Remotely Sensed Changes in Vegetation Cover Distribution and Groundwater along the Lower Gila River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Preprocessing
2.2.1. Aerial Multispectral Imagery
2.2.2. Light Detection and Ranging (LiDAR)
2.2.3. Drone and Field Data
2.2.4. Groundwater, Streamflow, Precipitation, and Water Equivalent Thickness Measurements
2.3. Methodology
2.3.1. Classification of Vegetation in 2016 Using Acquired LiDAR and Multispectral Data
2.3.2. Vegetation Segment Metrics
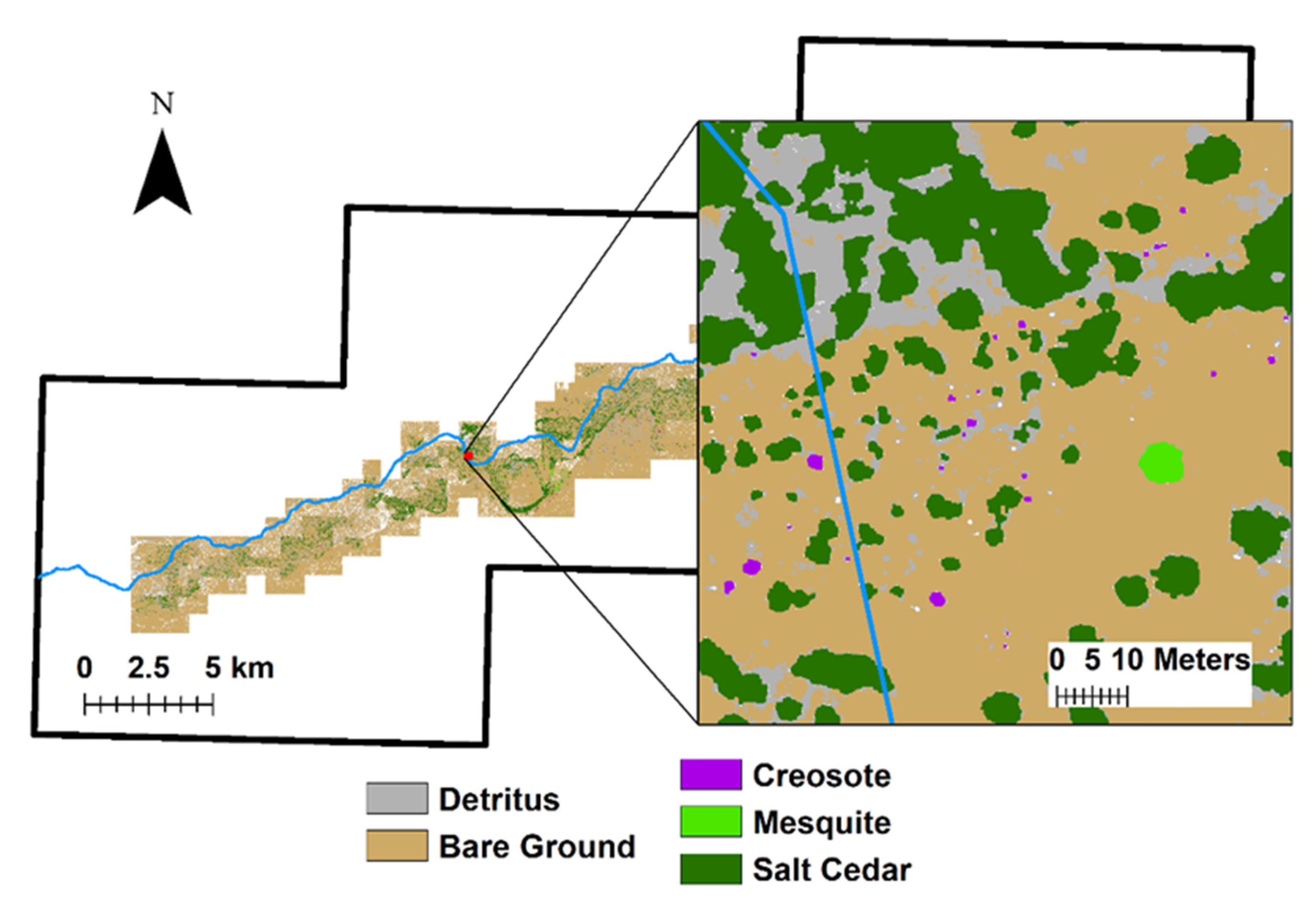
2.3.3. Classification of Vegetation Species
2.3.4. Classification of Vegetation in 1996, 2007, and 2019 Using NAPP and NAIP Data
2.3.5. Change Assessment
2.3.6. Buffer Analysis
3. Results
3.1. Living Vegetation Segmentation Classification
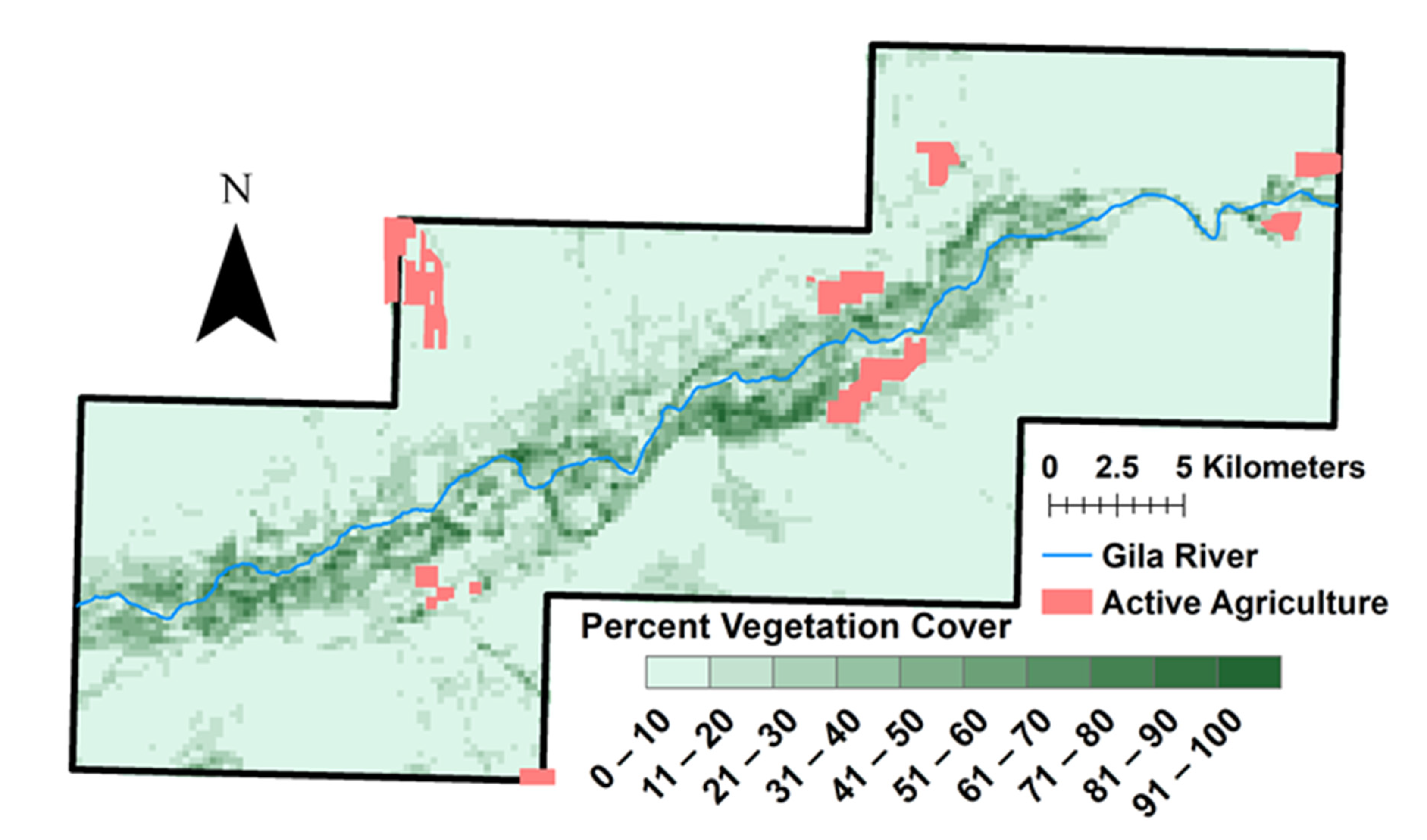
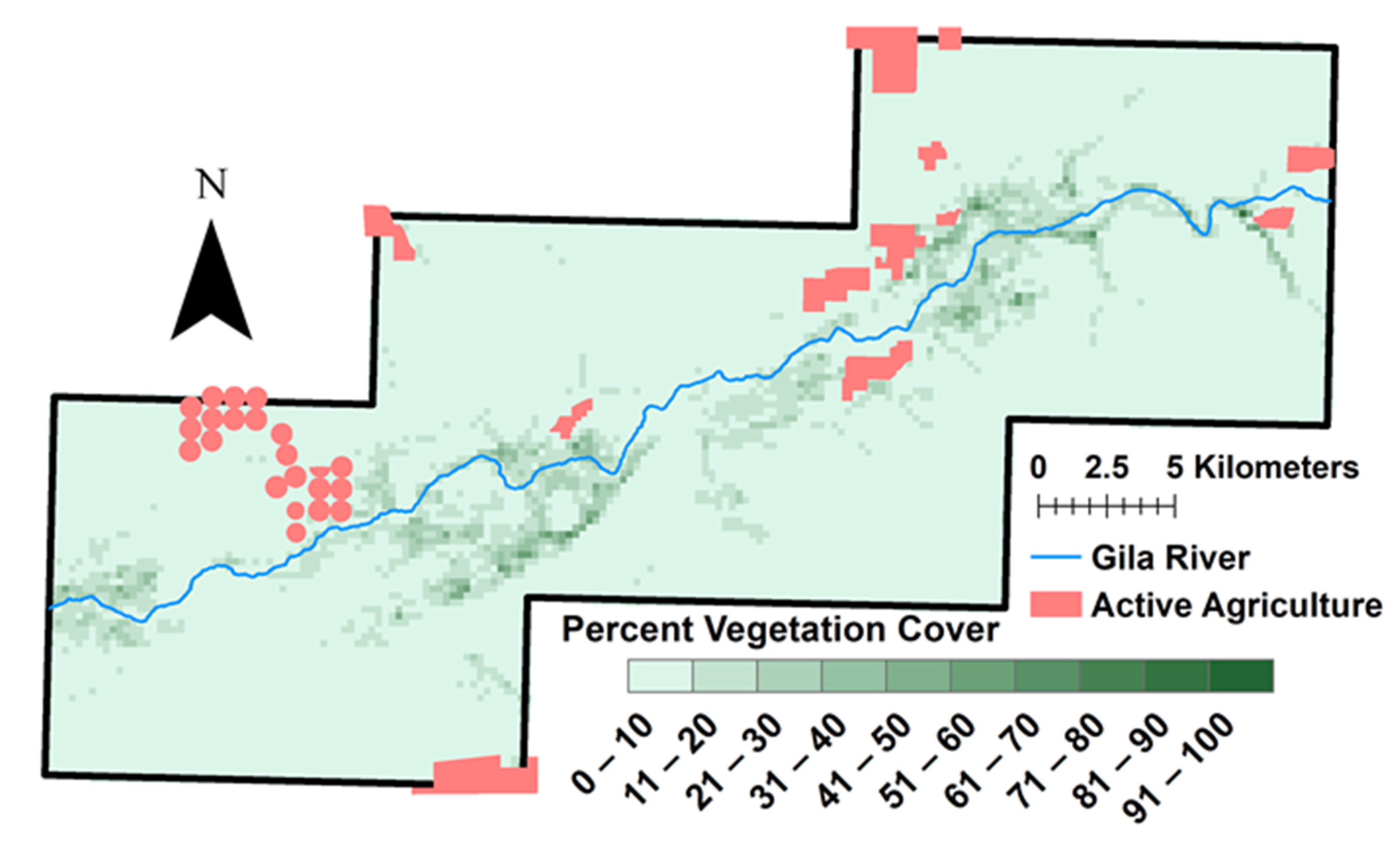
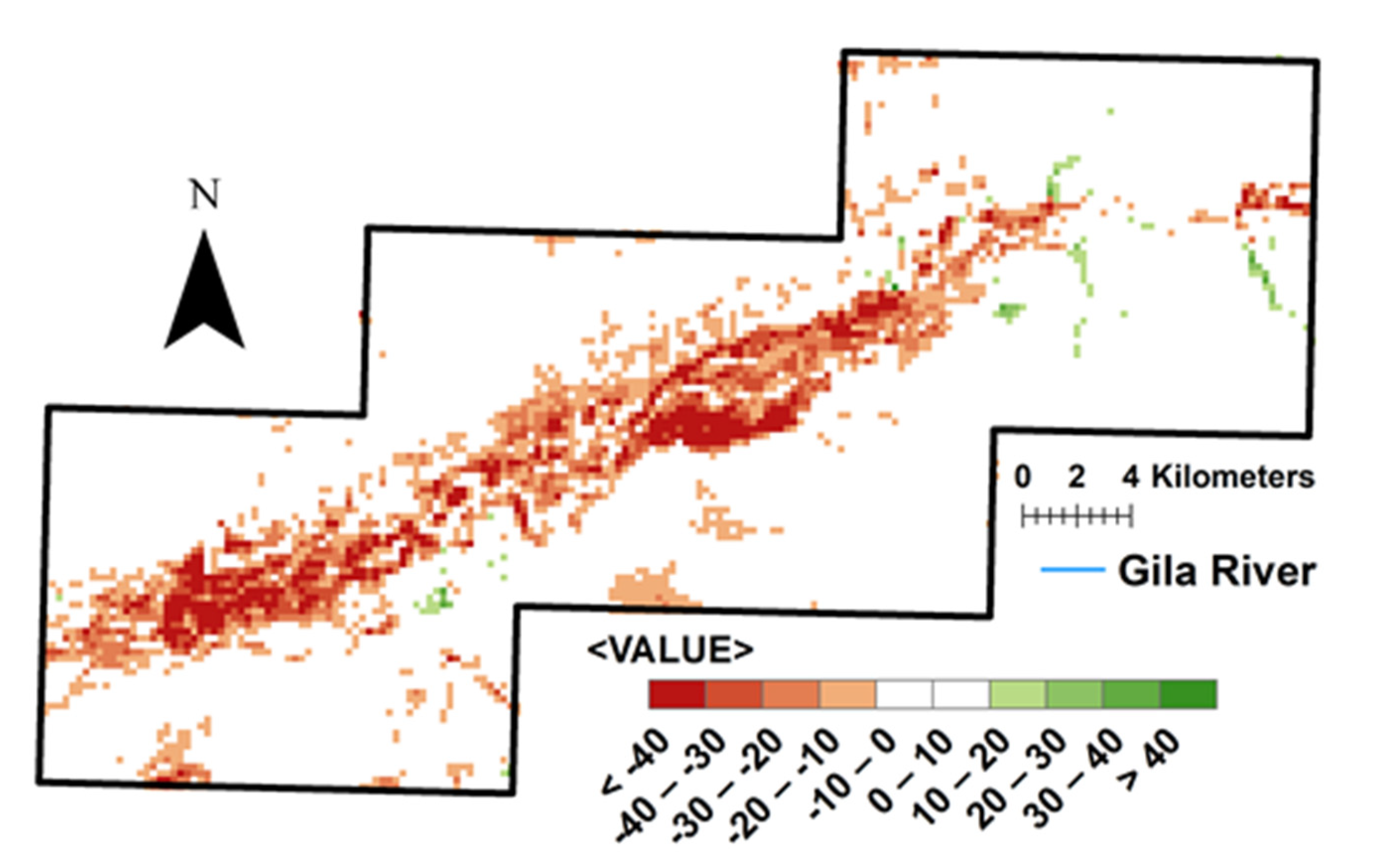
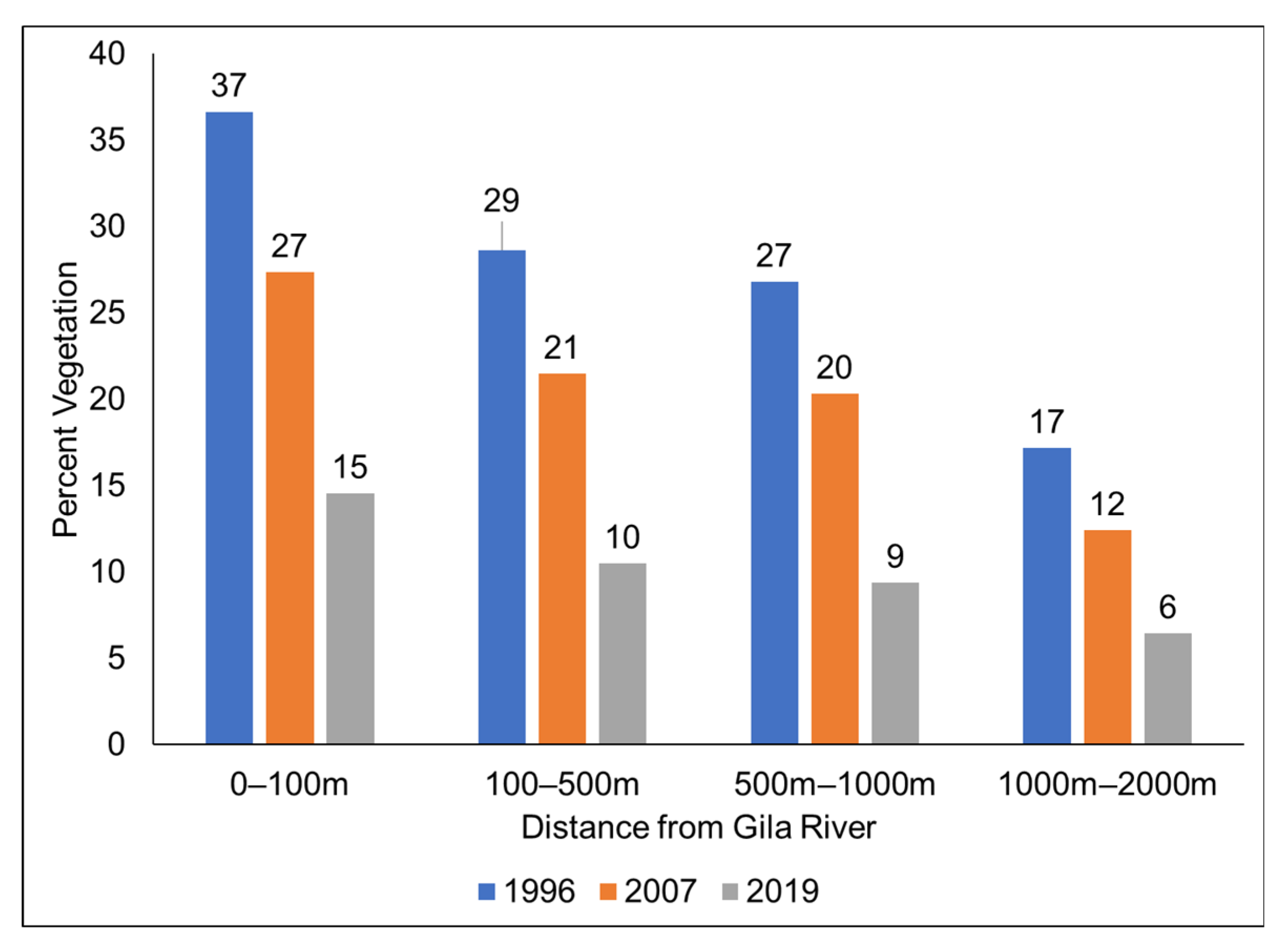
3.2. Vegetation Change and River Buffer Analysis
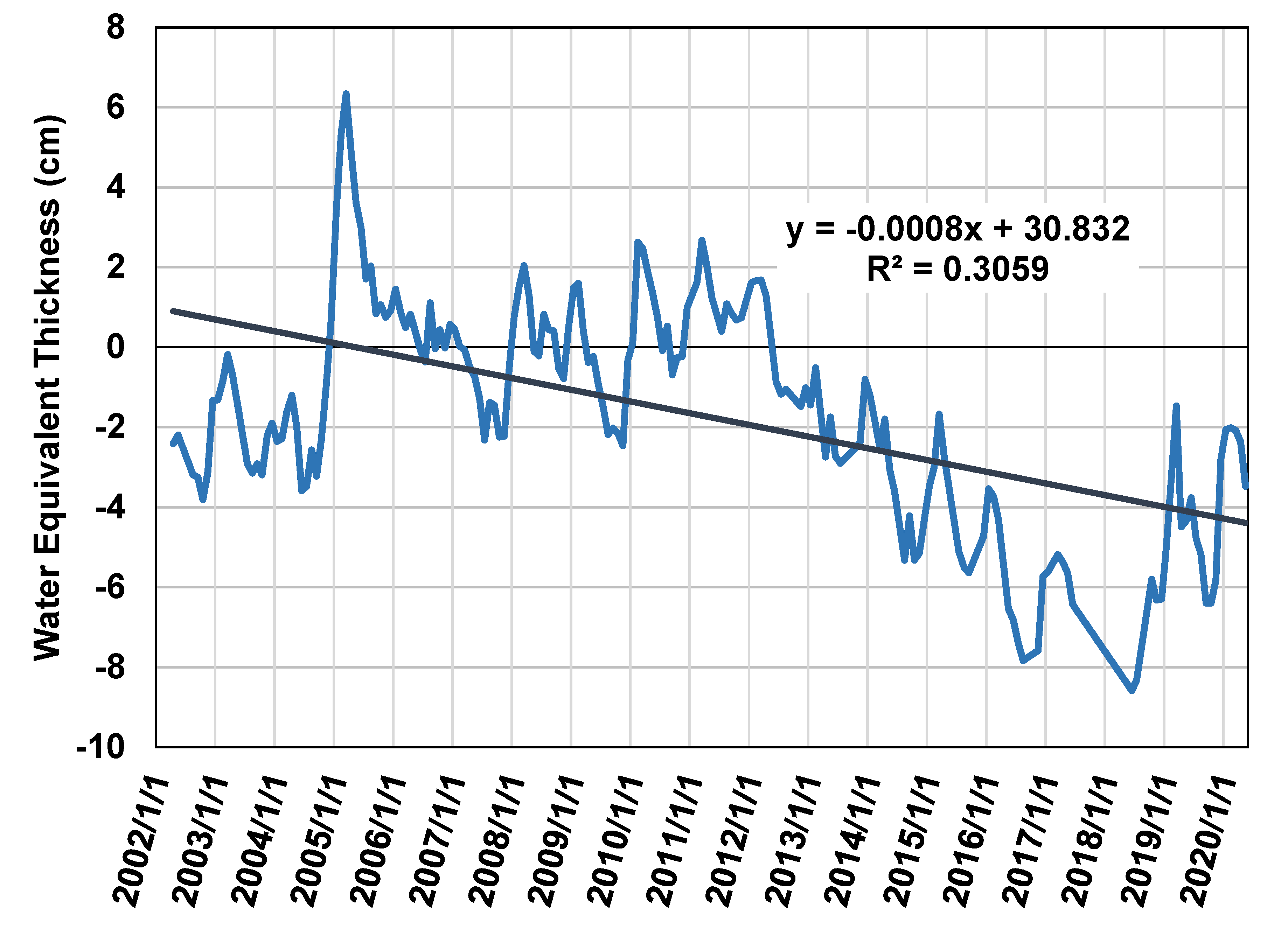
3.3. Analysis of Water Data
4. Discussion
4.1. Limitations and Improvements
4.2. Vegetation and Water
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Di Tomaso, J. Impact, biology, and ecology of saltcedar (tamarix spp.) in the southwestern united states. Weed Technol. 1998, 12, 326–336. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Taylor, J.; Smith, L.; Haukos, D. Seedling competition between native cottonwood and exotic saltcedar: Implications for restoration. Biol. Invasions 2009, 11, 1777–1787. [Google Scholar] [CrossRef] [Green Version]
- Stromberg, J. Functional equivalency of saltcedar (tamarix chinensis) and fremont cottonwood (populus fremonth) along a free-flowing river. Wetlands 1998, 18, 675–686. [Google Scholar] [CrossRef]
- Anderson, B.; Ohmart, R. Revegetation for Wildlife Enhancement along the Lower Colorado River; Arizona State University—Center for Environment Studies: Phoenix, AZ, USA, 1982. [Google Scholar]
- Anderson, B. Four Decades of Research on the Lower Colorado River; AVVAR Books: Blythe, CA, USA, 2011; p. 268. [Google Scholar]
- Sogge, M.; Paxton, E.; Tudor, A. Saltcedar and Southwestern Willow Flycatchers: Lessons from Long-Term Studies in Central Arizona; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006.
- Stromberg, J.; Beauchamp, V.; Dixon, M.; Lite, S.; Paradzick, C. Importance of low-flow and high-flow characteristics to restoration of riparian vegetation along rivers in arid south-western united states. Freshw. Biol. 2007, 52, 651–679. [Google Scholar] [CrossRef]
- Chew, M. The monstering of tamarisk: How scientists made a plant into a problem. J. Hist. Biol. 2009, 42, 231–266. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. The Development and Perpetuation of the Permanent Tamarisk Type in the Phreatophyte Zone of the Southwest; US Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1977.
- Haase, E. Survey of floodplain vegetation along the lower gila river in southwestern arizona. J. Ariz. Acad. Sci. 1972, 7, 75–81. [Google Scholar] [CrossRef]
- González, E.; Sher, A.; Anderson, R.; Bay, R.; Bean, D.; Bissonnete, G.; Cooper, D.; Dohrenwend, K.; Eichhorst, K.; El Waer, H.; et al. Secondary invasions of noxious weeds associated with control of invasive tamarix are frequent, idiosyncratic and persistent. Biol. Conserv. 2017, 213, 106–114. [Google Scholar] [CrossRef]
- Robinson, T. Introduction, Spread, and Areal Extent of Saltcedar (Tamarix) in the Western States; U.S. Government Printing Office: Washington, DC, USA, 1965.
- Nagler, P.; Glenn, E.; Jarnevich, C.; Shafroth, P. Distribution and abundance of saltcedar and russian olive in the western united states: Chapter 2. Saltcedar and Russian Olive Control Demonstration Act Science Assessment (Scientific Investigations Report 2009–5247); U.S. Geological Survey: Reston, VA, USA, 2010; pp. 11–31.
- Bedford, A.; Sankey, T.; Sankey, J.; Durning, L.; Ralston, B. Remote sensing of tamarisk beetle (diorhabda carinulata) impacts along 412 km of the colorado river in the grand canyon, arizona, USA. Ecol. Indic. 2018, 89, 365–375. [Google Scholar] [CrossRef]
- James, I.; O’Dell, R. Arizona’s Next Water Crisis Megafarms and Deeper Wells are Draining the Water Beneath Rural Arizona—Quietly, Irreversibly; The Arizona Republic: Phoenix, AZ, USA, 2019. [Google Scholar]
- Lahmers, T.; Eden, S. Water and Irrigated Agriculture in Arizona; University of Arizona Water Resources Research Center: Tucson, AZ, USA, 2018. [Google Scholar]
- Mendez-Estrella, R.; Romo-Leon, R.J.; Castellanos, E.A.; Gandarilla-Aizpuro, J.F.; Hartfield, K. Analyzing landscape trends on agriculture, introduced exotic grasslands and riparian ecosystems in arid regions of mexico. Remote Sens. 2016, 8, 664. [Google Scholar] [CrossRef] [Green Version]
- Romo-Leon, J.; van Leeuwen, W.; Castellanos-Villegas, A. Using remote sensing tools to assess land use transitions in unsustainable arid agro-ecosystems. J. Arid Environ. 2014, 106, 27–35. [Google Scholar] [CrossRef]
- Hultine, K.R.; Belnap, J.; van Riper, C.; Ehleringer, J.; Dennison, P.; Lee, M.; Nagler, P.; Snyder, K.; Uselman, S.; West, J. Tamarisk biocontrol in the western united states: Ecological and societal implications. Front. Ecol. Environ. 2010, 8, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.; McDaniel, K. Restoration of saltcedar (tamarix sp.)-infested floodplains on the bosque del apache national wildlife refuge. Weed Technol. 1998, 12, 345–352. [Google Scholar] [CrossRef]
- McGwire, K.; Minor, T.; Fenstermaker, L. Hyperspectral mixture modeling for quantifying sparse vegetation cover in arid environments. Remote Sens. Environ. 2000, 72, 360–374. [Google Scholar] [CrossRef]
- Akasheh, O.; Neale, C.; Jayanthi, H. Detailed mapping of riparian vegetation in the middle rio grande river using high resolution multi-spectral airborne remote sensing. J. Arid Environ. 2008, 72, 1734–1744. [Google Scholar] [CrossRef]
- Walker, J.; Briggs, J. An object-oriented approach to urban forest mapping in phoenix. Photogramm. Eng. Remote Sens. 2007, 73, 577–583. [Google Scholar] [CrossRef]
- Ghamisi, P.; Rasti, B.; Yokoya, N.; Wang, Q.; Hofle, B.; Bruzzone, L.; Bovolo, F.; Chi, M.; Anders, K.; Gloaguen, R.; et al. Multisource and multitemporal data fusion in remote sensing: A comprehensive review of the state of the art. IEEE Geosci. Remote Sens. Mag. 2019, 7, 6–39. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Cao, L. Tree-species classification in subtropical forests using airborne hyperspectral and lidar data. Remote Sens. 2017, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Sankey, T.; Sankey, J.; Horne, R.; Bedford, A. Remote sensing of tamarisk biomass, insect herbivory, and defoliation: Novel methods in the grand canyon region, arizona. Photogramm. Eng. Remote Sens. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Sankey, J.; Munson, S.; Webb, R.; Wallace, C.; Duran, C. Remote sensing of sonoran desert vegetation structure and phenology with ground-based lidar. Remote Sens. 2015, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Ustin, S.; Gamon, J. Remote sensing of plant functional types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef]
- John, R.; Chen, J.; Lu, N.; Guo, K.; Liang, C.; Wei, Y.; Noormets, A.; Ma, K.; Han, X. Predicting plant diversity based on remote sensing products in the semi-arid region of inner mongolia. Remote Sens. Environ. 2008, 112, 2018–2032. [Google Scholar] [CrossRef]
- McGaughey, R. Fusion/ldv Lidar Analysis and Visualization Software, 3.80; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2018.
- Landerer, F.; Swenson, S. Accuracy of scaled grace terrestrial water storage estimates. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Swenson, S.; Wahr, J. Post-processing removal of correlated errors in grace data. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Swenson, S. Grace Monthly Land Water Mass Grids Netcdf Release 5.0. Available online: http://dx.doi.org/10.5067/TELND-NC005 (accessed on 30 July 2020).
- Quinlan, J. C4.5 Programs for Machine Learning; Morgan Kaufmann Publishers: San Mateo, CA, USA, 1993. [Google Scholar]
- Kuhn, M. C5.0 Decision Trees and Rule-Based Models. Available online: https://cran.r-project.org/web/packages/C50/C50.pdf (accessed on 14 September 2020).
- Alonzo, M.; Bookhagen, B.; Roberts, D. Urban tree species mapping using hyperspectral and lidar data fusion. Remote Sens. Environ. 2014, 148, 70–83. [Google Scholar] [CrossRef]
- Maschler, J.; Atzberger, C.; Immitzer, M. Individual tree crown segmentation and classification of 13 tree species using airborne hyperspectral data. Remote Sens. 2018, 10, 1218. [Google Scholar] [CrossRef] [Green Version]
- Dalponte, M.; Bruzzone, L.; Gianelle, D. Fusion of Hyperspectral and Lidar Remote Sensing Data for Classification of Complex Forest Areas. IEEE Trans. Geosci. Remote Sens. 2008, 46, 1416–1427. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.B.; Heidebrecht, K. Spectral Unmixing of Vegetation, Soil and Dry Carbon in Arid Regions: Comparing Multispectral and Hyperspectral Observations. Int. J. Remote Sens. 2002, 23, 3939–3958. [Google Scholar] [CrossRef]
- US Army Corps of Engineers. Operation of Alamo and Painted Rock Dams during Jan—Feb 1993 Floods; Reservoir Regulation Section: Los Angeles, CA, USA, 1993.
- Bowling, J. Progress is Made to Restore the Gila River’s Flow in Metro Phoenix after Decades of Planning; The Arizona Republic: Phoenix, AZ, USA, 2020. [Google Scholar]
- McDonald, A.K.; Wilcox, B.P.; Moore, G.W.; Hart, C.R.; Sheng, Z.; Owens, M.K. Tamarix transpiration along a semiarid river has negligible impact on water resources. Water Resour. Res. 2015, 51, 5117–5127. [Google Scholar] [CrossRef] [Green Version]
- Pearce, C.M.; Smith, D.G. Saltcedar: Distribution, abundance, and dispersal mechanisms, northern montana, USA. Wetlands 2003, 23, 215–228. [Google Scholar] [CrossRef]
- Larmer, P. Tackling Tamarisk; High County News: Paonia, CO, USA, 1998. [Google Scholar]
- Krza, P. It’s ‘Bombs Away’ on New Mexico Saltcedar; High County News: Paonia, CO, USA, 2003. [Google Scholar]
- Larmer, P. Fighting Exoctics with Exotics; High County News: Paonia, CO, USA, 1998. [Google Scholar]
- DeLoach, C.J.; Lewis, P.A.; Herr, J.C.; Carruthers, R.I.; Tracy, J.L.; Johnson, J. Host specificity of the leaf beetle, diorhabda elongata deserticola (coleoptera: Chrysomelidae) from asia, a biological control agent for saltcedars (tamarix: Tamaricaceae) in the western united states. Biol. Control 2003, 27, 117–147. [Google Scholar] [CrossRef] [Green Version]
- Mast, K. The beetle, the bird and the tamarisk tree. In Discover; Kalmbach Media CO: Waukesha, WI, USA, 2018. [Google Scholar]
- Gonzalez, P.; Garfin, G.; Breshears, D.; Brooks, K.; Brown, H.; Elias, E.; Gunasekara, A.; Huntly, N.; Maldonado, J.; Mantua, N.; et al. Southwest. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume ii; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 1101–1184. [Google Scholar]
- Zhang, A.; Zheng, C.; Wang, S.; Yao, Y. Analysis of streamflow variations in the heihe river basin, northwest china: Trends, abrupt changes, driving factors and ecological influences. J. Hydrol. Reg. Stud. 2015, 3, 106–124. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Dong, Z.; Zhang, J.; Liu, L. Modern dust storms in china: An overview. J. Arid Environ. 2004, 58, 559–574. [Google Scholar] [CrossRef]



| Metric | Mean | Max | Min | Standard Deviation | Range | Sum |
|---|---|---|---|---|---|---|
| Blue Band | X | X | X | X | X | |
| Green Band | X | X | X | X | X | |
| Red Band | X | X | X | X | X | |
| NIR Band | X | X | X | X | X | |
| NDVI | X | X | X | X | X | |
| LiDAR Height | X | X | X | X | X | |
| LiDAR Intensity | X | X | X | X | X | |
| LiDAR Kurtosis | X | X | X | X | X | |
| LiDAR Density 0–1 m | X | |||||
| LiDAR Density 1–2 m | X | |||||
| LiDAR Density 2–3 m | X | |||||
| LiDAR Density 3–4 m | X | |||||
| LiDAR Density 4–5 m | X | |||||
| LiDAR Density 5–6 m | X | |||||
| LiDAR Skewness | X | X | X | X | X | |
| Pixel Count | X |
| Reference | ||||
|---|---|---|---|---|
| Creosote | Mesquite | Salt Cedar | ||
| Creosote | 8 | 0 | 2 | |
| Prediction | Mesquite | 0 | 2 | 2 |
| Salt Cedar | 4 | 1 | 125 | |
| Overall Accuracy: 94% Overall Kappa: 0.66 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartfield, K.; Leeuwen, W.J.D.v.; Gillan, J.K. Remotely Sensed Changes in Vegetation Cover Distribution and Groundwater along the Lower Gila River. Land 2020, 9, 326. https://doi.org/10.3390/land9090326
Hartfield K, Leeuwen WJDv, Gillan JK. Remotely Sensed Changes in Vegetation Cover Distribution and Groundwater along the Lower Gila River. Land. 2020; 9(9):326. https://doi.org/10.3390/land9090326
Chicago/Turabian StyleHartfield, Kyle, Willem J.D. van Leeuwen, and Jeffrey K. Gillan. 2020. "Remotely Sensed Changes in Vegetation Cover Distribution and Groundwater along the Lower Gila River" Land 9, no. 9: 326. https://doi.org/10.3390/land9090326







