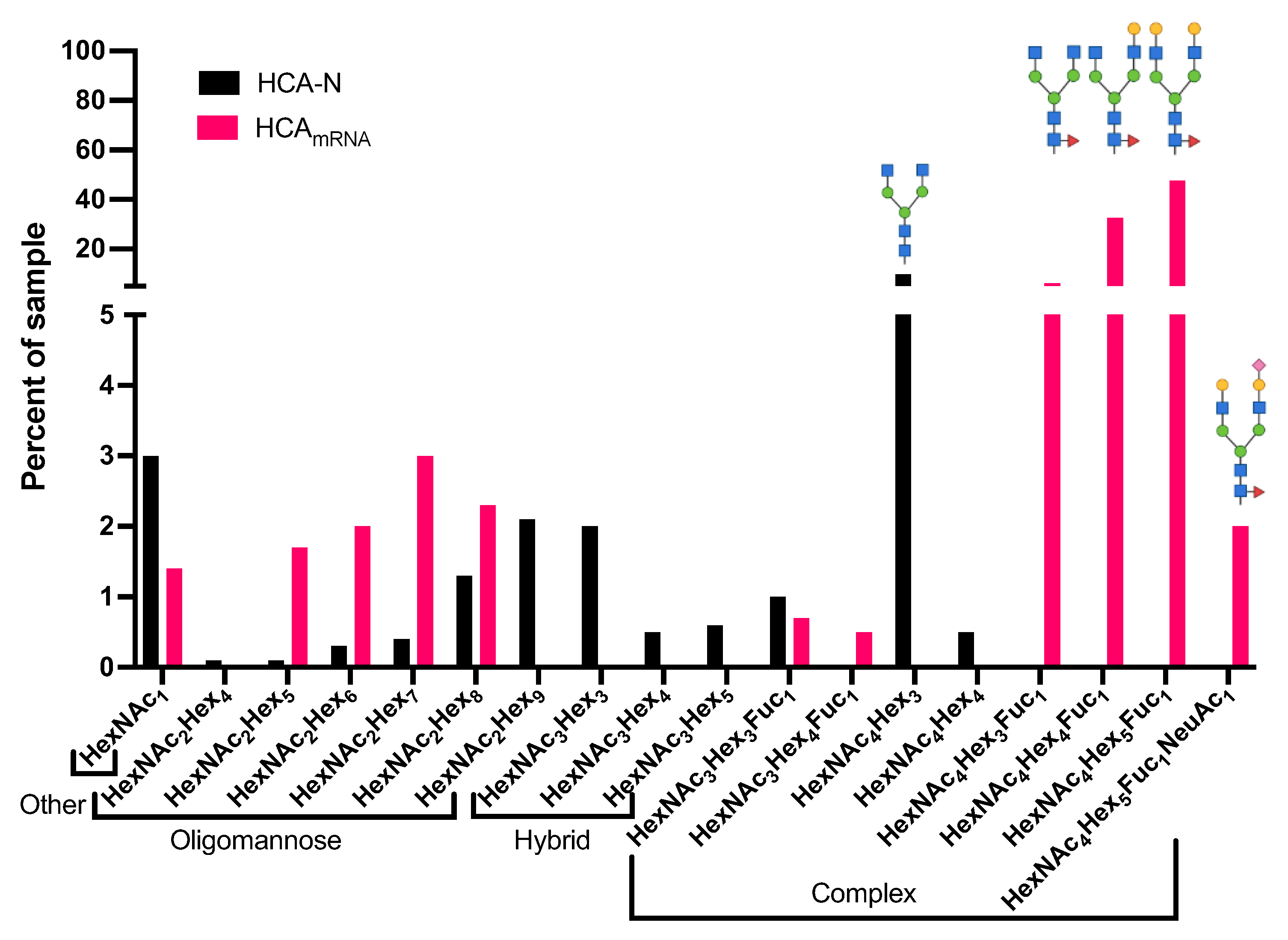
3.2. Compositional Differences in N-Glycan Profiles Were Observed between HCA-N and HCAmRNA
3.3. HCA-N and HCAmRNA Exhibit Different Levels of Sperm Phagocytosis Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramazi, S.; Zahiri, J. Post-translational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- Lee, H.S.; Im, W. Effects of N-Glycan Composition on Structure and Dynamics of IgG1 Fc and Their Implications for Antibody Engineering. Sci. Rep. 2017, 7, 12659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, J.; Lu, H. Antibody glycosylation: Impact on antibody drug characteristics and quality control. Appl. Microbiol. Biotechnol. 2020, 104, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Matsui, M.; Kawasaki, N. Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. mAbs 2019, 11, 350–372. [Google Scholar] [CrossRef]
- Macher, B.A.; Galili, U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: A carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-R.; Song, A.; Bergelson, S.; Arroll, T.; Parekh, B.; May, K.; Chung, S.; Strouse, R.; Mire-Sluis, A.; Schenerman, M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011, 10, 101–111. [Google Scholar] [CrossRef]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. mAbs 2023, 15, 2153410. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A. The history of IgG glycosylation and where we are now. Glycobiology 2020, 30, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Kizuka, Y. N-Glycosylation. In The Role of Glycosylation in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Varki, A.; Kornfeld, S. Historical Background and Overview. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R., Esko, J., Al, E., Eds.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022. [Google Scholar]
- Abès, R.; Teillaud, J.-L. Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals 2010, 3, 146–157. [Google Scholar] [CrossRef]
- Alter, G.; Ottenhoff, T.H.M.; Joosten, S.A. Antibody glycosylation in inflammation, disease and vaccination. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2018; Volume 39, pp. 102–110. [Google Scholar]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef]
- Peschke, B.; Keller, C.W.; Weber, P.; Quast, I.; Lünemann, J.D. Fc-Galactosylation of Human Immunoglobulin Gamma Isotypes Improves C1q Binding and Enhances Complement-Dependent Cytotoxicity. Front. Immunol. 2017, 8, 646. [Google Scholar] [CrossRef]
- Saunders, K.O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 2019, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Mausser, E.; Nador, E.; Politch, J.A.; Pauly, M.R.; Marathe, J.G.; Moench, T.R.; Zeitlin, L.; Whaley, K.J.; Anderson, D.J. LALAPG variant of the Human Contraception Antibody (HCA) reduces Fc-mediated effector functions while maintaining sperm agglutination activity. PLoS ONE 2023, 18, e0282147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.-J.; Kang, J.-H. Immune Cells in the Female Reproductive Tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, W. Plantibodies. Human antibodies produced by field crops enter clinical trials. Sci. Am. 1997, 227, 44. [Google Scholar] [CrossRef]
- Anderson, D.J.; Politch, J.A.; Cone, R.A.; Zeitlin, L.; Lai, S.K.; Santangelo, P.J.; Moench, T.R.; Whaley, K.J. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies. Biol. Reprod. 2020, 103, 275–285. [Google Scholar] [CrossRef]
- Politch, J.A.; Cu-Uvin, S.; Moench, T.R.; Tashima, K.T.; Marathe, J.G.; Guthrie, K.M.; Cabral, H.; Nyhuis, T.; Brennan, M.; Zeitlin, L.; et al. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLoS Med. 2021, 18, e1003495. [Google Scholar] [CrossRef]
- Thurman, A.R.; Moench, T.R.; Hoke, M.; Politch, J.A.; Cabral, H.; Mausser, E.; Nador, E.; Morton, J.; Hamorsky, K.; Swope, K.; et al. ZB-06, a vaginal film containing an engineered human contraceptive antibody (HC4-N), demonstrates safety and efficacy in a phase 1 postcoital test and safety study. Am. J. Obstet. Gynecol. 2023, 228, 716.e1–716.e12. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vanover, D.; Lindsay, K.E.; Bawage, S.S.; Kirschman, J.L.; Bhosle, S.; Lifland, A.W.; Zurla, C.; Santangelo, P.J. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat. Commun. 2018, 9, 3999. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Vanover, D.; Thoresen, M.; King, H.; Xiao, P.; Badial, P.; Araínga, M.; Bin Park, S.; Tiwari, P.M.; Peck, H.E.; et al. Aerosol Delivery of Synthetic mRNA to Vaginal Mucosa Leads to Durable Expression of Broadly Neutralizing Antibodies against HIV. Mol. Ther. 2020, 28, 805–819. [Google Scholar] [CrossRef]
- Fisher, P.; Thomas-Oates, J.; Wood, A.J.; Ungar, D. The N-Glycosylation Processing Potential of the Mammalian Golgi Apparatus. Front. Cell Dev. Biol. 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Rheinwald, J.G.; Anderson, D.J. Generation of Papillomavirus-Immortalized Cell Lines from Normal Human Ectocervical, Endocervical, and Vaginal Epithelium that Maintain Expression of Tissue-Specific Differentiation Proteins. Biol. Reprod. 1997, 57, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Swope, K.; Morton, J.; Pogue, G.P.; Hume, S.; Pauly, M.H.; Shepherd, J.; Simpson, C.A.; Bratcher, B.; Whaley, K.J.; Zeitlin, L.; et al. Manufacturing plant-made monoclonal antibodies for research or therapeutic applications. Methods Enzymol. 2021, 660, 239–263. [Google Scholar] [PubMed]
- Swope, K.; Morton, J.; Pogue, G.P.; Burden, L.; Partain, N.; Hume, S.; Shepherd, J.; Simpson, C.A.; Brennan, M.B.; Furman, T.C.; et al. Reproducibility and flexibility of monoclonal antibody production with Nicotiana benthamiana. mAbs 2022, 14, 2013594. [Google Scholar] [CrossRef]
- Strasser, R.; Stadlmann, J.; Schähs, M.; Stiegler, G.; Quendler, H.; Mach, L.; Glössl, J.; Weterings, K.; Pabst, M.; Steinkellner, H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008, 6, 392–402. [Google Scholar] [CrossRef]
- Oren-Benaroya, R.; Kipnis, J.; Eisenbach, M. Phagocytosis of human post-capacitated spermatozoa by macrophages. Hum. Reprod. 2007, 22, 2947–2955. [Google Scholar] [CrossRef][Green Version]
- Boylan, K.L.; Afiuni-Zadeh, S.; A Geller, M.; Hickey, K.; Griffin, T.J.; Pambuccian, S.E.; Skubitz, A.P. A feasibility study to identify proteins in the residual Pap test fluid of women with normal cytology by mass spectrometry-based proteomics. Clin. Proteom. 2014, 11, 30. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1998, 5, 397–409. [Google Scholar] [CrossRef]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-based biopharmaceutical engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Bussat, M.-C.; Lokteff, M.; Klinguer-Hamour, C.; Haeuw, J.-F.; Goetsch, L.; Wurch, T.; Dorsselaer, A.; Corvaia, N. Trends in Glycosylation, Glycoanalysis and Glycoengineering of Therapeutic Antibodies and Fc-Fusion Proteins. Curr. Pharm. Biotechnol. 2009, 9, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Sips, M.; Krykbaeva, M.; Diefenbach, T.; Ghebremichael, M.; Bowman, B.; Dugast, A.-S.; Boesch, A.; Streeck, H.; Kwon, D.; Ackerman, M.; et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016, 9, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Hossler, P.; Khattak, S.F.; Li, Z.J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009, 19, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Lood, R.; Nägeli, A. On enzymatic remodeling of IgG glycosylation; unique tools with broad applications. Glycobiology 2020, 30, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell 2018, 9, 47–62. [Google Scholar] [CrossRef]
- Wang, L.-X.; Tong, X.; Li, C.; Giddens, J.P.; Li, T. Glycoengineering of Antibodies for Modulating Functions. Annu. Rev. Biochem. 2019, 88, 433–459. [Google Scholar] [CrossRef]

| Protein | Sequence Coverage | Percentage of Total Sample (%) | |
|---|---|---|---|
| HCA-N | IgG heavy chain | 99.60 | 72 |
| IgG Lambda light chain | 80.80 | 24 | |
| HCAmRNA | IgG heavy chain | 93.84 | 10 |
| IgG Lambda light chain | 65.54 | 5 | |
| Serotransferrin | 80.69 | 66 | |
| Bovine Serum Albumin | 84.02 | 4 | |
| Glutathione S transferase | 84.69 | 2 | |
| Keratin, Type II Cytoskeletal | 45.10 | 2 | |
| Clostridial Collagenase | 40.10 | <1 | |
| Bovine Glutamate Dehydrogenase 1, Mitochondrial | 47.00 | <1 |
| HCA-N | HCAmRNA | |
|---|---|---|
| Occupied | 49.1% | 96.0% |
| Not occupied | 50.9% | 4.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nador, E.; Xia, C.; Santangelo, P.J.; Whaley, K.J.; Costello, C.E.; Anderson, D.J. Platform-Specific Fc N-Glycan Profiles of an Antisperm Antibody. Antibodies 2024, 13, 17. https://doi.org/10.3390/antib13010017
Nador E, Xia C, Santangelo PJ, Whaley KJ, Costello CE, Anderson DJ. Platform-Specific Fc N-Glycan Profiles of an Antisperm Antibody. Antibodies. 2024; 13(1):17. https://doi.org/10.3390/antib13010017
Chicago/Turabian StyleNador, Ellena, Chaoshuang Xia, Philip J. Santangelo, Kevin J. Whaley, Catherine E. Costello, and Deborah J. Anderson. 2024. "Platform-Specific Fc N-Glycan Profiles of an Antisperm Antibody" Antibodies 13, no. 1: 17. https://doi.org/10.3390/antib13010017
APA StyleNador, E., Xia, C., Santangelo, P. J., Whaley, K. J., Costello, C. E., & Anderson, D. J. (2024). Platform-Specific Fc N-Glycan Profiles of an Antisperm Antibody. Antibodies, 13(1), 17. https://doi.org/10.3390/antib13010017






