Abstract
Harnessing the immune system to combat disease has revolutionized medical treatment. Monoclonal antibodies (mAbs), in particular, have emerged as important immunotherapeutic agents with clinical relevance in treating a wide range of diseases, including allergies, autoimmune diseases, neurodegenerative disorders, cancer, and infectious diseases. These mAbs are developed from naturally occurring antibodies and target specific epitopes of single molecules, minimizing off-target effects. Antibodies can also be designed to target particular pathogens or modulate immune function by activating or suppressing certain pathways. Despite their benefit for patients, the production and administration of monoclonal antibody therapeutics are laborious, costly, and time-consuming. Administration often requires inpatient stays and repeated dosing to maintain therapeutic levels, limiting their use in underserved populations and developing countries. Researchers are developing alternate methods to deliver monoclonal antibodies, including synthetic nucleic acid-based delivery, to overcome these limitations. These methods allow for in vivo production of monoclonal antibodies, which would significantly reduce costs and simplify administration logistics. This review explores new methods for monoclonal antibody delivery, including synthetic nucleic acids, and their potential to increase the accessibility and utility of life-saving treatments for several diseases.
1. Introduction
Passive antibody therapy is the treatment of disease using infusions of antibodies made in the laboratory or in animals. This type of therapy differs from active immunity, which occurs when the immune system becomes activated after exposure to vaccines, pathogens, or cancer cells and subsequently generates antibodies within the host. The origins of passive antibody therapy can be traced back to the early 1890s with the work of Behring and Kitasato [1,2,3]. They demonstrated that serum from animals immunized against tetanus toxin could provide protection in other animals against tetanus, which became known as serum therapy. Before using antibiotics, this serum therapy was the first-line treatment for various infectious diseases, despite its associated side effects such as hypersensitivity reactions, serum sickness, and the risk of transmitting blood-borne pathogens [1,4]. However, due to the toxicity of serum therapy and its involved method of administration, its popularity declined in favor of antimicrobials, particularly in the context of infectious diseases. Nevertheless, with the emergence of monoclonal antibodies (mAbs), antibody-based therapy has been revitalized [5,6,7].
2. Monoclonal Antibodies: From Hybridoma to Humanized Transgenic Mice
Typically, foreign agents elicit a polyclonal antibody response in organisms where several different B lymphocytes produce antibodies that target multiple linear and/or conformational epitopes of the foreign molecule. In contrast, mAbs target a single epitope or antigen and are made in the lab by isolating the unique B cell that produces that antibody. Kohler and Milstein first introduced the generation of mAbs in 1975 using the hybridoma technique, which laid the foundation for developing mAbs-based therapeutics and diagnostic tools [8]. Briefly, this method involves the immunization of animals to generate a humoral immune response, followed by isolating the B cells from the immunized animals, usually from their spleen. These B cells are fused to immortal B cell cancer cells, myeloma, to produce hybridoma cells that are selected under hypoxanthine–aminopterin–thymidine (HAT) media to eliminate non-fused cells. The hybridomas are then serially diluted and screened to isolate single clones that all produce the same antibody to the determined target. This technique enables the production of antibodies of a defined specificity in large quantities ex vivo. Alternatively, more efficient methods of identifying mAbs have been explored, including various in vitro display technologies, in vivo phage display, and flow cytometry-based B cell screening and sorting [9,10,11]. The formats of mAbs have been improved over the years, from murine, chimeric, humanized, to fully human mAbs with successively decreasing degrees of immunogenicity in recipients [12]. These technologies have included the grafting of variable domains, the grafting of complementary determining regions, and the use of humanized transgenic mice.
3. Challenges of Making Monoclonal Antibody Therapies More Accessible: Current Limitations and Future Directions
Although mAb therapies are promising for treating various diseases, their accessibility to larger patient populations remains limited due to several challenges. One major challenge is that the current methods of generating high quantities of clinical-grade mAbs are arduous and extremely costly. The large size of mAbs precludes chemical synthesis methods, and the need for proper glycosylation and folding makes lower organism expression platforms unsuitable for commercial mAb production. Therefore, mammalian expression systems are needed to produce biologically functional mAbs. However, using mammalian expression systems is complicated and expensive due to the slow cell growth and low product yield. The most common cell culture line used for current synthetic mAb production is Chinese hamster ovary (CHO) cells, where genes encoding the mAb of interest are introduced into an expression vector that is subsequently stably transfected into CHO cells. Expressed mAbs are secreted into the supernatant and captured through affinity chromatography. However, the transfection, screening, and amplification processes using these cells are technically and logistically laborious, leading to increased production costs and making mAbs an expensive form of therapy [13].
Moreover, harsh conditions are often required to remove biological contaminants introduced by the expression system, which can result in product degradation, aggregation, and yield loss. Following manufacturing, careful formulation is required according to the solubility and intrinsic stability of the mAb product, further adding to the complexity of mAb therapeutics [14]. These challenges must be addressed before mAbs can be deployed on a wider scale and made more accessible to patients.
Recombinant mAbs are expensive therapeutics. For example, a full course of Campath® (Alemtuzumab), a mAb used for treating multiple sclerosis, can cost upwards of USD 60,000 [15]. In contrast, Keytruda® (Pembrolizumab), a Programmed cell death protein 1 (PD-1) inhibitor for treating various cancers, costs USD 10,897 for each dose every three weeks [16]. Additionally, most therapeutic mAbs are administered intravenously in a hospital setting, which can burden the healthcare system. To maintain efficacy, repeated administrations of these antibodies are typically required, and these therapies are frequently cost-prohibitive to patients. Although subcutaneous delivery of mAbs may address some of these shortcomings, not all mAbs can be formulated for this delivery method, as this approach is highly dependent on mAb solubility, viscosity, self-association, intrinsic stability, aggregation, and precipitation profiles [17]. All these barriers potentially limit the accessibility of these therapies to patients.
4. Approaches for Monoclonal Antibody Delivery: A Promising Alternative for Increased Accessibility and Efficiency
To overcome these challenges, gene therapy approaches have gained interest as an alternative method to produce mAb therapeutics. These methods involve delivering genes expressing mAbs of interest instead of the mAbs themselves, allowing in vivo mAb production in the body’s cells and eliminating the need for the production and purification processes currently used for mAbs, as mentioned above. The use of these gene therapy approaches for mAb administration can significantly reduce the costs of these treatments for several reasons. The manufacturing and purification of the viral vectors, DNA plasmids, or lipid nanoparticle-encapsulated mRNAs used to deliver the mAb genes can all be achieved more rapidly and with higher yields than protein-based mAbs. Gene therapy delivery platforms can also be administered easily into muscle or other tissues such as lung, spleen, lymph nodes, and bone marrow, reducing or eliminating the need for hospitalization [18]. Furthermore, depending on the platform, mAb genes may be expressed for several days or months, reducing the need for repeated delivery. In this review, we explore these new monoclonal antibody delivery methods, which can potentially increase the accessibility and utility of these life-saving treatments for several diseases.
5. Different Delivery Platforms for Monoclonal Antibodies
(i) Adeno-associated virus
Various methods are employed for gene delivery, including plasmid-coated gold particles, lipid–DNA complexes, naked DNA with electroporation, and viral vector-based delivery (Table 1). Among these, virus-based vectors are extensively researched, particularly adeno-associated virus (AAV) vectors. AAV is a small 25 nm non-enveloped virus that can infect both dividing and quiescent cells without integrating into the host cell genome. Due to its ability to enter target cells, transfer its genome to the nucleus, and maintain long-term expression, AAV is an attractive vector for gene therapy [19]. AAV-mediated gene delivery has shown a slow but persistent expression profile for several years compared to only several weeks with other viral vectors [20]. AAV also generates fewer adverse immune responses than other virus-based approaches because of its low uptake by antigen-presenting cells and limited presentation on major histocompatibility (MHC) complexes. This limits the host immune responses to both the vector itself as well as the transgene it is expressing [19].
Despite the advantages, AAV delivery has some limitations, such as the capacity to encapsulate only up to 3.3 kb of DNA, limiting the types of mAbs that can be delivered using this method. The use of alternative capsids and self-complementary AAV (scAAV) vectors, though increasing expression, further limits carrying capacity [21,22]. Additionally, pre-existing or host-generated anti-AAV immune responses can neutralize the virus, reducing its efficacy in repeat dosing. This concern, however, appears to be more of an issue with the use of adenovirus-based delivery vectors [23]. Studies have shown that 96% of patients given AAV therapy develop antibodies to the virus, and 32% had neutralizing antibodies in vitro. These neutralizing antibodies can limit AAV-mediated transduction in organs such as the liver and lung, but no limits were seen on gene delivery to muscle, brain, and retina [19]. Additionally, in many instances, long-term expression of the gene is not required and may, in fact, be harmful. Limiting the length of expression of these genes could be important to prevent any off-target effects and prevent long-term complications.
AAV-mediated delivery of monoclonal antibodies (mAbs) has been shown to be effective in both prophylactic and therapeutic applications in a variety of diseases, including Ebola, malaria, influenza, HIV, cancer, Alzheimer’s, and drug abuse [24,25,26,27,28,29,30,31]. The feasibility of this approach for mAb therapy was first demonstrated by Lewis et al. in an HIV model, where a recombinant AAV vector was used to deliver human antibody IgG1b12 to Rag1-deficient mice. The mice expressed IgG1b12 from muscle with gp120-binding specificity, and the antibody was able to neutralize both T cell line-adapted and primary HIV-1 isolates. After a single intramuscular (IM) administration, the recombinant AAV (rAAV) genome persisted for over 6 months [32]. Since then, the concept of antibody gene transfer using AAV was adapted to macaques by Johnson et al., who generated Simian immunodeficiency virus (SIV)-specific immunoadhesins, antibody-like molecules that fused the Fc region of an immunoglobulin and the functional domain of a binding protein and packaged them into AAV1 capsids. A single intramuscular injection of the AAV vector resulted in long-term (>1 year) continuous expression of a biologically active protein. Six out of nine immunized monkeys were protected against infection by the SIV challenge, and all nine were protected from AIDS. In contrast, all six of the controls became infected, and two-thirds (four of six) died over the course of the experiment [6]. Balazs et al. also demonstrated that AAV delivery of full-length human broadly neutralizing antibodies into humanized mice provided complete or partial protection from CD4 depletion following HIV infection [7].
Preclinical studies in mice and sheep have shown that AAV-based mAbs have good safety and tolerability profiles. No significant changes in blood chemistry or hematological parameters were observed, and only mild myositis was reported at the injection site, with no observed toxicity in major organs [33]. Two phase 1 clinical trials have been conducted to evaluate the safety and tolerability of AAV-delivered mAbs: one using broadly neutralizing HIV mAbs PG9 in healthy male adults and another using VRC07 in HIV-1 infected adults [34,35]. In both studies, intramuscular AAV-mediated mAb delivery was safe and well tolerated. In the PG9 study, vectored immunoprophylaxis using AAV1 resulted in serum neutralization in four of the twenty-one volunteers. While muscle biopsy confirmed PG9 expression, its expression level was too low to detect in circulation, directly suggesting very low expression. In contrast, in the clinical trial using AAV8-delivered VRC07, measurable mAb was detected in all three dose groups, with maximal concentrations exceeding 1 µg/mL in three individuals. Though target trough concentrations sufficient for protection have not been identified for VRC07, based on previous studies using the less potent mAb VRC01, exceeding 1 µg/mL with VRC07 is considered a reasonable therapeutic target [36]. Pseudoviral neutralization studies demonstrated the functionality of in vivo-generated VRC07 [35].
Although AAV-based mAb delivery has shown promise, several challenges still need to be addressed, some of which are common to other nucleic acid-based delivery methods. Immune responses to mAbs are a significant obstacle that needs to be overcome [6,37]. The formation of antibodies against the mAb of interest is known as the anti-drug antibody (ADA) response. Studies have shown that both heavy and light chains are targeted, mainly or exclusively, to variable regions, and reactivity to complementarity-determining region (CDR)-H3 peptide has been demonstrated. The magnitude of anti-antibody responses highly correlates with the degree of sequence divergence of the delivered antibody from the germline [38]. However, in some cases, ADA is not observed. For example, in an SIV challenge model study, one macaque maintained 240–350 μg/mL of anti-SIV antibody 5L7 for over six years with little to no anti-drug antibodies and remained uninfected [24]. Various strategies are being explored to overcome this challenge.
For instance, the liver is an immunologically tolerant organ; therefore, liver-directed expression may help limit ADAs [39]. Resident antigen-presenting cells such as dendritic cells, Kupffer cells, liver sinusoidal endothelial cells, hepatocytes, and hepatic stellate cells present antigens in a tolerogenic manner to T cells and express immunosuppressive cytokines, resulting in regulatory T cells (Tregs) expansion, effector T cell anergy or death, and type 1 regulatory cell induction [40]. Likewise, AAV-mediated gene therapy targeting the liver has been shown to induce antigen-specific Tregs [41,42,43,44,45]. A rhesus macaque study observed no ADA to 4L6 mAb when the AAV8 vector was administered intravenously using a liver-specific TBG promoter. Priming with AAV8-CMV or AAV8-TBG, followed by boosting with AAV1-CMV, significantly increased 4L6-IgG1, accompanied by a weak ADA response [46]. Although it has a relatively low immunogenicity profile compared to other viral vectors, AAV can still give rise to immune responses, making anti-vector immunity another hurdle. Cellular immune responses against AAV capsid limit transgene expression by eliminating transduced cells [47]. Notably, a non-human primate study has shown that the elicitation of capsid-specific CTLs is limited to AAV capsids that exhibit heparin-binding activity. Serotypes like AAV8, which lack heparin-binding activity, did not induce CTL responses [48]. Vector administration leads to seroconversion [49]. Humoral immune responses can generate AAV capsid-neutralizing antibodies, which block transduction and prevent re-administration of that particular gene therapy vector [50]. Transgene insertional mutagenesis risks also remain a concern. AAV integration has been shown to cause hepatocellular carcinoma (HCC) in mice. In dogs given AAV gene therapy for hemophilia, integration events were noted in or near genes associated with cell growth [51,52]. Identifying AAV genomes in tumor-associated genes of HCCs in patients raised safety concerns about the therapeutic use of AAVs [53]. However, whether AAV integrates into oncogenes in humans is not known and the risk thus far remains theoretical.
(ii) Plasmid DNA-based monoclonal antibody delivery
Given the challenges associated with viral vector-based synthetic mAb delivery approaches, researchers have explored alternative approaches, such as the use of “naked” DNA plasmids to deliver the necessary genes for in vivo production of immunotherapeutic mAbs. DNA-based mAb delivery originated from observations made during investigations of DNA vaccines. For example, in 1990, Wolff et al. reported protein expression from unformulated, naked DNA expression vectors injected into mouse skeletal muscle in vivo [5]. This observation led to the investigation of a DNA vaccine platform [54,55]. Although, at the same time, administration of DNA with a needle and syringe gave good expression in animal models, first-generation DNA vaccines induced weak cellular responses and weak or nonexistent antibody responses in many clinical studies [56,57,58,59,60,61,62].
Over the years, several strategies were discovered to enhance the expression of plasmid constructs. In the early 2000s, it was found that the delivery protocols incorporating “adaptive” in vivo electroporation (EP) can promote greater gene delivery into cells, leading to increased expression of the construct. This physical process exposes cells to a brief electrical field pulse that induces temporary pores in the cell membrane and promotes DNA electrophoresis, thus aiding DNA uptake. This technology was then applied to the in vivo production of mAbs in 2004 with the work of Tjelle et al. and Perez et al. [63,64]. EP would later be further refined through pulse pattern, array spacing, and voltage optimizations. Other methods of increasing expression, such as codon/RNA optimization to improve protein translation and RNA stability, were then established [65,66]. Additionally, leader sequences were added to genes to enhance translational efficiency, while strong promoters were used to drive expression.
Several advances were made to the delivery to improve in vivo expression of DNA-encoded mAbs (Table 1). Hyaluronidase treatment, which helps DNA move through the extracellular matrix and enhance plasmid uptake, would be administered either before DNA delivery or co-formulated with DNA [67,68]. Modifying the antibody to resemble the human parental germline antibody sequence would also increase overall production in vivo while preserving functionality [68,69]. Mutations such as triple Fc modification M252Y/S254T/T256E were introduced to promote neonatal Fc receptor-mediated recycling of IgG into circulation, thereby extending mAb half-life [70]. Incorporating these additional refinements would allow for in vivo mAb expression at biologically relevant levels in small animal models. The potential value of the DNA-based mAb delivery platform has thus far been demonstrated in many models of infectious diseases such as Ebola, Pseudomonas aeruginosa, influenza, chikungunya, dengue, Zika, and HIV, as well as cancer types including breast, ovarian, and prostate [68,71,72,73,74,75,76,77,78,79]. The equivalency of binding for in vivo delivered DNA-based mAbs to recombinant mAbs has been shown through epitope mapping [68]. In addition to their use alone, DNA-based mAbs can be combined with protective vaccines, providing both immediate and persistent protection and establishing vaccine-induced memory responses [74].
Non-viral DNA delivery offers several advantages over AAV-based antibody delivery, including increased gene insert size, avoiding immune responses directed against the AAV vector and thus avoiding host seroconversion, enabling repeat delivery, and ease of manufacture and manipulation. In addition, DNA is less immunogenic, and the temporal nature of the delivered DNA plasmid means there is no long-term risk from the delivery vehicle. Furthermore, the DNA-plasmid approach is highly cost-effective, as it can be produced in large quantities using established bacterial fermentation processes. Lastly, DNA is more stable at room temperature than proteins, viral vectors, and LNP-encapsulated mRNAs, further reducing costs by minimizing the need for cold chains during storage and transport. These advantages make DNA-delivered mAbs a more broadly applicable alternative to vector-based or traditional recombinant purified monoclonal antibodies.
DNA-based mAb delivery platforms offer several advantages but have some limitations; for instance, peak expression takes approximately 7–14 days, which may be too delayed for therapeutic purposes. In addition, EP devices are not standard equipment in most hospitals, so additional expenditure and training would be necessary to deploy this system. Even then, electroporation devices required for delivery may not be suitable for clinical settings due to their potential to cause muscle contractions, pain, and tissue damage [80,81]. Alternative delivery methods such as hydrodynamics injection and suction-based transfection are being investigated for DNA vaccines and may apply to DNA-encoded mAbs [82,83]. Though not seen in the clinic, theoretical risks of insertional mutagenesis and induction of autoimmune antibodies against DNA from repeat injections exist. As with AAV, anti-drug antibodies remain an issue, although redosing is possible as there is no vector immunity [71,73,76,84]. Transient T cell depletion has been used in immunocompetent mice to prevent animals from developing anti-antibody responses, enabling long-term expression. However, this may not be a translatable clinical strategy [68,76]. Two phase 1 clinical trials have been conducted for DNA-based mAbs using EP and hyaluronidase, one for the Zika virus (using INO-A002) and the other for preventing COVID-19. The INO-A002 study has been completed, but no results are available yet, while the COVID-19 study is actively recruiting.
(iii) mRNA-based mAb delivery
Advances in synthesis, purification, and delivery methods have renewed interest in messenger RNA (mRNA)-based delivery approaches. mRNA-based delivery of mAbs share many of the benefits of DNA-based approaches but have the advantage of faster protein expression (within hours), greater peak expression without mutational risks, and no anti-vector immunity allowing for repeated dosing [85,86,87,88,89]. Moreover, the short-lived nature of mRNA allows for controlled expression and minimizes the risk of multisystem inflammatory syndromes or other side effects associated with introducing foreign agents [90,91] (Figure 1).
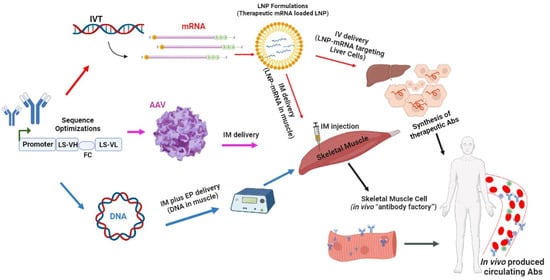
Figure 1.
An overview of mRNA-based delivery of mAb.
The manufacturing process for mRNA-encoded mAbs is highly efficient. Within weeks, clinical batches can be produced after obtaining the sequence encoding the mAbs of interest, and the process is scalable and cell-free. A facility dedicated to mRNA production could potentially manufacture mRNA-encoded mAbs against multiple targets with minimal adaptations to process and formulation [92,93,94].
The delivery of mRNA to the cytosol leads to the synthesis of the encoded mAbs, which is then subjected to post-translational modifications, resulting in a fully functional product delivered to the correct cellular compartments for proper function, usually within hours. The peak expression of mRNA-encoded antibodies typically occurs within days [95,96,97,98,99,100]. The genetic material carried by mRNA is expressed transiently until the mRNA is degraded. The durability of protein expression can range from hours to days depending on the dose of mRNA, the properties of the mRNA (optimizations), and the route of mRNA delivery [101]. The stability of mRNAs depends on various factors, including the 5′ cap, poly(A) tail, and various cis-elements, such as linear A + U- and C + U-rich motifs and stem-and-loop structures, which interact with trans-acting factors affecting the rate of decay [102]. The 5′ and 3′ UTRs that recruit RNA-binding proteins and microRNAs are crucial for stability and translation [103]. Additionally, circular RNAs can increase the half-life of protein production up to threefold compared to linear mRNA in vitro [104]. Like other nucleic acid-based approaches, mRNA can encode multiple proteins with different chemical and physical properties without major changes in their physiochemical properties, thus allowing for simple and cost-effective manufacturing.
The mRNA templates or byproducts from in vitro transcription can activate the innate immune system via recognition by pattern recognition receptors (PRRs), decreasing protein expression, reducing the mAbs’ longevity, and inducing adverse events in the host. Toll-like receptors 3, 7, and 8, retinoic acid-inducible gene 1 (RIG-I), and nucleotide-binding and oligomerization domain-containing protein 2 (NOD-2) are PRRs that recognize mRNA and its contaminants, leading to the production of proinflammatory cytokines and type I interferons [105,106]. To decrease the immunogenicity of mRNA, base modifications like pseudouridine or 5-methylcytidine, which are naturally occurring in RNA, can be incorporated [107,108,109,110]. Enriched GC content can also decrease immune responses while increasing expression several folds higher [111]. The immunogenicity of the platform can be further reduced by removing contaminants like short RNAs and double-stranded RNAs from the in vitro transcription process using techniques like high-performance liquid chromatography, anion exchange chromatography, affinity chromatography, size exclusion columns, cellulose purification, or RNase III treatment [86,87,88,112,113].
Due to its inherent instability, mRNA-encoding monoclonal antibodies require carrier assistance or delivery platforms to protect against nucleases and ensure effective delivery. Various strategies have been developed, including lipid-based, polymer-based, peptide-based, virus-like replicon particle, cationic nano-emulsion delivery, and direct injection into cells. Lipid nanoparticles (LNPs) are currently the most versatile and potent delivery system [101]. These nanoparticles are composed of ionizable amino lipids, polyethylene glycol, phospholipids, and cholesterol and are negatively charged nucleic acid delivery platforms [114]. The ionizable amino lipids facilitate self-assembly, cellular uptake of mRNA, and escape from the endosome by interacting with the endosomal membrane. Polyethylene glycol prolongs circulation time by preventing the binding of mRNA and plasma proteins, which would otherwise lead to clearance by the reticuloendothelial system. Phospholipids support the structure of the lipid bilayer, while cholesterol stabilizes the lipid nanoparticle structure [115]. These lipid nanoparticles provide two key advantages as a delivery platform for mRNA. Firstly, they protect mRNA from degradation by endosomal enzymes. Secondly, they can take advantage of existing cellular pathways to enhance mRNA expression. The first mechanism involves utilizing the Apolipoprotein E (ApoE)–Low-Density Lipoprotein Receptor (LDLR) pathway, a highly efficient delivery system [116]. Subsequently, the lipid nanoparticles are taken up through TLR4-mediated endocytosis, forming a vesicle that fuses with endosomes. Following this, the LNPs escape from endosomes, releasing mRNA into the cytoplasm to initiate protein synthesis.
LNPs are a popular delivery platform for mRNA due to their ability to protect mRNA from degradation and promote mRNA expression through endogenous cellular pathways. However, LNPs also have inherent immunogenicity that can lead to immune responses, which may not be desirable for in vivo expression of mAbs [117,118,119]. The size, surface charge, and repeat dosing of LNPs have all been shown to affect their immunogenicity [120,121,122]. Repeat dosing can also carry the risk of acute immune toxicity, known as complement activation-related pseudoallergy (CARPA), and can lead to the production of anti-polyethyleneglycol (PEG) antibodies, which are associated with both anaphylactic responses and accelerated blood clearance [123,124]. Cellular toxicities may also occur through the accumulation of lipids. These effects can be alleviated through the optimal formulation of mRNA and LNP. Aside from optimizing vesicle size and surface charge, other methodologies that have been explored include the incorporation of aliphatic ester prodrugs of anti-inflammatory steroids and the substitution of less immunogenic polymers [125]. Notably, CARPA and serious adverse events were not observed after the second dosing in non-human primate preclinical studies and a clinical trial assessing mRNA-encoded Chikungunya virus antibody [95,126].
Compared to viral vector- or DNA-based approaches, mRNA-based methods offer a faster route to protein production since the mRNA does not require nuclear localization to produce a functional protein. In a study by Pardi et al., VRC01 antibody expression was detected within hours and peaked at 24 h after IV injection of LNP-formulated m1Ψ-modified mRNA encoding the heavy and light chains of the antibody in BALB/c mice [97]. However, other studies have reported peak expression not occurring until seven days post-administration [127]. These expressed mRNA-delivered mAbs can then persist in the body for several months after administration [126,128]. Furthermore, mRNA-based approaches may potentially avoid antibody production against the encoded protein. Pardi et al. found no anti-EPO antibody production in BALB/c mice that received weekly intraperitoneal injections of 0.1 μg of murine erythropoietin (muEPO) encoding mRNA over five weeks [97] (Table 1).
One significant drawback of mRNA-encoded mAbs is that their administration is primarily limited to the intravenous (IV) route, with the liver being the target organ. This mode of administration is time-consuming and expensive, as mentioned earlier. However, Erasmus et al. demonstrated a novel approach that makes the intramuscular route a viable option, despite its limited number of target cells [129]. They combined self-amplification of an mRNA message encoding the antibody sequence with the co-expression of viral genes that antagonize the host’s innate immune response using alphavirus replicons. In their construct, alphavirus-derived self-amplifying replicon RNA (repRNA) maximized protein secretion on a per-cell basis. Serum concentrations of mRNA-expressed Zika virus (ZIKV)-117 antibody were over 30-fold higher than those of pseudouridine-modified or unmodified non-replicating mRNAs. Although self-amplifying mRNA may have also allowed for longer expression, this study did not investigate time points beyond 10 days. The high levels of ZIKV-117 mAb expression led to protection against lethal ZIKV infection in mice [129]. Despite the mAb levels being adequate for providing protection, it should be noted that double-stranded RNA intermediates formed during amplification may activate innate immunity, and the presence of replicon genes can also be immunogenic as well as lead to size constraints for the mAb gene [130,131,132].
Vanover et al. investigated an alternative administration route for mRNA-encoded mAbs in polyplexes by utilizing nebulized delivery of a glycophosphatidylinositol (GPI)-linked mRNA encoding a neutralizing mAb against SARS-CoV-2 infection [133]. The GPI-linked mAbs were anchored to the plasma membrane and induced retention in the lung. This approach increased the mAb half-life in lung tissue from 1.3 to 7.1 days. The mRNA-encoded mAbs (COV2–2832 or DH1041) prevented infection in a hamster SARS-CoV-2 prophylactic challenge model. In this study, mRNA-delivered mAbs protected hamsters from weight loss while reducing lung viral titers and loads. Viral nucleocapsid RNA detected by RNAscope, a commercially available in situ hybridization assay for detecting RNA in formalin-fixed paraffin-embedded tissue, showed decreased signal in the alveolar space but little effect on larger airways. However, reduced staining for SARS-CoV-2 spike protein was observed in both the airways and alveolar spaces. In addition, mRNA-treated hamsters had significantly lower lung and airway pathology scores. These findings indicate that localized delivery of mRNA-expressed mAbs can be highly effective. Furthermore, targeted delivery of mAbs to their site of action is favorable as it allows for lower doses of formulated mRNA to be utilized.
To date, only one clinical trial (led by Moderna) has investigated mRNA-encoded mAbs [126]. This trial involved the administration of mRNA-1944, which encodes the heavy and light chains of a Chikungunya virus (CHIKV)-specific monoclonal neutralizing antibody, CHKV-24, to healthy participants aged 18–50 years. Across doses, adverse effects were mild to moderate in severity and did not worsen with the addition of a second dose. At 12, 24, and 48 h after a single infusion of either 0.1, 0.3, or 0.6 mg/kg, dose-dependent levels of neutralizing CHKV-24-IgG were observed, which were predicted to be protective against CHIKV infection (≥1 µg/mL). These levels were sustained for ≥16 weeks at the 0.3 and 0.6 mg/kg doses, with a mean half-life of approximately 69 days. A second 0.3 mg/kg dose for the 0.3 mg/kg group administered one week after the first increased CHKV-24 IgG levels 1.8-fold. The ionizable lipid component of the lipid nanoparticle was readily eliminated, and CHKV-24 IgG mRNA was rapidly cleared within 48 h. Notably, no anti-PEG or anti-CHKV-24 IgG antibodies were detected in this study [126].
(iv) Nanobodies
Nanobodies are single-domain antibodies derived from camelids. Camelids produce antibodies that do not contain a light chain. The camelid antibody consists of two constant regions, a hinge region, and the antigen-binding domain variable heavy domain of heavy chain (VHH). Nanobodies are derived from the recombinant production of a VHH [134]. Nanobodies have binding affinities that are comparable to or better than conventional mAbs [135,136]. While the small size of nanobodies is advantageous for tissue penetration and passage through the blood–brain barrier, it also leads to rapid renal elimination and, therefore, a short serum half-life. Strategies to prolong the half-life of nanobodies include the addition of polyethylene glycol, albumin, or Fc fragment to the nanobody [137,138]. Rather than prolonging half-life, rapid clearance of nanobodies may be addressed by nucleic acid-based approaches which allow for long-term, stable production of nanobodies [139]. Like mAbs delivered in nucleic acid form, nanobodies delivered in this manner bypass in vitro production and characterization. Moreover, as before, AAV- or DNA-based delivery would avoid the need for repeated protein infusions and allow for stable concentrations of nanobody in vivo [140]. The delivery of nucleic acid-based nanobodies has also been explored in the context of circular RNAs, an area of increasing interest given their enhanced stability and more durable protein expression when compared to mRNA. Full-length mAb delivery using the circular RNA platform has yet to be reported, but Qu et al. demonstrated the feasibility of circular RNA therapeutics using a SARS-CoV-2 neutralizing nanobody [141]. In this study, supernatant of HEK293T cells transfected with the SARS-CoV-2 neutralizing nanobody circular RNA construct was able to effectively inhibit SARS-CoV-2 pseudovirus infection.

Table 1.
A comprehensive review of monoclonal antibody delivery platforms.
Table 1.
A comprehensive review of monoclonal antibody delivery platforms.
| AAV-Based Delivery | ||||
|---|---|---|---|---|
| Encoded mAb(s) | Modifications | Experimental Model | Mode of Delivery | Reference |
| anti-HIV-1 gp160 (IgG1b12) | dual promoter (pCMV/HC/EF1a/LC) | Rag1 mice | rAAV, IM | [32] |
| anti-human EGFR (14E1 and 14E1A, ablated CDR3) | nu/nu mice | AAV2/1, IM | [142] | |
| anti-HIV gp41 (4E10) and anti-HIV gp120 (b12) | Rag2−/−γc−/−, NSG, B6, Balb/C | AAV2/8, IM | [7] | |
| anti-Aβ | C57BL/6 mice | AAV1, IM | [29] | |
| anti-ganglioside GM3(Neu5Gc) (14F7, mouse IgG1) | BALB/c mice | AAV2/9, IM, IV | [143] | |
| anti-METH | scFv, self-complementary AAV | BALB/c mice | AAV8, IV | [30] |
| anti-HIV-1 gp41 (10E8), anti-HIV-1 gp120 (3BNC117, 10-1074) | “LS mutation” (M428L (Leucine)/N434S (Serine)) to increase half-life, vectors included specific miRNAs to promote transcriptional cleavage of transgene’s mRNA in APCs | rhesus macaques | AAV1, IM | [27] |
| anti-SIV gp120 and gp140 (5L7) | Mamu B*08-neg B*17-neg female Indian-origin rhesus macaque | AAV1, IM | [24] | |
| anti-PD-1 (Nb11) | nanobody (VHH) | C57BL/6 mice | AAV8, IV | [28] |
| anti-EBOV GP2 internal fusion loop (CA45) | F129L, Y445F, and Y731F mutations in the AAV6 capsid | BALB/c mice | AAV6.2FF, IM | [25] |
| anti-MARV GP (MR78, MR82 and MR191) | bicistronic, CASI promoter | BALB/c mice, Dorset lambs | AAV6.2FF, IM | [144] |
| DNA-Based Delivery | ||||
| Encoded mAb(s) | Modifications | Experimental Model | Mode of Delivery | Reference |
| anti-human thyroglobulin | tet-off, tet-on | C57BL/6 mice, C3H mice | IM + EP | [63] |
| anti-I-Ed, anti-IgDa, anti-NIP | C57BL/6 mice, BALB/c mice, BALB.B mice, C.B-17 mice | IM + EP | [64] | |
| anti-HIV Env (VRC01) | BALB/c mice | IM + EP | [65] | |
| anti-DENV nAb | LALA mutation | Foxn1/NuJ mice | IM + EP | [72] |
| anti-CHIKV envelope | B6.Cg-Foxn1nu/J mice | IM + EP | [74] | |
| anti-HER2 | modification of VL sequence by replacing asparagine at amino acid 65 with serine to remove potential N-glycosylation site | BALB/c mice | IM + EP | [145] |
| anti-Influenza (A and B) | BALB/c and CAnN.Cg-Foxn1Nu | IM + EP with hyaluronidase pretreatment | [71] | |
| anti-PSMA | B6.Cg-Foxn1 nu/J and C57BL/6J mice | IM + EP | [75] | |
| anti-CD4, anti-influenza, anti-Ebola | BALB/c mice | IM + EP, IM + EP with hyaluronidase pretx | [146] | |
| anti-Zaire ebolavirus glycoprotein | modification of N terminus amino acids back to germline | BALB/c (anti-CD4 and anti-CD8 transient depletion) | IM + EP with hyaluronidase | [68] |
| anti-CTLA-4 | Sequence modifications based on sequence alignment to the mouse germline sequence | C57Bl/6, BALB/c (anti-CD4 and anti-CD8 transient depletion) | IM + EP with hyaluronidase | [147] |
| anti-HER2 | BALB/c, athymic nude, RAG2−/−gc−/− | IM + EP with hyaluronidase pretx | [73] | |
| anti-ZIKV E protein DIII domain (DMAb-ZK190) | LALA mutation | C57BL/6 mice, Rhesus macaques | IM + EP with hyaluronidase pretreatment in mice, IM + EP only in rhesus macaques | [77] |
| anti-OspA Lyme | framework modification of the WT variant | C3H mice | IM + EP with hyaluronidase | [148] |
| anti-PCSK9 | C57BL/6J wild-type and nude B6.Cg-foxn1nu/J mice | IM + EP | [149] | |
| anti-HER2 | Nu/J mice | IM + EP with hyaluronidase | [78] | |
| anti-PD-1 | BALB/c mice | IM + EP | [150] | |
| anti-human CEA, anti-human EGFR, anti-HER2 | Swifter sheep, C57BL/6J RAG1 ko mice | IM + EP with hyaluronidase pretx | [151] | |
| multiple HIV-1-specific bNAbs | modification of the C- and N-terminus of the variable region to germline | BALB/c (anti-CD4 and anti-CD8 transient depletion), Rhesus macaques | IM + EP with hyaluronidase | [79] |
| anti-HBV | athymic nude CAnN.Cg-Foxn1nu/Crl mice | IM + EP | [152] | |
| anti-mCTLA-4 (9D9), anti-ratPD-1 | C57BL/6J mice | IM + EP with hyaluronidase pretx | [153] | |
| Intratumoral + EP | ||||
| anti-ZIKV envelope | B6.Cg-Foxn1nu/J mice | IM + EP | [154] | |
| 2C7, directed against a lipooligosaccharide glycan epitope, Neisseria gonorrhoeae | two complement enhancing variants, HC_E430G and HC_E345K, one complement abrogating variant HC_K322A/D270A | Jh mice, nude mice | IM + EP with hyaluronidase | [155] |
| anti-HER2 | BALB/c mice | IM + EP with hyaluronidase pretx | [156] | |
| anti-SARS-CoV2 spike | L234F/L235E/P331S; “TM” to ablate FcR and C1q binding, M252Y/S254T/T256E; “YTE” to promote FcRn-mediated recycling | BALB/c | IM + EP with hyaluronidase | [70] |
| mAb clones CIS43, 317, and L9, which target a junctional epitope, major repeat, and minor repeat of the Plasmodium falciparum circumsporozoite protein (CSP), respectively | reverting specific, non-essential residues in the framework region back to germline configuration | BALB/cJ (anti-CD4 and anti-CD8 transient depletion) | IM + EP with hyaluronidase | [69] |
| anti-human CEA | Swifter sheep | IM + EP with hyaluronidase | [157] | |
| mRNA-Based delivery | ||||
| Encoded mAb(s) | Modifications | Experimental Model | Mode of Delivery | Reference |
| anti-CD3/anti-claudin 6 (CLDN6), anti-CD3/anti-caludin 18.2 (CLDN18.2), anti-CD3/anti-epithelial cell adhesion molecule (EpCAM), anti-CD3/(anti-CLDN6)2 | 1-methylpseudouridine | NOD.Cg-Prkdscid IL2rgtm1Wjl/SzJ (NSG) mice | Formulation with TransIT-mRNA Transfection kit, IV | [99] |
| anti-HIV Env (VRC01) | 1-methylpseudouridine | BALB/c mice | LNP, IV | [97] |
| anti-Rabies glycoprotein G, anti-Botulinum neurotoxin serotype A (VHH-based neutralizing agent, VNA), anti-CD20, anti-HIV gp120, anti-Influenza B HA, anti-Shiga toxin 2 (VNA) | two fused VHHs complemented by an albumin-binding peptide | Swiss-Albino mice (rabies and influenza b) CD1 mice (VNAs) | LNP, IV | [100] |
| anti-Influenza A | 1-methylpseudouridine | cynomolgus monkeys | LNP, IV | [98] |
| anti-CHIKV E2 glycoprotein (CHIKV-24) | cynomolgus monkeys | LNP, IV | [95] | |
| anti-HER2 | C57BL/6 mice | cKK-E12 (also known as MD-1) lipid-like nanoparticles, IV | [127] | |
| Anti-ZIKV Env (ZIKV-117) | alphavirus replicon (replicating viral RNA that amplifies) 1-methylpseudouridine | C57BL/6 mice | Nanostructured lipid carrier, IM | [129] |
| anti-HIV GP120 (PGT121) | 1-methylpseudouridine | female Katahdin ewes | aerosol delivery of unformulated mRNA in water | [96] |
| anti-Influenza A matrix protein 2/anti-mouse Fcγ receptor IV | 1-methylpseudouridine | DOTAP (1,2-dioleoyl-3-trimethylammonium-propane)/cholesterol nanoparticles delivered intratracheally | [158] | |
| anti-CHIKV Env (mRNA-1944) | human | LNP, IV | [126] | |
| Poxviruses: Mature virion, c7D11 (anti-L1); Enveloped virion, c8A (anti-B5) and c6C (anti-A33) | New Zealand White rabbits | LNP, IM jet injection | [159] | |
| anti-PD-1 | 1-methylpseudouridine | C57BL/6 mice | LNP, IV | [128] |
| anti-SARS-CoV-2 | LS mutation (M428L/N434S) and GPI anchor 1-methylpseudouridine | Golden Syrian hamster | poly-beta amino thio ester (PBATE), nebulizer | [133] |
6. Summary and Conclusions
Monoclonal antibodies (mAbs) have demonstrated remarkable efficacy, offering target specificity and high affinity. However, their widespread use remains limited, especially in low- and middle-income countries. Gene-based approaches have emerged as promising alternatives to current synthetic production methods for mAbs to address this challenge. These approaches involve delivering nucleic acid encoding the desired mAb, which can significantly reduce production costs, resource requirements, and developmental time compared to protein-based mAb therapies. Moreover, gene-based strategies enable sustained delivery and in vivo generation of mAbs with proper post-translational modifications, eliminating the need for repeated infusions or injections. The implementation of gene-based approaches has the potential to enhance the accessibility and affordability of mAb therapies, making them more available to patients worldwide.
Furthermore, gene-based modalities such as viral vectors, DNA, and mRNA offer distinct advantages for mAb therapy (Table 1). mRNA-based mAb therapy provides immediate and transient expression, making it suitable for transient infectious diseases where prolonged mAb exposure is unnecessary. On the other hand, sustained mAb production, crucial for cancer or chronic infections, can be achieved using AAV- or DNA-based approaches. AAV is preferred for high mAb concentrations, while DNA delivery offers self-limited but repeatable administration, potentially increasing safety. Additionally, the advancement of nucleic acid technology has led to the exploration of circular RNAs, which exhibit enhanced stability compared to linear RNAs due to their resistance to exonucleases. This stability allows for higher and more durable protein expression. The use of nucleic acid-encoded mAb treatments holds tremendous promise in improving access to life-saving mAbs. Given their safety, efficacy, and scalability, translating nucleic acid technology into clinical practice and the routine use of nucleic acid-encoded mAbs in healthcare settings are highly likely.
7. Future Perspectives
The success of nucleic acid-based approaches, exemplified by the effective SARS-CoV-2 vaccines against COVID-19, has paved the way for their increasing utilization in the near future. These approaches offer advantages such as facile synthesis and expedited development and production scale-up, positioning them as a preferred platform for vaccine and therapeutic delivery. However, the use of an adeno-associated virus (AAV) as a delivery system may be hampered by pre-existing or acquired immunity to the vector, which would limit the ability for repeat dosing. DNA-based delivery platforms do not induce significant anti-vector responses and thus can be administered multiple times to the same patient. However, efficient in vivo uptake and expression of transgenes from DNA plasmids requires the use of electroporation-enhanced delivery, which poses challenges such as electroporation-induced muscle contractions and tissue damage. Additionally, electroporation machines are specialized equipment that are not commonly available and would require additional training for medical personnel before they can be used (Table 2).

Table 2.
Comprehensive table to summarize the different modes of delivery.
Conversely, mRNA and circular RNA-encoded monoclonal antibodies (mAbs) hold significant promise as relatively novel therapeutics due to their favorable safety profiles and repeatable dosing. Nevertheless, the development of lipid nanoparticle (LNP) formulations for mRNA delivery requires meticulous formulation to mitigate immune-mediated and cellular toxicities. Despite these obstacles, encouraging results from clinical trials underscore the need for further refinement and expansion of nucleic acid-based approaches. This burgeoning area of research bears tremendous potential, offering cost-effective medicines facilitated by nucleic acid-based mAbs.
The antibody is selected based on its binding capacity and functionality, such as neutralization, agonist/antagonist activity, or antibody-dependent cellular cytotoxicity. Next, the antibody’s VH and VL chain sequences are obtained and optimized to create a mAbs-encoding gene cassette. This cassette can then be used to create antibody-encoding (i) mRNA by in vitro transcription (IVT) using a DNA template (plasmid or PCR product); the transcribed mRNA is then formulated into lipid nanoparticles (LNP) and delivered into skeletal muscle cells via intramuscular (i.m.) injection; (ii) AAV particles by cloning the cassette into AAV genome construct and then transfecting this into a packaging cell line with a helper virus; recovered viruses are purified and concentrated and can be delivered into skeletal muscle cells via i.m injection; or (iii) DNA by cloning it into a DNA plasmid downstream of a strong eukaryotic promoter. The plasmid can be delivered into skeletal muscle cells via electroporation-enhanced i.m. injection. The muscle cells then transcribe and/or translate the delivered antibody gene cassette and produce antibodies that are secreted into the bloodstream and circulate throughout the body. Alternatively, the mRNA can be encapsulated in liver-targeting LNPs and delivered via intravenous (i.v.) injection, where it is transported through the bloodstream to the liver. In the liver, the protein (Abs) is synthesized in hepatocytes. The LS leader sequence is used to optimize protein secretion. VH represents the variable heavy chain, FC is the furin cleavage site, and VL stands for the variable light chain.
Author Contributions
Conceptual framework, writing, and editing: C.C., S.B.K., C.N.C., Y.K.P., Z.X., N.P., M.A.-M. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not. applicable.
Acknowledgments
Wonil Kim, Christine C. Roberts, and Joel N. Maslow are acknowledged for their helpful comments. The graphical picture in Figure 1 was created using BioRender.
Conflicts of Interest
K.M. has a patent application for the delivery of DNA-encoded monoclonal antibodies. C.C., S.B.K., C.N.C., Y.K.P. and K.M. are employees of GeneOne Life Science, Inc. Y.K.P. holds stock or stock options with a value exceeding USD 10,000. All other authors declare no competing financial interest.
References
- Casadevall, A.; Dadachova, E.; Pirofski, L.-A. Passive antibody therapy for infectious diseases. Nat. Rev. Genet. 2004, 2, 695–703. [Google Scholar] [CrossRef]
- Kitasato, S. Ueber den Tetanusbacillus. Med. Microbiol. Immunol. 1889, 7, 225–234. [Google Scholar] [CrossRef]
- Behring, E.V. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren; Philipps-University Marburg: Marburg, Germany, 1890. [Google Scholar]
- Casadevall, A.; Scharff, M.D. Serum therapy revisited: Animal models of infection and development of passive antibody therapy. Antimicrob. Agents Chemother. 1994, 38, 1695–1702. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.; Schnepp, B.C.; Zhang, J.; Connell, M.J.; Greene, S.M.; Yuste, E.; Desrosiers, R.C.; Clark, K.R. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 2009, 15, 901–906. [Google Scholar] [CrossRef]
- Balazs, A.B.; Chen, J.; Hong, C.M.; Rao, D.S.; Yang, L.; Baltimore, D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 2011, 481, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Bradbury, A.R.M.; Sidhu, S.; Dübel, S.; McCafferty, J. Beyond natural antibodies: The power of in vitro display technologies. Nat. Biotechnol. 2011, 29, 245–254. [Google Scholar] [CrossRef]
- Voigt, A.; Semenova, T.; Yamamoto, J.; Etienne, V.; Nguyen, C.Q. Therapeutic Antibody Discovery in Infectious Diseases Using Single-Cell Analysis. Adv. Exp. Med. Biol. 2018, 1068, 89–102. [Google Scholar] [CrossRef]
- André, A.S.; Moutinho, I.; Dias, J.N.R.; Aires-Da-Silva, F. In vivo Phage Display: A promising selection strategy for the improvement of antibody targeting and drug delivery properties. Front. Microbiol. 2022, 13, 962124. [Google Scholar] [CrossRef]
- Nissim, A.; Chernajovsky, Y. Historical Development of Monoclonal Antibody Therapeutics. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–18. [Google Scholar] [CrossRef]
- Birch, J.R.; Racher, A.J. Antibody production. Adv. Drug Deliv. Rev. 2006, 58, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Barolo, G.; Müller-Späth, T.; Wu, H.; Morbidelli, M. Aggregation Stability of a Monoclonal Antibody during Downstream Processing. Pharm. Res. 2011, 28, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, A.F. Monoclonal antibodies: Magic bullets with a hefty price tag. BMJ 2012, 345, e8346. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co., Inc. COST INFO & FINANCIAL HELP. 2023. Available online: https://www.keytruda.com/financial-support/ (accessed on 3 May 2023).
- Sifniotis, V.; Cruz, E.; Eroglu, B.; Kayser, V. Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies 2019, 8, 36. [Google Scholar] [CrossRef]
- Zhu, N.; Liggitt, D.; Liu, Y.; Debs, R. Systemic Gene Expression after Intravenous DNA Delivery into Adult Mice. Science 1993, 261, 209–211. [Google Scholar] [CrossRef]
- Daya, S.; Berns, K.I. Gene Therapy Using Adeno-Associated Virus Vectors. Clin. Microbiol. Rev. 2008, 21, 583–593. [Google Scholar] [CrossRef] [PubMed]
- De, B.P.; Hackett, N.R.; Crystal, R.G.; Boyer, J.L. Rapid/Sustained Anti-anthrax Passive Immunity Mediated by Co-administration of Ad/AAV. Mol. Ther. 2008, 16, 203–209. [Google Scholar] [CrossRef]
- Fang, J.; Qian, J.-J.; Yi, S.; Harding, T.C.; Tu, G.H.; VanRoey, M.; Jooss, K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol. 2005, 23, 584–590. [Google Scholar] [CrossRef]
- McCarty, D.M. Self-complementary AAV Vectors; Advances and Applications. Mol. Ther. 2008, 16, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Kawabata, K.; Sakurai, F.; Nakagawa, S.; Mizuguchi, H. Innate immune response induced by gene delivery vectors. Int. J. Pharm. 2008, 354, 9–15. [Google Scholar] [CrossRef]
- Martinez-Navio, J.M.; Fuchs, S.P.; Mendes, D.E.; Rakasz, E.G.; Gao, G.; Lifson, J.D.; Desrosiers, R.C. Long-Term Delivery of an Anti-SIV Monoclonal Antibody with AAV. Front. Immunol. 2020, 11, 449. [Google Scholar] [CrossRef]
- van Lieshout, L.P.; Rghei, A.D.; Cao, W.; He, S.; Soule, G.; Zhu, W.; Thomas, S.P.; Sorensen, D.; Frost, K.; Tierney, K.; et al. AAV-monoclonal antibody expression protects mice from Ebola virus without impeding the endogenous antibody response to heterologous challenge. Mol. Ther.-Methods Clin. Dev. 2022, 26, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, L.P.; Soule, G.; Sorensen, D.; Frost, K.L.; He, S.; Tierney, K.; Safronetz, D.; Booth, S.A.; Kobinger, G.P.; Qiu, X.; et al. Intramuscular Adeno-Associated Virus–Mediated Expression of Monoclonal Antibodies Provides 100% Protection against Ebola Virus Infection in Mice. J. Infect. Dis. 2018, 217, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Navio, J.M.; Fuchs, S.P.; Pantry, S.N.; Lauer, W.A.; Duggan, N.N.; Keele, B.F.; Rakasz, E.G.; Gao, G.; Lifson, J.D.; Desrosiers, R.C. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity 2019, 50, 567–575.e5. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Martisova, E.; Ballesteros-Briones, M.C.; Hervas-Stubbs, S.; Casares, N.; González-Sapienza, G.; Smerdou, C.; Vanrell, L. Long-Term Systemic Expression of a Novel PD-1 Blocking Nanobody from an AAV Vector Provides Antitumor Activity without Toxicity. Biomedicines 2020, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Abe, S.; Takahashi, T.; Shiozaki, K.; Okuda, M.; Mizukami, H.; Klinman, D.M.; Ozawa, K.; Okuda, K. Prophylaxis and Treatment of Alzheimer’s Disease by Delivery of an Adeno-Associated Virus Encoding a Monoclonal Antibody Targeting the Amyloid Beta Protein. PLoS ONE 2013, 8, e57606. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.E.; Iii, G.A.G.; Ewing, L.E.; Reichard, E.E.; Hambuchen, M.D.; Nanaware-Kharade, N.; Alam, S.; Bolden, C.T.; Owens, S.M.; Margaritis, P.; et al. Development and testing of AAV-delivered single-chain variable fragments for the treatment of methamphetamine abuse. PLoS ONE 2018, 13, e0200060. [Google Scholar] [CrossRef]
- Balazs, A.B.; Bloom, J.D.; Hong, C.M.; Rao, D.S.; Baltimore, D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat. Biotechnol. 2013, 31, 647–652. [Google Scholar] [CrossRef]
- Lewis, A.D.; Chen, R.; Montefiori, D.C.; Johnson, P.R.; Clark, K.R. Generation of Neutralizing Activity against Human Immunodeficiency Virus Type 1 in Serum by Antibody Gene Transfer. J. Virol. 2002, 76, 8769–8775. [Google Scholar] [CrossRef] [PubMed]
- Rghei, A.D.; van Lieshout, L.P.; McLeod, B.M.; Pei, Y.; Lopes, J.A.; Zielinska, N.; Baracuhy, E.M.; Stevens, B.A.Y.; Thomas, S.P.; Yates, J.G.E.; et al. Safety and Tolerability of the Adeno-Associated Virus Vector, AAV6.2FF, Expressing a Monoclonal Antibody in Murine and Ovine Animal Models. Biomedicines 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Priddy, F.H.; Lewis, D.J.M.; Gelderblom, H.C.; Hassanin, H.; Streatfield, C.; LaBranche, C.; Hare, J.; Cox, J.H.; Dally, L.; Bendel, D.; et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: A phase 1 randomised controlled trial. Lancet HIV 2019, 6, e230–e239. [Google Scholar] [CrossRef] [PubMed]
- Casazza, J.P.; Cale, E.M.; Narpala, S.; Yamshchikov, G.V.; Coates, E.E.; Hendel, C.S.; Novik, L.; Holman, L.A.; Widge, A.T.; Apte, P.; et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: A phase 1, dose-escalation trial. Nat. Med. 2022, 28, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, S.; Garrett, N.; Capparelli, E.V.; Osman, F.; Harkoo, I.; Yende-Zuma, N.; Gengiah, T.N.; Archary, D.; Samsunder, N.; Baxter, C.; et al. Safety and Pharmacokinetics of Monoclonal Antibodies VRC07-523LS and PGT121 Administered Subcutaneously for Human Immunodeficiency Virus Prevention. J. Infect. Dis. 2022, 226, 510–520. [Google Scholar] [CrossRef]
- Fuchs, S.P.; Martinez-Navio, J.M.; Piatak, M., Jr.; Lifson, J.D.; Gao, G.; Desrosiers, R.C. AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity. PLOS Pathog. 2015, 11, e1005090. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Navio, J.M.; Fuchs, S.P.; Pedreño-López, S.; Rakasz, E.G.; Gao, G.; Desrosiers, R.C. Host Anti-antibody Responses Following Adeno-associated Virus–mediated Delivery of Antibodies against HIV and SIV in Rhesus Monkeys. Mol. Ther. 2016, 24, 76–86. [Google Scholar] [CrossRef]
- Calne, R.Y.; Sells, R.A.; Pena, J.R.; Davis, D.R.; Millard, P.R.; Herbertson, B.M.; Binns, R.M.; Davies, D.A.L. Induction of Immunological Tolerance by Porcine Liver Allografts. Nature 1969, 223, 472–476. [Google Scholar] [CrossRef]
- Keeler, G.D.; Markusic, D.M.; Hoffman, B.E. Liver induced transgene tolerance with AAV vectors. Cell. Immunol. 2019, 342, 103728. [Google Scholar] [CrossRef]
- Markusic, D.M.; Hoffman, B.E.; Perrin, G.Q.; Nayak, S.; Wang, X.; LoDuca, P.A.; High, K.A.; Herzog, R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013, 5, 1698–1709. [Google Scholar] [CrossRef]
- Cao, O.; Dobrzynski, E.; Wang, L.; Nayak, S.; Mingle, B.; Terhorst, C.; Herzog, R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 2007, 110, 1132–1140. [Google Scholar] [CrossRef]
- Breous, E.; Somanathan, S.; Vandenberghe, L.H.; Wilson, J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 2009, 50, 612–621. [Google Scholar] [CrossRef]
- Martino, A.T.; Nayak, S.; Hoffman, B.E.; Cooper, M.; Liao, G.; Markusic, D.M.; Byrne, B.J.; Terhorst, C.; Herzog, R.W. Tolerance Induction to Cytoplasmic β-Galactosidase by Hepatic AAV Gene Transfer—Implications for Antigen Presentation and Immunotoxicity. PLoS ONE 2009, 4, e6376. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, E.; Mingozzi, F.; Liu, Y.-L.; Bendo, E.; Cao, O.; Wang, L.; Herzog, R.W. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 2004, 104, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.P.; Martinez-Navio, J.M.; Rakasz, E.G.; Gao, G.; Desrosiers, R.C. Liver-Directed but Not Muscle-Directed AAV-Antibody Gene Transfer Limits Humoral Immune Responses in Rhesus Monkeys. Mol. Ther.-Methods Clin. Dev. 2019, 16, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Welles, H.C.; Jennewein, M.F.; Mason, R.D.; Narpala, S.; Wang, L.; Cheng, C.; Zhang, Y.; Todd, J.-P.; Lifson, J.D.; Balazs, A.B.; et al. Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLOS Pathog. 2018, 14, e1007395. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.H.; Wang, L.; Somanathan, S.; Zhi, Y.; Figueredo, J.; Calcedo, R.; Sanmiguel, J.; Desai, R.; Chen, C.; Johnston, J.; et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006, 12, 967–971. [Google Scholar] [CrossRef]
- Tenenbaum, E.L.A.P.E.M.L.; Lehtonen, E.; Monahan, P.E. Evaluation of Risks Related to the Use of Adeno-Associated Virus-Based Vectors. Curr. Gene Ther. 2003, 3, 545–565. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Wright, J.F. Challenges Posed by Immune Responses to AAV Vectors: Addressing Root Causes. Front. Immunol. 2021, 12, 675897. [Google Scholar] [CrossRef]
- Davé, U.P.; Cornetta, K. AAV Joins the Rank of Genotoxic Vectors. Mol. Ther. 2021, 29, 418–419. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.-A.; Merricks, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021, 39, 47–55. [Google Scholar] [CrossRef]
- Nault, J.-C.; Datta, S.; Imbeaud, S.; Franconi, A.; Mallet, M.; Couchy, G.; Letouzé, E.; Pilati, C.; Verret, B.; Blanc, J.-F.; et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 2015, 47, 1187–1193. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Friedman, A.; et al. Heterologous Protection against Influenza by Injection of DNA Encoding a Viral Protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ugen, K.E.; Srikantan, V.; Agadjanyan, M.G.; Dang, K.; Refaeli, Y.; Sato, A.I.; Boyer, J.; Williams, W.V.; Weiner, D.B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1993, 90, 4156–4160. [Google Scholar] [CrossRef]
- Le, T.P.; Coonan, K.M.; Hedstrom, R.C.; Charoenvit, Y.; Sedegah, M.; Epstein, J.E.; Kumar, S.; Wang, R.; Doolan, D.; Maguire, J.D.; et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 2000, 18, 1893–1901. [Google Scholar] [CrossRef]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B.; et al. First Human Trial of a DNA-Based Vaccine for Treatment of Human Immunodeficiency Virus Type 1 Infection: Safety and Host Response. J. Infect. Dis. 1998, 178, 92–100. [Google Scholar] [CrossRef]
- MacGregor, R.R.; Boyer, J.D.; Ciccarelli, R.B.; Ginsberg, R.S.; Weiner, D.B. Safety and Immune Responses to a DNA-Based Human Immunodeficiency Virus (HIV) Type I Env/Rev Vaccine in HIV-Infected Recipients: Follow-up Data. J. Infect. Dis. 2000, 181, 406. [Google Scholar] [CrossRef] [PubMed]
- Calarota, S.A.; Leandersson, A.-C.; Bratt, G.; Hinkula, J.; Klinman, D.M.; Weinhold, K.J.; Sandström, E.; Wahren, B. Immune Responses in Asymptomatic HIV-1-Infected Patients After HIV-DNA Immunization Followed by Highly Active Antiretroviral Treatment. J. Immunol. 1999, 163, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Ugen, K.E.; Nyland, S.B.; Boyer, J.D.; Vidal, C.; Lera, L.; Rasheid, S.; Chattergoon, M.; Bagarazzi, M.L.; Ciccarelli, R.; Higgins, T.; et al. DNA vaccination with HIV-1 expressing constructs elicits immune responses in humans. Vaccine 1998, 16, 1818–1821. [Google Scholar] [CrossRef]
- Calarota, S.A.; Kjerrström, A.; Islam, K.B.; Wahren, B.; Wen, J.; Hao, W.; Fan, Y.; Du, J.; Du, B.; Qian, M.; et al. Gene Combination Raises Broad Human Immunodeficiency Virus-Specific Cytotoxicity. Hum. Gene Ther. 2001, 12, 1623–1637. [Google Scholar] [CrossRef]
- Wang, R.; Epstein, J.; Baraceros, F.M.; Gorak, E.J.; Charoenvit, Y.; Carucci, D.J.; Hedstrom, R.C.; Rahardjo, N.; Gay, T.; Hobart, P.; et al. Induction of CD4+T cell-dependent CD8+type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 2001, 98, 10817–10822. [Google Scholar] [CrossRef]
- Perez, N.; Bigey, P.; Scherman, D.; Danos, O.; Piechaczyk, M.; Pelegrin, M. Regulatable systemic production of monoclonal antibodies by in vivo muscle electroporation. Genet. Vaccines Ther. 2004, 2, 2. [Google Scholar] [CrossRef]
- Tjelle, T.E.; Corthay, A.; Lunde, E.; Sandlie, I.E.; Michaelsen, T.; Mathiesen, I.; Bogen, B. Monoclonal Antibodies Produced by Muscle after Plasmid Injection and Electroporation. Mol. Ther. 2004, 9, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, K.; Flingai, S.; Wise, M.; Tingey, C.; Ugen, K.E.; Weiner, D.B. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum. Vaccines Immunother. 2013, 9, 2253–2262. [Google Scholar] [CrossRef][Green Version]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine—Preliminary Report. N. Engl. J. Med. 2021, 385, e35. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.M.; Signori, E.; Wells, K.E.; Fazio, V.M.; Wells, D.J. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase-increased expression with reduced muscle damage. Gene Ther. 2001, 8, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Park, D.H.; Davis, C.W.; Smith, T.R.; Leung, A.; Tierney, K.; Bryan, A.; Davidson, E.; Yu, X.; Racine, T.; et al. In Vivo Delivery of Synthetic Human DNA-Encoded Monoclonal Antibodies Protect against Ebolavirus Infection in a Mouse Model. Cell Rep. 2018, 25, 1982–1993.e4. [Google Scholar] [CrossRef]
- Tursi, N.J.; Reeder, S.M.; Flores-Garcia, Y.; Bah, M.A.; Mathis-Torres, S.; Salgado-Jimenez, B.; Esquivel, R.; Xu, Z.; Chu, J.D.; Humeau, L.; et al. Engineered DNA-encoded monoclonal antibodies targeting Plasmodium falciparum circumsporozoite protein confer single dose protection in a murine malaria challenge model. Sci. Rep. 2022, 12, 14313. [Google Scholar] [CrossRef]
- Parzych, E.M.; Du, J.; Ali, A.R.; Schultheis, K.; Frase, D.; Smith, T.R.F.; Cui, J.; Chokkalingam, N.; Tursi, N.J.; Andrade, V.M.; et al. DNA-delivered antibody cocktail exhibits improved pharmacokinetics and confers prophylactic protection against SARS-CoV-2. Nat. Commun. 2022, 13, 5886. [Google Scholar] [CrossRef]
- Elliott, S.T.C.; Kallewaard, N.L.; Benjamin, E.; Wachter-Rosati, L.; McAuliffe, J.M.; Patel, A.; Smith, T.R.F.; Schultheis, K.; Park, D.H.; Flingai, S.; et al. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. NPJ Vaccines 2017, 2, 18. [Google Scholar] [CrossRef]
- Flingai, S.; Plummer, E.M.; Patel, A.; Shresta, S.; Mendoza, J.M.; Broderick, K.E.; Sardesai, N.Y.; Muthumani, K.; Weiner, D.B. Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Sci. Rep. 2015, 5, 12616. [Google Scholar] [CrossRef]
- Hollevoet, K.; De Smidt, E.; Geukens, N.; Declerck, P. Prolonged in vivo expression and anti-tumor response of DNA-based anti-HER2 antibodies. Oncotarget 2018, 9, 13623–13636. [Google Scholar] [CrossRef]
- Muthumani, K.; Block, P.; Flingai, S.; Muruganantham, N.; Chaaithanya, I.K.; Tingey, C.; Wise, M.; Reuschel, E.L.; Chung, C.; Muthumani, A.; et al. Rapid and Long-Term Immunity Elicited by DNA-Encoded Antibody Prophylaxis and DNA Vaccination against Chikungunya Virus. J. Infect. Dis. 2016, 214, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, K.; Marnin, L.; Kudchodkar, S.B.; Perales-Puchalt, A.; Choi, H.; Agarwal, S.; Scott, V.L.; Reuschel, E.L.; Zaidi, F.I.; Duperret, E.K.; et al. Novel prostate cancer immunotherapy with a DNA-encoded anti-prostate-specific membrane antigen monoclonal antibody. Cancer Immunol. Immunother. 2017, 66, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; DiGiandomenico, A.; Keller, A.E.; Smith, T.R.F.; Park, D.H.; Ramos, S.; Schultheis, K.; Elliott, S.T.C.; Mendoza, J.; Broderick, K.E.; et al. An engineered bispecific DNA-encoded IgG antibody protects against Pseudomonas aeruginosa in a pneumonia challenge model. Nat. Commun. 2017, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, R.N.; Patel, A.; Kudchodkar, S.B.; Park, D.H.; Stettler, K.; Beltramello, M.; Allen, J.W.; Mendoza, J.; Ramos, S.; Choi, H.; et al. In Vivo Delivery of a DNA-Encoded Monoclonal Antibody Protects Non-human Primates against Zika Virus. Mol. Ther. 2019, 27, 974–985. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Duperret, E.K.; Yang, X.; Hernandez, P.; Wojtak, K.; Zhu, X.; Jung, S.-H.; Tello-Ruiz, E.; Wise, M.C.; Montaner, L.J.; et al. DNA-encoded bispecific T cell engagers and antibodies present long-term antitumor activity. J. Clin. Investig. 2019, 4, e126086. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.C.; Xu, Z.; Tello-Ruiz, E.; Beck, C.; Trautz, A.; Patel, A.; Elliott, S.T.; Chokkalingam, N.; Kim, S.; Kerkau, M.G.; et al. In vivo delivery of synthetic DNA-encoded antibodies induces broad HIV-1-neutralizing activity. J. Clin. Investig. 2019, 130, 827–837. [Google Scholar] [CrossRef]
- Wallace, M.; Evans, B.; Woods, S.; Mogg, R.; Zhang, L.; Finnefrock, A.C.; Rabussay, D.; Fons, M.; Mallee, J.; Mehrotra, D.; et al. Tolerability of Two Sequential Electroporation Treatments Using MedPulser DNA Delivery System (DDS) in Healthy Adults. Mol. Ther. 2009, 17, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, K.; Smith, T.R.; Kiosses, W.B.; Kraynyak, K.A.; Wong, A.; Oh, J.; Broderick, K.E. Delineating the Cellular Mechanisms Associated with Skin Electroporation. Hum. Gene Ther. Methods 2018, 29, 177–188. [Google Scholar] [CrossRef]
- Lallow, E.O.; Jhumur, N.C.; Ahmed, I.; Kudchodkar, S.B.; Roberts, C.C.; Jeong, M.; Melnik, J.M.; Park, S.H.; Muthumani, K.; Shan, J.W.; et al. Novel suction-based in vivo cutaneous DNA transfection platform. Sci. Adv. 2021, 7, eabj0611. [Google Scholar] [CrossRef]
- Kitaguchi, K.; Toda, M.; Takekoshi, M.; Maeda, F.; Muramatsu, T.; Murai, A. Immune deficiency enhances expression of recombinant human antibody in mice after nonviral in vivo gene transfer. Int. J. Mol. Med. 2005, 16, 683–688. [Google Scholar]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Pardi, N.; Muramatsu, H.; Weissman, D.; Karikó, K. In Vitro Transcription of Long RNA Containing Modified Nucleosides. In Synthetic Messenger RNA and Cell Metabolism Modulation. Methods in Molecular Biology (Methods and Protocols); Rabinovich, P., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 969. [Google Scholar] [CrossRef]
- Weissman, D.; Pardi, N.; Muramatsu, H.; Karikó, K. HPLC Purification of In Vitro Transcribed Long RNA. Methods Mol. Biol . 2012, 969, 43–54. [Google Scholar] [CrossRef]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther.-Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Foster, J.B.; Choudhari, N.; Perazzelli, J.; Storm, J.; Hofmann, T.J.; Jain, P.; Storm, P.B.; Pardi, N.; Weissman, D.; Waanders, A.J.; et al. Purification of mRNA Encoding Chimeric Antigen Receptor Is Critical for Generation of a Robust T-Cell Response. Hum. Gene Ther. 2019, 30, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Probst, J.; Weide, B.; Scheel, B.; Pichler, B.J.; Hoerr, I.; Rammensee, H.-G.; Pascolo, S. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007, 14, 1175–1180. [Google Scholar] [CrossRef]
- Deal, C.E.; Carfi, A.; Plante, O.J. Advancements in mRNA Encoded Antibodies for Passive Immunotherapy. Vaccines 2021, 9, 108. [Google Scholar] [CrossRef]
- Bhat, B.; Karve, S.; Anderson, D.G. mRNA therapeutics: Beyond vaccine applications. Trends Mol. Med. 2021, 27, 923–924. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Na Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qian, S.-B. Therapeutic mRNA Engineering from Head to Tail. Accounts Chem. Res. 2021, 54, 4272–4282. [Google Scholar] [CrossRef] [PubMed]
- Kose, N.; Fox, J.M.; Sapparapu, G.; Bombardi, R.; Tennekoon, R.N.; de Silva, A.D.; Elbashir, S.M.; Theisen, M.A.; Humphris-Narayanan, E.; Ciaramella, G.; et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 2019, 4, eaaw6647. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.E.; Vanover, D.; Thoresen, M.; King, H.; Xiao, P.; Badial, P.; Araínga, M.; Bin Park, S.; Tiwari, P.M.; Peck, H.E.; et al. Aerosol Delivery of Synthetic mRNA to Vaginal Mucosa Leads to Durable Expression of Broadly Neutralizing Antibodies against HIV. Mol. Ther. 2020, 28, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Secreto, A.J.; Shan, X.; Debonera, F.; Glover, J.; Yi, Y.; Muramatsu, H.; Ni, H.; Mui, B.L.; Tam, Y.K.; et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017, 8, 14630. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Stadler, C.R.; Bähr-Mahmud, H.; Celik, L.; Hebich, B.; Roth, A.S.; Roth, R.P.; Karikó, K.; Türeci, Ö.; Sahin, U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017, 23, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Thran, M.; Mukherjee, J.; Pönisch, M.; Fiedler, K.; Thess, A.; Mui, B.L.; Hope, M.J.; Tam, Y.K.; Horscroft, N.; Heidenreich, R.; et al. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017, 9, 1434–1447. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Russell, J.E. Structural and Functional Analysis of an mRNP Complex That Mediates the High Stability of Human beta-globin mRNA. Mol. Cell. Biol. 2001, 21, 5879–5888. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Nelson, J.; Sorensen, E.W.; Mintri, S.; Rabideau, A.E.; Zheng, W.; Besin, G.; Khatwani, N.; Su, S.V.; Miracco, E.J.; Issa, W.J.; et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 2020, 6, eaaz6893. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.-J.; Huang, Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef]
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High Guanine and Cytosine Content Increases mRNA Levels in Mammalian Cells. PLoS Biol. 2006, 4, e180. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics with Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid Vesicle Size Determines the Th1 or Th2 Response to Entrapped Antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar] [CrossRef]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Fujiwara, H.; Hamaoka, T.; Mayumi, T. Positively Charged Liposome Functions as an Efficient Immunoadjuvant in Inducing Immune Responses to Soluble Proteins. Biochem. Biophys. Res. Commun. 1997, 240, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 2019, 17, 54. [Google Scholar] [CrossRef]
- Davies, N.; Hovdal, D.; Edmunds, N.; Nordberg, P.; Dahlén, A.; Dabkowska, A.; Arteta, M.Y.; Radulescu, A.; Kjellman, T.; Höijer, A.; et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Mol. Ther.-Nucleic Acids 2021, 24, 369–384. [Google Scholar] [CrossRef]
- August, A.; Attarwala, H.Z.; Himansu, S.; Kalidindi, S.; Lu, S.; Pajon, R.; Han, S.; Lecerf, J.-M.; Tomassini, J.E.; Hard, M.; et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat. Med. 2021, 27, 2224–2233. [Google Scholar] [CrossRef]
- Rybakova, Y.; Kowalski, P.S.; Huang, Y.; Gonzalez, J.T.; Heartlein, M.W.; DeRosa, F.; Delcassian, D.; Anderson, D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, W.; Tian, J.; Qi, C.; Cai, Z.; Yan, W.; Xuan, S.; Shang, A. Intravenous Delivery of RNA Encoding Anti-PD-1 Human Monoclonal Antibody for Treating Intestinal Cancer. J. Cancer 2022, 13, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.H.; Archer, J.; Fuerte-Stone, J.; Khandhar, A.P.; Voigt, E.; Granger, B.; Bombardi, R.G.; Govero, J.; Tan, Q.; Durnell, L.A.; et al. Intramuscular Delivery of Replicon RNA Encoding ZIKV-117 Human Monoclonal Antibody Protects against Zika Virus Infection. Mol. Ther.-Methods Clin. Dev. 2020, 18, 402–414. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H.; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Christensen, D.; Yus, B.I.; Aldon, Y.; Follmann, F.; Shattock, R.J. Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J. Control Release 2019, 304, 65–74. [Google Scholar] [CrossRef]
- Vanover, D.; Zurla, C.; Peck, H.E.; Orr-Burks, N.; Joo, J.Y.; Murray, J.; Holladay, N.; Hobbs, R.A.; Jung, Y.; Chaves, L.C.S.; et al. Nebulized mRNA-Encoded Antibodies Protect Hamsters from SARS-CoV-2 Infection. Adv. Sci. 2022, 9, e2202771. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.M.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef]
- Vuchelen, A.; O’day, E.; De Genst, E.; Pardon, E.; Wyns, L.; Dumoulin, M.; Dobson, C.M.; Christodoulou, J.; Hsu, S.-T.D. 1H, 13C and 15N assignments of a camelid nanobody directed against human α-synuclein. Biomol. NMR Assign. 2009, 3, 231–233. [Google Scholar] [CrossRef]
- Chapman, A.P.; Antoniw, P.; Spitali, M.; West, S.; Stephens, S.; King, D.J. Therapeutic antibody fragments with prolonged in vivo half-lives. Nat. Biotechnol. 1999, 17, 780–783. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Vercammen, J.; Kolkman, J.A.; Walsum, M.S.-V.; Revets, H.; van Dongen, G.A. Nanobodies Targeting the Hepatocyte Growth Factor: Potential New Drugs for Molecular Cancer Therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, G.; De Smidt, E.; Casteels, P.; Geukens, N.; Declerck, P.; Hollevoet, K. DNA-based delivery of anti-DR5 Nanobodies improves exposure and anti-tumor efficacy over protein-based administration. Cancer Gene Ther. 2020, 28, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Gondé, H.; Demeules, M.; Hardet, R.; Scarpitta, A.; Junge, M.; Pinto-Espinoza, C.; Varin, R.; Koch-Nolte, F.; Boyer, O.; Adriouch, S. A Methodological Approach Using rAAV Vectors Encoding Nanobody-Based Biologics to Evaluate ARTC2.2 and P2X7 In Vivo. Front. Immunol. 2021, 12, 704408. [Google Scholar] [CrossRef]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744.e16. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.T.; Wykoff-Clary, S.; Gross, C.S.; Schneider, D.; Jin, F.; Kretschmer, P.J.; Hermiston, T.W. Growth inhibition of an established A431 xenograft tumor by a full-length anti-EGFR antibody following gene delivery by AAV. Cancer Gene Ther. 2008, 16, 184–194. [Google Scholar] [CrossRef]
- Piperno, G.M.; Requena, A.L.; Predonzani, A.; Dorvignit, D.; Labrada, M.; Zentilin, L.; Burrone, O.R.; Cescogaspere, M. Recombinant AAV-mediated in vivo long-term expression and antitumour activity of an anti-ganglioside GM3(Neu5Gc) antibody. Gene Ther. 2015, 22, 960–967. [Google Scholar] [CrossRef]
- Rghei, A.D.; van Lieshout, L.P.; Cao, W.; He, S.; Tierney, K.; Lopes, J.A.; Zielinska, N.; Baracuhy, E.M.; Campbell, E.S.B.; Minott, J.A.; et al. Adeno-associated virus mediated expression of monoclonal antibody MR191 protects mice against Marburg virus and provides long-term expression in sheep. Gene Ther. 2022. [Google Scholar] [CrossRef]
- Kim, H.; Danishmalik, S.N.; Hwang, H.; Sin, J.-I.; Oh, J.; Cho, Y.; Lee, H.; Jeong, M.; Kim, S.-H.; Hong, H.J. Gene therapy using plasmid DNA-encoded anti-HER2 antibody for cancers that overexpress HER2. Cancer Gene Ther. 2016, 23, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.D.; Luo, Y.; Sun, M.; Yu, J.; Goff, A.J.; Glass, P.J.; Padte, N.N.; Huang, Y.; Ho, D.D. In Vivo Production of Monoclonal Antibodies by Gene Transfer via Electroporation Protects against Lethal Influenza and Ebola Infections. Mol. Ther.-Methods Clin. Dev. 2017, 7, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Trautz, A.; Stoltz, R.; Patel, A.; Wise, M.C.; Perales-Puchalt, A.; Smith, T.; Broderick, K.E.; Masteller, E.L.; Kim, J.J.; et al. Synthetic DNA-Encoded Monoclonal Antibody Delivery of Anti–CTLA-4 Antibodies Induces Tumor Shrinkage In Vivo. Cancer Res. 2018, 78, 6363–6370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Esquivel, R.; Flingai, S.; Schiller, Z.; Kern, A.; Agarwal, S.; Chu, J.; Patel, A.; Sullivan, K.; Wise, M.C.; et al. Anti-OspA DNA-Encoded Monoclonal Antibody Prevents Transmission of Spirochetes in Tick Challenge Providing Sterilizing Immunity in Mice. J. Infect. Dis. 2018, 219, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Khoshnejad, M.; Patel, A.; Wojtak, K.; Kudchodkar, S.B.; Humeau, L.; Lyssenko, N.N.; Rader, D.J.; Muthumani, K.; Weiner, D.B. Development of Novel DNA-Encoded PCSK9 Monoclonal Antibodies as Lipid-Lowering Therapeutics. Mol. Ther. 2018, 27, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Duperret, E.K.; Muthumani, K.; Weiner, D.B. Simplifying checkpoint inhibitor delivery through in vivo generation of synthetic DNA-encoded monoclonal antibodies (DMAbs). Oncotarget 2019, 10, 13–16. [Google Scholar] [CrossRef]
- Hollevoet, K.; De Vleeschauwer, S.; De Smidt, E.; Vermeire, G.; Geukens, N.; Declerck, P. Bridging the Clinical Gap for DNA-Based Antibody Therapy through Translational Studies in Sheep. Hum. Gene Ther. 2019, 30, 1431–1443. [Google Scholar] [CrossRef]
- Zankharia, U.S.; Kudchodkar, S.; Khoshnejad, M.; Perales-Puchalt, A.; Choi, H.; Ho, M.; Zaidi, F.; Ugen, K.E.; Kim, J.J.; Weiner, D.B.; et al. Neutralization of hepatitis B virus by a novel DNA-encoded monoclonal antibody. Hum. Vaccines Immunother. 2020, 16, 2156–2164. [Google Scholar] [CrossRef]
- Jacobs, L.; De Smidt, E.; Geukens, N.; Declerck, P.; Hollevoet, K. DNA-Based Delivery of Checkpoint Inhibitors in Muscle and Tumor Enables Long-Term Responses with Distinct Exposure. Mol. Ther. 2020, 28, 1068–1077. [Google Scholar] [CrossRef]
- Choi, H.; Kudchodkar, S.B.; Reuschel, E.L.; Asija, K.; Borole, P.; Agarwal, S.; Van Gorder, L.; Reed, C.C.; Gulendran, G.; Ramos, S.; et al. Synthetic nucleic acid antibody prophylaxis confers rapid and durable protective immunity against Zika virus challenge. Hum. Vaccines Immunother. 2019, 16, 907–918. [Google Scholar] [CrossRef]
- Parzych, E.M.; Gulati, S.; Zheng, B.; Bah, M.A.; Elliott, S.T.C.; Chu, J.D.; Nowak, N.; Reed, G.W.; Beurskens, F.J.; Schuurman, J.; et al. Synthetic DNA Delivery of an Optimized and Engineered Monoclonal Antibody Provides Rapid and Prolonged Protection against Experimental Gonococcal Infection. mBio 2021, 12, e00242-21. [Google Scholar] [CrossRef]
- Vermeire, G.; De Smidt, E.; Geukens, N.; Williams, J.A.; Declerck, P.; Hollevoet, K. Improved Potency and Safety of DNA-Encoded Antibody Therapeutics through Plasmid Backbone and Expression Cassette Engineering. Hum. Gene Ther. 2021, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Hollevoet, K.; Thomas, D.; Compernolle, G.; Vermeire, G.; De Smidt, E.; De Vleeschauwer, S.; Smith, T.R.F.; Fisher, P.D.; Dewilde, M.; Geukens, N.; et al. Clinically relevant dosing and pharmacokinetics of DNA-encoded antibody therapeutics in a sheep model. Front. Oncol. 2022, 12, 1017612. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Verbeke, R.; De Vlieger, D.; Dewitte, H.; Roose, K.; Van Nevel, S.; Krysko, O.; Bachert, C.; Schepens, B.; Lentacker, I.; et al. mRNA Encoding a Bispecific Single Domain Antibody Construct Protects against Influenza A Virus Infection in Mice. Mol. Ther.-Nucleic Acids 2020, 20, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Mucker, E.M.; Thiele-Suess, C.; Baumhof, P.; Hooper, J.W. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol. Ther.-Nucleic Acids 2022, 28, 847–858. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).