Habitat Features Influence Aquatic Macroinvertebrates in the Cruces Wetland, a Ramsar Site of Southern Chile
Abstract
1. Introduction
2. Materials and Methods
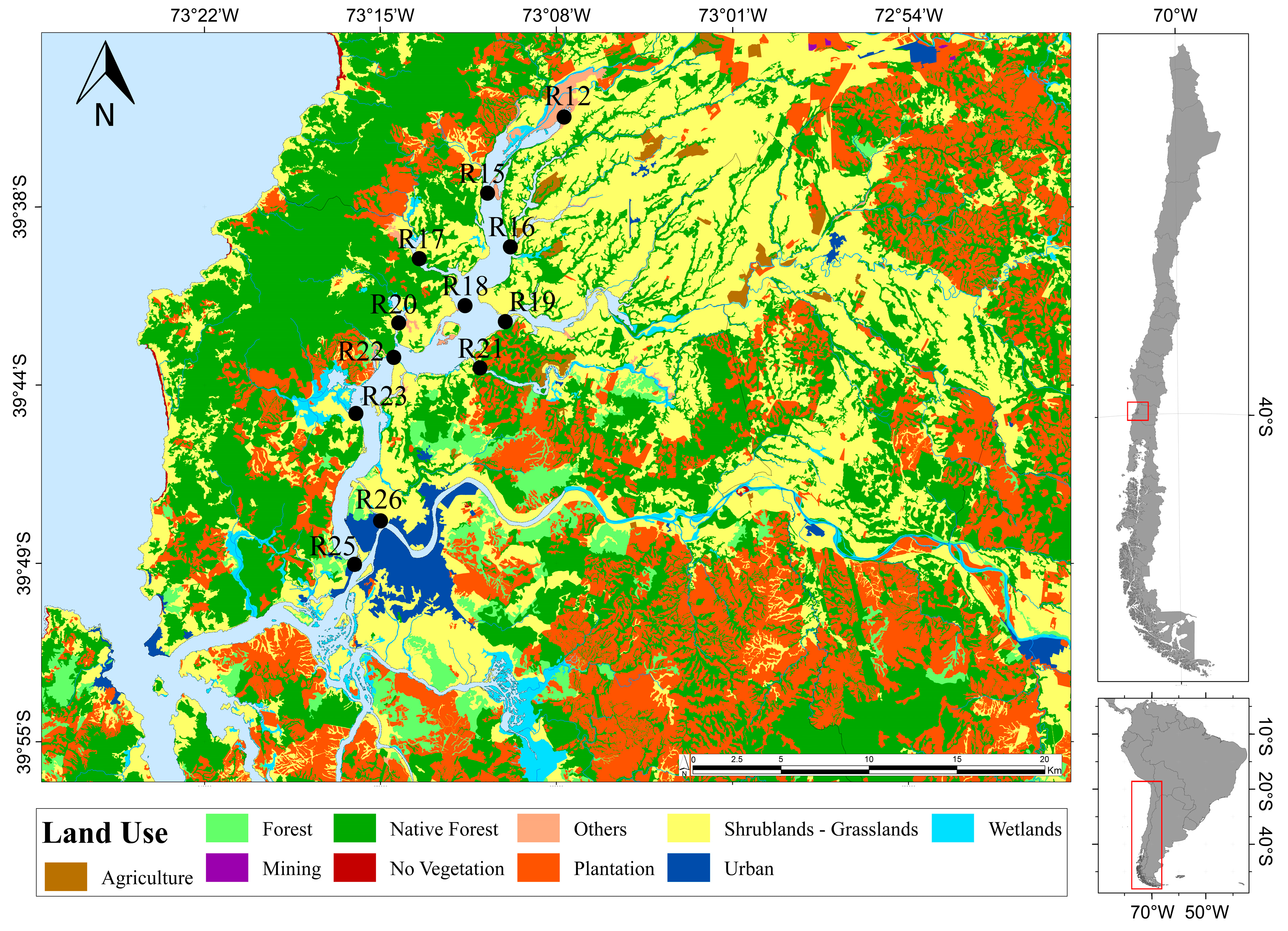
2.1. Study Area
2.2. Sampling Sites
2.3. Environmental Datasets
2.4. Macroinvertebrate Sampling and Processing
2.5. Sediment Analysis
2.6. Data Analysis
3. Results
3.1. Environmental Assessments
3.2. Benthic Macroinvertebrates
3.3. Sediment Characteristics
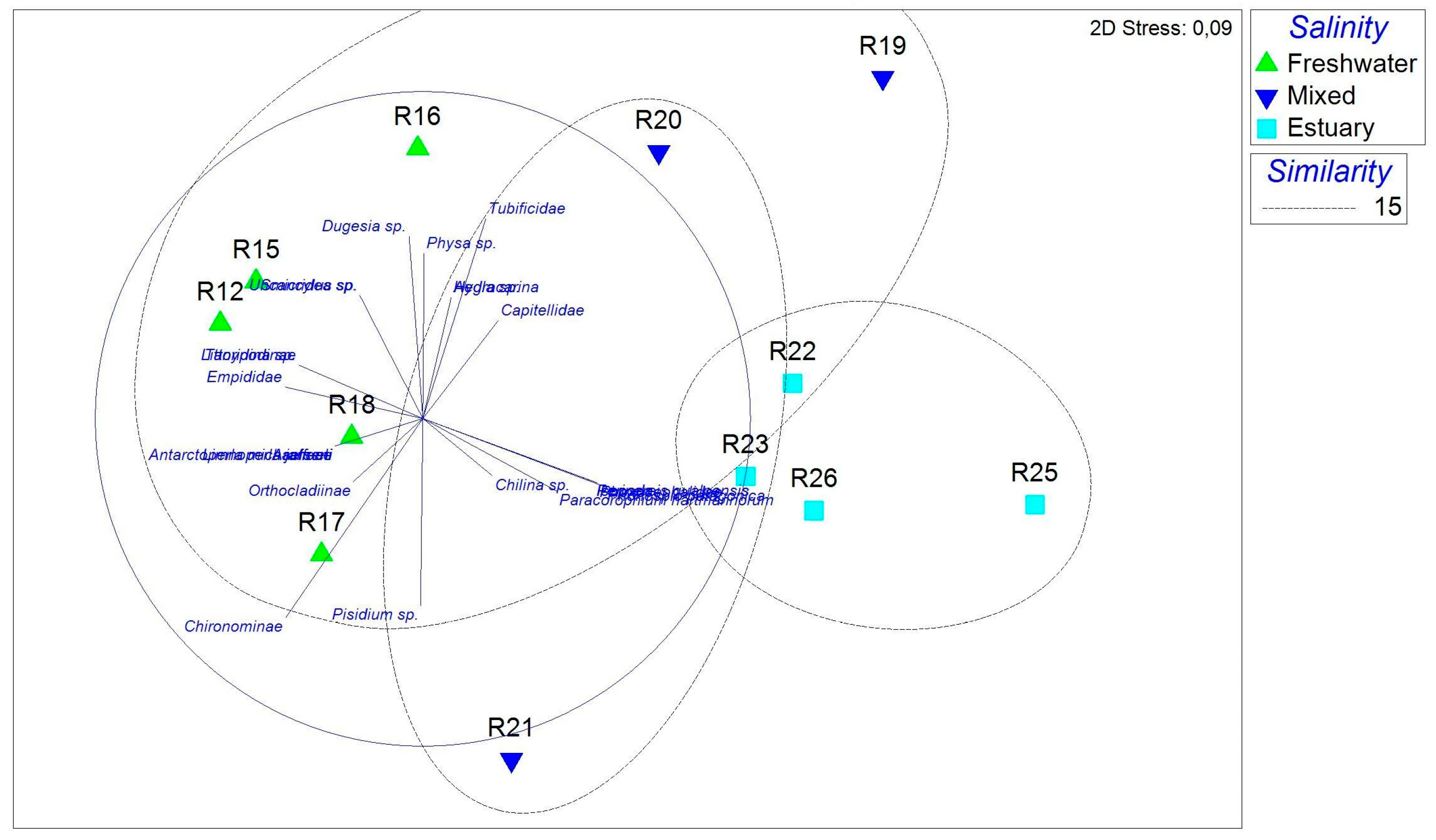
3.4. Relationship Between Environmental Variables and Macroinvertebrate Assemblages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRW | Cruces River wetland |
References
- Kennish, M.J. Environmental Threats and Environmental Future of Estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Barletta, M.; Lima, A.R.A.; Costa, M.F. Distribution, Sources and Consequences of Nutrients, Persistent Organic Pollutants, Metals and Microplastics in South American Estuaries. Sci. Total. Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, P.R.; Jima, M.; Philomina, J.; Bijoy Nandan, S. Assessment of Benthic Macroinvertebrate Response to Anthropogenic and Natural Disturbances in the Kodungallur-Azhikode Estuary, Southwest Coast of India. Environ. Monit. Assess. 2020, 192, 626. [Google Scholar] [CrossRef]
- Gupta, G.; Khan, J.; Upadhyay, A.K.; Singh, N.K. Wetland as a Sustainable Reservoir of Ecosystem Services: Prospects of Threat and Conservation. In Restoration of Wetland Ecosystem: A Trajectory Towards a Sustainable Environment; Springer: Singapore, 2020; pp. 31–43. [Google Scholar]
- Wallace, J.B.; Webster, J.R. The Role of Macroinvertebrates in Stream Ecosystem Function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Micael, J.; Navedo, J.G. Macrobenthic Communities at High Southern Latitudes: Food Supply for Long-distance Migratory Shorebirds. Austral Ecol. 2018, 43, 955–964. [Google Scholar] [CrossRef]
- Anderson, A.M.; Friis, C.; Gratto-Trevor, C.L.; Harris, C.M.; Love, O.P.; Morrison, R.I.G.; Prosser, S.W.J.; Nol, E.; Smith, P.A. Drought at a Coastal Wetland Affects Refuelling and Migration Strategies of Shorebirds. Oecologia 2021, 197, 661–674. [Google Scholar] [CrossRef]
- Selleslagh, J.; Amara, R. Environmental Factors Structuring Fish Composition and Assemblages in a Small Macrotidal Estuary (Eastern English Channel). Estuar. Coast. Shelf Sci. 2008, 79, 507–517. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Reis-Santos, P.; Maia, A.; Fonseca, V.; França, S.; Wouters, N.; Costa, M.J.; Cabral, H.N. Nursery Use Patterns of Commercially Important Marine Fish Species in Estuarine Systems along the Portuguese Coast. Estuar. Coast. Shelf Sci. 2010, 86, 613–624. [Google Scholar] [CrossRef]
- Fierro, P.; Bertran, C.; Martinez, D.; Valdovinos, C.; Vargas-Chacoff, L. Ontogenetic and Temporal Changes in the Diet of the Chilean Silverside Odontesthes regia (Atherinidae) in Southern Chile. Cah. Biol. Mar. 2014, 55, 323–332. [Google Scholar]
- Hart, E.A.; Lovvorn, J.R. Patterns of Macroinvertebrate Abundance in Inland Saline Wetlands: A Trophic Analysis. Hydrobiologia 2005, 541, 45–54. [Google Scholar] [CrossRef]
- Sargeant, B.L.; Gaiser, E.E.; Trexler, J.C. Indirect and Direct Controls of Macroinvertebrates and Small Fish by Abiotic Factors and Trophic Interactions in the Florida Everglades. Freshw. Biol. 2011, 56, 2334–2346. [Google Scholar] [CrossRef]
- Jayachandran, P.R.; Bijoy Nandan, S.; Jima, M.; Sreedevi, O.K.; Philomina, J.; Prabhakaran, M.P. Bioecology of Macrobenthic Communities in the Microtidal Monsoonal Kodungallur–Azhikode Estuary, Southwest Coast of India. Lakes Reserv. Res. Manag. 2019, 24, 372–390. [Google Scholar] [CrossRef]
- Gamboa-García, D.E.; Duque, G.; Cogua, P.; Marrugo-Negrete, J.L. Mercury Dynamics in Macroinvertebrates in Relation to Environmental Factors in a Highly Impacted Tropical Estuary: Buenaventura Bay, Colombian Pacific. Environ. Sci. Pollut. Res. Int. 2020, 27, 4044–4057. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xi, M.; Nicholaus, R.; Wang, Z.; Wang, X.; Kong, F.; Yu, Z. Behaviors and Biochemical Responses of Macroinvertebrate Corbicula Fluminea to Polystyrene Microplastics. Sci. Total. Environ. 2022, 813, 152617. [Google Scholar] [CrossRef]
- Hou, Y.; Kong, F.; Li, Y.; Xi, M.; Yu, Z. Key Factors of the Studies on Benthic Macroinvertebrate in Coastal Wetlands: Methods and Biodiversity. Ecohydrol. Hydrobiol. 2020, 20, 424–436. [Google Scholar] [CrossRef]
- Bertrán, C.; Fierro, P.; Encalada, E.; Peña-Cortés, F.; Tapia, J.; Hauenstein, E.; Vargas-Chacoff, L. Macrobenthos of the Coastal Budi Lagoon, Southern Chile: Changes Associated with Seasonal Environmental Variation. Braz. J. Oceanogr. 2016, 64, 239–248. [Google Scholar] [CrossRef]
- Sandoval, N.; Zarges, C.V.; Pablo, O.J.; Vásquez, D. Impacts of Coseismic Uplift Caused by the 2010 8.8 Mw Earthquake on the Macrobenthic Community of the Tubul-Raqui Saltmarsh (Chile). Estuar. Coast. Shelf Sci. 2019, 226, 106278. [Google Scholar] [CrossRef]
- Novoa, V.; Rojas, O.; Ahumada-Rudolph, R.; Sáez, K.; Fierro, P.; Rojas, C. Coastal Wetlands: Ecosystems Affected by Urbanization? Water 2020, 12, 698. [Google Scholar] [CrossRef]
- Martínez-Curci, N.S.; Fierro, P.; Navedo, J.G. Does Experimental Seaweed Cultivation Affect Benthic Communities and Shorebirds? Applications for Extensive Aquaculture. Ecol. Appl. 2023, 33, e2799. [Google Scholar] [CrossRef]
- Rivera, C.; Quiroga, E.; Meza, V.; Pastene, M. Evaluation of Water Quality and Heavy Metal Concentrations in the RAMSAR Wetland El Yali (Central Chile, 33°45′S). Mar. Pollut. Bull. 2019, 145, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.; Bertrán, C.; Araya, C.; Astudillo, M.J.; Vargas-Chacoff, L.; Carrasco, G.; Vaderrama, A.; Letelier, L. Study of the copper, chromium and lead content in Mugil cephalus and Eleginops maclovinus obtained in the mouths of the Maule and Mataquito rivers (Maule region, Chile). J. Chil. Chem. Soc. 2009, 54, 36–39. [Google Scholar] [CrossRef]
- Gaete, H.; Álvarez, M.; Lobos, G.; Soto, E.; Jara-Gutiérrez, C. Assessment of Oxidative Stress and Bioaccumulation of the Metals Cu, Fe, Zn, Pb, Cd in the Polychaete Perinereis Gualpensis from Estuaries of Central Chile. Ecotoxicol. Environ. Saf. 2017, 145, 653–658. [Google Scholar] [CrossRef]
- Fierro, P.; Tapia, J.; Bertrán, C.; Acuña, C.; Vargas-Chacoff, L. Assessment of Heavy Metal Contamination in Two Edible Fish Species and Water from North Patagonia Estuary. Appl. Sci. 2021, 11, 2492. [Google Scholar] [CrossRef]
- Wildsmith, M.D.; Rose, T.H.; Potter, I.C.; Warwick, R.M.; Clarke, K.R. Benthic Macroinvertebrates as Indicators of Environmental Deterioration in a Large Microtidal Estuary. Mar. Pollut. Bull. 2011, 62, 525–538. [Google Scholar] [CrossRef]
- Steven, R.; Morrison, C.; Arthur, J.M.; Castley, J.G. Avitourism and Australian Important Bird and Biodiversity Areas. PLoS ONE 2015, 10, e0144445. [Google Scholar] [CrossRef]
- Nimptsch, J.; Fierro, P.; Górski, K.; Colin, N.; Muñoz, J.L. Rivers Flowing to the Southern Pacific. In Rivers of South America; Elsevier: Amsterdam, The Netherlands, 2025; pp. 863–902. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Nimptsch, J.; Woelfl, S.; Osorio, S.; Valenzuela, J.; Ebersbach, P.; von Tuempling, W.; Palma, R.; Encina, F.; Figueroa, D.; Kamjunke, N.; et al. Tracing Dissolved Organic Matter (DOM) from Land-Based Aquaculture Systems in North Patagonian Streams. Sci. Total. Environ. 2015, 537, 129–138. [Google Scholar] [CrossRef]
- Mages, M.; Woelfl, S.; Óvári, M.; Jun, W.V.T. The Use of a Portable Total Reflection X-Ray Fluorescence Spectrometer for Field Investigation. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 2129–2138. [Google Scholar] [CrossRef]
- Domínguez, E.; Fernández, H.R. Macroinvertebrados Bentónicos Sudamericanos. Sist. Y Biol. 2009, 656. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Marin, V.H.; Delgado, L.E.; Vila, I.; Tironi, A.; Barrera, V.; Ibanez, C. Regime Shifts of Cruces River Wetland Ecosystem: Current Conditions, Future Uncertainties. Lat. Am. J. Aquat. Res. 2014, 42, 160–171. [Google Scholar] [CrossRef]
- Schaefer, K.; Einax, J.W. Analytical and Chemometric Characterization of the Cruces River in South Chile. Environ. Sci. Pollut. Res. Int. 2010, 17, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Onyena, A.P.; Nkwoji, J.A.; Chukwu, L.O. Evaluation of Hydrochemistry and Benthic Macroinvertebrates in Chanomi Creek, Niger Delta Nigeria. Reg. Stud. Mar. Sci. 2021, 46, 101907. [Google Scholar] [CrossRef]
- Alavaisha, E.; Lyon, S.; Lindborg, R. Assessment of Water Quality Across Irrigation Schemes: A Case Study of Wetland Agriculture Impacts in Kilombero Valley, Tanzania. Water 2019, 11, 671. [Google Scholar] [CrossRef]
- Suárez, B.; Barrios, M.; Teixeira de Mello, F. Macroinvertebrates’ Response to Different Land Use in Lowland Streams from Uruguay: Use of Artificial Substrates for Biomonitoring. Neotrop. Biodivers. 2022, 8, 136–146. [Google Scholar] [CrossRef]
- Phillips, J.D. Coastal Wetlands, Sea Level, and the Dimensions of Geomorphic Resilience. Geomorphology 2018, 305, 173–184. [Google Scholar] [CrossRef]
- Jaramillo, E.; Contreras, H.; Quijón, P. Seasonal and Interannual Variability in Population Abundances of the Intertidal Macroinfauna of Queule River Estuary, South-Central Chile. Rev. Chil. Hist. Nat. 2001, 74, 455–468. [Google Scholar] [CrossRef][Green Version]
- Bertrán, C.; Arenas, J.; Parra, O. Macrofauna Del Curso Inferior y Estuario Del Río Biobío (Chile): Cambios Asociados a Variabilidad Estacional Del Caudal Hídrico. Rev. Chil. Hist. Nat. 2001, 74, 331–340. [Google Scholar] [CrossRef]
- Garcés-Vargas, J.; Schneider, W.; Pinochet, A.; Piñones, A.; Olguin, F.; Brieva, D.; Wan, Y. Tidally Forced Saltwater Intrusions Might Impact the Quality of Drinking Water, the Valdivia River (40° S), Chile Estuary Case. Water 2020, 12, 2387. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.B.; Thrush, S.F.; Probert, P.K. The Interacting Effect of Hydrodynamics and Organic Matter on Colonization: A Soft-Sediment Example. Estuar. Coast. Shelf Sci. 2001, 52, 705–714. [Google Scholar] [CrossRef]
- Kim, C.; Kang, H.Y.; Lee, Y.-J.; Yun, S.-G.; Kang, C.-K. Isotopic Variation of Macroinvertebrates and Their Sources of Organic Matter Along an Estuarine Gradient. Estuar. Coast. 2020, 43, 496–511. [Google Scholar] [CrossRef]
- Battle, J.; Golladay, S.W. Water Quality and Macroinvertebrate Assemblages in Three Types of Seasonally Inundated Limesink Wetlands in Southwest Georgia. J. Freshw. Ecol. 2001, 16, 189–207. [Google Scholar] [CrossRef]
- Barnes, R.S.K. Chapter 11 Macrofaunal Community Structure and Life Histories in Coastal Lagoons. In Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1994; pp. 311–362. [Google Scholar]
- Pequeño, G.; Pavés, H.; Bertrán, C.; Vargas Ch, L. Seasonal Limnetic Feeding Regime of the “Robalo” Eleginops Maclvinus (Valenciennes 1830), in the Valdivia River, Chile. Gayana 2010, 74, 47–56. [Google Scholar]
- Bridges, T.S.; Levin, L.A.; Cabrera, D.; Plaia, G. Effects of Sediment Amended with Sewage, Algae, or Hydrocarbons on Growth and Reproduction in Two Opportunistic Polychaetes. J. Exp. Mar. Biol. Ecol. 1994, 177, 99–119. [Google Scholar] [CrossRef]
- Giere, O.; Preusse, J.-H.; Dubilier, N. Tubificoides Benedii (Tubificidae, Oligochaeta)—a Pioneer in Hypoxic and Sulfidic Environments. An Overview of Adaptive Pathways. In Aquatic Oligochaetes; Springer: Dordrecht, The Netherlands, 1999; pp. 235–241. [Google Scholar]
- Rîsnoveanu, G.; Vadineanu, A. Observations on the Population Dynamics of Potamothrix hammoniensis (Michaelsen, 1901) (Tubificidae, Oligochaeta) in Lake Isacova in the Danube Delta. Hydrobiologia 2002, 479, 23–30. [Google Scholar] [CrossRef]
- Hirst, A.; Alpine, J.; Crawford, C. Benthic Macro Invertebrate Communities of High Conservation Value Thirsty and Little Thirsty Lagoons, Cape Barren Island, Tasmania. Pap. Proc. R. Soc. Tasman. 2006, 140, 17–24. [Google Scholar] [CrossRef]
- Barletta, M.; Lima, A.R.A. Systematic Review of Fish Ecology and Anthropogenic Impacts in South American Estuaries: Setting Priorities for Ecosystem Conservation. Front. Mar. Sci. 2019, 6, 237. [Google Scholar] [CrossRef]
- Vásquez, D.; Sandoval, N.; Fierro, P.; Valdovinos, C. Morphological Impacts of the Chilean Megathrust Earthquake Mw 8.8 on Coastal Wetlands of High Conservation Value. Estuar. Coast. Shelf Sci. 2020, 245, 106922. [Google Scholar] [CrossRef]
- Aazami, J.; Esmaili Sari, A.; Abdoli, A.; Sohrabi, H.; Van den Brink, P.J. Assessment of Ecological Quality of the Tajan River in Iran Using a Multimetric Macroinvertebrate Index and Species Traits. Environ. Manag. 2015, 56, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Bhatta, B.; Talchabhadel, R.; Virdis, S.G.P. Integrated Assessment of the Landuse Change and Climate Change Impacts on the Sediment Yield in the Songkhram River Basin, Thailand. Catena 2022, 209, 105859. [Google Scholar] [CrossRef]
- Coccia, C.; Vega, C.; Fierro, P. Macroinvertebrate-Based Biomonitoring of Coastal Wetlands in Mediterranean Chile: Testing Potential Metrics Able to Detect Anthropogenic Impacts. Water 2022, 14, 3449. [Google Scholar] [CrossRef]
- Garcés-Vargas, J.; Ruiz, M.; Pardo, L.M.; Nuñez, S.; Pérez-Santos, I. Caracterización Hidrográfica Del Estuario Del Río Valdivia, Centro-Sur de Chile. Lat. Am. J. Aquat. Res. 2013, 41, 113–125. [Google Scholar] [CrossRef]



| Station | Abbreviation | R12 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | R22 | R23 | R25 | R26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | T° | 19.5 | 21.6 | 23.4 | 24.5 | 19.4 | 23.4 | 23.8 | 23.3 | 22.8 | 22.2 | 22.2 | 20.4 |
| Conductivity (µS/cm) | Cond | 131.4 | 118.8 | 111.4 | 69.9 | 129.1 | 49.9 | 276.0 | 159.4 | 349.0 | 822.0 | 822.0 | 384.0 |
| pH | pH | 7.3 | 7.4 | 7.8 | 7.6 | 7.9 | 7.6 | 7.0 | 6.9 | 7.6 | 7.3 | 7.3 | 7.2 |
| Dissolved oxygen (mg/L) | DO | 8.6 | 8.6 | 9.0 | 8.8 | 9.4 | 8.9 | 9.2 | 8.2 | 9.0 | 9.1 | 9.1 | 8.7 |
| Oxygen saturation (%) | DOS | 93.0 | 98.0 | 105.7 | 105.3 | 103.7 | 106.1 | 109.3 | 95.3 | 107.8 | 104.7 | 104.7 | 96.8 |
| Salinity (PSU) | Sal | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.3 | 0.1 |
| Turbidity (NTU) | Tur | 0.0 | 3.3 | 2.7 | 3.9 | 3.9 | 5.8 | 4.2 | 5.0 | 4.9 | 5.9 | 5.9 | 2.2 |
| Color (CU Pt Co) | Col | 9.2 | 14.6 | 16.1 | 21.5 | 16.9 | 28.4 | 23.0 | 19.2 | 14.6 | 12.3 | 12.3 | 6.9 |
| DIC (mg/L) | DIC | 3.5 | 2.6 | 2.0 | 0.9 | 2.1 | 1.2 | 1.0 | 1.6 | 1.2 | 1.1 | 2.1 | 1.6 |
| DOC (mg/L) | DOC | 0.9 | 0.9 | 1.3 | 1.2 | 1.1 | 1.0 | 1.2 | 1.2 | 1.2 | 1.1 | 0.8 | 0.7 |
| DC (mg/L) | DC | 4.4 | 3.6 | 3.2 | 2.1 | 3.2 | 2.2 | 2.2 | 2.8 | 2.5 | 2.3 | 2.9 | 2.3 |
| TIC (mg/L) | TIC | 4.0 | 3.5 | 2.4 | 1.0 | 2.7 | 1.1 | 1.3 | 1.3 | 1.6 | 1.6 | 1.9 | 1.7 |
| TOC (mg/L) | TOC | 1.0 | 1.2 | 1.4 | 1.3 | 1.3 | 1.0 | 1.3 | 1.3 | 1.2 | 1.1 | 0.7 | 0.8 |
| TC (mg/L) | TC | 5.0 | 4.6 | 3.8 | 2.3 | 4.0 | 2.1 | 2.5 | 2.6 | 2.8 | 2.7 | 2.7 | 2.5 |
| POC (mg/L) | POC | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| DQO (mg/L) | DQO | 46.2 | 7.1 | 13.2 | 10.2 | 9.3 | 8.7 | 8.8 | 11.3 | 10.8 | 11.4 | 14.8 | 5.9 |
| N-NH4 (µg/L) | NH4 | 11.1 | 10.6 | 13.3 | 10.6 | 6.2 | 9.0 | 3.7 | 3.0 | 4.4 | 4.4 | 13.8 | 7.2 |
| N-NO3 (µg/L) | NO3 | 57.8 | 29.8 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 4.1 |
| N-NO2 µg/L | NO2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| N-TOTAL (µg/L) | N | 195.2 | 207.2 | 216.4 | 233.9 | 214.9 | 224.7 | 220.8 | 250.0 | 229.0 | 243.6 | 255.3 | 133.3 |
| P-PO4 (µg/L) | PO4 | 2.0 | 2.4 | 2.9 | 3.2 | 3.3 | 3.3 | 2.2 | 3.4 | 3.6 | 2.7 | 4.5 | 3.3 |
| P-TOTAL (µg/L) | P | 15.9 | 15.1 | 19.2 | 18.5 | 20.8 | 24.9 | 19.6 | 23.6 | 23.4 | 23.5 | 33.4 | 15.3 |
| Ca (µg/L) | Ca | 960 | 792 | 2534 | 1011 | 2461 | 2436 | 1451 | 1616 | 2073 | 1462 | 3169 | 3574 |
| Mn (µg/L) | Mn | 3.5 | 3.5 | 15.6 | 4.8 | 13.5 | 13.3 | 10.7 | 8.3 | 12.4 | 2.8 | 8.5 | 5.7 |
| Fe (µg/L) | Fe | 91 | 51 | 273 | 107 | 114 | 176 | 120 | 155 | 147 | 159 | 106 | 46 |
| Cu (µg/L) | Cu | 3.8 | 0.7 | 0.8 | 0.9 | 3.2 | 1.9 | 1.2 | 1.7 | 1.5 | 3.2 | 1.1 | 1.7 |
| Pb (µg/L) | Pb | 0.8 | 0.7 | 0.5 | 0.8 | 1.3 | 0.6 | 0.9 | 0.8 | 0.8 | 2.2 | 0.6 | 1.0 |
| R12 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | R22 | R23 | R25 | R26 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polychaeta | ||||||||||||
| Prionospio patagonica | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 107 | 2640 | 173 |
| Perinereis gualpensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 |
| Capitellidae | 0 | 0 | 13 | 0 | 0 | 560 | 67 | 0 | 80 | 0 | 0 | 0 |
| Oligochaeta | ||||||||||||
| Tubificidae | 27 | 40 | 187 | 13 | 80 | 0 | 0 | 0 | 0 | 13 | 0 | 0 |
| Crustacea | ||||||||||||
| Paracorophium hartmannorum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1920 | 787 | 93 | 3933 |
| Phoxorgia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 1520 | 27 |
| Aegla sp. | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 |
| Malacostraca | ||||||||||||
| Isopoda | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 27 | 0 |
| Arachnida | ||||||||||||
| Hydracarina | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 |
| Araneae | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insecta | ||||||||||||
| Antarctoperla michaelseni | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Limnoperla jaffueli | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Smicridea sp. | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Orthocladiinae | 0 | 0 | 0 | 27 | 187 | 0 | 0 | 0 | 0 | 0 | 0 | 67 |
| Tanypodinae | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chironominae | 0 | 0 | 0 | 13 | 13 | 0 | 0 | 13 | 0 | 0 | 0 | 0 |
| Empididae | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastropoda | ||||||||||||
| Physa sp. | 0 | 0 | 13 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 |
| Chilina sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 40 | 0 | 0 | 0 |
| Uncancylus sp. | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Littoridina sp. | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bivalvia | ||||||||||||
| Pisidium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 80 | 13 | 13 | 0 | 0 |
| Turbellaria | ||||||||||||
| Dugesia sp. | 0 | 0 | 27 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taxa Richness | 2 | 3 | 6 | 3 | 6 | 3 | 5 | 3 | 5 | 4 | 5 | 4 |
| Total abundance (N/m2) | 40 | 66 | 266 | 53 | 319 | 600 | 133 | 106 | 2080 | 920 | 4293 | 4200 |
| Evenness (J′) | 0.9 | 0.9 | 0.6 | 0.9 | 0.7 | 0.3 | 0.8 | 0.7 | 0.2 | 0.4 | 0.5 | 0.2 |
| Shannon Diversity (H′) | 0.6 | 0.9 | 1.1 | 1 | 1 | 0.3 | 1 | 0.7 | 0.4 | 0.5 | 0.8 | 0.3 |
| Stations | Fraction (%) | SD | OM (%) | SD | |||
|---|---|---|---|---|---|---|---|
| Station R12 | gravel | 0.85 | ± | 0.40 | 88.40 | ± | 1.59 |
| sand | 85.35 | ± | 0.89 | 6.54 | ± | 0.43 | |
| mud | 13.80 | ± | 1.19 | 12.12 | ± | 0.34 | |
| Station R15 | gravel | 3.51 | ± | 2.52 | 75.42 | ± | 10.12 |
| sand | 59.05 | ± | 2.74 | 12.15 | ± | 2.20 | |
| mud | 37.43 | ± | 4.60 | 16.33 | ± | 0.06 | |
| Station R16 | gravel | 0.26 | ± | 0.04 | 67.63 | ± | 17.04 |
| sand | 46.49 | ± | 11.36 | 22.58 | ± | 1.42 | |
| mud | 53.24 | ± | 11.37 | 16.57 | ± | 0.30 | |
| Station R17 | gravel | 5.25 | ± | 3.29 | 80.18 | ± | 3.13 |
| sand | 63.85 | ± | 25.00 | 16.16 | ± | 1.21 | |
| mud | 30.90 | ± | 26.94 | 20.59 | ± | 0.62 | |
| Station R18 | gravel | 33.41 | ± | 48.29 | 54.02 | ± | 44.65 |
| sand | 45.51 | ± | 30.52 | 14.93 | ± | 2.02 | |
| mud | 21.07 | ± | 18.37 | 46.66 | ± | 44.73 | |
| Station R19 | gravel | 0.11 | ± | 0.06 | 63.73 | ± | 34.42 |
| sand | 89.02 | ± | 6.13 | 5.30 | ± | 0.18 | |
| mud | 10.87 | ± | 6.14 | 10.50 | ± | 0.15 | |
| Station R20 | gravel | 58.44 | ± | 2.97 | 3.30 | ± | 1.11 |
| sand | 34.62 | ± | 3.46 | 7.65 | ± | 2.33 | |
| mud | 6.95 | ± | 2.19 | 19.45 | ± | 1.15 | |
| Station R21 | gravel | 0.02 | ± | 0.03 | 3.70 | ± | 6.42 |
| sand | 10.19 | ± | 0.46 | 7.09 | ± | 0.29 | |
| mud | 89.79 | ± | 0.44 | 9.08 | ± | 0.80 | |
| Station R22 | gravel | 0.56 | ± | 0.24 | 33.13 | ± | 5.49 |
| sand | 51.38 | ± | 3.91 | 20.92 | ± | 0.29 | |
| mud | 48.05 | ± | 3.83 | 16.59 | ± | 0.15 | |
| Station R23 | gravel | 0.22 | ± | 0.15 | 57.73 | ± | 19.11 |
| sand | 52.72 | ± | 3.52 | 9.51 | ± | 0.37 | |
| mud | 47.07 | ± | 3.41 | 10.38 | ± | 5.13 | |
| Station R25 | gravel | 3.92 | ± | 0.16 | 1.11 | ± | 0.07 |
| sand | 95.90 | ± | 6.13 | 5.30 | ± | 0.05 | |
| mud | 0.18 | ± | 6.14 | 10.50 | ± | 2.54 | |
| Station R26 | gravel | 17.09 | ± | 0.71 | 0.83 | ± | 0.05 |
| sand | 79.37 | ± | 25.00 | 1.18 | ± | 0.03 | |
| mud | 3.54 | ± | 26.94 | 12.99 | ± | 0.50 |
| Stations | Total Organic Matter (%) |
|---|---|
| R12 | 8.00 ± 0.60 |
| R15 | 16.05 ± 2.93 |
| R16 | 19.42 ± 0.34 |
| R17 | 20.93 ± 3.21 |
| R18 | 15.31 ± 10.49 |
| R19 | 3.76 ± 0.26 |
| R20 | 5.89 ± 1.69 |
| R21 | 8.87 ± 0.75 |
| R22 | 18.91 ± 0.28 |
| R23 | 9.99 ± 2.34 |
| R25 | 1.01 ± 0.05 |
| R26 | 1.54 ± 0.04 |
| Variable | Sum of Squares (Trace) | Pseudo-F | p-Value | Cumulative Proportion |
|---|---|---|---|---|
| Salinity | 11,580.0 | 3.688 | 0.001 | 0.269 |
| TC | 6984.9 | 2.575 | 0.013 | 0.432 |
| NH4 | 3882.4 | 1.513 | 0.146 | 0.522 |
| Mn | 3526.1 | 1.451 | 0.196 | 0.604 |
| PO4 | 5090.3 | 2.563 | 0.038 | 0.723 |
| DC | 3163.5 | 1.807 | 0.123 | 0.796 |
| Tur | 2406.3 | 1.517 | 0.222 | 0.852 |
| NO3 | 2276.4 | 1.678 | 0.224 | 0.905 |
| Pb | 1720.8 | 1.465 | 0.312 | 0.945 |
| Cu | 1493.0 | 1.745 | 0.371 | 0.980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierro, P.; Rodríguez-Jorquera, I.; Lara, C.; Woelfl, S.; Machuca-Sepúlveda, J.; Vega, C.; Nimptsch, J. Habitat Features Influence Aquatic Macroinvertebrates in the Cruces Wetland, a Ramsar Site of Southern Chile. Land 2025, 14, 1890. https://doi.org/10.3390/land14091890
Fierro P, Rodríguez-Jorquera I, Lara C, Woelfl S, Machuca-Sepúlveda J, Vega C, Nimptsch J. Habitat Features Influence Aquatic Macroinvertebrates in the Cruces Wetland, a Ramsar Site of Southern Chile. Land. 2025; 14(9):1890. https://doi.org/10.3390/land14091890
Chicago/Turabian StyleFierro, Pablo, Ignacio Rodríguez-Jorquera, Carlos Lara, Stefan Woelfl, Jorge Machuca-Sepúlveda, Carlos Vega, and Jorge Nimptsch. 2025. "Habitat Features Influence Aquatic Macroinvertebrates in the Cruces Wetland, a Ramsar Site of Southern Chile" Land 14, no. 9: 1890. https://doi.org/10.3390/land14091890
APA StyleFierro, P., Rodríguez-Jorquera, I., Lara, C., Woelfl, S., Machuca-Sepúlveda, J., Vega, C., & Nimptsch, J. (2025). Habitat Features Influence Aquatic Macroinvertebrates in the Cruces Wetland, a Ramsar Site of Southern Chile. Land, 14(9), 1890. https://doi.org/10.3390/land14091890








