Variation in Soil Organic Carbon and Total Nitrogen Stocks Across Elevation Gradients and Soil Depths in the Mount Kenya East Forest
Abstract
1. Introduction
2. Materials and Methods
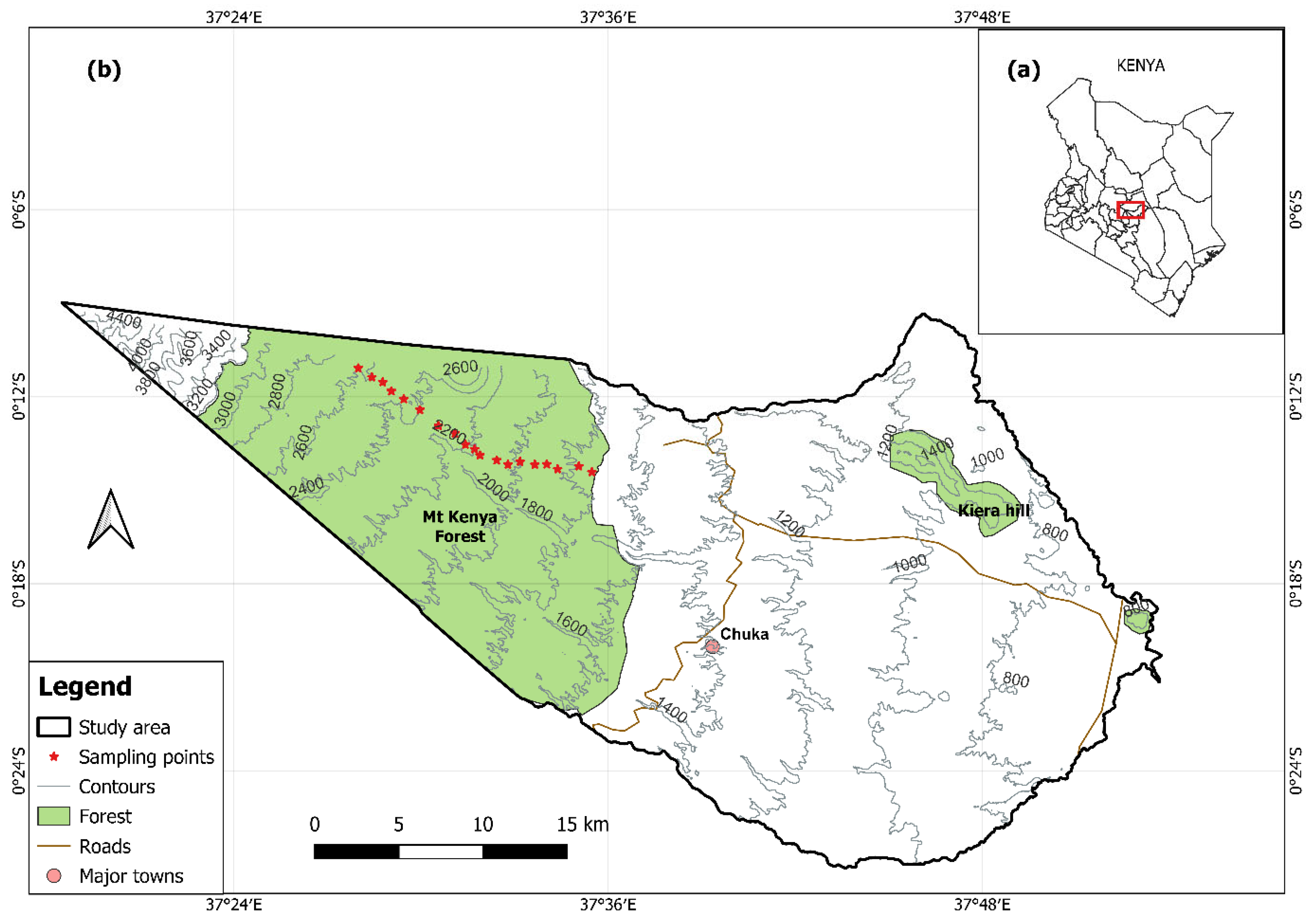
2.1. Study Area
2.1.1. Geographical Location
2.1.2. Topography, Geology, and Soils
2.1.3. Climate
2.1.4. Water Resources
2.1.5. Biodiversity
| Elevation Gradient (m) | Land Use | Vegetation Type | Sampling Locations (n) | Soil Type | Mean Annual Temperature (°C) | Total Annual Rainfall (mm) |
|---|---|---|---|---|---|---|
| 2350–2650 (Upper Forest) | Forestland | Natural forest and bamboo thickets
| 6 |
| 15.6–13.6 | 1907–1916 |
| 2000–2350 (Middle Forest) | Forestland | Natural forest
| 7 |
| 18–15.6 | 1900–1907 |
| 1700–2000 (Lower Forest) | Forestland | Natural forest with fragments of plantation forest
| 6 |
| 19.2–18 | 1800–1900 |
2.1.6. Demographic and Socio-Economic Characteristics
2.2. Sampling Design
2.3. Soil Sampling
2.4. Preparation and Pre-Treatment of Samples
2.5. Soil Physicochemical Analysis
2.6. Calculation of Bulk Density, Soil Organic Carbon Stocks, and Total Nitrogen Stocks
2.7. Statistical Analysis
3. Results
3.1. Selected Soil Physicochemical Properties Within Different Ranges of Elevation Gradients and Soil Depths
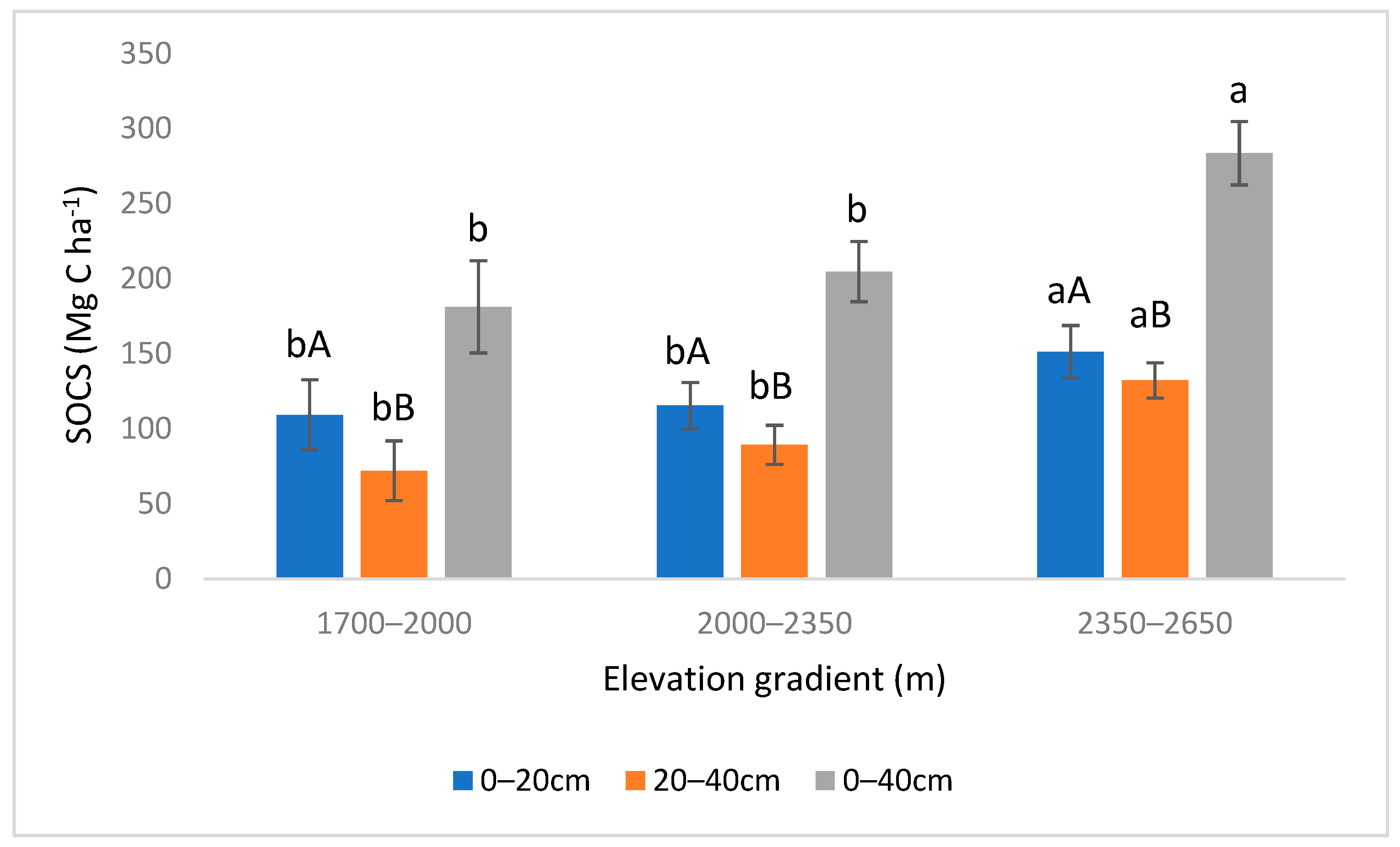
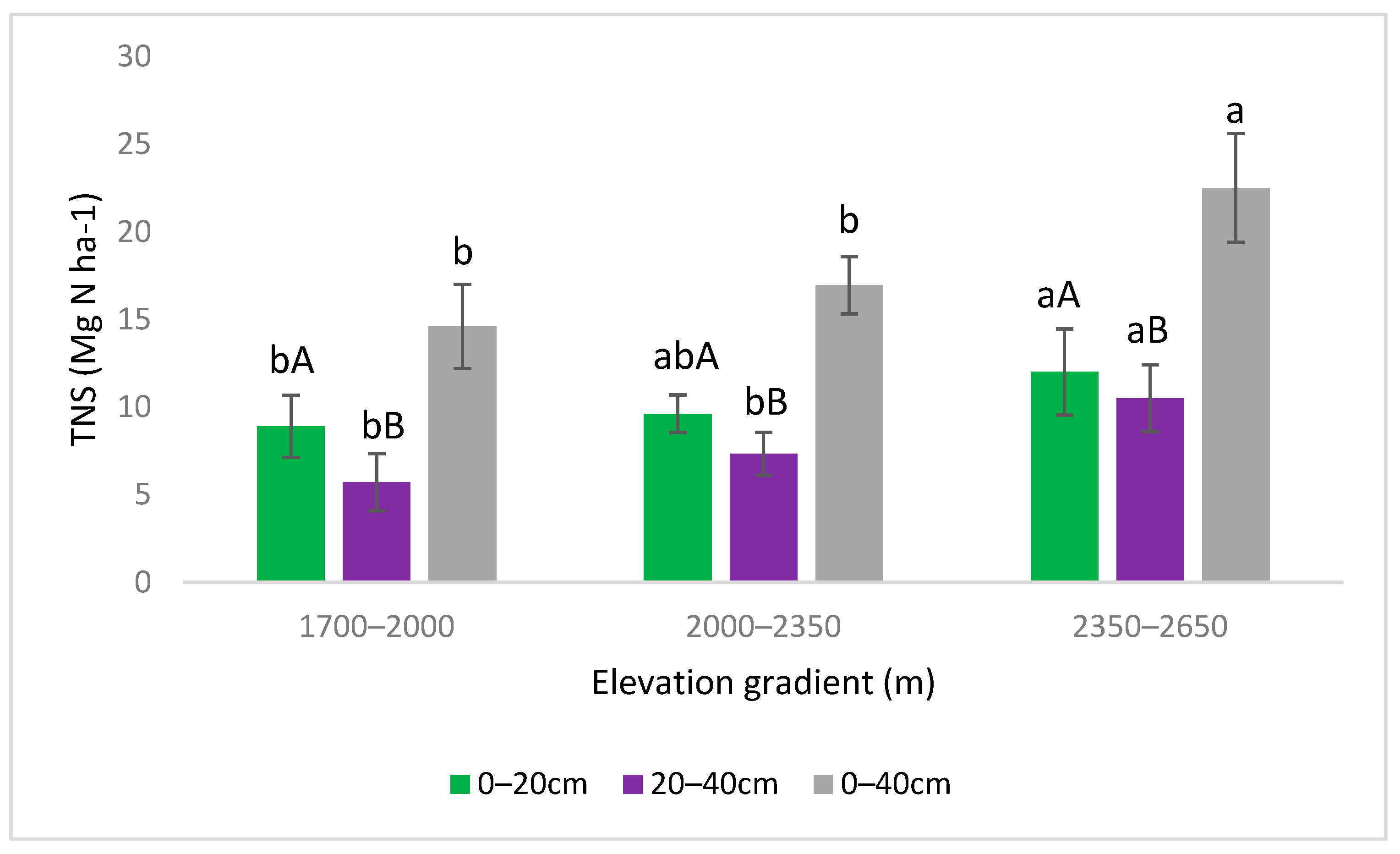
3.2. Variations in SOCS and TNS with Elevation Gradient and Soil Depth
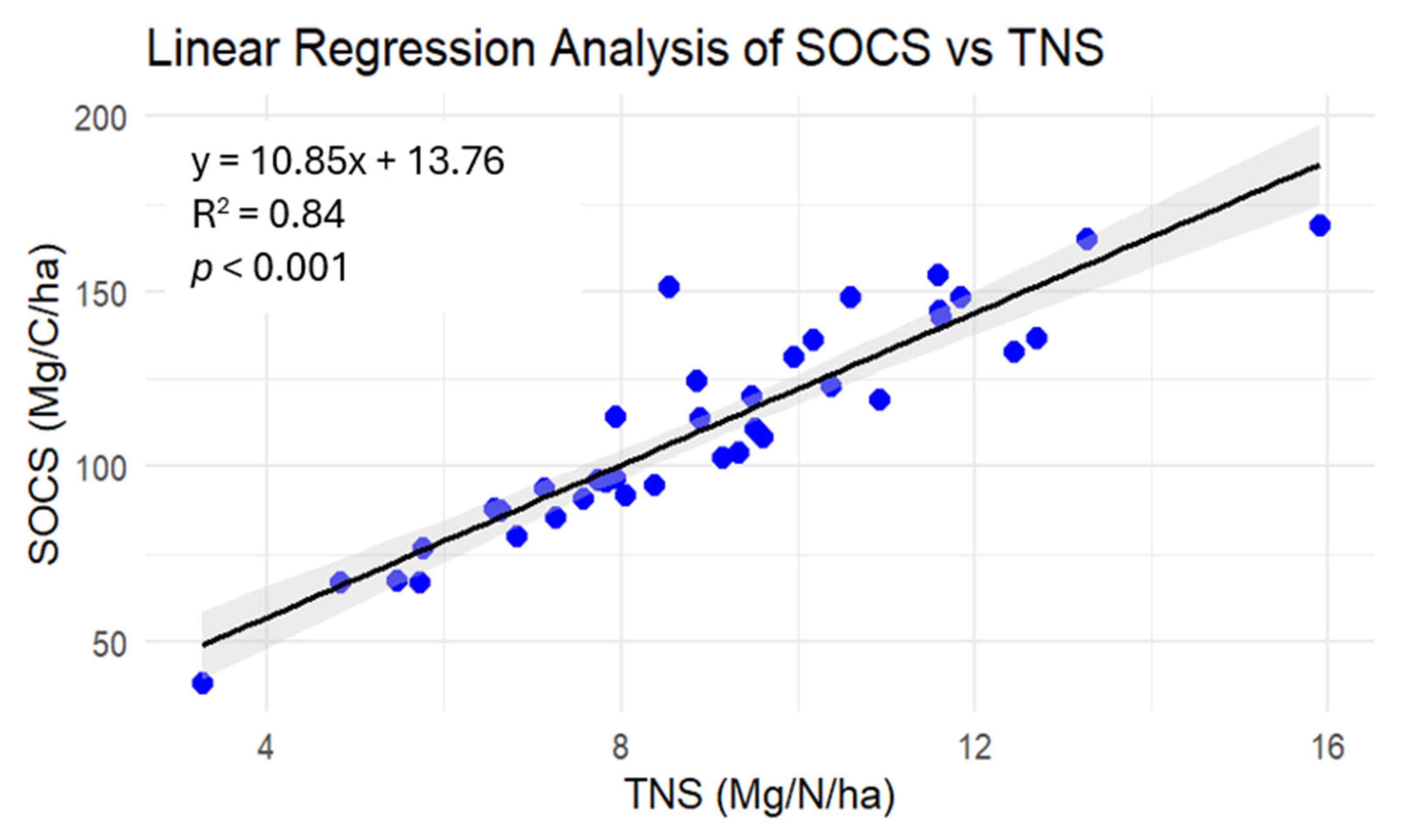
3.3. Relationship Between SOCS and TNS
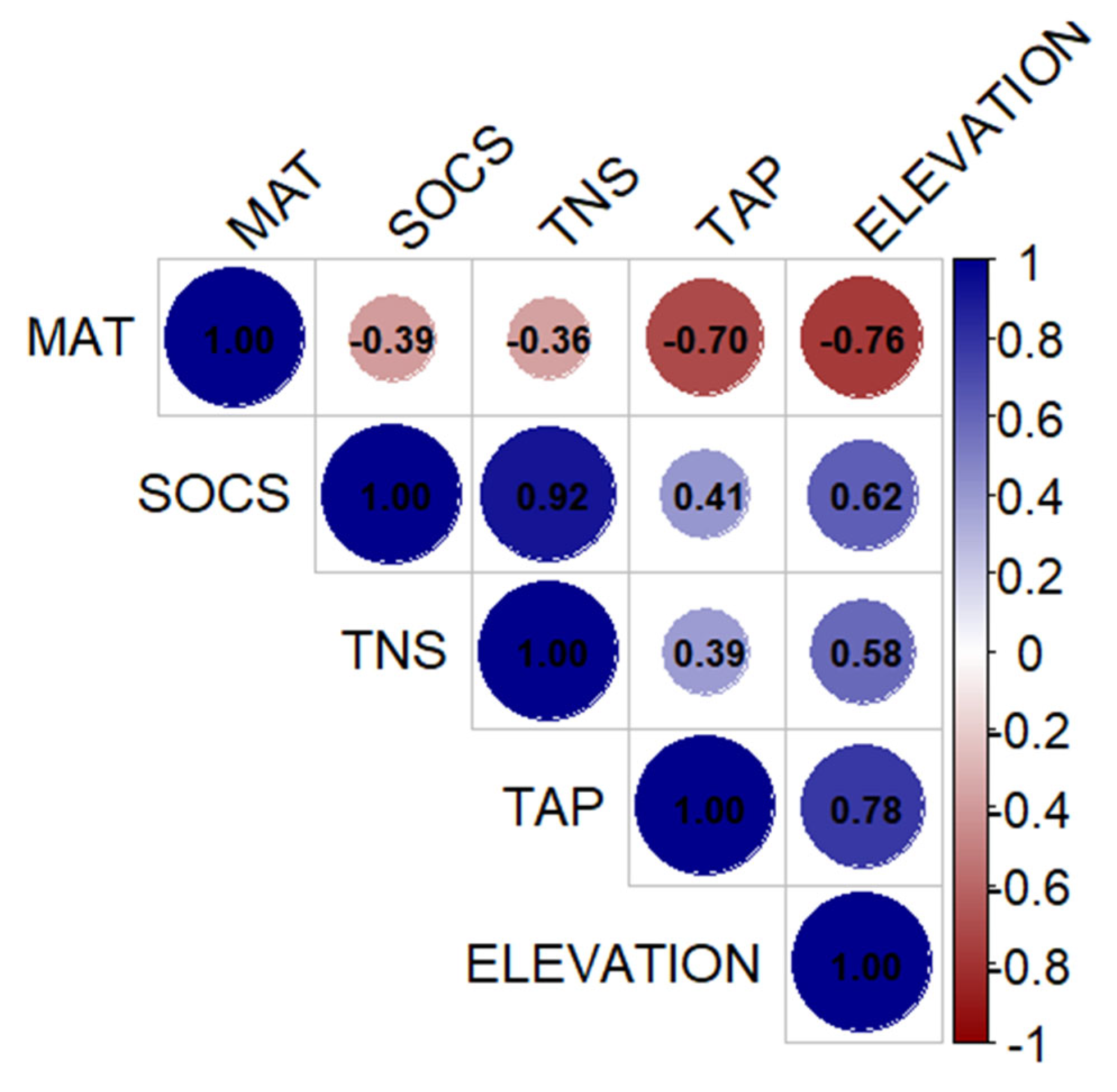
3.4. Correlations Between SOCS and TNS and Other Soil Properties and Environmental Variables
3.4.1. Correlations Between SOCS and TNS and Other Soil Properties
3.4.2. Correlation Between SOCS and TNS and Environmental Variables
4. Discussion
4.1. Overview of Selected Physicochemical Properties of Study Area Soils
4.2. SOCS and TNS Variation with Elevation Gradient and Soil Depth
4.3. Relationships Between SOCS and TNS and Environmental Variables and Other Soil Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BD | Bulk Density |
| MAT | Mean Annual Temperature |
| MKEF | Mount Kenya East Forest |
| SOC | Soil Organic Carbon |
| SOCS | Soil Organic Carbon Stocks |
| SOM | Soil Organic Matter |
| TAP | Total Annual Precipitation |
| TN | Total Nitrogen |
| TNS | Total Nitrogen Stocks |
References
- Zhao, Z.; Zhang, X.; Dong, S.; Wu, Y.; Liu, S.; Su, X.; Wang, X.; Zhang, Y.; Tang, L. Soil Organic Carbon and Total Nitrogen Stocks in Alpine Ecosystems of Altun Mountain National Nature Reserve in Dry China. Environ. Monit. Assess. 2019, 191, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Basile-Doelsch, I.; Chevallier, T.; Dignac, M.-F.; Erktan, A. Carbon Storage in Soils, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; ISBN 9780128229743. [Google Scholar]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial Distribution of Soil Organic Carbon Stocks in France. Biogeosciences 2011, 8, 1053–1065. [Google Scholar] [CrossRef]
- Xin, Z.; Qin, Y.; Yu, X. Spatial Variability in Soil Organic Carbon and Its Influencing Factors in a Hilly Watershed of the Loess Plateau, China. Catena 2016, 137, 660–669. [Google Scholar] [CrossRef]
- Bangroo, S.A.; Najar, G.R.; Rasool, A. Effect of Altitude and Aspect on Soil Organic Carbon and Nitrogen Stocks in the Himalayan Mawer Forest Range. Catena 2017, 158, 63–68. [Google Scholar] [CrossRef]
- Ahmad Dar, J.; Somaiah, S. Altitudinal Variation of Soil Organic Carbon Stocks in Temperate Forests of Kashmir Himalayas, India. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ontl, T.A.; Schulte, L.A. Soil Carbon Storage. Nat. Educ. Knowl. 2012, 3, 35. [Google Scholar]
- Lal, R. Challenges and Opportunities in Soil Organic Matter Research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Lal, R. Beyond COP21: Potential and Challenges of the “4 per Thousand” Initiative. J. Soil Water Conserv. 2016, 71, 20A–25A. [Google Scholar] [CrossRef]
- Schrumpf, M.; Schulze, E.D.; Kaiser, K.; Schumacher, J. How Accurately Can Soil Organic Carbon Stocks and Stock Changes Be Quantified by Soil Inventories? Biogeosciences 2011, 8, 1193–1212. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; Mcbratney, A.B.; Remy, V.D.; Courcelles, D.; et al. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil Carbon Sequestration and Land-Use Change: Processes and Potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, H.; Guo, S. Soil Organic Carbon as a Function of Land Use and Topography on the Loess Plateau of China. Ecol. Eng. 2015, 83, 249–257. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, M.; Wang, Y. Effect of Environmental Factors on Regional Soil Organic Carbon Stocks across the Loess Plateau Region, China. Agric. Ecosyst. Environ. 2011, 142, 184–194. [Google Scholar] [CrossRef]
- Amanuel, W.; Yimer, F.; Karltun, E. Soil Organic Carbon Variation in Relation to Land Use Changes: The Case of Birr Watershed, Upper Blue Nile River Basin, Ethiopia. J. Ecol. Environ. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Tsui, C.C.; Chen, Z.S.; Hsieh, C.F. Relationships between Soil Properties and Slope Position in a Lowland Rain Forest of Southern Taiwan. Geoderma 2004, 123, 131–142. [Google Scholar] [CrossRef]
- Tsui, C.C.; Tsai, C.C.; Chen, Z.S. Soil Organic Carbon Stocks in Relation to Elevation Gradients in Volcanic Ash Soils of Taiwan. Geoderma 2013, 209–210, 119–127. [Google Scholar] [CrossRef]
- Kong, J.; He, Z.; Chen, L.; Zhang, S.; Yang, R.; Du, J. Elevational Variability in and Controls on the Temperature Sensitivity of Soil Organic Matter Decomposition in Alpine Forests. Ecosphere 2022, 13, e4010. [Google Scholar] [CrossRef]
- Blackburn, K.W.; Libohova, Z.; Adhikari, K.; Kome, C.; Maness, X.; Silman, M.R. Influence of Land Use and Topographic Factors on Soil Organic Carbon Stocks and Their Spatial and Vertical Distribution. Remote Sens. 2022, 14, 2846. [Google Scholar] [CrossRef]
- Ali, S.; Begum, F.; Hayat, R.; Bohannan, B.J.M. Variation in Soil Organic Carbon Stock in Different Land Uses and Altitudes in Bagrot Valley, Northern Karakoram. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 551–561. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Righelato, R. Tropical Montane Forests Are a Larger than Expected Global Carbon Store. Biogeosciences 2014, 11, 2741–2754. [Google Scholar] [CrossRef]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil Carbon and Nitrogen Stocks in Forests along an Altitudinal Gradient in the Eastern Himalayas and a Meta-Analysis of Global Data. Glob. Chang. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef]
- Zech, M.; Hörold, C.; Leiber-Sauheitl, K.; Kühnel, A.; Hemp, A.; Zech, W. Buried Black Soils on the Slopes of Mt. Kilimanjaro as a Regional Carbon Storage Hotspot. Catena 2014, 112, 125–130. [Google Scholar] [CrossRef]
- County Government of Tharaka Nithi. Third County Integrated Development Plan (2023–2027); County Government of Tharaka Nithi: Kathwana, Kenya, 2023. [Google Scholar]
- Rotich, B.; Maket, I.; Kipkulei, H.; Melenya, C.; Nsima, P.; Ahmed, M.; Csorba, A.; Micheli, E. Determinants of Soil and Water Conservation Practices Adoption by Smallholder Farmers in the Central Highlands of Kenya. Farming Syst. 2024, 2, 100081. [Google Scholar] [CrossRef]
- Nature Kenya. Ecosystem Service Assessment for the Restoration of Mount Kenya Forest; Nature Kenya: Nairobi, Kenya, 2019. [Google Scholar]
- Otieno, T.A.; Otieno, L.A.; Rotich, B.; Löhr, K.; Kipkulei, H.K. Modeling Climate Change Impacts and Predicting Future Vulnerability in the Mount Kenya Forest Ecosystem Using Remote Sensing and Machine Learning. Environ. Monit. Assess. 2025, 197, 1–21. [Google Scholar] [CrossRef]
- Bussmann, R.W. Destruction and Management of Mount Kenya’s Forests. Ambio 1996, 25, 314–317. [Google Scholar]
- Akotsi, E.F.N.; Ndirangu, J.K.; Gachanja, M. Changes in Forest Cover in Kenya’s Five “Water Towers” 2003–2005; Department of Resource Surveys and Remote Sensing: Nairobi, Kenya, 2006. [Google Scholar]
- Rotich, B.; Ahmed, A.; Kinyili, B.; Kipkulei, H. Historical and Projected Forest Cover Changes in the Mount Kenya Ecosystem: Implications for Sustainable Forest Management. Environ. Sustain. Indic. 2025, 26, 100628. [Google Scholar] [CrossRef]
- Bussmann, R.W. Vegetation Zonation and Nomenclature of African Mountains—An Overview. Lyonia 2006, 11, 41–66. [Google Scholar]
- Muchena, F.N.; Gachene, C.K.K. Soils of the Highland and Mountainous Areas of Kenya with Special Emphasis on Agricultural Soils. Mt. Res. Dev. 1988, 8, 183–191. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Resolution Adopted by the General Assembly on 25 September 2015, 42809, 1–13; United Nations: New York, NY, USA, 2015. [Google Scholar]
- County Government of Tharaka Nithi. Tharaka Nithi County Integrated Development Plan 2018–2022; County Government of Tharaka Nithi: Kathwana, Kenya, 2018. [Google Scholar]
- Dijkshoorn, J.A. Soil and Terrain Database for Kenya (Ver. 2.0) (KENSOTER); ISRIC-World Soil Information: Wageningen, The Netherlands, 2007. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kenya Wildlife Service. Mt. Kenya Ecosystem Management Plan 2010–2020; Kenya Wildlife Service: Nairobi, Kenya, 2010. [Google Scholar]
- Kenya Forest Service. Mt. Kenya Forest Reserve Management Plan 2010–2019; Kenya Wildlife Service: Nairobi, Kenya, 2010; pp. 4–9. [Google Scholar]
- Jaetzold, R.; Schmidt, H.; Hornet, Z.B.; Shisanya, C. Farm Management Handbook of Kenya, 2nd ed.; Natural Conditions and Farm Information, Volume 11/C; Eastern Province, Ministry of Agriculture/GTZ: Nairobi, Kenya, 2007. [Google Scholar]
- Mairura, F.S.; Musafiri, C.M.; Kiboi, M.N.; Macharia, J.M.; Ng’etich, O.K.; Shisanya, C.A.; Okeyo, J.M.; Okwuosa, E.A.; Ngetich, F.K. Homogeneous Land-Use Sequences in Heterogeneous Small-Scale Systems of Central Kenya: Land-Use Categorization for Enhanced Greenhouse Gas Emission Estimation. Ecol. Indic. 2022, 136, 108677. [Google Scholar] [CrossRef]
- Labeyrie, V.; Deu, M.; Barnaud, A.; Calatayud, C.; Buiron, M.; Wambugu, P.; Manel, S.; Glaszmann, J.C.; Leclerc, C. Influence of Ethnolinguistic Diversity on the Sorghum Genetic Patterns in Subsistence Farming Systems in Eastern Kenya. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kenya Forest Service. Chuka Participatory Forest Management Plan 2015–2019; Kenya Forest Service: Nairobi, Kenya, 2015. [Google Scholar]
- Lawrence, P.G.; Roper, W.; Morris, T.F.; Guillard, K. Guiding Soil Sampling Strategies Using Classical and Spatial Statistics: A Review. Agron. J. 2020, 112, 493–510. [Google Scholar] [CrossRef]
- Huising, E.J.; Mesele, S. Protocol for Field Survey—Guidelines for Field Surveyors on Soil Sample Collection and Field Assessment of Agricultural Lands in Africa; Soils4Africa Project Report D4.2A. 2022. Available online: https://cordis.europa.eu/project/id/862900 (accessed on 15 April 2025).
- Burgos Hernández, T.D.; Deiss, L.; Slater, B.K.; Demyan, M.S.; Shaffer, J.M. High-Throughput Assessment of Soil Carbonate Minerals in Urban Environments. Geoderma 2021, 382, 114778. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; Volume 9, pp. 363–375. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Paul, L.W. Laboratory Methods of Soil and Plant Analysis: A Working Manual the Second Edition; Sacred Africa Nairobi: Nairobi, Kenya, 2002. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar] [CrossRef]
- Búzas, I. (Ed.) Manual of Soil and Agrochemical Analysis: II Physico-Chemical and Chemical Methods of Soil Analysis; Mezőgazda Kiadó: Budapest, Hungary, 1988. (In Hungarian) [Google Scholar]
- Makó, A.; Tóth, G.; Weynants, M.; Rajkai, K.; Hermann, T.; Tóth, B. Pedotransfer Functions for Converting Laser Diffraction Particle-Size Data to Conventional Values. Eur. J. Soil Sci. 2017, 68, 769–782. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; pp. 67–71. [Google Scholar]
- Tadese, S.; Soromessa, T.; Aneseye, A.B.; Gebeyehu, G.; Noszczyk, T.; Kindu, M. The Impact of Land Cover Change on the Carbon Stock of Moist Afromontane Forests in the Majang Forest Biosphere Reserve. Carbon Balance Manag. 2023, 18, 24. [Google Scholar] [CrossRef]
- Mishra, G.; Giri, K.; Jangir, A.; Francaviglia, R. Projected Trends of Soil Organic Carbon Stocks in Meghalaya State of Northeast Himalayas, India. Implications for a Policy Perspective. Sci. Total Environ. 2020, 698, 134266. [Google Scholar] [CrossRef]
- Alvarez-Castellanos, M.P.; Escudero-Campos, L.; Mongil-Manso, J.; San Jose, F.J.; Jiménez-Sánchez, A.; Jiménez-Ballesta, R. Organic Carbon Storage in Waterlogging Soils in Ávila, Spain: A Traditional Agrosilvopastoral Region. Land 2024, 13, 1630. [Google Scholar] [CrossRef]
- Batjes, N.H.H. Total Carbon and Nitrogen in the Soils of the World N.H. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- FAO. Measuring and Modeling Soil Carbon Stocks and Stock Changes in Livestock Production Systems: Guidelines for Assessment (Version 1); Livestock Environmental Assessment and Performance (LEAP) Partnership; FAO: Rome, Italy, 2019. [Google Scholar]
- Veldkamp, E. Organic Carbon Turnover in Three Tropical Soils under Pasture after Deforestation. Soil Sci. Soc. Am. J. 1994, 58, 175–180. [Google Scholar] [CrossRef]
- Wang, T.; Kang, F.; Cheng, X.; Han, H.; Ji, W. Soil Organic Carbon and Total Nitrogen Stocks under Different Land Uses in a Hilly Ecological Restoration Area of North China. Soil Tillage Res. 2016, 163, 176–184. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Toru, T.; Kibret, K. Carbon Stock under Major Land Use/Land Cover Types of Hades Sub-Watershed, Eastern Ethiopia. Carbon Balance Manag. 2019, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Geremew, B.; Tadesse, T.; Bedadi, B.; Gollany, H.T.; Tesfaye, K.; Aschalew, A. Impact of Land Use/Cover Change and Slope Gradient on Soil Organic Carbon Stock in Anjeni Watershed, Northwest Ethiopia. Environ. Monit. Assess. 2023, 195, 971. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.N.; Tsheko, R. Spatial and Temporal Variation of Soil Properties and Soil Organic Carbon in Semi-Arid Areas of Sub-Sahara Africa. Geoderma Reg. 2024, 36, e00770. [Google Scholar] [CrossRef]
- Ghimire, P.; Lamichhane, U.; Bolakhe, S.; Lee, C.J. Impact of Land Use Types on Soil Organic Carbon and Nitrogen Stocks: A Study from the Lal Bakaiya Watershed in Central Nepal. Int. J. For. Res. 2023, 2023, 9356474. [Google Scholar] [CrossRef]
- Muktar, M.; Bobe, B.; Kibebew, K.; Yared, M. Soil Organic Carbon Stock under Different Land Use Types in Kersa Sub Watershed, Eastern Ethiopia. African J. Agric. Res. 2018, 13, 1248–1256. [Google Scholar] [CrossRef]
- Kanyanjua, S.M.; Ireri, L.; Wambua, S.; Nandwa, S.M. Acidic Soils in Kenya: Constraints and Remedial Options; Kenya Agricultural Research Institute: Nairobi, Kenya, 2002; 27p. [Google Scholar]
- Batjes, N.H. A Global Data Set of Soil PH Properties. Technical Paper 27; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 1995; ISBN 9066720492. [Google Scholar]
- Aweto, A.O.; Moleele, N.M. Impact of Eucalyptus Camaldulensis Plantation on an Alluvial Soil in South Eastern Botswana. Int. J. Environ. Stud. 2005, 62, 163–170. [Google Scholar] [CrossRef]
- Soumare, A.; Manga, A.; Fall, S.; Hafidi, M.; Ndoye, I.; Duponnois, R. Effect of Eucalyptus Camaldulensis Amendment on Soil Chemical Properties, Enzymatic Activity, Acacia Species Growth and Roots Symbioses. Agrofor. Syst. 2015, 89, 97–106. [Google Scholar] [CrossRef]
- Tsozué, D.; Nghonda, J.P.; Tematio, P.; Basga, S.D. Changes in Soil Properties and Soil Organic Carbon Stocks along an Elevation Gradient at Mount Bambouto, Central Africa. Catena 2019, 175, 251–262. [Google Scholar] [CrossRef]
- Landon, J.R. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics; Routledge: Milton Park, UK, 2014. [Google Scholar]
- Hailemariam, M.B.; Woldu, Z.; Asfaw, Z.; Lulekal, E. Impact of Elevation Change on the Physicochemical Properties of Forest Soil in South Omo Zone, Southern Ethiopia. Appl. Environ. Soil Sci. 2023, 2023, 7305618. [Google Scholar] [CrossRef]
- Njeru, C.M.; Ekesi, S.; Mohamed, S.A.; Kinyamario, J.I.; Kiboi, S.; Maeda, E.E. Assessing Stock and Thresholds Detection of Soil Organic Carbon and Nitrogen along an Altitude Gradient in an East Africa Mountain Ecosystem. Geoderma Reg. 2017, 10, 29–38. [Google Scholar] [CrossRef]
- Asrat, F.; Soromessa, T.; Bekele, T.; Kurakalva, R.M.; Guddeti, S.S.; Smart, D.R.; Steger, K. Effects of Environmental Factors on Carbon Stocks of Dry Evergreen Afromontane Forests of the Choke Mountain Ecosystem, Northwestern Ethiopia. Int. J. For. Res. 2022, 2022, 9447946. [Google Scholar] [CrossRef]
- Adiyah, F.; Michéli, E.; Csorba, A.; Gebremeskel Weldmichael, T.; Gyuricza, C.; Ocansey, C.M.; Dawoe, E.; Owusu, S.; Fuchs, M. Effects of Landuse Change and Topography on the Quantity and Distribution of Soil Organic Carbon Stocks on Acrisol Catenas in Tropical Small-Scale Shade Cocoa Systems of the Ashanti Region of Ghana. Catena 2022, 216, 106366. [Google Scholar] [CrossRef]
- Dad, J.M. Organic Carbon Stocks in Mountain Grassland Soils of Northwestern Kashmir Himalaya: Spatial Distribution and Effects of Altitude, Plant Diversity and Land Use. Carbon Manag. 2019, 10, 149–162. [Google Scholar] [CrossRef]
- Tan, Z.X.; Lal, R.; Smeck, N.E.; Calhoun, F.G. Relationships between Surface Soil Organic Carbon Pool and Site Variables. Geoderma 2004, 121, 187–195. [Google Scholar] [CrossRef]
- Wang, F.P.; Wang, X.C.; Yao, B.Q.; Zhang, Z.H.; Shi, G.X.; Ma, Z.; Chen, Z.; Zhou, H.K. Effects of Land-Use Types on Soil Organic Carbon Stocks: A Case Study across an Altitudinal Gradient within a Farm-Pastoral Area on the Eastern Qinghai-Tibetan Plateau, China. J. Mt. Sci. 2018, 15, 2693–2702. [Google Scholar] [CrossRef]
- Becker, J.N.; Dippold, M.A.; Hemp, A.; Kuzyakov, Y. Ashes to Ashes: Characterization of Organic Matter in Andosols along a 3400 m Elevation Transect at Mount Kilimanjaro Using Analytical Pyrolysis. Catena 2019, 180, 271–281. [Google Scholar] [CrossRef]
- Rotich, B.; Makindi, S.; Esilaba, M. Communities Attitudes and Perceptions towards the Status, Use and Management of Kapolet Forest Reserve in Kenya. Int. J. Biodivers. Conserv. 2020, 12, 363–374. [Google Scholar] [CrossRef]
- Mariye, M.; Jianhua, L.; Maryo, M. Land Use and Land Cover Change, and Analysis of Its Drivers in Ojoje Watershed, Southern Ethiopia. Heliyon 2022, 8, e09267. [Google Scholar] [CrossRef]
- Muhati, G.L.; Olago, D.; Olaka, L. Quantification of Carbon Stocks in Mount Marsabit Forest Reserve, a Sub-Humid Montane Forest in Northern Kenya under Anthropogenic Disturbance. Glob. Ecol. Conserv. 2018, 14, e00383. [Google Scholar] [CrossRef]
- Assefa, D.; Rewald, B.; Sandén, H.; Rosinger, C.; Abiyu, A.; Yitaferu, B.; Godbold, D.L. Catena Deforestation and Land Use Strongly Effect Soil Organic Carbon and Nitrogen Stock in Northwest Ethiopia. Catena 2017, 153, 89–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil Organic Carbon and Total Nitrogen Stocks as Affected by Vegetation Types and Altitude across the Mountainous Regions in the Yunnan Province, South-Western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Wang, X.D.; Lu, X.Y.; Yan, Y.; Fan, J.H. Soil Organic Carbon and Nutrients along an Alpine Grassland Transect across Northern Tibet. J. Mt. Sci. 2013, 10, 564–573. [Google Scholar] [CrossRef]
- Bonnett, S.A.F.; Ostle, N.; Freeman, C. Seasonal Variations in Decomposition Processes in a Valley-Bottom Riparian Peatland. Sci. Total Environ. 2006, 370, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, G.; Soromessa, T.; Bekele, T.; Teketay, D. Carbon Stocks and Factors Affecting Their Storage in Dry Afromontane Forests of Awi Zone, Northwestern Ethiopia. J. Ecol. Environ. 2019, 43, 1–18. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, Z.; Xu, Y.; Ren, C.; Yang, G.; Han, X.; Ren, G.; Feng, Y. Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China. Forests 2018, 9, 598. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep Soil Organic Matter-a Key but Poorly Understood Component of Terrestrial C Cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Neba, S.C.; Tsheko, R.; Kayombo, B.; Moroke, S.T. Variation of Soil Organic Carbon across Different Land Covers and Land Uses in the Greater Gaborone Region of Botswana. World J. Adv. Eng. Technol. Sci. 2022, 7, 97–112. [Google Scholar] [CrossRef]

| Soil Property | Soil Depth (cm) | Elevation Gradient (m) | ||
|---|---|---|---|---|
| 1700–2000 | 2000–2350 | 2350–2650 | ||
| BD (g cm−3) | 0–20 | 0.70 ± 0.07 aA | 0.63 ± 0.05 aB | 0.48 ± 0.05 bB |
| 20–40 | 0.75 ± 0.05 aA | 0.73 ± 0.07 aA | 0.55 ± 0.06 bA | |
| 0–40 | 0.73 ± 0.06 a | 0.68 ± 0.08 a | 0.52 ± 0.06 b | |
| SOC (%) | 0–20 | 7.96 ± 2.44 bA | 9.31 ± 1.64 bA | 15.70 ± 1.74 aA |
| 20–40 | 4.77 ± 1.25 bB | 6.17 ± 1.02 bB | 12.02 ± 1.45 aB | |
| 0–40 | 6.37 ± 2.49 b | 7.74 ± 2.09 b | 13.88 ± 2.47 a | |
| TN (%) | 0–20 | 0.65 ± 0.18 bA | 0.78 ± 0.13 bA | 1.26 ± 0.27 aA |
| 20–40 | 0.38 ± 0.10 bB | 0.51 ± 0.09 bB | 0.94 ± 0.13 aB | |
| 0–40 | 0.51 ± 0.20 b | 0.64 ± 0.18 b | 1.10 ± 0.26 a | |
| pH | 0–20 | 4.08 ± 0.23 bA | 4.29 ± 0.41 bA | 5.02 ± 0.47 aA |
| 20–40 | 4.28 ± 0.17 bA | 4.26 ± 0.31 bA | 5.17 ± 0.54 aA | |
| 0–40 | 4.18 ± 0.22 b | 4.27 ± 0.35 b | 5. 09 ± 0.49 a | |
| Sand (%) | 0–20 | 24.98 ± 6.33 bA | 35.18 ± 7.44 bA | 58.90 ± 20.21 aA |
| 20–40 | 10.16 ± 3.99 bB | 16.52 ± 5.22 bB | 46.38 ± 13.99 aA | |
| 0–40 | 17.57 ± 9.24 b | 25.85 ± 11.48 b | 52.64 ± 17.82 a | |
| Silt (%) | 0–20 | 55.42 ± 2.16 aA | 51.55 ± 5.73 aA | 35.86 ± 16.96 bA |
| 20–40 | 56.00 ± 2.64 abA | 56.85 ± 3.86 aA | 45.74 ± 11.41 bA | |
| 0–40 | 55.71 ± 2.32 a | 54.20 ± 5.44 a | 40.80 ± 14.71 b | |
| Clay (%) | 0–20 | 19.61 ± 4.76 aB | 13.27 ± 2.47 bB | 5.24 ± 3.34 cA |
| 20–40 | 33.84 ± 3.40 aA | 26.62 ± 5.33 bA | 7.89 ± 2.65 cA | |
| 0–40 | 26.72 ± 8.41 a | 19.94 ± 7.99 b | 6.56 ± 3.19 c | |
| Texture | 0–20 | Silt Loam | Silt Loam | Sandy Loam |
| 20–40 | Silty Clay Loam | Silt Loam | Loam | |
| 0–40 | Silt Loam | Silt Loam | Sandy Loam | |
| Variables | SOCS (Mg C ha−1) | TNS (Mg N ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| DF | F Value | p-Value | Significance | DF | F Value | p-Value | Significance | |
| Elevation Gradient | 2 | 17.82 | 4.6 × 10−6 | *** | 2 | 11.50 | 0.000145 | *** |
| Depth | 1 | 9.429 | 0.00405 | ** | 1 | 9.592 | 0.00378 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotich, B.; Szegi, T.; Gelsleichter, Y.A.; Fuchs, M.; Ocansey, C.M.; Phenson, J.N.; Abdulkadir, M.; Kipkulei, H.; Wawire, A.; Mutuma, E.; et al. Variation in Soil Organic Carbon and Total Nitrogen Stocks Across Elevation Gradients and Soil Depths in the Mount Kenya East Forest. Land 2025, 14, 1217. https://doi.org/10.3390/land14061217
Rotich B, Szegi T, Gelsleichter YA, Fuchs M, Ocansey CM, Phenson JN, Abdulkadir M, Kipkulei H, Wawire A, Mutuma E, et al. Variation in Soil Organic Carbon and Total Nitrogen Stocks Across Elevation Gradients and Soil Depths in the Mount Kenya East Forest. Land. 2025; 14(6):1217. https://doi.org/10.3390/land14061217
Chicago/Turabian StyleRotich, Brian, Tamás Szegi, Yuri Andrei Gelsleichter, Márta Fuchs, Caleb Melenya Ocansey, Justine Nsima Phenson, Mustapha Abdulkadir, Harison Kipkulei, Amos Wawire, Evans Mutuma, and et al. 2025. "Variation in Soil Organic Carbon and Total Nitrogen Stocks Across Elevation Gradients and Soil Depths in the Mount Kenya East Forest" Land 14, no. 6: 1217. https://doi.org/10.3390/land14061217
APA StyleRotich, B., Szegi, T., Gelsleichter, Y. A., Fuchs, M., Ocansey, C. M., Phenson, J. N., Abdulkadir, M., Kipkulei, H., Wawire, A., Mutuma, E., Mesele, S. A., Michéli, E., & Csorba, Á. (2025). Variation in Soil Organic Carbon and Total Nitrogen Stocks Across Elevation Gradients and Soil Depths in the Mount Kenya East Forest. Land, 14(6), 1217. https://doi.org/10.3390/land14061217






