The Different Colors of mAbs in Solution
Abstract
1. Introduction
2. Spectrophotometric Properties of Proteins in Solution in the Visible Range
3. Compendial and Characterization Methods for Determination of the Color of Protein Solutions
4. Color of a Protein Solution Is a Critical Quality Attribute Potentially Caused by Variants or Impurities
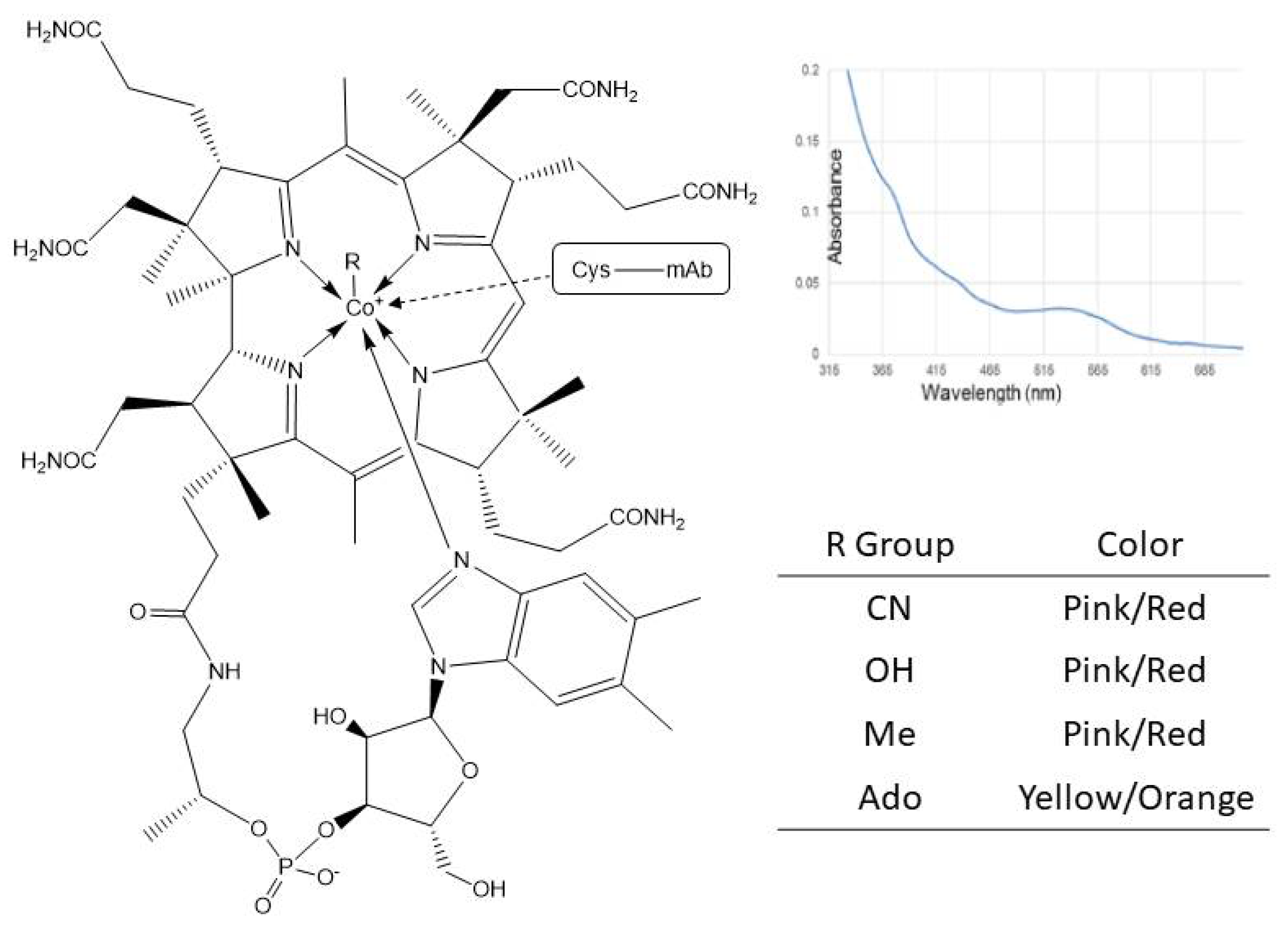
4.1. Postranslational Modifications Resulting in an Intrinsic Chromophore
4.2. Formation of Covalent Adducts Resulting in Extrinsic Chromophores
4.2.1. Formation of Advanced Glycation End Products Extrinsic Chromophores
4.2.2. Formation of Extrinsic Chromophores Originating from Cell Culture Components
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic Tryptophan Fluorescence in the Detection and Analysis of Proteins: A Focus on Förster Resonance Energy Transfer Techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef]
- Polozova, A. Origins of Color Change in Biopharmaceuticals: Identification of Protein and Excipient-Related Factors WCBP; MedImmune: Gaithersburg, MD, USA, 2013. [Google Scholar]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef]
- Li, Y.; Polozova, A.; Gruia, F.; Feng, J. Characterization of the Degradation Products of a Color-Changed Monoclonal Antibody: Tryptophan-Derived Chromophores. Anal. Chem. 2014, 86, 6850–6857. [Google Scholar] [CrossRef]
- Sreedhara, A.; Yin, J.; Joyce, M.; Lau, K.; Wecksler, A.T.; Deperalta, G.; Yi, L.; Wang, Y.J.; Kabakoff, B.; Kishore, R.S. Effect of ambient light on IgG1 monoclonal antibodies during drug product processing and development. Eur. J. Pharm. Biopharm. 2016, 100, 38–46. [Google Scholar] [CrossRef]
- Xu, J.; Jin, M.; Song, H.; Huang, C.; Xu, X.; Tian, J.; Qian, N.-X.; Steger, K.; Lewen, N.S.; Tao, L.; et al. Brown drug substance color investigation in cell culture manufacturing using chemically defined media: A case study. Process. Biochem. 2014, 49, 130–139. [Google Scholar] [CrossRef]
- Vijayasankaran, N.; Varma, S.; Yang, Y.; Mun, M.; Arevalo, S.; Gawlitzek, M.; Swartz, T.; Lim, A.; Li, F.; Zhang, B.; et al. Effect of cell culture medium components on color of formulated monoclonal antibody drug substance. Biotechnol. Prog. 2013, 29, 1270–1277. [Google Scholar] [CrossRef]
- Prentice, K.M.; Gillespie, R.; Lewis, N.; Fujimori, K.; McCoy, R.; Bach, J.; Connell-Crowley, L.; Eakin, C.M. Hydroxocobalamin association during cell culture results in pink therapeutic proteins. MAbs 2013, 5, 974–981. [Google Scholar] [CrossRef][Green Version]
- Derfus, G.E.; Dizon-Maspat, J.; Broddrick, J.T.; Velayo, A.C.; Toschi, J.D.; Santuray, R.T.; Hsu, S.K.; Winter, C.M.; Krishnan, R.; Amanullah, A. Red colored IgG4 caused by vitamin B12 from cell culture media combined with disulfide reduction at harvest. MAbs 2014, 6, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Martin, R.; Huang, Y.; Borwankar, A.; Tan, Z.; West, J.; Singh, N.; Borys, M.; Ghose, S.; Ludwig, R.; et al. Vitamin B 12 association with mAbs: Mechanism and potential mitigation strategies. Biotechnol. Bioeng. 2018, 115, 900–909. [Google Scholar] [CrossRef]
- Innis, W.S.; McCormick, D.B.; Merrill, A.H., Jr. Variations in riboflavin binding by human plasma: Identification of immunoglobulins as the major proteins responsible. Biochem. Med. 1985, 34, 151–165. [Google Scholar] [CrossRef]
- Watson, C.D.; Ford, H.C. High-affinity binding of riboflavin and FAD by immunoglobulins from normal human serum. Biochem. Int. 1988, 16, 1067–1074. [Google Scholar]
- Zhu, X.; Wentworth, P.; Kyle, R.A.; Lerner, R.A.; Wilson, I.A. Cofactor-containing antibodies: Crystal structure of the original yellow antibody. Proc. Natl. Acad. Sci. USA 2006, 103, 3581–3585. [Google Scholar] [CrossRef]
- Butko, M.; Pallat, H.; Cordoba, A.; Yu, X.C. Recombinant Antibody Color Resulting from Advanced Glycation End Product Modifications. Anal. Chem. 2014, 86, 9816–9823. [Google Scholar] [CrossRef]
- Wei, B.; Berning, K.; Quan, C.; Zhang, Y.T. Glycation of antibodies: Modification, methods and potential effects on biological functions. MAbs 2017, 9, 586–594. [Google Scholar] [CrossRef]
- Chumsae, C.; Gifford, K.; Lian, W.; Liu, H.; Radziejewski, C.H.; Zhou, Z.S. Arginine Modifications by Methylglyoxal: Discovery in a Recombinant Monoclonal Antibody and Contribution to Acidic Species. Anal. Chem. 2013, 85, 11401–11409. [Google Scholar] [CrossRef] [PubMed]
- Chumsae, C.; Hossler, P.; Raharimampionona, H.; Zhou, Y.; McDermott, S.; Racicot, C.; Radziejewski, C.; Zhou, Z.S. When Good Intentions Go Awry: Modification of a Recombinant Monoclonal Antibody in Chemically Defined Cell Culture by Xylosone, an Oxidative Product of Ascorbic Acid. Anal. Chem. 2015, 87, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Reneker, L.W.; Obrenovich, M.E.; Strauch, C.; Cheng, R.; Jarvis, S.M.; Ortwerth, B.J.; Monnier, V.M. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 16912–16917. [Google Scholar] [CrossRef]
- Lam, X.M.; Lai, W.G.; Chan, E.K.; Ling, V.; Hsu, C.C. Site-Specific Tryptophan Oxidation Induced by Autocatalytic Reaction of Polysorbate 20 in Protein Formulation. Pharm. Res. 2011, 28, 2543–2555. [Google Scholar] [CrossRef]
- Fischer, S.; Hoernschemeyer, J.; Mahler, H.-C. Glycation during storage and administration of monoclonal antibody formulations. Eur. J. Pharm. Biopharm. 2008, 70, 42–50. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia. USP-24 Monograph 631 Color and Achromaticity; United States Pharmacopoeia: Rockville, MD, USA, 2000; pp. 1926–1927. Available online: www.usp.org (accessed on 23 May 2021).
- European Pharmacopoeia. 2.2.2 Degree of Coloration of Liquids; European Pharmacopoeia: Strasbourg, France, 1997; pp. 15–16. Available online: www.pheur.org (accessed on 23 May 2021).
- Ministry of Health, Labour and Welfare of Japan. Methods for Color Matching JPXVII; Ministry of Health, Labour and Welfare of Japan: Tokyo, Japan, 2016.
- Swartz, T.E.; Yin, J.; Patapoff, T.W.; Horst, T.; Skieresz, S.M.; Leggett, G.; Morgan, C.J.; Rahimi, K.; Marhoul, J.; Kabakoff, B. A Spectral Method for Color Quantitation of a Protein Drug Solution. PDA J. Pharm. Sci. Technol. 2016, 70, 361–381. [Google Scholar] [CrossRef]
- ICHQ1B. Stability Testing: Photostability of New Drug Substances and Products. Available online: https://www.ich.org/fileadmin/Public.ICH.Q1B.Q1B_Guideline.pdf (accessed on 23 May 2021).
- Manual of Policies and Procedure Acceptability of Standards from Alternative Compendia (BP/EP/JP). Available online: https://www.fda.gov/media/72412/download (accessed on 23 May 2021).
- Pack, B.W.; Montgomery, L.L.; Hetrick, E.M. Modernization of Physical Appearance and Solution Color Tests Using Quantitative Tristimulus Colorimetry: Advantages, Harmonization, and Validation Strategies. J. Pharm. Sci. 2015, 104, 3299–3313. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia. USP Color—Instrumental Measurement; United States Pharmacopoeia: Rockville, MD, USA, 2016; Available online: https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c1061.pdf (accessed on 23 May 2021).
- Elder, D. ICH Q4 Pharmacopeial harmonization and evaluation and recommendation of pharamacopeial texts for use in the ICH regions. In ICH Quality Guidelines, an Implementation Quide; Teasdale, A., Elder, D., Nims, R.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Council of Europe. European Pharmacopoeia Revises General Chapter on Degree of Coloration of Liquids; Council of Europe: Strasbourg, France, 2019; Available online: https://www.edqm.eu/en/news/european-pharmacopoeia-revises-general-chapter-degree-coloration-liquids (accessed on 23 May 2021).
- Hetrick, E.M.; Vannoy, J.; Montgomery, L.L.; Pack, B.W. Integrating Tristimulus Colorimetry into Pharmaceutical Development for Color Selection and Physical Appearance Control: A Quality-by-Design Approach. J. Pharm. Sci. 2013, 102, 2608–2621. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Swartz, T.E.; Zhang, J.; Kabakoff, B.; Patapoff, T.W.; Chen, B.; Marhoul, J.; Shih, N.; Rahimi, K. Validation of a Spectral Method for Quantitative Measurement of Color in Protein Drug Solutions. PDA J. Pharm. Sci. Technol. 2016, 70, 382–391. [Google Scholar] [CrossRef]
- ICH Q5 Quality of Biotechnological Products. Available online: https://www.ich.org/products/guidelines/quality/article/quality-guidelines.html (accessed on 9 November 2020).
- ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q6B/Step4/Q6B_Guideline.pdf (accessed on 23 May 2021).
- Liu, H.; Nowak, C.; Shao, M.; Ponniah, G.; Neill, A. Impact of cell culture on recombinant monoclonal antibody product heterogeneity. Biotechnol. Prog. 2016, 32, 1103–1112. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Gong, R. Engineering of Fc Fragments with Optimized Physicochemical Properties Implying Improvement of Clinical Potentials for Fc-Based Therapeutics. Front. Immunol. 2018, 8, 1860. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.K.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef]
- Dreaden, T.M.; Chen, J.; Rexroth, S.; Barry, B.A. N-Formylkynurenine as a Marker of High Light Stress in Photosynthesis. J. Biol. Chem. 2011, 286, 22632–22641. [Google Scholar] [CrossRef]
- Tessier, F.; Obrenovich, M.; Monnier, V.M. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 1999, 274, 20796–20804. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts. Int. J. Mol. Sci. 2017, 18, 2557. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Cerami, A. Nonenzymatic browning in vivo: Possible process for aging of long-lived proteins. Science 1981, 211, 491–493. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Gaar, J.; Naffa, R.; Brimble, M.A. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Nowak, C.; Cheung, J.K.; Dellatore, S.M.; Katiyar, A.; Bhat, R.; Sun, J.; Ponniah, G.; Neill, A.; Mason, B.; Beck, A.; et al. Forced degradation of recombinant monoclonal antibodies: A practical guide. MAbs 2017, 9, 1217–1230. [Google Scholar] [CrossRef]
- Das, B.K.; Sun, T.X.; Akhtar, N.J.; Chylack, L.T., Jr.; Liang, J.J. Fluorescence and immunochemical studies of advanced glycation-related lens pigments. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2058–2066. [Google Scholar]
- Ranjan, M.; Beedu, S.R. Spectroscopic and biochemical correlations during the course of human lens aging. BMC Ophthalmol. 2006, 6, 10. [Google Scholar] [CrossRef]
- Takeuchi, M.; Makita, Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr. Mol. Med. 2001, 1, 305–315. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ahmad, S.; Choi, I.; Ahmad, N.; Farhan, M.; Tatyana, G.; Shahab, U. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life 2015, 67, 897–913. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Tsekovska, R.; Sredovska-Bozhinov, A.; Niwa, T.; Ivanov, I.; Mironova, R.; Tsekovska, A.S.-B.R. Maillard reaction and immunogenicity of protein therapeutics. World J. Immunol. 2016, 6, 19–38. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yuk, I.; Pai, R.; McKay, P.; Eigenbrot, C.; Dennis, M.; Katta, V.; Francissen, K.C. Unveiling a Glycation Hot Spot in a Recombinant Humanized Monoclonal Antibody. Anal. Chem. 2008, 80, 2379–2390. [Google Scholar] [CrossRef]
- Münch, G.; Keis, R.; Wessels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.-D.; Schinzel, R.; Weßels, A. Determination of Advanced Glycation End Products in Serum by Fluorescence Spectroscopy and Competitive ELISA. Clin. Chem. Lab. Med. 1997, 35, 669–678. [Google Scholar] [CrossRef]
- Quan, C.; Alcala, E.; Petkovska, I.; Matthews, D.; Canova-Davis, E.; Taticek, R.; Ma, S. A study in glycation of a therapeutic recombinant humanized monoclonal antibody: Where it is, how it got there, and how it affects charge-based behavior. Anal. Biochem. 2008, 373, 179–191. [Google Scholar] [CrossRef]
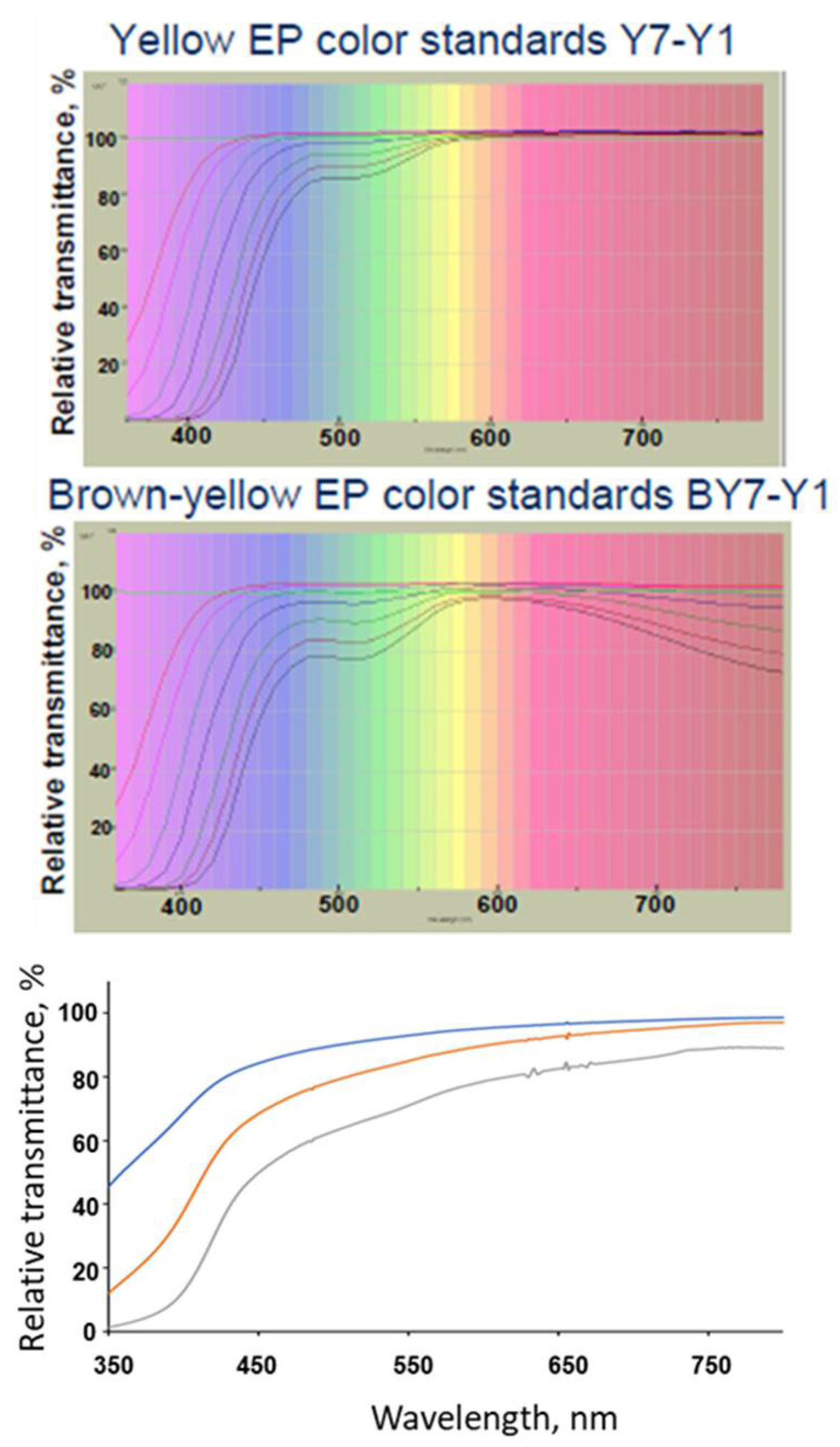
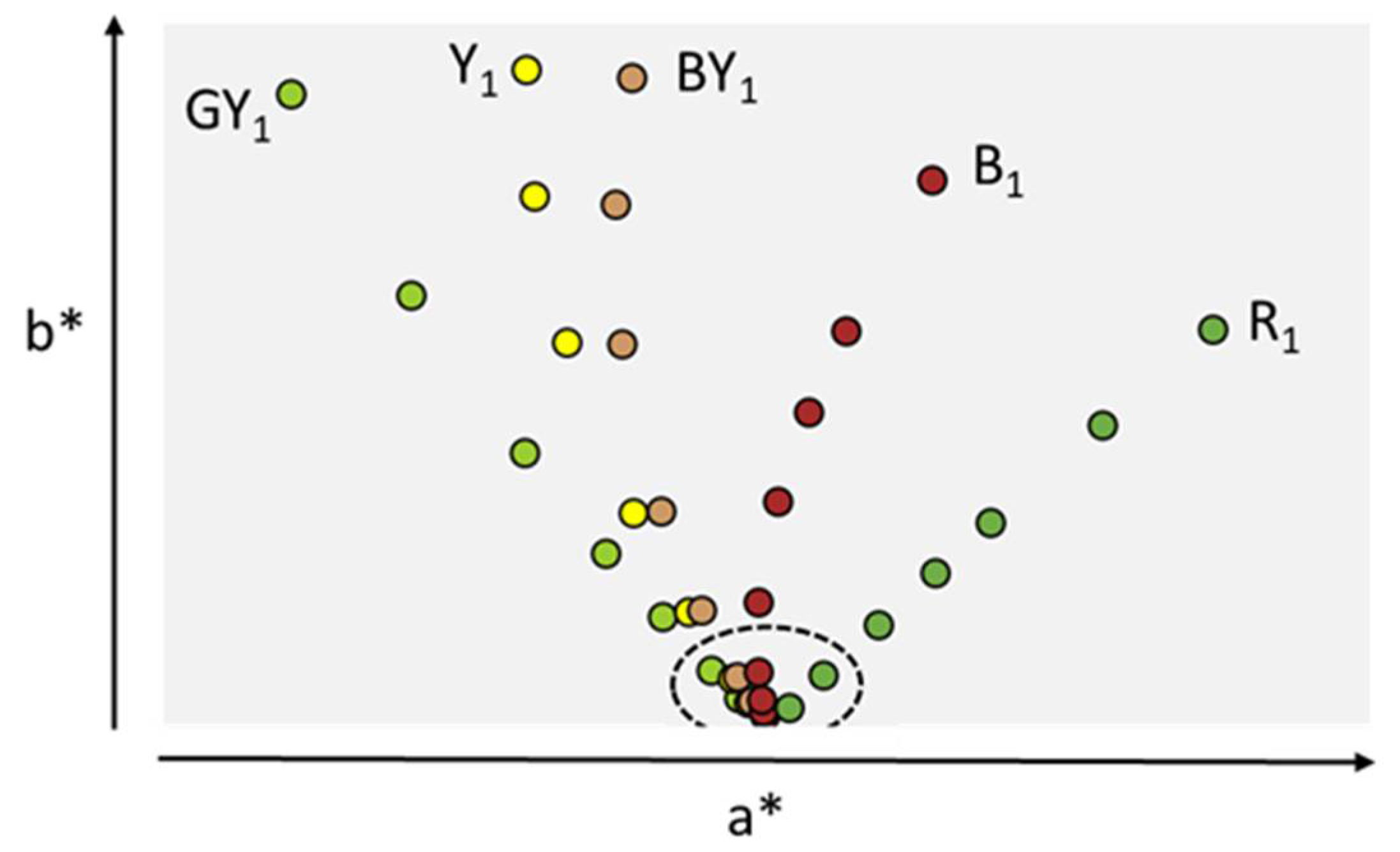
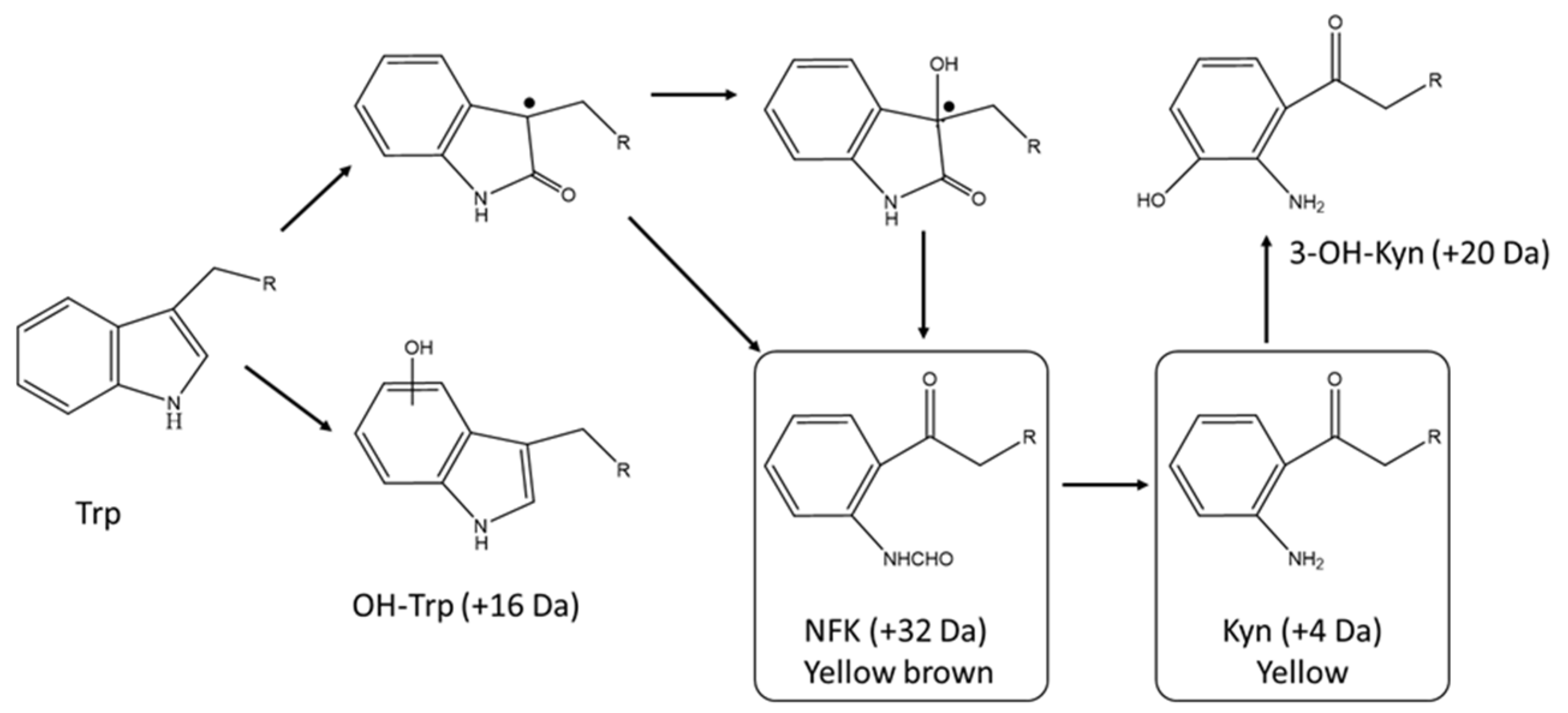
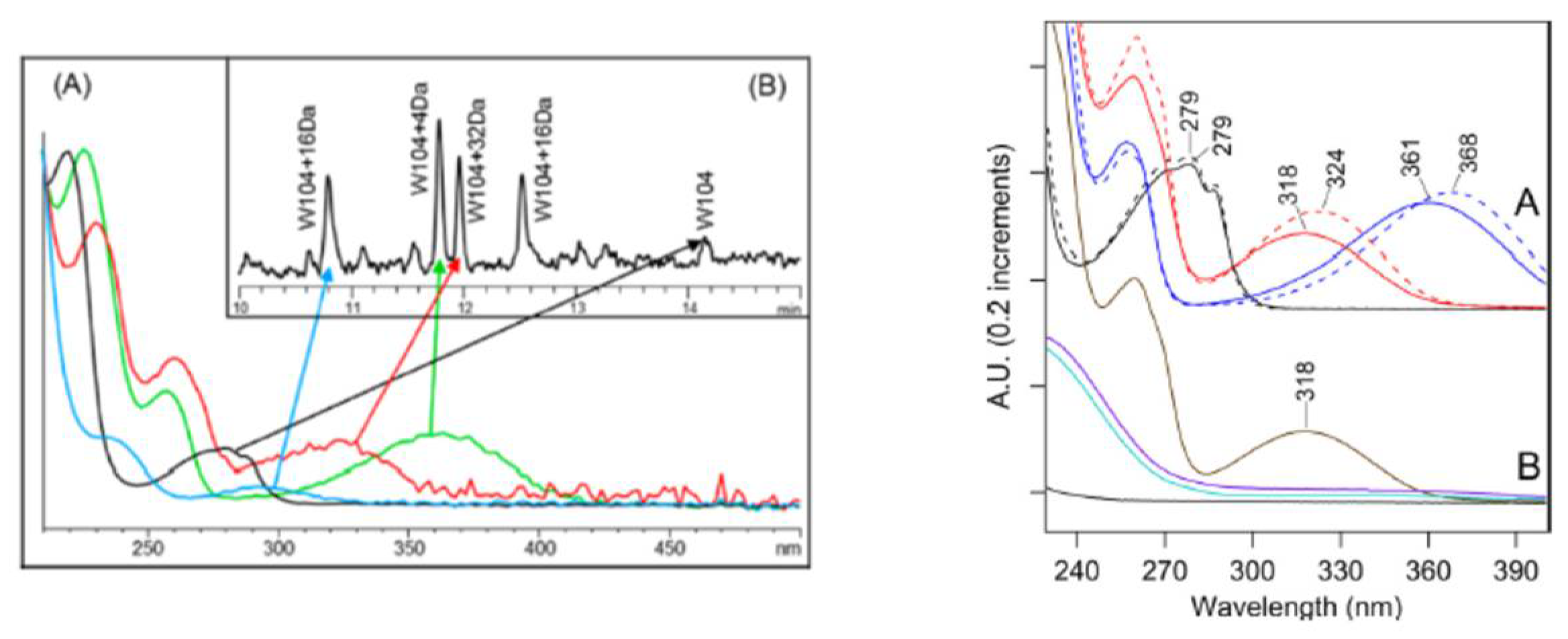

| Factor | Degradation Observed | Color Observed | Reference |
|---|---|---|---|
| UV light exposure | Tryptophan oxidation | Yellow/Yellow Brown | [6,7] |
| Copper/Iron in cell culture | Tryptophan oxidation and AGE products 1 | Yellow/Yellow Brown | [8,9] |
| Vitamin B12 (hydroxocobalamin) in cell culture | Adduct | Red/Pink | [8,10,11,12] |
| Vitamin B2 (riboflavin) in cell culture | Adduct | Yellow | [13,14,15] |
| Glucose/Ribose and dicarbonyls in cell culture | AGE products | Brown | [16,17,18] |
| Vitamin C in cell culture | AGE products | Brown | [19,20] |
| Sucrose in formulation | AGE products 1 | Brown 2 | [17] |
| Polysorbate excipient in formulation | Tryptophan oxidation | Yellow/Yellow Brown 2 | [6,21] |
| Dextrose during infusion | AGE products 1 | Brown | [22] |
| Excitation Maximum Wavelength | Emission Maximum Wavelength | Reference | |
|---|---|---|---|
| Tryptophan | 280 nm | 348 nm | [2,40] |
| Kynurenine (Kyn) | 330 nm and 380 nm | 480 nm | [40] |
| N-formylkynurenine (NFK) | 325 nm | 434 nm | [16] |
| Vesperlysine | 380 nm | 440 nm | [16,42] |
| AGE products | 360 nm | 430 nm | [43,44,45] |
| Pentosidine | 335 nm | 385 nm | [46] |
| Vesperlysine A, B | 366 nm | 442 nm | [46] |
| Absorbance Maximum Wavelength | |||
| Tryptophan | 280 nm | [6,40,41] | |
| Kynurenine (Kyn) | 260 nm and 365 nm | [6,40,41] | |
| N-formylkynurenine (NFK) | 260 nm and 320 nm | [6,41] | |
| Hydroxy tryprophan | 240 nm and 295 nm | [6] | |
| Vesperlysine | 302 nm and 363 nm | [16,42] | |
| AGE products | 330 nm | [42,43,44] | |
| Vitamin B12 | 360, 420, 550 nm | [12] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogelly, A. The Different Colors of mAbs in Solution. Antibodies 2021, 10, 21. https://doi.org/10.3390/antib10020021
Ambrogelly A. The Different Colors of mAbs in Solution. Antibodies. 2021; 10(2):21. https://doi.org/10.3390/antib10020021
Chicago/Turabian StyleAmbrogelly, Alexandre. 2021. "The Different Colors of mAbs in Solution" Antibodies 10, no. 2: 21. https://doi.org/10.3390/antib10020021
APA StyleAmbrogelly, A. (2021). The Different Colors of mAbs in Solution. Antibodies, 10(2), 21. https://doi.org/10.3390/antib10020021





