Effects of Biochar on Soil Organic Carbon Stability in Degraded Alpine Grasslands—A Study on Arid Regions in Central Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Classification of Grassland Degradation Degree
2.3. Basic Physicochemical Properties of Cotton Straw Biochar and Degraded Soil
2.4. Experimental Design
2.5. Sampling Methods and Measurement Indicators
2.6. Data Analysis
3. Results
3.1. Effects of Biochar Addition on Soil and Plant Organic Carbon in Degraded Grassland
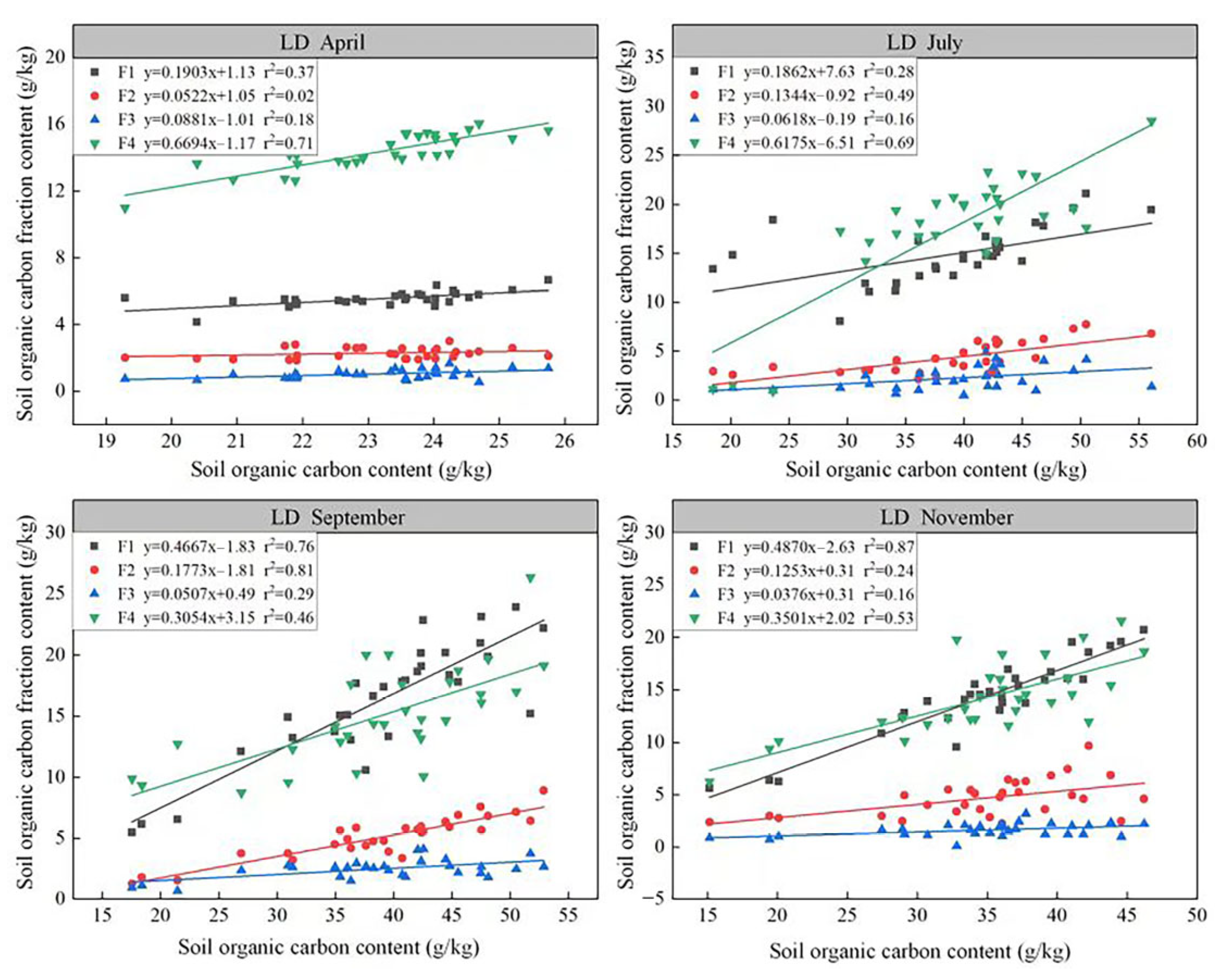
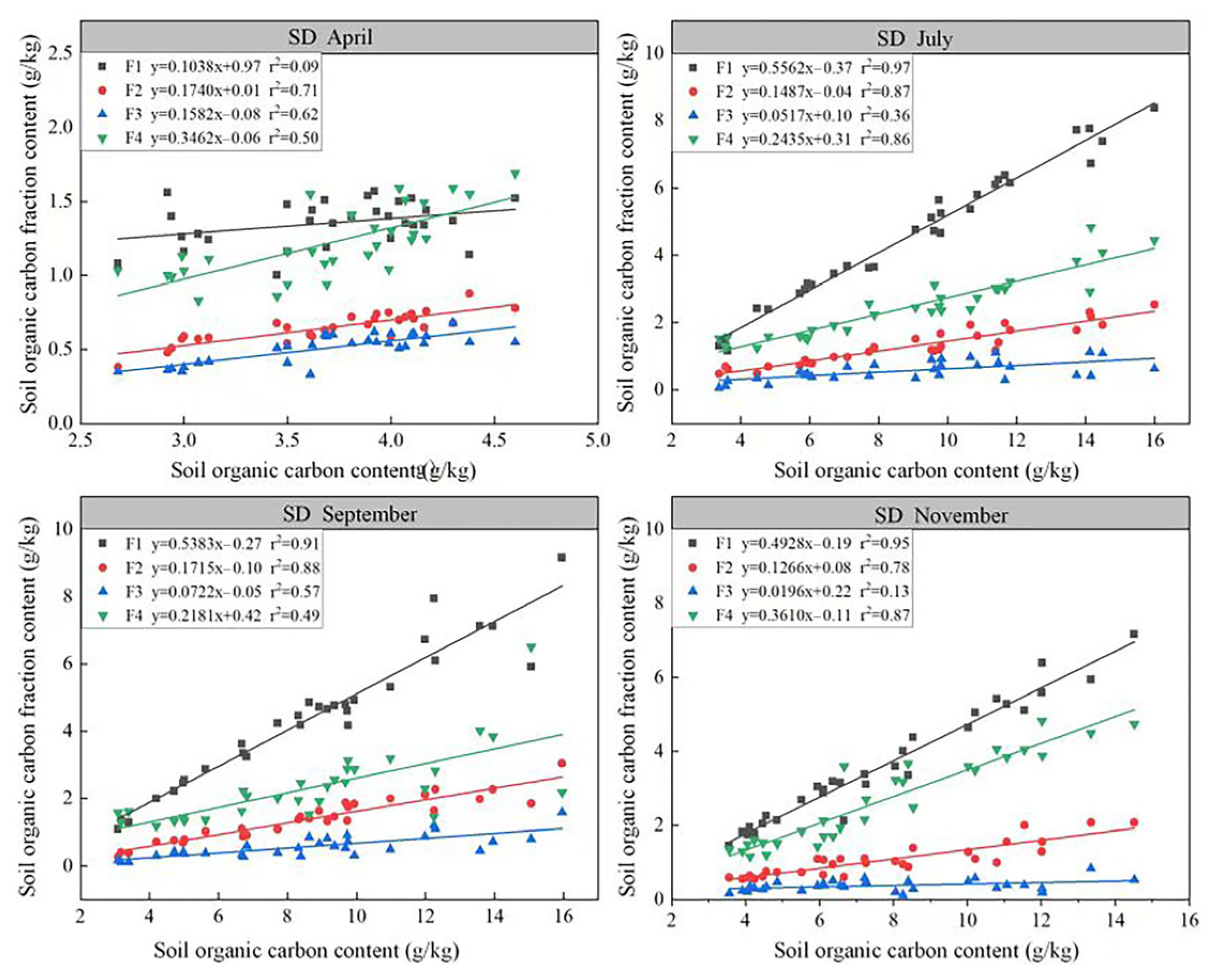
3.2. Effect of Biochar Addition on Soil Organic Carbon Fractions in Degraded Grasslands
3.3. Effects of Biochar Addition on the Stability of Soil Organic Carbon Pools in Degraded Grasslands
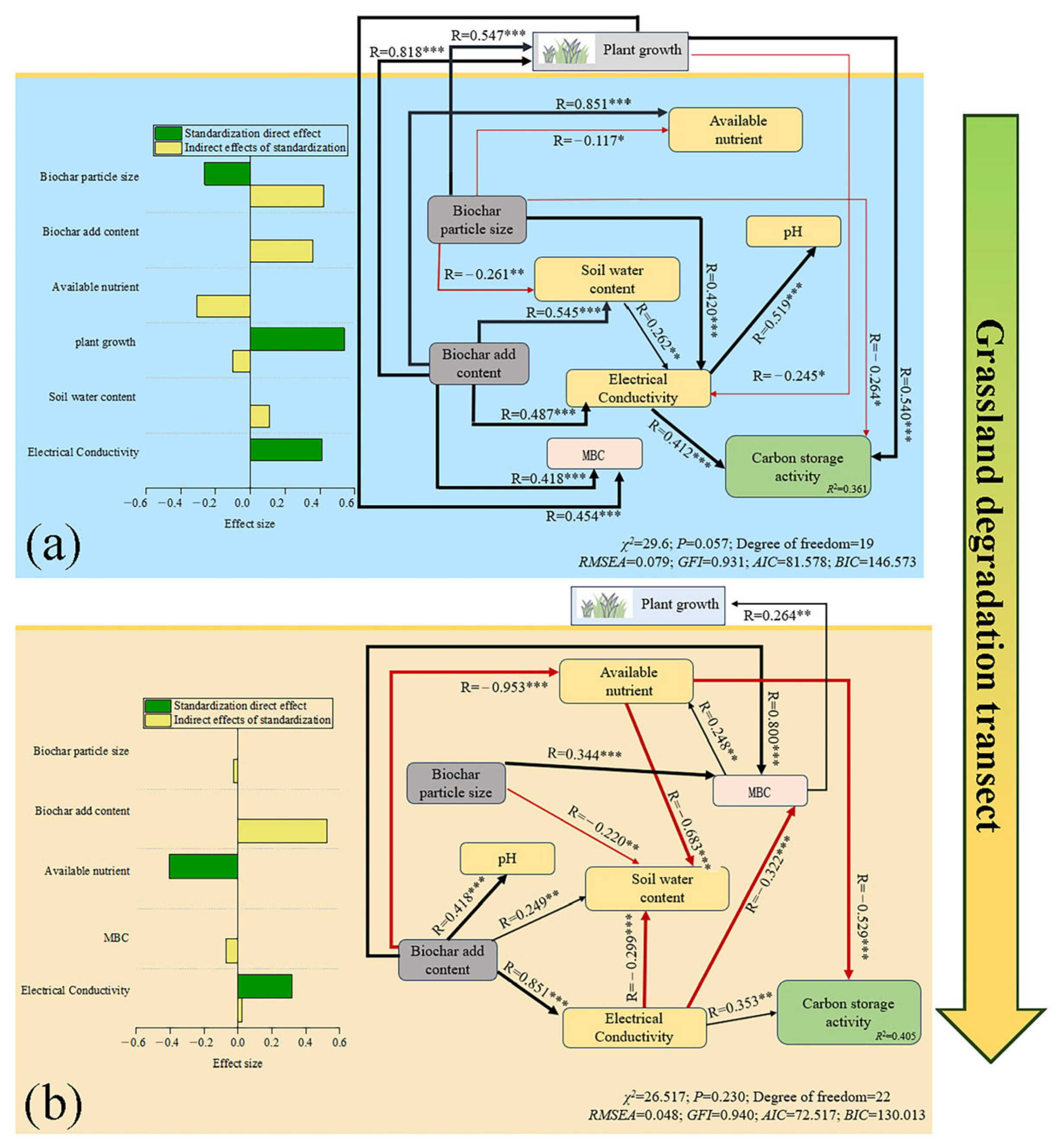
3.4. Relationship of Indicators to Changes in Carbon Pool Stability
4. Discussion
4.1. Direct Impact of Biochar on Soil Carbon Pools
4.2. The Indirect Effects of Biochar and Soil Environmental Feedback
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, L.H.X.; Zhang, Z.; Osbert, J. Grassland ecosystems in China: Review of current knowledge and research advancement. Phil. Trans. R. Soc. B 2007, 362, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, J.; Hall, D. The global carbon sink: A grassland perspective. Global Change Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Fang, J.; Geng, X.; Zhang, X.; Shen, H.; Hu, H. How many areas of grasslands are there in China. Chin. Sci. Bull. 2018, 63, 1731–1739. [Google Scholar] [CrossRef]
- Shen, H.; Zhu, Y.; Zhao, X.; Di, X.; Gao, S.; Fang, J. Analysis of current grassland resources in China. Chin. Sci. Bull. 2016, 61, 139–154. [Google Scholar]
- Bardgett, R.; Bullock, J.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Env. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhang, X.; Li, L.; Wang, X. Biochar application significantly increases soil organic carbon under conservation tillage: An 11-year field experiment. Biochar 2023, 5, 28. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Rong, X.; Zhou, X.; Fei, J.; Peng, J.; Luo, J. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality. Biochar 2024, 6, 3. [Google Scholar] [CrossRef]
- Qambrani, N.; Rahman, M.; Won, S.; Shim, A.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2008, 41, 210–219. [Google Scholar] [CrossRef]
- Nguyen, T.; Xu, C.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.; Bai, S. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; Nobili, M.; Lin, Q.; Devonshire, B.; Brookes, P. Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biol. Biochem. 2013, 57, 513–523. [Google Scholar] [CrossRef]
- Reed, E.; Chadwick, D.; Hill, P.; Jones, D. Critical comparison of the impact of biochar and wood ash on soil organic matter cycling and grassland productivity. Soil Biol. Biochem. 2017, 110, 134–142. [Google Scholar] [CrossRef]
- Han, F.; Ren, L.; Zhang, X. Effect of biochar on the soil nutrients about different grasslands in the Loess Plateau. Catena 2016, 137, 554–562. [Google Scholar] [CrossRef]
- Biederman, L.; Phelps, J.; Ross, B.; Polzin, M.; Harpole, W. Biochar and manure alter few aspects of prairie development: A field test. Agric. Ecosyst. Environ. 2017, 236, 78–87. [Google Scholar] [CrossRef]
- Gebhardt, M.; Fehmi, J.; Rasmussen, C.; Gallery, R. Soil amendments alter plant biomass and soil microbial activity in a semidesert grassland. Plant Soil 2017, 419, 53–70. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, Y.; Liu, Y.; Gong, Y.; Zhang, W.; Liu, W.; Ailiewutalipu, A. A study on soil biological properties of artificial grassland over different cultivation times in Bayanbulak. Acta Pratacult. Sin. 2010, 19, 218–226. [Google Scholar]
- Lu, J.; Hu, Y.; Yue, P.; Li, K. Assessment on the health of alpine steppe in Bayinbuluk based on CVOR index. Arid. Zone Res. 2017, 34, 862–869. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Hu, Y.; Abulaizi, M.; Tian, Y.; Chen, M.; Jia, H.; Zhou, J.; Kou, T.; Jia, Y. Effects of Short-term Biochar Addition of Cotton Straw on the Growth of Carex liparocarpos and Festuca arundinacea Schreb in Alpine Meadow. J. Agro Environ. Sci. 2024, 43, 1–12. Available online: http://kns.cnki.net/kcms/detail/12.1347.S.20240528.1251.002.html (accessed on 29 May 2024).
- Yu, P.; Li, Y.; Liu, S.; Ding, Z.; Zhang, A.; Tang, X. The quantity and stability of soil organic carbon following vegetation degradation in a salt-affected region of Northeastern China. Catena 2022, 211, 105984. [Google Scholar] [CrossRef]
- Lin, Q. Evaluation of the Methods for Measuring Soil Microbial Biomass. J. China Agric. Univ. 1997, (Suppl. S2), 1–11. Available online: https://kns.cnki.net/kcms2/article/abstract?v=Bo5Zm1RyAikijGtrdCuSY0-erNfikOitCsKG_pmTPd_idgnSCy3PCwKrvuIrfFRlRGTp46E7E1GUct4QBnpSwUohTQ0nn-sav73c4eygy4bVs1YN56WC6i2qQkRnWrMEGbFQE-pYob9C1I_dEF43N0E1D3VzvuL0Kw4oC7hMfH_Z-MXDbYTubDlaadOZlFa3&uniplatform=NZKPT&language=CHS (accessed on 30 April 2025).
- Hu, Y.; Yu, G.; Zhou, J.; Li, K.; Chen, M.; Abulaizi, M.; Cong, M.; Yang, Z.; Zhu, X.; Jia, H. Grazing and reclamation-induced microbiome alter ations drive organic carb on stability with in soil aggregates in alpine steppes. Catena 2023, 231, 107306. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Liang, F.; Liu, W.; Wang, Y.; Cao, W.; Song, H.; Chen, J.; Guo, J. Global integrative meta-analysis of the responses in soil organic carbon stock to biochar amendment. J. Environ. Manag. 2024, 351, 119745. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Bahar, M.; Sarkar, B.; Donne, S.; Ok, Y.; Palansooriya, K.; Kirikham, M.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Preiser, J.; Widyastuti, R.; Scheu, S.; Potapov, A. Plant roots fuel tropical soil animal communities. Ecol. Lett. 2023, 26, 742–753. [Google Scholar] [CrossRef]
- Kumar, S.; Nakajima, T.; Mbonimpa, E.; Gautam, S.; Somireddy, U.; Kadono, A.; Rafique, R.; Fausey, N. Long-term tillage and drainage influences on soil organic carbon dynamics, aggregate stability and corn yield. Soil Sci. Plant Nutr. 2014, 60, 108–118. [Google Scholar] [CrossRef]
- Meng, Y. Effects of Biochar on Soil Organic Carbon Pools in Phaeozem. Northeast. Agric. Univ. 2018, 40, 1340–1347. [Google Scholar]
- Zhao, S.; Yu, X.; Li, Z.; Yan, Y.; Zhang, X.; Wang, X.; Zhang, A. Effects of Biochar Pyrolyzed at Varying Temperatures on Soil Organic Carbon and Its Components: Influence on the Soil Active Organic Carbon. Environ. Sci. 2017, 38, 333–342. [Google Scholar] [CrossRef]
- Hua, L.; Jin, S.; Tang, Z. Effect of Bio-charcoal on Release of Carbon Dioxide in Soil. J. Anhui Agric. Sci. 2012, 40, 6501–6503, 6540. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Yang, Z.; Abulaizi, M.; Tian, Y.; Jia, H.; Kou, T.; Jia, Y. Effect of Snow Removal on Stability of Soil Organic Carbon Pool During Freeze-Thaw Period in Bayinbuluk Alpine Wetland. Bull. Soil Water Conserv. 2024, 44, 326–334. [Google Scholar] [CrossRef]
- Soong, J.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.; Janssens, I.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Global Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Yang, S.; Tang, F.; Yang, H.; Zhang, Y.; Peng, X.; Huang, Y. Effects of peach tree planting patterns on soil organic carbon fractions and carbon pool management index in southern Yunnan. Acta Ecol. Sin. 2023, 43, 14. [Google Scholar]
- Jeffery, S.; Voorde, T.; Harris, W.; Mommer, L.; Groenigen j Deyn, G.; Ekelund, F.; Briones, M.; Bezemer, T. Biochar application differentially affects soil micro-, meso-macro-fauna and plant productivity within a nature restoration grassland. Soil Biol. Biochem. 2022, 174, 108789. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2023, 909, 168627. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Ma, X.; Gong, L. Characteristics and Driving Factors of Soil Organic Carbon Fractions under Different Vegetation Types of the mid-Northern Piedmont of the Tianshan Mountains, Xinjiang. Ecol. Environ. Sci. 2022, 31, 1124–1131. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, R.; Sun, J.; Sun, Y.; Xu, G.; Wang, F. Microplastics change soil properties, plant performance, and bacterial communities in salt-affected soils. J. Hazard. Mater. 2024, 471, 134333. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Li, Z.; Wang, X.; Hou, N.; Li, D. Remediation of soda-saline-alkali soil through soil amendments: Microbially mediated carbon and nitrogen cycles and remediation mechanisms. Sci. Total Environ. 2024, 924, 171641. [Google Scholar] [CrossRef]
- Song, X.; Wang, P.; Zwieten, L.; Wang, H.; Li, X.; Cheng, K.; Yang, Y.; Wang, M.; Liu, T.; Li, F. Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation. Carbon Res. 2022, 1, 5. [Google Scholar] [CrossRef]
- Belenguer-Manzanedo, M.; Rochera, C.; Alcaraz, C.; Martínez-Eixarch, M.; Camacho, A. Disentangling drivers of soil organic carbon storage in deltaic rice paddies from the Ebro Delta. Catena 2023, 228, 107131. [Google Scholar] [CrossRef]
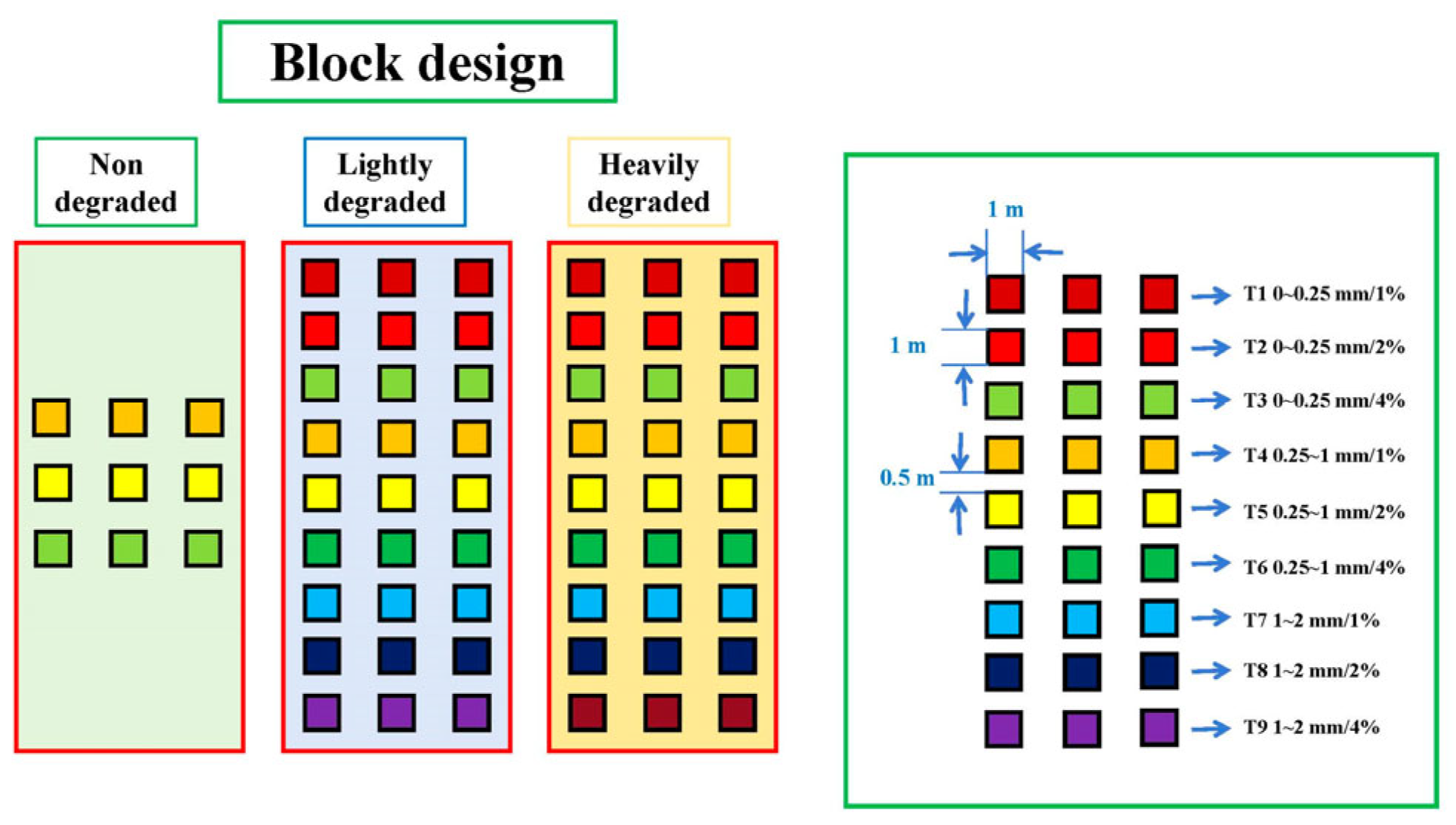

| Degradation Level | Dominant Species | Pioneer Species | Degradation Indicator Species | Soil Organic Carbon | Total Nitrogen | Soil Sand Content | Grassland Degradation Index |
|---|---|---|---|---|---|---|---|
| Relative Cover | Relative Cover | Relative Cover | |||||
| ND | 0.61 | 0.27 | 0.12 | 3.55 | 0.47 | 12 | 0.96 |
| LD | 0.44 | 0.35 | 0.21 | 2.3 | 0.31 | 34 | 0.68 |
| SD | 0 | 0 | 1 | 0.04 | 0.09 | 76 | 0.25 |
| pH | Electrical Conductivity (EC) | Soil Water Content (WC) | Soil Organic Carbon (SOC) | Total Nitrogen (TN) | Available Nitrogen (AN) | Available Phosphorus (AP) | Available Potassium (AK) | |
|---|---|---|---|---|---|---|---|---|
| (μS/cm) | (%) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||
| ND | 7.26 ± 0.18 b | 619.36 ± 8.15 a | 54.75 ± 0.72 a | 35.48 ± 1.70 a | 4.67 ± 0.21 a | 66.93 ± 0.77 a | 18.14 ± 1.21 b | 236.67 ± 12.34 a |
| LD | 7.48 ± 0.05 b | 513.87 ± 24.27 b | 30.85 ± 1.97 b | 23.42 ± 0.35 b | 3.13 ± 0.10 b | 41.99 ± 2.26 b | 21.87 ± 0.69 a | 190.93 ± 9.95 b |
| SD | 8.34 ± 0.09 a | 282.45 ± 8.46 c | 10.74 ± 0.53 c | 4.01 ± 0.08 c | 0.94 ± 0.03 c | 19.77 ± 1.28 c | 6.25 ± 1.13 c | 79.03 ± 8.10 c |
| Application Rate and Particle Size | 1% | 2% | 4% | 0 |
|---|---|---|---|---|
| 0~0.25 mm | T1 | T2 | T3 | CK |
| 0.25~1 mm | T4 | T5 | T6 | |
| 1~2 mm | T7 | T8 | T9 |
| Soil Organic Carbon Accumulation (kg/m2) | Above-Ground Plant Organic Carbon Accumulation (kg/m2) | Grassland Organic Carbon Stocks (kg/m2) | Carbon Stock Enhancement (kg/m2) | |||||
|---|---|---|---|---|---|---|---|---|
| LD | SD | LD | SD | LD | SD | LD | SD | |
| T1 | 4.04 | 1.38 | 0.06 | 0.01 | 4.10 | 1.24 | 1.86 | 0.78 |
| T2 | 4.14 | 1.23 | 0.07 | 0.01 | 4.20 | 1.56 | 1.96 | 0.64 |
| T3 | 3.99 | 1.55 | 0.06 | 0.01 | 4.05 | 0.73 | 1.81 | 0.95 |
| T4 | 3.5 | 0.72 | 0.06 | 0.01 | 3.56 | 1.01 | 1.32 | 0.12 |
| T5 | 4.2 | 1.00 | 0.07 | 0.01 | 4.27 | 1.8 | 2.03 | 0.40 |
| T6 | 3.43 | 1.79 | 0.07 | 0.01 | 3.51 | 0.69 | 1.27 | 1.19 |
| T7 | 3.52 | 0.69 | 0.08 | 0.01 | 3.60 | 0.77 | 1.36 | 0.09 |
| T8 | 3.54 | 0.76 | 0.07 | 0.01 | 3.61 | 0.88 | 1.37 | 0.16 |
| T9 | 3.37 | 0.87 | 0.09 | 0.08 | 3.46 | 2.57 | 1.22 | 0.28 |
| ND | 2.50 | 2.50 | 0.08 | <0.01 | 2.57 | 0.61 | 0.33 | 1.97 |
| TCK | 2.19 | 0.60 | 0.05 | 0.05 | 2.24 | 2.24 | ||
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | CK | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apr. | F1 | 5.43 ± 0.23 a | 5.23 ± 0.07 a | 5.61 ± 0.09 a | 5.45 ± 0.16 a | 5.61 ± 0.07 a | 5.64 ± 0.20 a | 5.74 ± 0.15 a | 5.38 ± 0.72 a | 5.84 ± 0.27 a | 5.41 ± 0.17 a |
| F2 | 2.05 ± 0.15 a | 2.23 ± 0.22 a | 2.19 ± 0.18 a | 2.5 ± 0.26 a | 2.38 ± 0.24 a | 2.32 ± 0.23 a | 2.28 ± 0.17 a | 2.24 ± 0.2 a | 2.11 ± 0.22 a | 2.4 ± 0.1 a | |
| F3 | 0.91 ± 0.15 a | 0.97 ± 0.11 a | 1.17 ± 0.16 a | 1.03 ± 0.33 a | 0.94 ± 0.23 a | 1.05 ± 0.09 a | 1.06 ± 0.21 a | 1.02 ± 0.21 a | 0.94 ± 0.07 a | 1.17 ± 0.11 a | |
| F4 | 14.85 ± 0.37 a | 14.29 ± 0.26 a | 14.85 ± 0.47 a | 14.63 ± 0.72 a | 14.12 ± 0.79 a | 13.91 ± 0.78 a | 13.94 ± 1.49 a | 14.3 ± 0.66 a | 14.03 ± 0.76 a | 14.43 ± 0.49 a | |
| Jul. | F1 | 13.52 ± 1.24 bc | 17.29 ± 0.65 ab | 18.92 ± 1.47 a | 14.7 ± 1 bc | 13.37 ± 1.45 bc | 16.68 ± 1.38 ab | 11.36 ± 1.68 c | 13.52 ± 1.19 bc | 13.75 ± 0.53 bc | 5.51 ± 0.46 d |
| F2 | 3.94 ± 0.52 bc | 5.56 ± 0.65 ab | 6.97 ± 0.55 a | 5.22 ± 0.53 b | 3.07 ± 0.56 c | 5.46 ± 0.86 ab | 2.61 ± 0.19 c | 2.82 ± 0.11 c | 4.36 ± 0.75 bc | 2.63 ± 0.16 c | |
| F3 | 1.52 ± 0.58 bc | 3.08 ± 1.07 abc | 3.21 ± 0.47 ab | 3.76 ± 0.59 a | 1.55 ± 0.47 bc | 2.13 ± 0.76 abc | 1.93 ± 0.41 abc | 1.81 ± 0.82 abc | 1.96 ± 0.08 abc | 1.1 ± 0.08 c | |
| F4 | 18.95 ± 1.42 abc | 19.34 ± 1.9 abc | 18.52 ± 0.57 bc | 16.54 ± 0.85 c | 15.97 ± 0.9 c | 23.06 ± 2.73 a | 18.51 ± 0.85 bc | 21.44 ± 1.14 ab | 21.28 ± 0.95 ab | 11.51 ± 1.38 d | |
| Sep. | F1 | 13.63 ± 0.86 d | 19.72 ± 1.23 abc | 20.2 ± 1.5 ab | 15.28 ± 1.37 cd | 13.03 ± 1.35 d | 19.57 ± 0.89 abc | 15.72 ± 1.74 bcd | 19.18 ± 2 abc | 20.49 ± 1.89 abc | 6.07 ± 0.31 e |
| F2 | 4.4 ± 0.35 b | 6.87 ± 1.03 a | 5.79 ± 0.13 ab | 5.58 ± 0.79 ab | 4.91 ± 0.79 ab | 6.76 ± 0.51 a | 4.15 ± 0.68 b | 4.6 ± 0.67 b | 6.12 ± 0.7 ab | 1.53 ± 0.16 c | |
| F3 | 2.52 ± 0.09 abc | 3.14 ± 0.46 ab | 3.38 ± 0.36 a | 1.82 ± 0.19 c | 2.93 ± 0.42 ab | 1.9 ± 0.11 c | 2.82 ± 0.14 ab | 2.38 ± 0.2 bc | 2.8 ± 0.24 ab | 0.9 ± 0.12 d | |
| F4 | 12.09 ± 1.7 de | 16.86 ± 1.65 bc | 11.19 ± 0.99 e | 16.41 ± 1.78 bcd | 22.11 ± 2.11 a | 17.3 ± 1.24 b | 12.2 ± 1.49 cde | 16.01 ± 0.92 bcd | 15.32 ± 0.84 bcde | 10.64 ± 1.05 e | |
| Nov. | F1 | 12.86 ± 1.86 b | 15.59 ± 2.6 ab | 16.9 ± 1.28 ab | 13.76 ± 1.51 ab | 18.38 ± 1.16 a | 15.63 ± 0.91 ab | 14.55 ± 0.52 ab | 15.01 ± 1.84 ab | 14.24 ± 0.76 ab | 6.07 ± 0.22 c |
| F2 | 4.27 ± 0.43 a | 5.46 ± 0.48 a | 5.51 ± 1.32 a | 3.4 ± 0.21 a | 4.53 ± 1.09 a | 5.96 ± 1.02 a | 4.19 ± 0.96 a | 5.4 ± 2.18 a | 5.21 ± 0.15 a | 2.7 ± 0.17 a | |
| F3 | 1.11 ± 0.56 bc | 2.5 ± 0.35 a | 1.97 ± 0.32 ab | 1.62 ± 0.23 abc | 1.58 ± 0.31 abc | 1.28 ± 0.13 bc | 1.47 ± 0.31 bc | 1.95 ± 0.13 ab | 1.78 ± 0.36 abc | 0.87 ± 0.08 c | |
| F4 | 18.63 ± 1.3 a | 15.18 ± 1.87 ab | 15.15 ± 0.69 ab | 14.92 ± 1.88 ab | 16.4 ± 2.62 ab | 13.13 ± 1.47 bc | 15.24 ± 1.79 ab | 12.5 ± 0.37 bc | 12.16 ± 1.16 bc | 8.58 ± 1.18 c |
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | CK | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apr. | F1 | 1.16 ± 0.1 a | 1.16 ± 0.12 a | 1.19 ± 0.19 a | 1.44 ± 0.05 a | 1.35 ± 0.01 a | 1.54 ± 0.02 a | 1.4 ± 0.15 a | 1.25 ± 0.14 a | 1.34 ± 0.11 a | 1.43 ± 0.09 a |
| F2 | 0.56 ± 0.0 de | 0.59 ± 0.05 cde | 0.49 ± 0.06 e | 0.63 ± 0.02 bcd | 0.68 ± 0.02 abc | 0.73 ± 0.03 ab | 0.78 ± 0.05 a | 0.62 ± 0.04 bcd | 0.65 ± 0.03 bcd | 0.74 ± 0.01 ab | |
| F3 | 0.43 ± 0.05 ab | 0.42 ± 0.05 b | 0.43 ± 0.08 ab | 0.49 ± 0.04 ab | 0.6 ± 0.05 a | 0.57 ± 0.02 ab | 0.54 ± 0 ab | 0.51 ± 0.05 ab | 0.51 ± 0.09 ab | 0.59 ± 0.02 ab | |
| F4 | 1.04 ± 0.11 ab | 0.96 ± 0.05 b | 0.99 ± 0.03 ab | 1.2 ± 0.16 ab | 1.4 ± 0.15 a | 1.36 ± 0.17 ab | 1.33 ± 0.15 ab | 1.33 ± 0.14 ab | 1.3 ± 0.14 ab | 1.26 ± 0.03 ab | |
| Jul. | F1 | 5.09 ± 0.35 b | 6.16 ± 0.41 ab | 6.86 ± 0.87 a | 3.37 ± 0.2 c | 5.33 ± 0.42 b | 7.3 ± 0.64 a | 3.21 ± 0.22 c | 2.65 ± 0.24 cd | 5.18 ± 0.79 b | 1.31 ± 0.09 d |
| F2 | 1.42 ± 0.13 bc | 2.03 ± 0.08 a | 1.76 ± 0.33 ab | 0.94 ± 0.03 cde | 1.38 ± 0.15 bcd | 2.08 ± 0.23 a | 0.89 ± 0.12 de | 0.65 ± 0.09 e | 1.29 ± 0.06 bcd | 0.59 ± 0.06 e | |
| F3 | 0.56 ± 0.11 abcd | 0.56 ± 0.21 abcd | 0.82 ± 0.2 ab | 0.49 ± 0.1 abcd | 0.92 ± 0.11 a | 0.8 ± 0.15 ab | 0.47 ± 0.04 bcd | 0.29 ± 0.07 cd | 0.66 ± 0.12 abc | 0.14 ± 0.06 d | |
| F4 | 2.77 ± 0.19 bc | 3.41 ± 0.73 ab | 3.03 ± 0.44 ab | 1.75 ± 0.1 cde | 2.71 ± 0.19 bc | 3.91 ± 0.37 a | 1.89 ± 0.34 cde | 1.53 ± 0.16 de | 2.57 ± 0.23 bcd | 1.47 ± 0.09 e | |
| Sep. | F1 | 4.73 ± 0.31 c | 5.19 ± 0.36 c | 7.94 ± 0.7 a | 3.2 ± 0.5 d | 4.52 ± 0.22 c | 6.78 ± 0.34 b | 3.14 ± 0.35 d | 2.48 ± 0.39 d | 4.7 ± 0.05 c | 1.25 ± 0.09 e |
| F2 | 1.46 ± 0.27 cde | 1.71 ± 0.16 abc | 2.27 ± 0.41 a | 1.08 ± 0.2 def | 1.65 ± 0.14 bcd | 2.17 ± 0.1 ab | 0.89 ± 0.12 efg | 0.8 ± 0.06 fg | 1.47 ± 0.08 cde | 0.35 ± 0.04 g | |
| F3 | 0.57 ± 0.13 bc | 0.73 ± 0.1 bc | 1.22 ± 0.21 a | 0.34 ± 0.03 cd | 0.52 ± 0.12 bc | 0.76 ± 0.19 b | 0.34 ± 0.04 cd | 0.44 ± 0.08 bcd | 0.72 ± 0.1 bc | 0.13 ± 0.02 d | |
| F4 | 2.52 ± 0.35 ab | 3.5 ± 1.53 a | 1.97 ± 0.26 ab | 1.71 ± 0.37 ab | 2.64 ± 0.36 ab | 3.56 ± 0.37 a | 1.76 ± 0.24 ab | 1.53 ± 0.27 b | 2.45 ± 0.28 ab | 1.49 ± 0.1 b | |
| Nov. | F1 | 4.43 ± 0.57 c | 4.6 ± 0.42 bc | 5.68 ± 0.39 ab | 2.32 ± 0.42 de | 2.94 ± 0.43 de | 6.07 ± 0.6 a | 2.37 ± 0.27 de | 2.67 ± 0.31 de | 3.13 ± 0.13 d | 1.67 ± 0.11 e |
| F2 | 1.24 ± 0.2 bc | 1.11 ± 0.14 bcd | 1.32 ± 0.13 b | 0.76 ± 0.17 de | 0.88 ± 0.14 cde | 2.05 ± 0.03 a | 0.69 ± 0.04 e | 0.81 ± 0.07 de | 1.09 ± 0.01 bcd | 0.59 ± 0.01 e | |
| F3 | 0.46 ± 0.03 ab | 0.24 ± 0.06 bc | 0.36 ± 0.12 abc | 0.32 ± 0.03 bc | 0.34 ± 0.08 bc | 0.59 ± 0.13 a | 0.38 ± 0.03 abc | 0.41 ± 0.08 abc | 0.44 ± 0.07 abc | 0.2 ± 0.02 c | |
| F4 | 3.7 ± 0.07 ab | 3.24 ± 0.46 b | 4.06 ± 0.4 a | 1.69 ± 0.12 c | 3.17 ± 0.26 b | 4.43 ± 0.21 a | 1.49 ± 0.31 c | 1.69 ± 0.1 c | 1.76 ± 0.21 c | 1.38 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Abulaizi, M.; Tian, Y.; Hu, Y.; Chen, M.; Li, K.; Jia, Y.; Kou, T.; Zhou, J.; Jia, H. Effects of Biochar on Soil Organic Carbon Stability in Degraded Alpine Grasslands—A Study on Arid Regions in Central Asia. Land 2025, 14, 1018. https://doi.org/10.3390/land14051018
Hu Y, Abulaizi M, Tian Y, Hu Y, Chen M, Li K, Jia Y, Kou T, Zhou J, Jia H. Effects of Biochar on Soil Organic Carbon Stability in Degraded Alpine Grasslands—A Study on Arid Regions in Central Asia. Land. 2025; 14(5):1018. https://doi.org/10.3390/land14051018
Chicago/Turabian StyleHu, Yunpeng, Maidinuer Abulaizi, Yuxin Tian, Yang Hu, Mo Chen, Keyi Li, Yuanbin Jia, Tianle Kou, Jianqin Zhou, and Hongtao Jia. 2025. "Effects of Biochar on Soil Organic Carbon Stability in Degraded Alpine Grasslands—A Study on Arid Regions in Central Asia" Land 14, no. 5: 1018. https://doi.org/10.3390/land14051018
APA StyleHu, Y., Abulaizi, M., Tian, Y., Hu, Y., Chen, M., Li, K., Jia, Y., Kou, T., Zhou, J., & Jia, H. (2025). Effects of Biochar on Soil Organic Carbon Stability in Degraded Alpine Grasslands—A Study on Arid Regions in Central Asia. Land, 14(5), 1018. https://doi.org/10.3390/land14051018






